Abstract
L-selectin initiates lymphocyte interactions with high endothelial venules (HEVs) of lymphoid organs through binding to ligands with specific glycosylation modifications. 6-Sulfo sLex, a sulfated carbohydrate determinant for L-selectin, is carried on core 2 and extended core 1 O-glycans of HEV-expressed glycoproteins. The MECA-79 monoclonal antibody recognizes sulfated extended core 1 O-glycans and partially blocks lymphocyte-HEV interactions in lymphoid organs. Recent evidence has identified the contribution of 6-sulfo sLex carried on N-glycans to lymphocyte homing in mice. Here, we characterize CL40, a novel IgG monoclonal antibody. CL40 equaled or surpassed MECA-79 as a histochemical staining reagent for HEVs and HEV-like vessels in mouse and human. Using synthetic carbohydrates, we found that CL40 bound to 6-sulfo sLex structures, on both core 2 and extended core 1 structures, with an absolute dependency on 6-O-sulfation. Using transfected CHO cells and gene-targeted mice, we observed that CL40 bound its epitope on both N-glycans and O-glycans. Consistent with its broader glycan-binding, CL40 was superior to MECA-79 in blocking lymphocyte-HEV interactions in both wild-type mice and mice deficient in forming O-glycans. This superiority was more marked in human, as CL40 completely blocked lymphocyte binding to tonsillar HEVs, whereas MECA-79 inhibited only 60%. These findings extend the evidence for the importance of N-glycans in lymphocyte homing in mouse and indicate that this dependency also applies to human lymphoid organs.
High endothelial venules (HEVs) in secondary lymphoid organs, such as lymph nodes, Peyer's patches, and tonsils, are essential for immune surveillance by supporting the recruitment, or homing, of lymphocytes from the blood.1–5 Homing consists of multiple steps: tethering and rolling of lymphocytes on HEVs, chemokine-mediated activation of lymphocyte integrins, firm arrest of the lymphocytes, and transendothelial migration.1,6 L-selectin is a C-type lectin present on the cell surface of lymphocytes.1,2,6 Through its interactions with specific carbohydrate-based ligands expressed on HEVs,5 L-selectin is essential for the tethering and rolling steps. The known HEV ligands are glycoproteins, all of which have mucin segments with a characteristic high density of O-linked glycans. As identified in mice and/or humans, these ligands include CD34, podocalyxin, endomucin, MAdCAM-1, and nepmucin.4,5,7 These ligands are also defined by a monoclonal antibody (mAb), MECA-79, and the complex of reactive glycoproteins is given the name PNAd (for peripheral lymph node addressin).8,9 MECA-79 stains HEVs of human, mouse, and other species; it is function-blocking, in that it inhibits in vitro adherence of lymphocytes to HEVs in lymphoid organ sections, short-term homing of lymphocytes to lymph nodes in mice, and rolling of lymphocytes along HEVs in murine lymph nodes.8–10 The minimal L-selectin recognition determinant found on PNAd components is 6-sulfo sialyl Lewis X (6-sulfo sLex),11,12 comprised of sialyl Lewis X, modified with a sulfate ester on the C-6 position of GlcNAc (Figure 1). Ligand O-glycans can present this structure on the terminus of either a core 2 branch or an extended core 1 branch, or on both branches (Figure 1).11,13–15 Although α2–3 sialylation, α1–3 fucosylation, and 6-O-sulfation are required for optimal L-selectin interaction,5 6-O-sulfation in the context of an extended core 1 O-glycan (Figure 1) is essential for the MECA-79 epitope.13 The sulfation modifications, recognized by L-selectin and MECA-79, are generated cooperatively by two GlcNAc-6-O sulfotransferases, GlcNAc6ST-116 and GlcNAc6ST-217,18 (hereafter referred to as ST-1 and ST-2). The importance of these enzymes in lymphocyte homing has been shown in studies using single- and double-knockout mice.19–24 ST-1/ST-2 doubly null mice show an approximately 75% deficiency in L-selectin-dependent lymphocyte homing to lymph nodes and the complete absence of MECA-79 staining of HEVs.21,22
Figure 1.
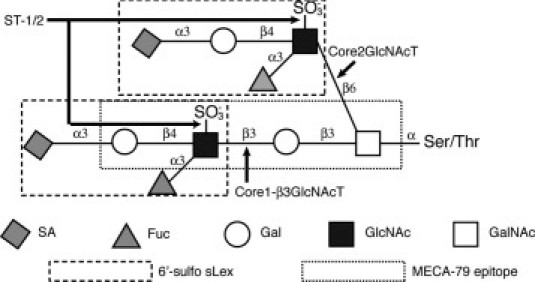
Disulfated biantennary O-glycan found in L-selectin ligands. Shown is a prototypical structure of the biantennary O-glycan, which decorates CD34 and other protein scaffolds within peripheral lymph node addressin (PNAd). Both branches are terminated by 6-sulfo sialyl Lewis x. The lower branch consists of the core 1 structure, which is extended by Core1-β3GlcNAcT with sulfation on the 6-position of GlcNAc by the sulfotransferases GlcNAc6ST-1 (ST-1) and GlcNAc6ST-2 (ST-2). The formation of the core 2 branch (upper) is initiated by the action of Core2GlcNAcT. Sulfation of this branch also occurs through the action of ST-1 and ST-2. The boxes indicate 6-sulfo sLex and the MECA-79 epitope. Chemically synthesized oligosaccharides based on the two chains were tested for antibody reactivity in Figure 2.
MECA-79 positive vessels not only are present in normal lymphoid organs but also are induced in some instances of infection and inflammation.5,7,25–28 The positive vessels are frequently, but not always, high-walled and are found either in organized lymphoid aggregates (tertiary lymphoid organs) or within diffuse lymphoid aggregates.26,28 Human diseases in which MECA-79-positive vessels occur include Crohn's disease, ulcerative colitis, gastritis associated with Helicobacter pylori infection, heart and kidney allograft rejection, bronchial asthma, myocarditis, rheumatoid arthritis, Hashimoto's thyroiditis, and Graves' disease. Therapeutic effects of intravenously injected MECA-79 have been found in a sheep model of asthma.29
MECA-79 is only partially effective in blocking lymphocyte adherence to HEVs in mouse lymph nodes,8 and more notably in human tonsils.25,30 Contrary to the prevailing view that only O-glycans are essential for L-selectin determinants, N-glycans of murine CD34 can present 6-sulfo sLex to L-selectin.31 A structural analysis of human tonsillar PNAd is compatible with the existence of similarly modified N-glycans.15 The importance of these N-glycans for homing to lymph nodes was explored in mice lacking the glycosyltransferases that elaborate the relevant core 2 (Core2GlcNAcT) and extended core 1 branches (Core1-β3GlcNAcT) on O-glycans31 (Figure 1). MECA-79 staining was abolished on lymph node HEVs in these mice, yet approximately 50% of L-selectin-dependent homing persisted. N-glycanase treatment of HEVs eliminated the residual ligand activity, thus directly implicating N-glycans. The biological significance of the N-glycans was further indicated by the finding that tomato lectin, which binds to complex N-glycans, partially reduces lymphocyte homing in wild-type mice.
To validate these findings and explore their generality in human and other species, it would be desirable to have antibodies that recognize the 6-sulfo sLex structure on both N- and O-glycans. So far, two murine IgM antibodies, known as G72 and G152, have been shown to bind this determinant.12 Although these antibodies block staining of human lymph node HEVs by an L-selectin-IgG chimera, their activities in lymphocyte-HEV binding assays have not been determined. Moreover, these antibodies do not react with HEVs in mouse or rat,12 probably because of variation in sialic acid forms between species.32 Therefore, these antibodies are not useful in small-animal models of disease. With respect to MECA-79, its reactivity is limited to a subset of O-glycans, as explained above, and its function-blocking activity is incomplete. Furthermore, it is an IgM and has limited usefulness in small animal models of chronic inflammation (S.H., unpublished observations).
In the present study, we characterized a newly generated mouse IgG mAb, designated CL40. We demonstrate the reactivity of CL40 for both O-glycan and N-glycan chains that terminate with 6-sulfo sLex. CL40 stains HEVs in humans and rodents, as well as HEV-like vessels at sites of inflammation. Consistent with this broad reactivity, this mAb is superior to MECA-79 in blocking lymphocyte binding to HEVs, especially in human. The new findings expand our knowledge about the glycosylation and sulfation requirements for L-selectin ligands.
