Abstract
Cutaneous neurofibromas are the hallmarks of neurofibromatosis type 1 (NF1). They are composed of multiple cell types, and traditionally they are believed to arise from small nerve tributaries of the skin. A key finding in the context of this view has been that subpopulations of tumor Schwann cells harbor biallelic inactivation of the NF1 gene (NF1−/−). In the present study, our aim was to clarify further the pathogenesis of cutaneous neurofibromas. First, we detected cells expressing multipotency-associated biomarkers in cutaneous neurofibromas. Second, we developed a method for isolating and expanding multipotent neurofibroma-derived precursor cells (NFPs) from dissociated human cutaneous neurofibromas and used it to analyze their growth and differentiation potential. In analogy to solitary cells resident in neurofibromas, NFPs were found to express nestin and had the potential to differentiate to, at least, Schwann cells, neurons, epithelial cells, and adipocytes. Mutation analysis of the NFPs revealed that their genotype was NF1+/−. The results led us to speculate that the development of cutaneous neurofibromas includes the recruitment of multipotent NF1+/− precursor cells. These cells may be derived from the multipotent cells of the hair roots, which often are intimately associated with microscopic neurofibromas.
Neurofibromatosis type 1 (NF1) is caused by mutations in the NF1 gene that encodes the tumor suppressor protein neurofibromin, an inactivator of Ras. Clinical diagnosis of the disease is based on the presence of café-au-lait macules, axillary freckling, hamartomas of the iris (Lisch nodules), optic pathway gliomas, distinctive osseous lesions such as sphenoid wing dysplasia or pseudarthrosis, and neurofibromas.1 Neurofibromas can be classified according to their anatomical location: cutaneous, subcutaneous, intraneural, and plexiform.2 Plexiform neurofibromas are congenital tumor masses involving nerve trunks and often extend to the skin, whereas cutaneous neurofibromas are not detectable at birth and usually appear during adolescence.
Histologically, neurofibromas are mixed tumors consisting of cells with divergent differentiation characteristics. The use of traditional histological stains, as well as immunohistochemistry with a variety of biomarkers and electron microscopy, has been taken as proof for the involvement of Schwann cells, perineurial cells, and fibroblasts.3–5 Neurofibromas also contain numerous mast cells and axonal processes, all of which are embedded in an abundant collagenous extracellular matrix.4,6 Unlike plexiform neurofibromas, which carry a risk for malignant transformation and may form tumor masses of several kilograms, cutaneous neurofibromas invariably retain their benign phenotype. Their neoplastic cells never undergo malignant transformation and the tumor diameter usually varies from millimeters to 2 cm, rarely exceeding 3 cm.
In light of previous reports and the current study, a feasible explanation for neurofibroma development involves a biallelic inactivation of the NF1 gene.7,8 This inactivation has been detected in cultured cells displaying characteristics typical of Schwann cells, including a bipolar morphology and the expression of S100 protein. Previous studies have observed diploinsufficiency in 29 of 29 cutaneous neurofibromas in which two separate mutations were found in 26 of 29 tumors and loss of heterozygosity was found in 3 of 297 It should be noted that each of the second mutations was unique. This finding indicates that the NF1−/− cell population is clonal and suggests that the NF1−/− genotype provides these cells with a growth advantage.
Using a bioengineered mouse model, Le et al9 have shown that the implantation of skin-derived precursor cells with an Nf1−/− genotype into mice with an Nf1+/− background results in the development of neurofibromas. However, recent studies have characterized skin-derived precursor cells which potentially reside in hair roots of mice and have the potential to generate subpopulations of cells expressing neuronal, glial, smooth muscle, and adipocyte markers.10–12 Earlier mouse studies demonstrated that Nf1−/− Schwann cells are necessary, but not sufficient, for tumor formation to occur. It thus emerges that tumor progression requires complex interactions between Nf1−/− Schwann cells and Nf1+/− cell lineages in the tumor microenvironment.13 In this context, a recent study has shown that mouse Nf1+/− mast cells not only are recruited to neurofibromas from bone marrow but also that the neurofibromas fail to develop if the recruitment of the Nf1+/− cells is hindered, and the tumors begin to shrink when they are depleted of the Nf1+/− mast cells.14
The present study points to the possible existence of yet another category of players in the development of cutaneous neurofibromas: multipotent NF1+/−precursor cells that can give rise to some of the different cell types found in neurofibromas. Our previous histological analyses of apparently normal skin from patients with NF1 revealed minute neurofibromas, presumably in the early stages of development, in the immediate vicinity of the hair follicular apparatus15 and the finding that hair follicles, together with their adjacent tissue, have been shown to serve as reservoirs of multipotent progenitor cell populations16 led us to study the potential association of hair root with cutaneous neurofibromas. This concept is further supported by the clinical observation that cutaneous neurofibromas are found only rarely in densely innervated areas devoid of hair, such as the fingertips and lips, whereas less densely innervated areas, such as the skin of the back, frequently host numerous tumors.