Materials and Methods
Antibodies and Synthetic Oligosaccharides
CL40 (murine IgG1) was provided to us by Dyax Corporation (Cambridge, MA). The antibody was originally produced at Thios Pharmaceuticals (Emeryville, CA), by immunizing ST-1/ST-2 doubly null mice21 with an extended core 1 structure terminating with 6-sulfo sLex (Figure 1). Murine hybridoma supernatants were screened for binding to the glycan immunogen, and CL40 was selected. G72 was a kind gift of Dr. Reiji Kannagi. The MECA-79 hybridoma was provided by Dr. Eugene Butcher. The extended core 1 and core 2 branches were synthesized by Matt Pratt and Carolyn Bertozzi33 and provided to us by Thios Pharmaceuticals.
Cell Line
300.19L cells34 (mouse pre-B cell lymphoma stably transfected with full-length human L-selectin cDNA) were provided by Dr. Geoffrey Kansas.
Mice
Mice deficient in GlcNAc6ST-1 (ST-1),23 GlcNAc6ST-2 (ST-2),22 and both ST-1 and ST-222 (all on the C57BL/6 background) were used. Mice doubly null for α(1,3)fucosyltransferase-IV and -VII (FTIV and FTVII)35 were provided by the Consortium for Functional Glycomics. Mice deficient in both core 1 extension and core 2 branching enzymes (Core1βGlcNAcT and Core2GlcNAcT)36 were crossed onto the BALB/c background.
Rheumatoid Arthritis and Ulcerative Colitis Specimens
FFPE blocks containing synovia from patients with rheumatoid arthritis (RA) or osteoarthritis and colons from patients with ulcerative colitis (UC) or noninflammatory conditions were obtained from surgical specimens archived in the Department of Pathology at the University of California, San Francisco. The tissue samples were obtained under Committee on Human Research approval (CHR H1060-28724). Specimens from patients with a diagnosis of RA were evaluated, and samples that demonstrated the classic features of rheumatoid arthritis including papillary hyperplasia and lymphoplasmacytic infiltration of the synovium were selected. Similarly, total colectomy specimens from patients with a diagnosis of UC were evaluated, and samples that demonstrated chronic colitis with acute inflammation and mucosal ulceration were selected.
Enzyme-Linked Immunosorbent Assay with Synthetic Sugars
Extended core 1 and core 2 O-glycans were chemically synthesized with or without 6-O- sulfation and conjugated with biotin at their reducing termini.33 The enzyme-linked immunosorbent assay (ELISA) plates (Thermo Scientific, Waltham, MA) were coated overnight with 10 μg/ml streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS. The plates were washed and incubated with twofold serial dilutions of 50 nmol/L synthetic sugars diluted in PBST (PBS containing 0.1% Tween-20). Antibodies were applied at the following final concentrations: CL40 at 5 μg/ml and MECA-79 (rat IgM), G72 supernatant12 (mouse IgM), and HECA-452 (rat IgM; BD Pharmingen, San Jose, CA) at 1 μg/ml. Bound antibodies were detected with alkaline phosphatase (ALP)-conjugated secondary Abs (Jackson ImmunoResearch) with PNPP as substrate (Thermo Scientific).
Transfection and Analysis of CHO Cells
CHO cells were transiently transfected with various combinations of cDNAs encoding glycosyltransferases and sulfotransferases as previously described.13,31,37 Parental CHO cells lack Core1βGlcNAcT, Core2GlcNAcT, FTIV, and FTVII.13,18,37 CHO cells transfected with cDNAs for CD34, FTVII, ST-1, and/or ST-2 reconstitute 6-sulfo sLex on N-glycans, whereas CHO cells transfected with cDNAs for Core1βGlcNAcT and Core2GlcNAcT, in addition to those for CD34, FTVII, ST-1/ST-2, can elaborate 6-sulfo sLex on O-glycans.31 CHO cells were cotransfected with a cDNA for EGFP-N1 (enhanced green fluorescent protein), which allowed gating on cells that were productively transfected. CHO cells (2 × 106 cells/sample) were stained with CL40, MECA-79, G72, or their isotype controls at 10 μg/ml in PBS with 2% bovine serum albumin at 4°C. The cells were treated successively with biotin-conjugated secondary Abs (Jackson ImmunoResearch laboratories, Inc.), and allophycocyanin (APC)-conjugated streptavidin (Caltag, Carlsbad, CA) and were analyzed by flow cytometry on a FACSort system (BD Biosciences, San Jose, CA). Desialylation was achieved by incubation of the cells for 2 hours at 37°C with 100 mU/ml Arthrobacter ureafaciens neuraminidase (EMD Chemicals, Gibbstown, NJ) in PBS.
Immunostaining
Fresh human tonsils, mouse peripheral lymph nodes (PLN), and rat PLN were embedded in O.C.T. compound (Sakura Finetek, Torrance, CA) and frozen. Sections (10 μm thick) were cut in a Leica Microsystems (Bannockburn, IL) cryostat and transferred onto Superfrost-Plus slides (Fisher Scientific, Pittsburgh, PA). The dried slides were fixed in 2% paraformaldehyde for 20 minutes, then washed and stained with CL40 or MECA-79 (5 μg/ml) and either anti-human CD31 (goat IgG; Santa Cruz Biotechnology, Santa Cruz, CA), anti-mouse CD31 (rat IgG2a; BD Pharmingen), or anti-rat CD31 (mouse IgG1; Chemicon, Billerica, MA). MECA-79 was detected with Cy3-conjugated anti-rat IgM, and CL40 was detected with biotin-conjugated anti-mouse IgG1, followed by Cy3-conjugated streptavidin. All secondary/tertiary antibodies were from Jackson ImmunoResearch Laboratories. Cryostat sections from pancreata of 12-week-old NOD mice and 10-week-old RIP-BLC mice and from ankle joints of B10 mice with collagen-induced arthritis were stained with CL40 or MECA-79 and anti-mouse CD31. The collagen-induced arthritis was induced in 6- to 8-week-old female B10RIII mice,38 and ankle tissues were cryosectioned with a Cryo-Jane system (Instrumedics, St. Louis, MO). To test for N-glycans in the CL40 epitope, adjacent tissue sections of murine PLN were digested with N-glycosidase F (EMD Chemicals) at 100 U/ml or treated with buffer alone and then stained with CL40. For CL40 staining of human samples, the secondary antibody used was horseradish peroxidase-conjugated anti-mouse EnVision+ (DAKO, Carpinteria, CA) in conjunction with NovaRED (Vector Laboratories, Burlingame, CA) as substrate. Images were captured using an Optiphot microscope and AxioCam camera (Nikon, Yokohama, Japan). All photographic images were taken at 20× magnification.
A tissue microarray of formalin-fixed normal murine tissues was obtained from Imgenex (Cat. no. IMH-335; San Diego, CA). For these and the RA and UC samples, the slides were deparaffinized with Histoclear II (National Diagnostics, Atlanta, GA), rehydrated through a graded series of ethanol/water, and subjected to pressure cooker epitope retrieval. CL40 staining was performed with the EnVision+ system as described above. MECA-79 staining was performed by a three-step procedure in which the primary antibody was followed by biotinylated mouse anti-rat kappa (Caltag; Invitrogen, Carlsbad, CA) and streptavidin-horseradish peroxidase (Jackson ImmunoResearch) with NovaRED as substrate and hematoxylin for counterstaining. The primary antibodies were applied at 1 μg/ml.
Immunoprecipitation and Western Blotting
PNAd was isolated from detergent lysates of human tonsils with MECA-79 coupled to cyanogen bromide-activated Sepharose 4B (Sigma-Aldrich, St. Louis, MO).9 Human CD34 was isolated from PNAd with anti-human CD34 (clone 581; BD Pharmingen) conjugated to protein G-Sepharose 4B (Zymed; Invitrogen, Carlsbad, CA). Stroma of mouse PLN was prepared as previously described.22 Mouse CD34 was immunoprecipitated from a detergent lysate of stroma with anti-mouse CD34 (RAM34 mAb; BD Pharmingen). Desialylation of PNAd and PLN stroma was achieved by incubation with 50 mU Arthrobacter ureafaciens neuraminidase (EMD Chemicals) in PBS with protease inhibitor cocktail (Sigma-Aldrich) for 16 hours at 37°C. Immunoprecipitated human and murine CD34 were digested with N-glycosidase F (EMD Chemicals) after SDS denaturation. Samples were analyzed by SDS-polyacrylamide gel electrophoresis with reduction and transferred to polyvinylidene difluoride membranes (Applied Biosystems, Foster City, CA). Membranes were blotted with CL40, MECA-79, HECA-452, and isotype control immunoglobulins (5 μg/ml), followed by horseradish peroxidase-conjugated secondary antibodies with enhanced chemiluminescence detection (Amersham, Pittsburgh, PA). Band intensity was determined by Multi Gauge software (Fujifilm, Valhalla, NY).