Materials and Methods
Patients and Neurofibroma Tissue
Using a CO2 laser, we removed 50 cutaneous neurofibromas from 10 patients who had a diagnosis of NF1.1 Patients had given informed consent and we had received approval from the local ethical committee. None of the patients had a clinical phenotype correlating to the microdeletion of the NF1 gene (large number of neurofibromas, facial dysmorphism, and low IQ).17 Paraffin-embedded neurofibromas were obtained from the Department of Pathology, Turku University Hospital.
Three-Dimensional Structure of Cutaneous Neurofibromas
We cut six paraffin-embedded cutaneous neurofibromas from three separate patients into 5-μm sections and stained them with hematoxylin and van Gieson stains. For detection of mast cells, the sections were stained with toluidine blue. The sections were imaged with an Olympus BX51 virtual microscope (Olympus, Tokyo, Japan) equipped with an Olympus U-CMAD3 camera and dotSlide2.1 software (Olympus). The pictures from sequential sections were adjusted and rotated using Adobe Photoshop Version 8.0, and the three-dimensional models were created using 3D-DOCTOR software (Able Software Corp., Lexington, MA).
Culturing of Neurofibroma-Derived Precursor Cells
The cell cultures were initiated as previously described.7,8,18 The skin covering the neurofibroma was removed and the tumor tissue was cut into small pieces and put into preincubation medium containing Dulbecco's modified Eagle's medium (GIBCO, Grand Island, NY) with 10% fetal calf serum (Invitrogen, Eugene, OR), 2 μmol/L forskolin (Sigma-Aldrich, St. Louis, MO), and antibiotics for 5 to 10 days. The tumors were then enzymatically dissociated and the cells suspended.18 The cell suspension was cultured overnight in 25-cm2 culture flasks in proliferation medium with forskolin.8 The medium was then collected and the unattached cells spun down. The cells were resuspended in medium favoring stem cell growth.19 This stem cell growth medium consisted of Dulbecco's modified Eagle's medium (GIBCO) and F12 (GIBCO) media in 1:1 ratio, containing B27 supplement, 2 mmol/L L-glutamine, 10 ng/ml basic fibroblast growth factor, 20 ng/ml human recombinant epidermal growth factor (all from Invitrogen), 15 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (Sigma-Aldrich), and antibiotics. Every 3 to 4 days, 50% of the medium was centrifuged and the cells were resuspended in fresh medium. After 1 to 2 weeks, the cells started to form cell spheres. Once the latter had attached to the culture surface, the cells comprising the spheres began to divide rapidly, their number increasing dramatically within 1 to 2 days, forming sheets. The cells in these sheets were characterized by using indirect immunolabeling and Western blot analysis for different stem cell markers, as described previously.20
For the NF1 mutation analysis and further differentiation of the cells, single floating cells (or cell spheres) were captured with a micropipette under inverted light microscope and seeded onto 10-cm2 culture plates containing previously defined stem cell growth medium.
To induce differentiation of the cells into Schwann cells, the medium was replaced with the Schwann cell proliferation medium.8 For adipogenesis, cells were incubated in stem cell growth medium supplemented with 1.7 μmol/L insulin (Sigma-Aldrich), 0.5 mmol/L 1-Methyl-3-isobutylxanthine (Sigma-Aldrich), 100 μmol/L indomethacin (Sigma-Aldrich), and 1 μmol/L dexamethasone (Sigma-Aldrich). After 8 days, the cells were fixed with 10% formalin and stained with Oil Red O stain (Cambrex Bio Science, Walkersville, MD).
Antibodies
Neurofibroma tissue sections were labeled and cultured cells characterized by indirect immunolabeling with a panel of commercial antibodies. The following preparations were used: mouse monoclonal antibodies to cow S100b protein (ab11179; Abcam, Cambridge, UK), human collagen type IV (C1926; Sigma-Aldrich), human nestin (ab22035; Abcam), human integrin alpha 4 (ab220; Abcam), cytokeratin 14 (C8791; Sigma-Aldrich), and rat class III β-tubulin, (MMS-435P; Covance, Princeton, NJ); rabbit polyclonal antibodies to cow S100 protein (18–0046; Chemicon International, Temecula, CA), human nestin (ab5968; Abcam), human neurofibromin (sc-67; Santa Cruz Biotechnology, Santa Cruz, CA), and human fibronectin (A245; Dako, Glostrup, Denmark). Mouse antibodies to CD9, E-cadherin, and stage-specific embryonic antigen 1 (SSEA1) were from Human Embryonic Stem Cell Marker Antibody Panel Plus (sc009; R&D systems, Minneapolis, MN).