Stamper-Woodruff Assay
In vitro adherence of lymphocytes to lymphoid organs was performed with a modified Stamper-Woodruff assay39: 10-μm-thick cryostat-cut sections of lymphoid organs were air-dried and fixed in 2% paraformaldehyde, and sections were preincubated with CL40, MECA-79, and isotype controls (at 100 μg/ml). The antibodies were decanted and 300.19L cells (2 × 107 in 100 μL) in RPMI-1640 (1 mg/ml bovine serum albumin) were applied (7°C). The slides were gyrated for 30 minutes at 90 rpm. After gentle decanting, the slides were fixed in 2.5% glutaraldehyde, stained with 0.5% Toluidine Blue, and mounted. L-selectin was inhibited with 10 mmol/L EDTA or 5 μg/ml anti L-selectin Ab (DREG-56; BD Pharmingen).
In Vivo Lymphocyte Homing Assay
Splenocytes from 6- to 8-week-old CD-1 mice were labeled with 5 μmol/L 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen). Then, 5 × 107 cells in 100 μL PBS with 200 μg Abs (CL40, MECA-79 or their isotype controls) were injected intravenously into mutant mice or age-matched wild-type controls (6- to 8-week-old female mice). At 1 hour after injection, lymphoid organs were mechanically dispersed and CMFDA+ cells were counted by flow cytometry as a percentage of total lymphocyte number.22
Results
Characterization of Glycan-Binding Specificity of CL40
CL40 mAb, a murine IgG1, was obtained by immunizing ST-1/ST-2 doubly null mice with an extended core 1 structure terminating with 6-sulfo sLex (Figure 1). We tested the reactivity of CL40 against 6-O-sulfated oligosaccharides corresponding to core 1 and core 2 branched O-glycans that are found in L-selectin ligands (Figure 1). We also evaluated the same glycans without the 6-O-sulfate modifications. The synthetic sugars possessing biotin at their reducing termini were immobilized onto streptavidin-coated plates. CL40 reacted with both extended core 1 and core 2 chains that terminated with 6-sulfo sLex (Figure 2A). There was no reactivity with either oligosaccharide branch if 6-O-sulfate was not present. G72 showed an identical binding profile. MECA-79 reacted only with the extended core 1 structure and showed an absolute requirement for 6-O-sulfate, in agreement with previous findings.13
Figure 2.
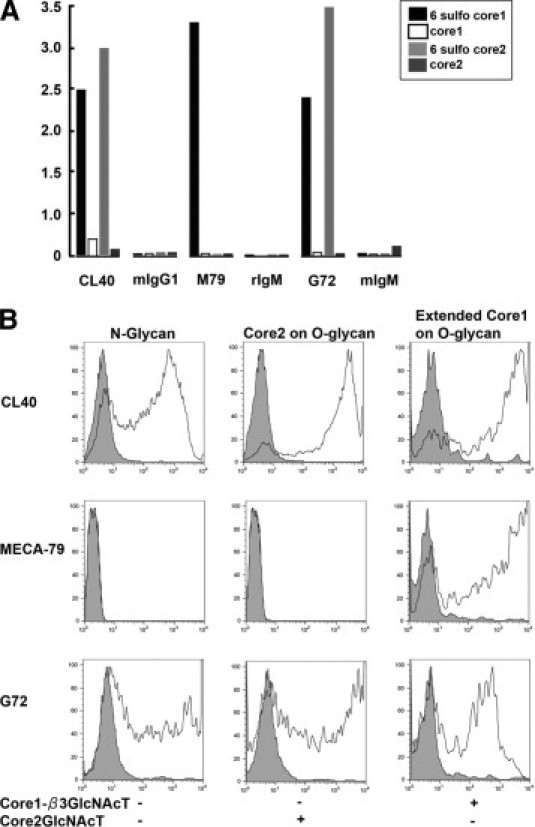
Glycan-binding specificity of CL40. A: Enzyme-linked immunosorbent assays were performed with sLex-terminating extended core 1 and core 2 O-glycans with or without sulfation (as denoted by the legend) on the C-6 position of GlcNAc (Figure 1). The binding of the indicated antibodies and class-matched control immunoglobulins is shown. Data are representative of three independent experiments. B: CHO cells were transfecting with CD34, FTVII, ST-1, and ST-2, with or without Core1βGlcNAcT and Core2GlcNAcT, as indicated in the figure) or isotype control (gray filled profiles) and analyzed by flow cytometry. The types of glycans that are formed in CHO cells transfected with the various combinations of glycosyltransferases are indicated at the tops of the three panels. Data are representative of three independent experiments.
To further characterize the CL40 epitope, we transfected CHO cells with (1) CD34 cDNA to provide a scaffold protein; (2) core 1 extension enzyme (Core1-β3GlcNAcT) cDNA or a core 2 branching enzyme (Core2GlcNAcT) cDNA to provide different O-glycan branches; and (3) FTVII cDNA and ST-1/2 cDNAs to generate the 6-sulfo sLex capping structure. We compared the staining of the cells with MECA-79, G72, and CL40. It was previously shown that CHO cells transfected with FTVII/ST-2 cDNAs are able to produce 6-sulfo sLex, but only on N-glycans.31 We found that CD34/FTVII/ST-1/2 transfected cells reacted with both G72 and CL40, suggesting that these antibodies can recognize 6-sulfo sLex on N-glycans (Figure 2B). However, MECA-79 failed to react, as expected because of the exclusive occurrence of its epitope on O-glycans. When the CHO cells were additionally transfected with either Core1-β3GlcNAcT or Core2GlcNAcT cDNA, the staining with G72 and CL40 increased, consistent with the presentation of 6-sulfo sLex on both types of O-glycan branches (Figure 2B). For MECA-79, reactivity was present only when Core1-β3GlcNAcT was introduced, as expected.13
Immunohistochemical and Western Blotting Analyses with CL40
We surveyed the staining reactivity of CL40 on a mouse multitissue array containing the following FFPE tissues from 8-week-old CD-1 mice: skin, spleen, skeletal muscle, lung, heart, tongue, salivary gland, liver, pancreas, stomach, small intestine, colon, kidney, urinary bladder, seminal vesicle, testis, epididymis, uterus, ovary, thymus, cerebrum, pons, and cerebellum. Comparably fixed lymph nodes from 8-week-old CD-1 mice were also embedded in paraffin and sectioned. We detected staining only in lymph nodes, where the staining was restricted to HEVs (Figure 3A). CL40 reacted with HEVs in human tonsil and lymph nodes of rat and mouse with the same basic pattern as MECA-79 (Figure 3B). We also compared CL40 and MECA-79 at the biochemical level and observed that they recognized the same components in both mouse and human lymphoid organs, as determined by Western blotting of organ stroma (Figure 3C). This result indicates that the same array of protein scaffolds carry both determinants.
Figure 3.
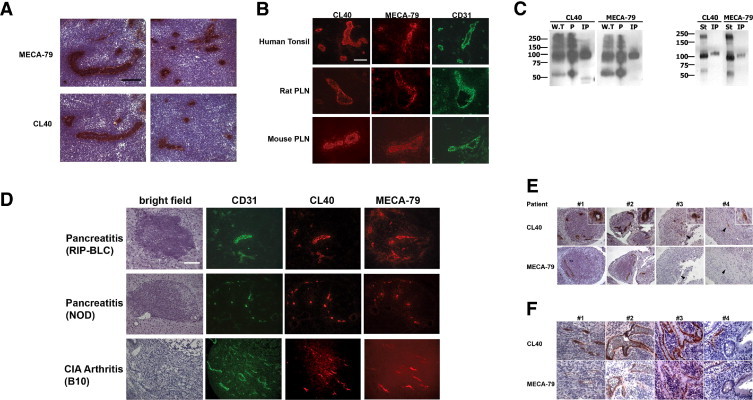
CL40 reactivity with high endothelial venules (HEVs) and HEV-like vessels and with PNAd components. A: Murine peripheral lymph node (PLN) sections were stained with MECA-79 and CL40 using horseradish peroxidase immunohistochemistry. B: Adjacent sections of human tonsil, rat PLN, and murine PLN were stained by with CL40 (red), MECA-79 (red), and a CD31 antibody (green, to reveal vessels) using fluorescence immunohistochemistry. C: Left: Human whole tonsil (W.T), peripheral lymph node addressin or PNAd (designated P) and immunoprecipitated human CD34 (IP) were electrophoresed. Right: Stroma from mouse PLN (St) and murine CD34 isolated by immunoprecipitation from a detergent lysate of stroma (IP) with anti-mouse CD34 was electrophoresed. The transferred membranes were blotted with MECA-79 or CL40 at 5 μg/ml. Data are representative of three independent experiments. D: Sections of pancreata from RIP-BLC and NOD mice, and the synovium from mice with collagen-induced arthritis (B10 mice) were sectioned. Consecutive sections were stained with hematoxylin (bright field), CL40 (red), MECA-79 (red), and a CD31 antibody (green). E: Adjacent sections of paraffin-embedded synovium from four patients with rheumatoid arthritis were stained with CL40 and MECA-79. Patient 4 exhibited CL40+ vessels that were not stained with MECA-79. In other patients, in addition to double-positive vessels, individual CL40+MECA-79− vessels are evident. Insets: Higher magnification views of CL40+ vessels. Arrowheads indicate corresponding vessels in adjacent sections of individual patients. F: Adjacent sections of paraffin-embedded colonic mucosa from patients with ulcerative colitis were stained with CL40 and MECA-79. All scale bars = 100 μm. Original magnification, 20×.
CL40 stained HEV-like vessels in tertiary lymphoid organs in the pancreata of RIP-BLC transgenic mice and NOD mice in a pattern indistinguishable from that obtained with MECA-7927 (Figure 3D). We also found identical staining by MECA-79 and CL40 of synovial vessels in a murine collagen-induced arthritis model (Figure 3D).
MECA-79 is known to stain vessels in the inflamed synovium of rheumatoid arthritis patients.40,41 We examined synovial samples from 11 patients with RA and compared these with samples from 9 osteoarthritis patients as a control. The RA samples were preselected to have classic features of rheumatoid arthritis including lymphoplasmacytic infiltration of the synovium. Of the 11 samples, 10 showed positive MECA-79 vessels (Figure 3E). The vessels were HEV-like, and occurred in association with loose mononuclear cells or in dense lymphoid aggregates. In serial sections, we found that the MECA-79+ vessels were also CL40+. Notably, the one case that was negative for MECA-79 staining showed several HEV-like vessels that stained with CL40 (Figure 3E). In the other cases, we also found CL40+ vessels that were negative for MECA-79. Such vessels occurred where the lymphoid cells were loosely organized. These presumably correspond to vessels previously described in RA that are negative for MECA-79 but express ST-2 protein.41 The osteoarthritis samples were negative for both MECA-7941 and CL40.
Nakayama and coworkers42 reported MECA-79+ vessels in the inflamed colonic mucosa of ulcerative colitis patients. The number of positive vessels increased in patients with active disease, compared with patients in remission. We examined tissue samples from seven patients with ulcerative colitis and found that four had vessels in the inflamed mucosa that were positive with both MECA-79 and CL40, whereas in the other three cases there was no vascular staining with either antibody. In serial sections, every MECA-79+ vessel stained with CL40 (Figure 3F). Notably, for both UC and RA specimens, CL40 staining of a given vessel was generally stronger than by MECA-79 in the adjacent section, even when the amplification steps were carefully matched (done for UC).
Critical Post-Translational Modifications For CL40 Epitope
Post-translational modifications (fucosylation, sialylation, and sulfation) are critical for optimal L-selectin recognition of its HEV- ligands.5 We wanted to determine which post-translational modifications are required for CL40 reactivity. We first performed transfection experiments with CHO cells to define the CL40 epitope. Transfection with cDNAs for CD34, Core2GlcNAcT, ST-1/2, and FTVII permits the reconstitution of 6-sulfo sLex on core 2 O-glycans on transfected cells.13,36 CL40 stained such cells (Figure 4A). When we treated CD34/FTVII/Core2GlcNAcT/ST1/2 CHO cells with sialidase, CL40 reactivity was lost, indicating a requirement for sialylation (Figure 4A). We confirmed this result for actual lymph node glycoproteins by treating lymph node stroma with sialidase. This treatment eliminated the Western blotting signal with CL40 (Figure 4B), but had no effect on the MECA-79 pattern, in agreement with previous findings.43 Sialidase treatment or mild periodate treatment of human tonsil sections reduced CL40 staining of HEVs, further supporting the requirement for sialylation (see Supplemental Figure S1 at http://ajp.amjpathol.org). Fucosylation appeared to be dispensable for the epitope, since CL40 staining of HEVs in PLN of FTIV/FTVII doubly null mice was indistinguishable from that in wild-type mice (see Supplemental Figure S2 at http://ajp.amjpathol.org). We confirmed the fucosylation independence in a reconstitution experiment in which we prepared CD34/Core2GlcNAcT/ST1/2 CHO cells with or without transfection with FTVII cDNA. We found that CL40 stained the two cell populations equivalently (Figure 4E). Thus, the CL40 epitope, like the MECA-79 epitope, does not require fucosylation but is permissive to it.
Figure 4.
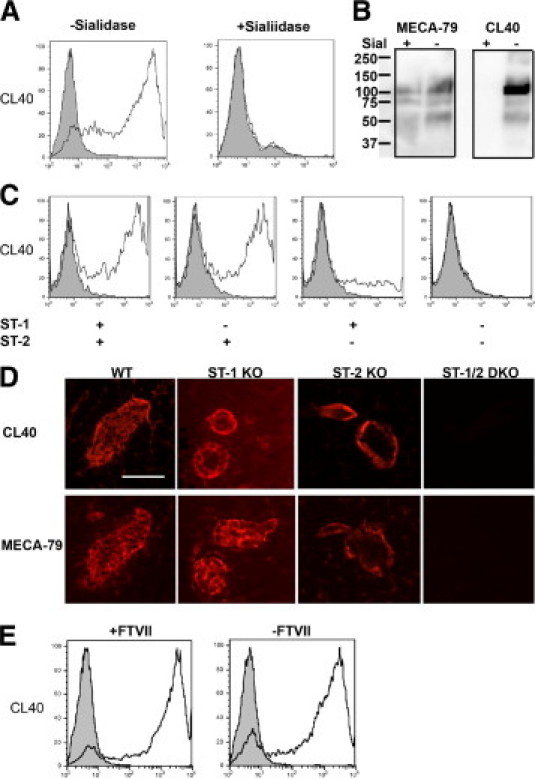
Sialylation and sulfation requirements for CL40. A: CHO cells were transfected with CD34, Core2GlcNAcT, FTVII, ST-1, and ST-2 and were treated with either 100 mU/ml neuraminidase (Sialidase) (middle) or PBS (left) at 37°C for 2 hours. The cells were stained with CL40 (open profiles) or isotype control (gray filled profiles) and were analyzed by flow cytometry. B: Murine PLN stroma were treated with 50 mU neuraminidase (Sial) or PBS for 16 hours at 37°C and were analyzed by Western blotting with CL40. The membrane was stripped and reblotted with MECA-79. C: CHO cells were transfected with CD34, Core2GlcNAcT, and FTVII, either with or without ST-1 and ST-2, as indicated. Cells were stained with CL40 (open profiles) or isotype control (gray filled profiles) and analyzed as above. D: Consecutive sections from PLNs of wild-type (WT) mice, ST-1 knockout (KO) mice, ST-2 KO mice and ST-1/2 doubly null mice (ST-DKO) were stained with CL40 or MECA-79. E: CHO cells were transfected with CD34, C2GnT, ST-1, and ST-2 with or without FTVII. Cells were stained with CL40 (open profiles) or isotype control (gray filled profiles) and analyzed as above. All data were representative of three independent experiments. Original magnification, 20×. All scale bars = 50 μm.