Secondary antibodies were Alexa Fluor 488 conjugated goat anti-mouse IgG (A11029) and Alexa Fluor 568 conjugated goat anti-rabbit IgG (A11011) (both from Molecular Probes, Eugene, OR). Hoechst nucleic acid stain (H3570; Invitrogen) was used to visualize nuclei.
Immunolabeling of the Cultured Cells and Neurofibromas
Immunolabeling of cell cultures and neurofibroma tissues was performed as previously described.20 The cells were photographed with Leica DMRB fluorescence microscope (Leica, Wetzlar, Germany). Confocal laser scanning microscopy was carried out using Zeiss LSM 510 META confocal microscope (Zeiss, Jena, Germany) and LSM 3.0 software (Zeiss).
Reverse Transcription–Polymerase Chain Reaction
Total RNA was isolated from frozen neurofibroma tissue and cultured NFPs using RNeasy Mini Kit (74104; Qiagen, Venlo, The Netherlands) according to the protocol provided by the manufacturer. Total RNA was transcribed into single-stranded DNA in a 20 μL reaction using Phusion RT-PCR kit according to the protocol provided by the manufacturer (F-546L; Finnzymes, Espoo, Finland). cDNA was used as a template for PCR in a 20 μL reaction containing 20 pmol of primers specific for different OCT4 isoforms,21 0.2 mmol/L of each of the four deoxynucleotides, and 0.4 U of Phusion Hot Start DNA Polymerase (Finnzymes). Amplification was performed in 35 cycles of denaturation (20 seconds at 98°C), annealing (20 seconds at 64°C), and extension (30 seconds at 72°C). In the negative control samples, the template was omitted. The PCR products were analyzed electrophoretically on 1.5% agarose gels and stained with ethidium bromide.
Mutation Analysis
Mutation analysis of the NF1 gene was performed as described.22,23 Two parallel cell cultures per tumor were established: one for RNA isolation and one for DNA isolation. Cultures subjected to RNA isolation were treated with 200 μg/ml of puromycin (Sigma-Aldrich) for 4 hours before isolation, to circumvent the nonsense-mediated RNA decay. The entire coding region was amplified in three overlapping cDNA fragments of ∼4kb, which were sequenced using BigDye Terminator Cycle Sequencing Kit (4337456 v3.1; Applied Biosystems, Foster City, CA). Multiplex ligation-dependent probe amplification analysis was performed using the P081 and P082 assay (MRC-Holland, Amsterdam, The Netherlands) to search for any copy-number changes that might have escaped detection by direct sequencing at the cDNA level.24 All alterations found at the cDNA level were confirmed at the gDNA level. To verify the presence of the second hit mutation, Schwann cells derived from the nine tumors from three patients cultured under conditions favoring NF1−/− genotype8 were subjected to mutation screening.
Results
Association of Neurofibromas with Hair Follicles
We first approached the spatial relationship of neurofibromas and hair by creating three-dimensional histology models of six typical cutaneous neurofibromas with a diameter of ∼10 mm. All six tumors, as well as ten other less stringently analyzed tumors, were found to contain elements of the follicular apparatus, including the hair and its follicle, erector pili muscle, and sebaceous gland, although these structures often were separated from each other by typical neurofibroma tissue (Figure 1A).
Figure 1.
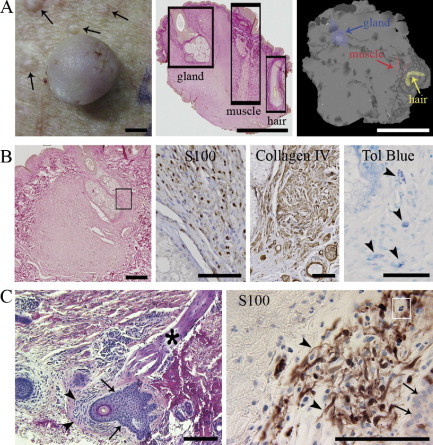
Association of hair follicles with neurofibromas at different stages. A: Cutaneous neurofibroma (diameter ∼7 mm) and numerous small, yet visible, tumor growths (left, arrows); all neurofibromas analyzed contained elements of a hair follicle (middle); three-dimensional model of a neurofibroma (right). Scale bars = 2 mm. B: Microscopic neurofibroma ensheathing a hair root (left). The rectangle depicts the approximate area further immunolabled for S100 and collagen IV; mast cells (arrowheads) are visualized by toluidine blue staining (right). Scale bars: 250 μm (left); 100 μm (S100, Collagen IV, and Tol Blue). C: Minute S100-positive neurofibroma intimately associated with hair follicle in apparently healthy looking skin of a patient with NF1. Tumor mass (arrowheads), follicular epithelium (arrows), erector pili muscle (asterisk), and mast cell (white rectangle) are shown. Scale bars = 200 μm.