We further examined the sulfation requirement for CL40 by using reconstituted CHO cells. Consistent with the ELISA results obtained with synthetic sugars (Figure 2A), GlcNAc-6-O-sulfation was required. Transfection of CD34/FTVII/Core2GlcNAcT CHO cells with either a ST-1 or ST-2 cDNA resulted in positive CL40 staining, with a stronger signal from the latter enzyme (Figure 4C). Furthermore, the CL40 staining pattern in PLN of ST knockout mice closely resembled that for MECA-79 (Figure 4D). Lymph node HEVs from ST-2 null mice showed greatly reduced staining with both antibodies, with the residual staining being predominantly abluminal. In lymph nodes of ST-1/ST-2 double-null mice, HEVs were devoid of staining (Figure 4D). In summary, the post-translational requirements for CL40 binding closely parallel those for L-selectin, with the exception of fucosylation. CL40 could be compromised as a ligand reporter antibody because of its indifference to fucosylation. Nonetheless, as we show below, CL40 does recognize functionally relevant epitopes in HEVs of murine and human lymphoid organs.
Functional Evaluation of CL40 in Mouse
To examine the functional activity of CL40 and to compare it with MECA-79, we first performed the Stamper-Woodruff adhesion assay, which measures the binding of lymphocytes to HEVs in cryostat-cut sections of lymphoid organs.39 We wanted to compare the antibodies at saturating levels, so that the epitopes would be completely occupied by antibody. To determine the saturating levels, we performed immunostaining of mouse lymph node HEVs over a range of CL40 and MECA-79 concentrations. The staining IC50 values for CL40 and MECA-79 were approximately 0.3 μg/ml and 1.2 μg/ml, respectively (see Supplemental Figure S3 at http://ajp.amjpathol.org). Given that CL40 is an IgG and MECA-79 an IgM, these results indicate comparable affinities for their binding to HEVs. The adhesion assays used a murine pre-B lymphoma line (300.19 cells), which was stably transfected with a human L-selectin cDNA (designated as 300.19L).34 We applied these cells to murine PLN sections, in the presence of MECA-79 or CL40 at 100 μg/ml, which greatly exceeds the IC50 values for both. Both antibodies inhibited binding, relative to medium alone (Figure 5A), with CL40 producing stronger inhibition (94.0 ± 2.3%) than MECA-79 (72.3 ± 8.8%) (P < 0.01 by one-way analysis of variance with Tukey's post hoc test). We verified that the binding to PLN HEV was completely sensitive to EDTA and to DREG-56, a function-blocking antibody against L-selectin (data not shown).
Figure 5.
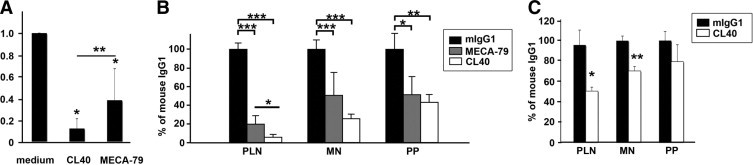
Inhibition of in vitro lymphocyte attachment to HEVs and lymphocyte homing by CL40. A: Attachment of 300.19L cells to HEVs in murine PLN cryosections was determined in the presence of 100 μg/ml of the indicated antibody or in medium. Data were obtained from 6 independent experiments with means and SEM values indicated. The results were analyzed statistically using one-way analysis of variance with P values determined by the Tukey's post hoc test. *P < 0.005 for either of the antibodies versus medium and **P < 0.01 between CL40 and MECA-79. B: Short-term homing of splenocytes to lymphoid organs was determined 1 hour after intravenous injection of labeled lymphocytes plus 200 μg of the indicated antibody or nonspecific mIgG1. Homing indices are computed as a percentage relative to that obtained with mIgG1. Means and SEM values based on 3 experiments are shown. As determined by one-way analysis of variance with Tukey's post hoc test, *P < 0.05, **P < 0.01, and ***P < 0.005. PN, MN, and PP denote peripheral lymph nodes, mesenteric lymph nodes, and Peyer's patches, respectively. C: Short-term homing was determined in Core2GlcNAcT/Core1-β3GlcNAcT double-knockout mice. The data shown are pooled from three experiments. *P < 0.05 and **P < 0.005 between CL40 and mouse IgG1 as determined by the two-way Student's t-test. The fact that CL40 produced only partial inhibition of homing in these O-glycan-deficient mice further points to the existence of determinants other than 6-sulfo sLex, as do the results shown in Supplemental Figure S4 (http://ajp.amjpathol.org).
In short-term homing assays in mice, CL40 was more potent than MECA-79 in inhibiting lymphocyte homing to PLN. With injection of 200 μg of antibody, CL40 inhibited homing by 96.3 ± 0.77%, whereas MECA-79 inhibited by 87.0 ± 3.72% (P < 0.05 between MECA-79 and CL40) (Figure 5B). At 25 μg antibody per mouse, CL40 inhibited homing by 79.7 ± 15.5% and MECA-79 inhibited by 16.5± 4.8% (P < 0.01).
The results of Figure 2 indicate that CL40 can recognize 6-sulfo sLex on N-linked glycans, which distinguishes it from MECA-79, which recognizes 6-sulfo sLex only on O-glycans.13 To investigate whether CL40 could interact with the N-glycans that are essential for homing in the Core2GlcNAcT/Core1-β3GlcNAcT double-knockout mice,36 we conduced homing studies in these mice. CL40 inhibited approximately 50% of the homing to PLN and 30% of that to MN in the double-knockout mice (Figure 5C). Corroborating these findings, we observed that CL40 stained HEVs in PLNs of these mice, albeit requiring a high concentration of antibody (see Supplemental Figure S4A at http://ajp.amjpathol.org). In agreement with the previous characterization,36 there was no staining with MECA-79 at the same concentration (see Supplemental Figure S4A at http://ajp.amjpathol.org). We also evaluated the contribution of N-glycans to the CL40 epitope in wild-type mice by using N-glycanase.36 We treated adjacent PLN sections with N-glycanase or buffer alone. N-glycanase reduced CL40 staining of HEVs, but had no effect on staining of HEVs for CD31 (see Supplemental Figure S4B at http://ajp.amjpathol.org). In a quantitative assessment, 43% of HEVs (50/115) showed clearly reduced staining after N-glycanase treatment, whereas CD31 staining of HEVs was not affected.
We also tested the effects of CL40 on homing in the sulfotransferase doubly null mice (ST-1/ST-2 double-knockout). Homing to PLN in these mutant mice was approximately 30% of the wild-type level (see Supplemental Figure S5 at http://ajp.amjpathol.org), as was found in previous characterizations.21,22 Consistent with the inability of CL40 to stain HEVs in these mice (Figure 4D), CL40 did not inhibit this residual homing (see Supplemental Figure S5 at http://ajp.amjpathol.org).
CL40 Recognition of N-Glycans In Human Tonsillar PNAd
We next examined whether CL40 recognized N-glycans on a bona fide ligand from a human lymphoid organ. We focused on CD34, which is the predominant ligand component in human tonsillar PNAd.44 We isolated PNAd by affinity chromatography on a MECA-79 column and then purified CD34 by immunoprecipitation with a CD34 mAb (Figure 6A). As expected, N-glycanase treatment of CD34 had no effect on the Western blotting signal with MECA-79 or CD34 mAb (Clone 581), although the bands shifted down in molecular weight due to the loss of N-glycans (Figure 6A). In contrast, this treatment reduced the CL40 Western blot signal by 70%. Blotting by HECA-452 was reduced by 90%, consistent with a previous report that this antibody is capable of recognizing sLex-related moieties on N-glycans.45 N-glycanase digestion of murine lymph node stroma and lymph node CD34 produced similar reductions in CL40 blotting (data not shown).
Figure 6.
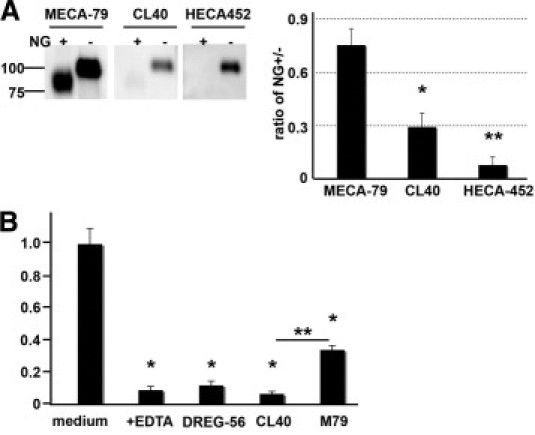
Contribution of N-glycans to CL40 reactivity. A: CD34 was immunoprecipitated from human tonsillar PNAd, treated with N-glycosidase F (NG) or PBS at 37°C for 42 hours, and then analyzed by Western blotting with the indicated antibodies. The band intensities were calculated as the ratios of NG-treated over untreated (right panel) using Multi Gauge software as described under Materials and Methods. The indicated means and SEM values were derived from three independent experiments. *P < 0.01 and **P < 0.005 against MECA-79, as determined by one-way analysis of variance and Tukey's post hoc test. B: Attachment of 300.19L cells to HEVs in cryosections of human tonsil was determined in the presence of 100 μg/ml of the indicated antibodies or medium alone. Isotype controls were also used in these experiments, although data are not shown because the results were the same as with medium only. As determined by one-way analysis of variance and Tukey's post hoc test, *P < 0.005 for the difference between the indicated treatment and medium alone and **P < 0.01 for the difference between CL40 and MECA-79. There was a significant difference between MECA-79 and EDTA at P < 0.01, and between MECA-79 and DREG-56 at P < 0.01.