A minute, clinically undetectable cutaneous neurofibroma is shown in Figure 1B. The tumor is in close contact with the hair follicle apparatus, displays positivity for S100 and collagen IV, and contains numerous mast cells. All this is consistent with a typical cutaneous neurofibroma. Figure 1C shows an even smaller tumor growth in an apparently healthy area of skin of a patient with NF1. The minute tumor mass displays histological and immunohistological characteristics, including the presence of mast cells, again consistent with those of a neurofibroma. The tumor mass is intimately associated with the follicular epithelium. The consistent occurrence of the minute neurofibromas in association with hair follicles suggests that their spatial proximity is not a coincidence.
Biomarkers for Multipotent Precursors Are Expressed by a Subpopulation of Neurofibroma Cells
Nestin, a marker for neural stem cells, is expressed by cells that are present in the hair follicles and can differentiate into both Schwann cells and blood vessels.25,26 Collectively, our results show that neurofibromas host nestin-positive small blood vessels and isolated, nestin-positive spindle-shaped tumor cells (Figure 2C). To further study whether multipotent cells were present in cutaneous neurofibromas, we analyzed multipotency and cell differentiation markers alpha 4 integrin, E-cadherin, CD9, and SSEA1. All four markers were detected in a number of cells distributed throughout the diffuse tumor tissue and, in some cases, in close proximity to remnants of hair follicles (Figure 2, A–F). In addition, OCT4B isoform was detected using isoform specific RT-PCR (not shown).21 However, the isoform A, specific for embryonic stem cells, was not detectable. These results indicate that neurofibromas contain multipotent cells that may contribute to the tumor formation.
Figure 2.
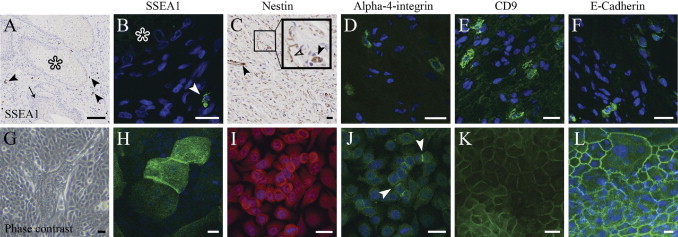
Expression of SSEA1, nestin, alpha 4 integrin, CD9, and E-cadherin in neurofibroma tissue and cultured NFPs. A: Low magnification of an avidin-biotin immunolabeling shows glandular epithelium (asterisk) surrounded by neurofibroma tissue. Arrow points to the follicular epithelium within neurofibroma tissue. Arrowheads point to the SSEA1-positive cells. B: Indirect immunofluorescence labeling for SSEA1 of the same area as in A. Glandular epithelium is marked with an asterisk, and the arrowhead points to the SSEA1-positive cell. C: Small blood vessels (closed arrowheads) and a subpopulation of spindle-shaped tumor cells (open arrowhead) are positive for nestin in cutaneous neurofibroma. D: Alpha 4 integrin–positive cells shown within neurofibroma tissue. E: CD9 is expressed by a subpopulation of neurofibroma cells. F: Immunoreaction for E-cadherin. G: Phase contrast image of NFPs maintained in stem cell medium. H–L: NFPs express SSEA1, nestin, alpha 4 integrin, CD9, and E-cadherin. Arrowheads point to the positive immunoreaction for alpha 4 integrin. Collectively, the results attest to the low level of differentiation of these cells. For all fluorescence images, cell nuclei are visualized with Hoechst nuclear stain (blue). Scale bars = 100 μm (A); 20 μm (B–L).
Multipotent NFPs in Culture
To determine whether neurofibromas can be a source of cultures of multipotent cells, we developed a method for isolating and expanding multipotent NFPs from dissociated human cutaneous neurofibromas and used it to analyze their growth and differentiation potential. The cells were first cultured under conditions described to favor stem cell proliferation,19 and NFPs were found to express nestin, alpha 4 integrin, SSEA1, CD9, and E-cadherin (Figure 2, H–L), all of which have been shown to be expressed by multipotent cells at different stages of their cellular differentiation.27,28 In addition, the presence of nestin protein was demonstrated by Western transfer analysis (not shown). Although a subpopulation of cells in neurofibromas and NFPs express similar multipotency markers, these results do not provide direct evidence that the tumor cells and cultured cells are identical or related.
A characteristic feature of all NFP cultures described here was that a single cell, or an isolated sphere of NFPs consisting of ∼20 cells, gave rise to a colony occupying several square centimeters (see Supplemental Figure S1 at http://ajp.amjpathol.org.). The estimated population doubling time was 19 hours, as estimated from the increase of growth area and by a time-lapse video recording with 10-minute exposure intervals (see Supplemental Videos S1 and S2 at http://ajp.amjpathol.org.). The latter showed that, while dividing, the cells lose contact both with the adjacent cells and with the substratum, assuming a spherical morphology, and then rapidly undergo division. The daughter cells then reintegrate into the monolayer, attach to the substratum, and regain their original shape and cell contacts. It is noteworthy that the growth of the cells is not restricted to the horizontal surface of the culture dish, as is usually observed in cell culture set-ups but also frequently observed on the vertical surfaces, up to the level of the culture medium.
Differentiation Potential of NFPs
To further evaluate the differentiation potential of NFPs, the cultured cells were analyzed using several differentiation biomarkers. Despite the absence of known Schwann cell growth factors or serum,8,18 a number of the cells produced by the NFPs cultured in stem cell medium19 exhibited a Schwann cell–like phenotype in that they were bipolar and S100 positive (Figure 3, A–B). This highlights two important issues: that axonal contact is not a prerequisite for Schwann cell differentiation and, secondly, that heterozygous mutation in the NF1 gene may favor what appears to be a spontaneous differentiation of progenitor cells toward the Schwann cell lineage. In addition to Schwann cells, some NFP cultures also featured the appearance of cytokeratin-positive epithelial cells and class III β-tubulin–positive neurons (Figure 3, D and G). Furthermore, when cultured in media favoring differentiation toward the adipocyte or Schwann cell lineages, the NFPs yielded the corresponding phenotypes (Figure 3, C and F). The neuronal differentiation occurred spontaneously in NFP cultures in stem cell growth medium. (Figure 3, G and H).
Figure 3.
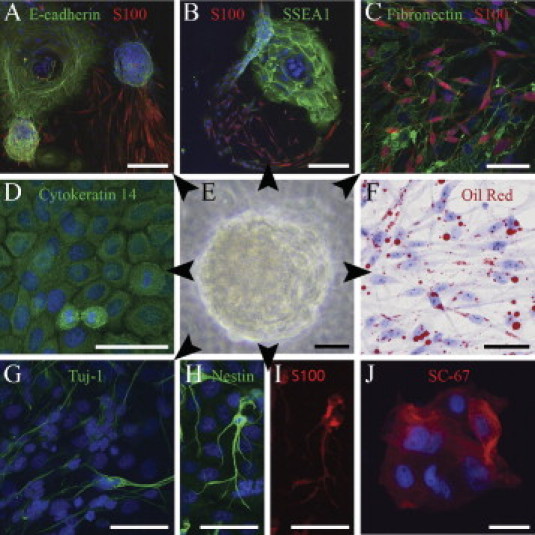
Multipotency of an NFP sphere (E) and its differentiation to mesenchymal, neural, and epithelial cells (arrowheads). A–B: Sprouting of spindle-shaped, S100-positive Schwann cells from E-cadherin–positive and SSEA1-positive NFPs. C: S100 and fibronectin labeling attest to Schwann cell and mesenchymal differentiation of NFPs. D: Cytokeratin 14 indicates epithelial differentiation. F: Differentiation to adipocytes detected with Oil Red O stain. G-I: Class III β-tubulin, nestin, and S100 indicate neuronal differentiation. J: Positive immunoreaction for neurofibromin is in line with the mutation analysis indicating the NF1+/− genotype of these cells. Schwann cells and adipocytes (C and F) were maintained in their respective differentiation mediums, whereas all other cultures were maintained in the stem cell medium. Cell nuclei are visualized with Hoechst nuclear stain. Scale bars = 200 μm (A and B); 50 μm (C–J).