Because CL40 can recognize its epitope on both N-glycans and O-glycans of tonsillar CD34 (Figures 2A and 6A) as well as its O-glycans (Figure 2A), whereas MECA-79 can recognize only the latter, we compared the two antibodies for inhibition of lymphocyte adherence to HEVs in human tonsils, again using concentrations of antibody well in excess of the IC50 values. We continued to use 300.19L cells as the lymphocyte population. The requirement for L-selectin in binding was confirmed by >90% inhibition of cell attachment with the addition of EDTA or the L-selectin mAb DREG-56 (Figure 6B). Consistent with a previous report, MECA-79 produced partial inhibition (60%) of lymphocyte binding to HEVs.25 CL40 reduced binding to the background level (Figure 6B).
Discussion
MECA-79 has proven to be an invaluable tool in deciphering the mechanisms of lymphocyte homing.5,8,9 The PNAd complex, defined by MECA-79, is clearly essential for L-selectin-mediated lymphocyte interactions with HEVs in lymphoid organs. Because of its utility in immunohistochemistry and its function-blocking activity, MECA-79 has enjoyed widespread use in the identification of L-selectin ligands in vascular beds. MECA-79 is nevertheless an imperfect surrogate for L-selectin. Discrepancies first emerged when it was found that MECA-79 was only partially effective in blocking L-selectin-dependent adhesion to HEVs or to purified PNAd.8,9,25,30 In addition, a variety of in vitro and in vivo systems exhibit L-selectin ligands on vascular endothelium that are not reactive with MECA-79.46–48
Through further analyses of ligand glycoproteins, including the structural definition of PNAd glycans, it is now clear that the carbohydrate-binding repertoire of L-selectin includes, but is broader than, the MECA-79 epitope. The 6-sulfo-sLex structure has emerged as a more general recognition epitope for L-selectin. The MECA-79 epitope overlaps with this determinant when it occurs on extended core 1 glycans, thus likely accounting for the function blocking activity of the antibody (Figure 1). Steric inhibition of 6-sulfo-sLex on core 2 O-glycans probably contributes indirectly to the inhibitory efficacy of MECA-79.36 The greatest limitation of MECA-79 as a staining reagent or as the basis for a therapeutic antibody may be its inability to recognize N-glycans.31
The present study evaluated CL40 as an alternative to MECA-79 and exploited its advantages to gain further information about L-selectin ligands. As an immunohistochemical reagent for secondary lymphoid organs, CL40 was equivalent to MECA-79 in selectively staining HEVs in murine lymph nodes and human tonsils (Figure 3, A and B). Furthermore, the same complex of glycoprotein scaffolds appears to carry the MECA-79 and CL40 epitopes in lymphoid organs, based on the highly similar patterns seen in Western blotting of organ stroma (Figure 3C).
Clear distinctions emerged between the two antibodies when we compared the glycan requirements for their epitopes. Using synthetic glycans in ELISA, we found that CL40 bound to two types of 6-sulfo sLex terminating O-glycans, both of which are present in L-selectin ligands (Figures 1 and 2A). We confirmed that MECA-79 recognized only the one based on an extended core 1 O-glycan. Reconstitution studies in CHO cells substantiated these results (Figure 2B). Further characterization of the CL40 epitope showed that sialylation was required (Figure 4, A and B; see Supplemental Figure S1 at http://ajp.amjpathol.org), but fucosylation was dispensable (Figure 4E; Supplemental Figure S2 at http://ajp.amjpathol.org), a characteristic that could lead to false positives. With respect to sulfation, CL40 binding to both of the O-glycans showed an absolute requirement for GlcNAc-6-O-sulfation (Figure 2A and Figure 4, C and D). The reconstitution studies established that both ST-1 and ST-2 could satisfy the sulfation requirements for CL40 binding, but the contribution of ST-2 appeared greater than that of ST-1 (Figure 4C). Analysis of ST-1 and ST-2 null mice verified that, in vivo, these enzymes collaborate to produce the CL40 epitope in HEVs, with the latter enzyme making the stronger contribution (Figure 4D). As with MECA-79,21,22 CL40 staining was completely abrogated in lymph node HEVs in ST-1/ST-2 double-deficient mice. Consistent with the absence of HEV staining, CL40 was not able to inhibit the approximately 25% residual homing present in these mice (see Supplemental Figure S5 at http://ajp.amjpathol.org). The structural basis for this MECA-79/CL40 noninhibitable, yet L-selectin-dependent homing21,22 remains an enigma. A determinant other than 6-sulfo sLex would appear to be involved. Nonetheless, our results with CL40 extend the evidence for the critical contribution of GlcNAc-6-O-sulfation to L-selectin ligands.
The most striking difference in binding specificity between the two antibodies concerns the contribution of N-glycans. Whereas MECA-79 can bind only to O-glycans, CL40 was able to recognize its epitope in the context of either O-glycans or N-glycans. This characteristic was first suggested in the CHO reconstitution experiments (Figure 2B) and was confirmed by showing that (1) N-glycanase treatment reduced CL40 binding to CD34 within human tonsillar PNAd (Figure 6A) and (2) N-glycanase treatment of murine lymph nodes sections reduced CL40 staining of HEVs (see Supplemental Figure S4 at http://ajp.amjpathol.org).
Not unexpectedly, the broader binding specificity of CL40 correlated with superior function-blocking activity. Thus, CL40 completely inhibited the binding of 300.19L cells to human tonsillar HEVs in the Stamper-Woodruff in vitro adhesion assay. In contrast, MECA-79 produced only partial inhibition (Figure 6B). CL40 was also more potent than MECA-79 in blocking in vitro attachment of 300.19L cells to HEVs in sections of murine lymph node (Figure 5A) and in inhibiting in vivo homing of lymphocytes to lymph nodes in mice (Figure 5B). To test the possibility that the superior inhibitory activity of CL40 could be attributed to its N-glycan binding, we used Core2GlcNAcT/Core1-β3GlcNAcT double-knockout mice.31 Residual homing in these mice depends on 6-sulfo sLex determinants carried by N-glycans.31 CL40 (but not MECA-79) was able to stain PLN HEVs in these mice and inhibited homing by approximately 50%, substantiating the ability of CL40 to recognize N-glycans in a biological context. The use of CL40 in combination with these double-knockout mice may be valuable in dissecting other cases of N-glycan-dependent L-selectin ligands in normal or disease settings.
The dramatic superiority of CL40 over MECA-79 in blocking lymphocyte adherence to tonsillar HEVs argues for the cooperation of both N-glycan- and O-glycan-borne determinants in constituting L-selectin ligands in a human lymphoid organ. This finding provides independent support for previous studies using different approaches.15,30 It should be noted that a precedent exists for a L-selectin ligand that is exclusively based on N-glycans, namely, the HCELL glycoform of CD44 on human hematopoietic progenitor cells.49 However, this ligand would not be expected to be recognized by CL40, because the ligand activity does not require sulfation.
Our investigation of inflammatory models in mouse and inflammatory diseases in human validates the utility of CL40 as an alternative to MECA-79 for immunohistochemistry. In three murine models, we found indistinguishable staining of HEV-like vessels by the two antibodies. In our survey of human RA and UC cases, every HEV-like vessel that stained with MECA-79 was also positive for CL40. Notably, the staining reaction was generally stronger for CL40, and in the case of RA we found vessels that stained with CL40 but not MECA-79. It is plausible that the enhanced staining by CL40 reflects L-selectin ligands that are based on N-glycans. It remains to be determined whether the MECA-79 negative ligands reported for certain endothelial cells47,48 rely on N-glycans and would be reactive with CL40. In conclusion, we believe that CL40 is an improved ligand reporter antibody compared with MECA-79, the standard in the field. Furthermore, CL40 may have utility as a therapeutic agent in animal models of chronic inflammation.