NF1 Mutation Analysis
A comprehensive NF1 mutation analysis, carried out as described by Messiaen et al,22,23 was performed on NFP cultures established from three different patients with NF1, including one 75-year-old male (patient A) and two females aged 37 and 35 years (patients B and C, respectively). Two NFP cultures from each patient were used for mutation analysis: one culture was used for RNA isolation and the other for DNA isolation. Both NFP cultures derived from patient A revealed recurrent previously reported splice mutation c.1466A>G; [r.1466_1527del], but no second hit mutation was found. NFP cultures derived from patient B showed the presence of a nonsense mutation: c.3868A>T; p.Lys1290X, but again no second hit mutation was found. NFP cultures derived from patient C presented a frameshift mutation: c.232_244del. Again, no second hit mutation was observed. NFPs also expressed neurofibromin (Figure 3J), further attesting to the presence of a healthy NF1 allele in these cells. The results thus showed that the NFP culture protocol yields purely haploinsufficient cell populations.
For comparison, we analyzed NF1 mutations of Schwann cells cultured from nine tumors from three patients using conditions as previously described.7,8 In each case, NF1 diploinsufficiency was found in the cells cultured without forskolin. The cause of the diploinsufficiency was a unique somatic mutation in 7 of 9 cases and loss of heterozygosity in 2 of 9 cases. It should be noted also that a tumor from patient A served as a source for both NFPs, and under different culture conditions for Schwann cells.7,8 In the latter cells, a second hit in the NF1 gene was readily detectable (c.4084C>T; p.Arg1362X).
Discussion
Because most studies on neurofibroma tumorigenesis have been carried out in mice and the results on mice may not always be directly applicable to humans, the present study concentrated on human cutaneous neurofibromas. The use of human samples, however, excludes several experimental approaches (eg, lineage tracing, prospective isolation of tumor stem cells).
The merits of the current study include the successful isolation and in vitro enrichment of multipotent NF1+/− cells with the ability to differentiate into several cell types found in human cutaneous neurofibromas. Although it has been demonstrated previously that neurofibromas contain cells with both NF1+/− and NF1−/− genotypes,7,8 it was not unexpected to identify only one genotype in each of the NFP cultures analyzed, because they originated from one NFP sphere having a very limited number of cells. The current culture conditions thus favored multipotent NFPs, which are heterozygous for an NF1 mutation. We also showed that it was possible to enrich NF1−/− Schwann cells from the very same tissue.
Furthermore, our data show that NFPs with NF1+/− genotype have a tendency to undergo spontaneous differentiation into cells with Schwann cell phenotype. This leads us to speculate that even the biallelically mutated Schwann cells within the neurofibromas may have arisen by spontaneous differentiation from multipotent precursor cells that have lost their healthy NF1 allele.
Our study shows that neurofibromas contain multipotent NF1+/− precursor cells. Indirect evidence leads us to speculate that the hair follicle apparatus, including nerve tributaries, is a potential source of these cells. We also suggest that the recruitment of multipotent NF1+/− precursor cells may be an important factor in the development of human cutaneous neurofibromas.
Footnotes
Supported by grants from the Academy of Finland, Turku University Foundation, Turku Graduate School of Biomedical Sciences, and Southwest Finland Hospital District.
Supplemental material for this article can be found on http://ajp.amjpathol.org or at doi:10.1016/j.ajpath.2010.10.041.
Supplementary data
Supplemental Figure S1.
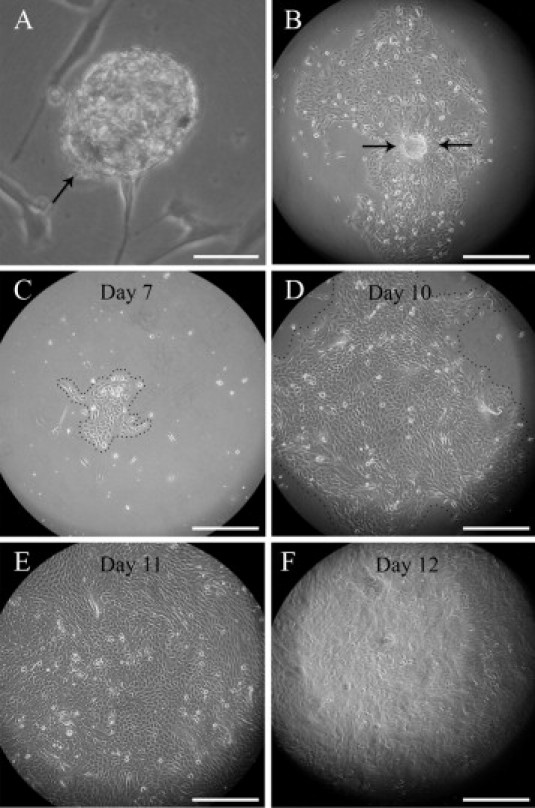
Proliferation of neurofibroma-derived cells in stem cell medium. A: A sphere of NFPs (arrow) anchored to the bottom of the culture disk. B: A single sphere has initiated the fast spreading cell sheet. C–F: A 7- to 12-day time series illustrates the fast proliferation rate of progenitor cells in a growing cell sheet. At day 7 (C), the cells are still confined to a limited area, but by day 10 they fill almost the entire optical field (D), clearly exceeding it by day 11 (E). On day 12 (F), the culture displays a marked difference of appearance as it becomes multilayered. See also Supplemental Videos S1 and S2. Scale bars: 50 μm (A); 300 μm (others).
NFP spheres moving on a cell layer and seeding new cells into the layer. Three spheres are followed with arrows. The recording time is 4 hours with 5-minute intervals between frames. The video has been generated using Olympus IX71 microscope equipped with Cell^R 2.6 software.
Growth of multipotent NFPs. Note the “popping up” of small spherical cells on the top of the cell layer and their subsequent division, which in turn is followed by merging of the cells back to the cell sheet. Also note that one of the dividing cells is followed with an arrow, which shows that during the 17-hour recording, the cell enters to the mitosis second time and attests to the short generation time of these cells. The video has been generated using Olympus IX71 microscope equipped with Cell^R 2.6 software.
References
- 1.Stumpf D., Alksne J., Annegers J., Brown S., Conneally P., Housman D., Leppert M., Miller J., Moss M., Pileggi A., Rapin I., Strohman R., Swanson L., Zimmerman A. Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45:575–578. [PubMed] [Google Scholar]
- 2.Evans D., Komminoth P., Scheihauer B., Peltonen J. Neurofibromatosis type 1. Pathology and Genetics of Tumours of Endocrine Organs: World Health Organization Classification of Tumours. In: DeLellis R., Lioyd R., Heitz P., Eng C., editors. IARC Press; Lyon, France: 2004. pp. 243–248. [Google Scholar]
- 3.Lassmann H., Jurecka W., Lassmann G., Gebhart W., Matras H., Watzek G. Different types of benign nerve sheath tumors: Light microscopy, electron microscopy and autoradiography. Virchows Arch A Pathol Anat Histopathol. 1977;375:197–210. doi: 10.1007/BF01102988. [DOI] [PubMed] [Google Scholar]
- 4.Peltonen J., Jaakkola S., Lebwohl M., Renvall S., Risteli L., Virtanen I., Uitto J. Cellular differentiation and expression of matrix genes in type 1 neurofibromatosis. Lab Invest. 1988;59:760–771. [PubMed] [Google Scholar]
- 5.Pummi K., Aho H., Laato M., Peltonen J., Peltonen S. Tight junction proteins and perineurial cells in neurofibromas. J Histochem Cytochem. 2006;54:53–61. doi: 10.1369/jhc.5A6671.2005. [DOI] [PubMed] [Google Scholar]
- 6.Jaakkola S., Peltonen J., Riccardi V., Chu M., Uitto J. Type 1 neurofibromatosis: selective expression of extracellular matrix genes by Schwann cells, perineurial cells, and fibroblasts in mixed cultures. J Clin Invest. 1989;84:253–261. doi: 10.1172/JCI114148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maertens O., Brems H., Vandesompele J., De Raedt T., Heyns I., Rosenbaum T., De Schepper S., De Paepe A., Mortier G., Janssens S., Speleman F., Legius E., Messiaen L. Comprehensive NF1 screening on cultured Schwann cells from neurofibromas. Hum Mutat. 2006;27:1030–1040. doi: 10.1002/humu.20389. [DOI] [PubMed] [Google Scholar]
- 8.Serra E., Rosenbaum T., Winner U., Aledo R., Ars E., Estivill X., Lenard H., Lázaro C. Schwann cells harbor the somatic NF1 mutation in neurofibromas: evidence of two different Schwann cell subpopulations. Hum Mol Genet. 2000;9:3055–3064. doi: 10.1093/hmg/9.20.3055. [DOI] [PubMed] [Google Scholar]
- 9.Le L., Shipman T., Burns D., Parada L. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes K., McKenzie I., Mill P., Smith K., Akhavan M., Barnabé-Heider F., Biernaskie J., Junek A., Kobayashi N., Toma J., Kaplan D., Labosky P., Rafuse V., Hui C., Miller F. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 11.Fernandes K., Toma J., Miller F. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci. 2008;363:185–198. doi: 10.1098/rstb.2006.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toma J., McKenzie I., Bagli D., Miller F. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y., Ghosh P., Charnay P., Burns D., Parada L. Neurofibromas in NF1: schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang F., Ingram D., Chen S., Zhu Y., Yuan J., Li X., Yang X., Knowles S., Horn W., Li Y., Zhang S., Yang Y., Vakili S., Yu M., Burns D., Robertson K., Hutchins G., Parada L., Clapp D. Nf1-dependent tumors require a microenvironment containing Nf1+/– and c-kit-dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karvonen S., Kallioinen M., YlÄ-Outinen H., Pöyhönen M., Oikarinen A., Peltonen J. Occult neurofibroma and increased S100 protein in the skin of patients with neurofibromatosis type 1: new insight to the etiopathomechanism of neurofibromas. Arch Dermatol. 2000;136:1207–1209. doi: 10.1001/archderm.136.10.1207. [DOI] [PubMed] [Google Scholar]