Note Added in Proof
While the present manuscript was being prepared for publication, Hirakawa et al50 reported a similar antibody, which was also generated by immunizing ST-1/ST-2 double deficient mice.
Acknowledgments
We thank Dr. Clive Wood of Dyax Corporation for providing CL40 and Bruce Hironaka of Thios Pharmaceuticals for the synthetic O-glycans. We thank Ted Yednock for valuable advice. The FTIV/FTVII double-knockout mice were provided by Core F of the Consortium for Functional Glycomics.
Footnotes
Supported by grants from the National Institutes of Health (R01-GM57411 and R01-GM23547) (S.D.R.); by grants (P01- CA71932 and R01-CA33000) (M.F.); by a postdoctoral fellowship from the National Arthritis Foundation (A105190/Fund 86075) (H.A.-K.); and by a fellowship from the Toyobo Biotechnology Foundation (Y.I.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi:10.1016/j.ajpath.2010.11.009.
Supplementary data
Sialic acid requirement for CL40 staining of high endothelial venules (HEVs). A: Sections of murine peripheral lymph node (PLN) were treated with mild periodate or with buffer control, and stained with CL40, MECA-79, or L-selectin/Fc chimera as indicated. Mild periodate oxidation oxidizes the side chain of sialic acid and has been shown to enhance staining of PLN HEVs by L-selectin/Fc chimera (Norgard et al.; Proc Natl Acad Sci USA 1993, 90:1068–1072). B: Sections of murine PLN were treated with neuraminidase or buffer control and stained as above. Scale bar = 100 μm.
Fucosylation independence for CL40 staining of HEVs. Sections of murine PLN from wild-type C57BL/6 mice and FTIV/FTVII double-knockout mice on the same background were stained with CL40 or MECA-79. Scale bar = 50 μm.
Staining of HEVs by CL40 and MECA-79 at different concentrations. Sections of murine PLN from wild-type C57BL/6 mice were stained with MECA-79 or CL40 at the indicated final concentrations. Two-step staining was used in both bases with a Cy3-conjugated goat anti-mouse IgG1 for CL40 and a Cy3-conjugated goat anti-rat IgM for MECA-79.
CL40 staining of HEVs in mice lacking O-glycan branches and effect of N-glycanase on CL40 staining. A: Consecutive sections of PLN from Core1GlcNAcT/Core2GlcNAcT double-knockout mice were stained with the indicated antibodies or isotype control immunoglobulins at 100 μg/mL. Scale bar = 100 μm. B: Consecutive sections of PLN from wild-type mice were treated with N-glycan or control buffer and stained with CL40.
Effects of CL40 on lymphocyte homing in ST-1/ST-2 double-knockout mice. Short-term lymphocyte homing was performed in the presence of 200 μg per mouse CL40 or isotype matched IgG1. The level of homing to PLN was approximately 32% of that seen in wild-type mouse, consistent with previous observations.22
References
- 1.Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Vestweber D., Blanks J.E. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79:181–213. doi: 10.1152/physrev.1999.79.1.181. [Erratum appears in Physiol Rev 2000, 80: follow i] [DOI] [PubMed] [Google Scholar]
- 3.von Andrian U.H., Mempel T.R. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 4.Miyasaka M., Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol. 2004;4:360–370. doi: 10.1038/nri1354. [DOI] [PubMed] [Google Scholar]
- 5.Rosen S.D. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 6.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 7.Uchimura K., Rosen S.D. Sulfated L-selectin ligands as a therapeutic target in chronic inflammation. Trends Immunol. 2006;27:559–565. doi: 10.1016/j.it.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Streeter P.R., Rouse B.T.N., Butcher E.C. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg E.L., Robinson M.K., Warnock R.A., Butcher E.C. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Andrian U.H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation. 1996;3:287–300. doi: 10.3109/10739689609148303. [DOI] [PubMed] [Google Scholar]
- 11.Hemmerich S., Leffler H., Rosen S.D. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- 12.Mitsuoka C., Sawada-Kasugai M., Ando-Furui K., Izawa M., Nakanishi H., Nakamura S., Ishida H., Kiso M., Kannagi R. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis X. J Biol Chem. 1998;273:11225–11233. doi: 10.1074/jbc.273.18.11225. [DOI] [PubMed] [Google Scholar]
- 13.Yeh J.C., Hiraoka N., Petryniak B., Nakayama J., Ellies L.G., Rabuka D., Hindsgaul O., Marth J.D., Lowe J.B., Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension beta 1,3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka N., Kawashima H., Petryniak B., Nakayama J., Mitoma J., Marth J.D., Lowe J.B., Fukuda M. Core 2 branching beta1,6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J Biol Chem. 2004;279:3058–3067. doi: 10.1074/jbc.M311150200. [DOI] [PubMed] [Google Scholar]
- 15.Mir G.H., Helin J., Skarp K.-P., Cummings R.D., Makitie A., Renkonen R., Leppanen A. Glycoforms of human endothelial CD34 that bind L-selectin carry sulfated sialyl Lewis x capped O- and N-glycans. Blood. 2009;114:733–741. doi: 10.1182/blood-2009-03-210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchimura K., Muramatsu H., Kadomatsu K., Fan Q.W., Kurosawa N., Mitsuoka C., Kannagi R., Habuchi O., Muramatsu T. Molecular cloning and characterization of an N-acetylglucosamine-6-O- sulfotransferase. J Biol Chem. 1998;273:22577–22583. doi: 10.1074/jbc.273.35.22577. [DOI] [PubMed] [Google Scholar]
- 17.Bistrup A., Bhakta S., Lee J.K., Belov Y.Y., Gunn M.D., Zuo F.R., Huang C.C., Kannagi R., Rosen S.D., Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high-endothelial-cell ligands for L-selectin. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiraoka N., Petryniak B., Nakayama J., Tsuboi S., Suzuki M., Yeh J.-C., Izawa D., Tanaka T., Miyasaka M., Lowe J.B., Fukuda M. A novel, high endothelial venule-specific sulfotransferase expresses 6-sulfo sialyl Lewisx, an L-selectin ligand displayed by CD34. Immunity. 1999;11:79–89. doi: 10.1016/s1074-7613(00)80083-7. [DOI] [PubMed] [Google Scholar]
- 19.Gauguet J.M., Rosen S.D., Marth J.D., von Andrian U.H. Core 2 branching beta1,6-N-acetylglucosaminyltransferase and high endothelial cell N-acetylglucosamine-6-sulfotransferase exert differential control over B- and T-lymphocyte homing to peripheral lymph nodes. Blood. 2004;104:4104–4112. doi: 10.1182/blood-2004-05-1986. [DOI] [PubMed] [Google Scholar]
- 20.Hemmerich S., Bistrup A., Singer M.S., van Zante A., Lee J.K., Tsay D., Peters M., Carminati J.L., Brennan T.J., Carver-Moore K., Leviten M., Fuentes M.E., Ruddle N.H., Rosen S.D. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 21.Kawashima H., Petryniak B., Hiraoka N., Mitoma J., Huckaby V., Nakayama J., Uchimura K., Kadomatsu K., Muramatsu T., Lowe J.B., Fukuda M. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol. 2005;6:1096–1104. doi: 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- 22.Uchimura K., Gauguet J.M., Singer M.S., Tsay D., Kannagi R., Muramatsu T., von Andrian U.H., Rosen S.D. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol. 2005;6:1105–1113. doi: 10.1038/ni1258. [DOI] [PubMed] [Google Scholar]
- 23.Uchimura K., Kadomatsu K., El-Fasakhany F.M., Singer M.S., Izawa M., Kannagi R., Takeda N., Rosen S.D., Muramatsu T. N-acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing. J Biol Chem. 2004;279:35001–35008. doi: 10.1074/jbc.M404456200. [DOI] [PubMed] [Google Scholar]
- 24.van Zante A., Gauguet J.M., Bistrup A., Tsay D., von Andrian U.H., Rosen S.D. Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse. J Exp Med. 2003;198:1289–1300. doi: 10.1084/jem.20030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michie S.A., Streeter P.R., Bolt P.A., Butcher E.C., Picker L.J. The human peripheral lymph node vascular addressin. Am J Pathol. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]