- 16.Tiede S., Kloepper J., Bodò E., Tiwari S., Kruse C., Paus R. Hair follicle stem cells: walking the maze. Eur J Cell Biol. 2007;86:355–376. doi: 10.1016/j.ejcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Tinschert S. Clinical phenotypes in patients with NF1 microdeletions. In: Kaufmann D., editor. Neurofibromatoses. Karger; Basel, Switzerland: 2008. pp. 78–88. [Google Scholar]
- 18.Rosenbaum T., Rosenbaum C., Winner U., Müller H., Lenard H., Hanemann C. Long-term culture and characterization of human neurofibroma-derived Schwann cells. J Neurosci Res. 2000;61:524–532. doi: 10.1002/1097-4547(20000901)61:5<524::AID-JNR7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Castrén M., Tervonen T., Kärkkäinen V., Heinonen S., Castrén E., Larsson K., Bakker C., Oostra B., Akerman K. Altered differentiation of neural stem cells in fragile X syndrome. Proc Natl Acad Sci USA. 2005;102:17834–17839. doi: 10.1073/pnas.0508995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jouhilahti E., Peltonen S., Peltonen J. Class III beta-tubulin is a component of the mitotic spindle in multiple cell types. J Histochem Cytochem. 2008;56:1113–1119. doi: 10.1369/jhc.2008.952002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atlasi Y., Mowla S., Ziaee S., Gokhale P., Andrews P. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008;26:3068–3074. doi: 10.1634/stemcells.2008-0530. [DOI] [PubMed] [Google Scholar]
- 22.Messiaen L., Callens T., Mortier G., Beysen D., Vandenbroucke I., Van Roy N., Speleman F., Paepe A. Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat. 2000;15:541–555. doi: 10.1002/1098-1004(200006)15:6<541::AID-HUMU6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Messiaen L., Wimmer K. NF1 mutational spectrum. In: Kaufmann D., editor. Neurofibromatoses. Karger; Basel, Switzerland: 2008. pp. 63–77. [Google Scholar]
- 24.Wimmer K., Yao S., Claes K., Kehrer-Sawatzki H., Tinschert S., De Raedt T., Legius E., Callens T., Beiglböck H., Maertens O., Messiaen L. Spectrum of single- and multiexon NF1 copy number changes in a cohort of 1,100 unselected NF1 patients. Genes Chromosomes Cancer. 2006;45:265–276. doi: 10.1002/gcc.20289. [DOI] [PubMed] [Google Scholar]
- 25.Amoh Y., Li L., Yang M., Moossa A., Katsuoka K., Penman S., Hoffman R. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci U S A. 2004;101:13291–13295. doi: 10.1073/pnas.0405250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amoh Y., Li L., Campillo R., Kawahara K., Katsuoka K., Penman S., Hoffman R. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A. 2005;102:17734–17738. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roth T., Ramamurthy P., Ebisu F., Lisak R., Bealmear B., Barald K. A mouse embryonic stem cell model of Schwann cell differentiation for studies of the role of neurofibromatosis type 1 in Schwann cell development and tumor formation. Glia. 2007;55:1123–1133. doi: 10.1002/glia.20534. [DOI] [PubMed] [Google Scholar]
- 28.Son M., Woolard K., Nam D., Lee J., Fine H. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NFP spheres moving on a cell layer and seeding new cells into the layer. Three spheres are followed with arrows. The recording time is 4 hours with 5-minute intervals between frames. The video has been generated using Olympus IX71 microscope equipped with Cell^R 2.6 software.
Growth of multipotent NFPs. Note the “popping up” of small spherical cells on the top of the cell layer and their subsequent division, which in turn is followed by merging of the cells back to the cell sheet. Also note that one of the dividing cells is followed with an arrow, which shows that during the 17-hour recording, the cell enters to the mitosis second time and attests to the short generation time of these cells. The video has been generated using Olympus IX71 microscope equipped with Cell^R 2.6 software.