- 26.Renkonen J., Tynninen O., Häyry P., Paavonen T., Renkonen R. Glycosylation might provide endothelial zip codes for organ-specific leukocyte traffic into inflammatory sites. Am J Pathol. 2002;161:543–550. doi: 10.1016/S0002-9440(10)64210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bistrup A., Tsay D., Shenoy P., Singer M.S., Bangia N., Luther S.A., Cyster J.G., Ruddle N.H., Rosen S.D. Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in HEV-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope. Am J Pathol. 2004;164:1635–1644. doi: 10.1016/S0002-9440(10)63722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drayton D.L., Liao S., Mounzer R.H., Ruddle N.H. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 29.Rosen S.D., Tsay D., Singer M.S., Hemmerich S., Abraham W.M. Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma. Am J Pathol. 2005;166:935–944. doi: 10.1016/S0002-9440(10)62313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark R.A., Fuhlbrigge R.C., Springer T.A. L-Selectin ligands that are O-glycoprotease resistant and distinct from MECA-79 antigen are sufficient for tethering and rolling of lymphocytes on human high endothelial venules. J Cell Biol. 1998;140:721–731. doi: 10.1083/jcb.140.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitoma J., Bao X., Petryanik B., Schaerli P., Gauguet J.M., Yu S.Y., Kawashima H., Saito H., Ohtsubo K., Marth J.D., Khoo K.H., von Andrian U.H., Lowe J.B., Fukuda M. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–418. doi: 10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- 32.Mitoma J., Miyazaki T., Sutton-Smith M., Suzuki M., Saito H., Yeh J.C., Kawano T., Hindsgaul O., Seeberger P.H., Panico M., Haslam S.M., Morris H.R., Cummings R.D., Dell A., Fukuda M. The N-glycolyl form of mouse sialyl Lewis X is recognized by selectins but not by HECA-452 and FH6 antibodies that were raised against human cells. Glycoconj J. 2009;26:511–523. doi: 10.1007/s10719-008-9207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratt M.R., Bertozzi C.R. Syntheses of 6-sulfo sialyl Lewis X glycans corresponding to the L-selectin ligand “sulfoadhesin”. Org Lett. 2004;6:2345–2348. doi: 10.1021/ol0493195. [DOI] [PubMed] [Google Scholar]
- 34.Kansas G.S., Ley K., Munro J.M., Tedder T.F. Regulation of leukocyte rolling and adhesion to high endothelial venules through the cytoplasmic domain of L-selectin. J Exp Med. 1993;177:833–838. doi: 10.1084/jem.177.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MalÝ P., Thall A.D., Petryniak B., Rogers C.E., Smith P.L., Marks R.M., Kelly R.J., Gersten K.M., Cheng G., Saunders T.L., Camper S.A., Camphausen R.T., Sullivan F.X., Isogai Y., Hindsgaul O., von Andrian U.H., Lowe J.B. The α(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E- and P-selectin ligand biosynthesis. Cell. 1996;86:643–653. doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- 36.Mitoma J., Petryniak B., Hiraoka N., Yeh J.C., Lowe J.B., Fukuda M. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis x-type L-selectin ligand activity. J Biol Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. [DOI] [PubMed] [Google Scholar]
- 37.Bierhuizen M.F., Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1-3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci USA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J., Rosen S.D., Bendele P., Hemmerich S. Induction of PNAd and N-acetylglucosamine 6-O-sulfotransferases 1 and 2 in mouse collagen-induced arthritis. BMC Immunol. 2006;7:12. doi: 10.1186/1471-2172-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamper H.B., Jr, Woodruff J.J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144:828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salmi M., Rajala P., Jalkanen S. Homing of mucosal leukocytes to joints: Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J Clin Invest. 1997;99:2165–2172. doi: 10.1172/JCI119389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pablos J.L., Santiago B., Tsay D., Singer M.S., Palao G., Galindo M., Rosen S.D. A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-alpha in cultured endothelial cells. BMC Immunol. 2005;6:6. doi: 10.1186/1471-2172-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzawa K., Kobayashi M., Sakai Y., Hoshino H., Watanabe M., Harada O., Ohtani H., Fukuda M., Nakayama J. Preferential induction of peripheral lymph node addressin on high endothelial venule-like vessels in the active phase of ulcerative colitis. Am J Gastroenterol. 2007;102:1499–1509. doi: 10.1111/j.1572-0241.2007.01189.x. [DOI] [PubMed] [Google Scholar]
- 43.Hemmerich S., Butcher E.C., Rosen S.D. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and [sic] adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Puri K.D., Finger E.B., Gaudernack G., Springer T.A. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai S., Davis A.E., 3rd Complement regulatory protein C1 inhibitor binds to selectins and interferes with endothelial-leukocyte adhesion. J Immunol. 2003;171:4786–4791. doi: 10.4049/jimmunol.171.9.4786. [DOI] [PubMed] [Google Scholar]
- 46.Giuffrè L., Cordey A.S., Monai N., Tardy Y., Schapira M., Spertini O. Monocyte adhesion to activated aortic endothelium: role of L-selectin and heparan sulfate proteoglycans. J Cell Biol. 1997;136:945–956. doi: 10.1083/jcb.136.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tu L., Delahunty M.D., Ding H., Luscinskas F.W., Tedder T.F. The cutaneous lymphocyte antigen is an essential component of the L-selectin ligand induced on human vascular endothelial cells. J Exp Med. 1999;189:241–252. doi: 10.1084/jem.189.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shigeta A., Matsumoto M., Tedder T.F., Lowe J.B., Miyasaka M., Hirata T. An L-selectin ligand distinct from P-selectin glycoprotein ligand-1 is expressed on endothelial cells and promotes neutrophil rolling in inflammation. Blood. 2008;112:4915–4923. doi: 10.1182/blood-2008-04-153866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sackstein R., Dimitroff C.J. A hematopoietic cell L-selectin ligand that is distinct from PSGL-1 and displays N-glycan-dependent binding activity. Blood. 2000;96:2765–2774. [PubMed] [Google Scholar]
- 50.Hirakawa J., Tsuboi K., Sato K., Kobayashi M., Watanabe S., Takakura A., Imai Y., Ito Y., Fukuda M., Kawashima H. Novel anti-carbohydrate antibodies reveal the cooperative function of sulfated N- and O-glycans in lymphocyte homing. J Biol Chem. 2010 doi: 10.1074/jbc.M110.167296. doi:10.1074/jbc.M110.167296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sialic acid requirement for CL40 staining of high endothelial venules (HEVs). A: Sections of murine peripheral lymph node (PLN) were treated with mild periodate or with buffer control, and stained with CL40, MECA-79, or L-selectin/Fc chimera as indicated. Mild periodate oxidation oxidizes the side chain of sialic acid and has been shown to enhance staining of PLN HEVs by L-selectin/Fc chimera (Norgard et al.; Proc Natl Acad Sci USA 1993, 90:1068–1072). B: Sections of murine PLN were treated with neuraminidase or buffer control and stained as above. Scale bar = 100 μm.
Fucosylation independence for CL40 staining of HEVs. Sections of murine PLN from wild-type C57BL/6 mice and FTIV/FTVII double-knockout mice on the same background were stained with CL40 or MECA-79. Scale bar = 50 μm.
Staining of HEVs by CL40 and MECA-79 at different concentrations. Sections of murine PLN from wild-type C57BL/6 mice were stained with MECA-79 or CL40 at the indicated final concentrations. Two-step staining was used in both bases with a Cy3-conjugated goat anti-mouse IgG1 for CL40 and a Cy3-conjugated goat anti-rat IgM for MECA-79.
CL40 staining of HEVs in mice lacking O-glycan branches and effect of N-glycanase on CL40 staining. A: Consecutive sections of PLN from Core1GlcNAcT/Core2GlcNAcT double-knockout mice were stained with the indicated antibodies or isotype control immunoglobulins at 100 μg/mL. Scale bar = 100 μm. B: Consecutive sections of PLN from wild-type mice were treated with N-glycan or control buffer and stained with CL40.
Effects of CL40 on lymphocyte homing in ST-1/ST-2 double-knockout mice. Short-term lymphocyte homing was performed in the presence of 200 μg per mouse CL40 or isotype matched IgG1. The level of homing to PLN was approximately 32% of that seen in wild-type mouse, consistent with previous observations.22


