Abstract
Evaluation of the pattern of smoking prevalence over time could provide insight for disease prevention and tobacco control policy. Examination of assortative mating is important in assessing the relative contribution of genetic and environmental influences in behavior genetics. A significant spousal concordance for smoking could imply assortative mating behavior and have potential influence on the risk for offspring smoking. Using intergenerational data from large scale adult twin panels in Australia, the present study examined the patterns of prevalence and spousal concordance of lifetime smoking across a wide range of birth cohorts. Data were collected for more than 16,000 twins, their spouses, parents, and parents-reported grandparents. The measurement models were used to calculate prevalence and spousal concordance for smoking. Prevalence of lifetime smoking for females increased significantly over successive cohorts, and was higher for twins (0.28–0.49) than for their parents or grandparents (0.06–0.42). Smoking prevalence was still higher in males than in females for the twins (0.49–0.69), and parental or grandparental (0.62–0.77) generations, but the gender differences largely decreased in younger cohorts. Moderate but significant spousal correlations were found across cohorts and generations, which were higher for twins (0.39–0.55) than for their parents and grandparents (0.19–0.49). Despite using a simplified smoking assessment, findings in this study demonstrated changes of lifetime smoking patterns across birth cohorts and the presence of assortative mating for smoking behavior.
Keywords: lifetime smoking, spousal concordance, assortative mating, multiple generations
1. Introduction
Tobacco use has become a serious global public health issue. It is the leading but preventable cause of disease in developing and developed regions (Ezzati and Lopez, 2003; Proctor, 2001). Deaths caused by tobacco use have increased by more than 1 million between 1990 and 2000 (Ezzati et al., 2002), but global tobacco consumption continues to increase (Ezzati and Lopez, 2003). In 1998 the World Health Organization reported that world-wide smoking prevalence was higher overall for men (47.9%) than for women (12.4%) (Corrao et al., 2000). Nevertheless, the current increase in prevalence appears to be primarily driven by increased smoking uptake by young people and women (Twombly, 2003). Overall smoking rates have decreased in many developed countries in recent years (Bray et al., 2004), which may be partially attributed to the implementation of tobacco control strategies (White et al., 2003). However, several studies in Europe have found that male smoking prevalence decreases across successive birth cohorts, while the female prevalence increases (Osler et al., 1998; Laaksonen et al., 1999; Kemm, 2001; Fernandez et al., 2003).
Based on information of smoking patterns from many countries, Lopez and colleagues (1994) proposed a model with four distinct stages to characterize smoking epidemic over time. In brief, stage I is a beginning of the smoking epidemic. Stage II is a prevalence increasing period; male smoking rises rapidly reaching a high peak and female smoking increases, too. In stage III, male smoking prevalence begins to decline and female smoking keeps increasing till reaching a plateau. In stage IV, smoking prevalence declines slowly for both genders and reaches a similar level of around 30% at the end. Considering the significantly different phases of the cigarette epidemic across time span, few previous studies have attempted to examine changes in smoking prevalence using data collected across wide range of birth cohorts. Studies to date include those from Great Britain (birth cohort 1897–1966) (Kemm, 2001), Finland (birth cohort 1913–1972) (Laaksonen et al., 1999), Denmark (aged from 30 to more than 70 years old) (Osler et al., 1998), and Canada (birth cohort 1940–1975) (Birkett, 1997). Trends are not consistent across countries for smoking prevalence in males and females. Results from Great Britain and Canada demonstrated a continuing decline of smoking prevalence in males over time, whereas for females both studies found that smoking prevalence increased from the oldest cohorts, and then decreased to a similar magnitude as men in younger cohorts. However, the birth cohort with the highest smoking prevalence for females was much younger in the Canadian sample (born around 1960), than in the Great Britain sample (born 1922–1926), showing that there may be some factors influencing secular trends in tobacco use in different populations (e.g. changes in tobacco control policies or tobacco marketing). Cross-generational tobacco use could document these influences in a single population over time without confounding trends in tobacco use with larger demographic changes in the population. Thus, examining changes of prevalence in different generations across several birth cohorts helps to control for possible demographic shifts in the population over time.
In the smoking epidemic model introduced above (Lopez et al., 1994), females have a one- to two-decade lag for the peak in smoking prevalence compared to males. After reaching a plateau, female smoking prevalence starts to decrease slightly at the end of stage III, but yet the high female smoking rate could last for decades. Prior to this stage, female smoking prevalence will having increase dramatically during stage II. The increase in smoking amongst women may increase the magnitude of spousal concordance (i.e. higher similarities for behavioral traits between spouses) for smoking, thus increasing the likelihood of smoking exposure for their offspring. Parental and sibling smoking have been associated with smoking in offspring (Tyas and Pederson, 1998) and having two parents who smoke regularly increases risk for tobacco use in the next generation (Komro et al., 2003). This increased risk of smoking in offspring is due at least in part to environmental factors transmitted from parents. If, for example, both parents are regular smokers, children tend to have higher exposure to a smoking environment than if neither parent smokes. Conversely, parental smoking cessation reduces this environmental exposure and also helps lower the risk for adolescent smoking (Chassin et al., 2002).
On the other hand, spousal smoking patterns may also reflect a genetic influence on smoking behavior. Tobacco use has substantial genetic influences, with genetic factors explaining 39–49% of the variance in smoking persistence among adults (Madden et al., 1999), and 39% (0–68%) and 86% (70–94%) of the variation in smoking initiation and smoking quantity among youth, respectively (Koopmans et al., 1999). Assortative mating based on phenotypic assortment (wherein people tend to marry those who have similar traits to themselves) is an especially important element in assessing the relative contribution of genetic and environmental influences on variation in a trait. If phenotypic assortment strongly affects the observed spousal genetic correlation for smoking, offspring are at increased risk for smoking due to genetic factors, in addition to the environmental risks described above (Kearsey and Pooni, 1996).
Assortative mating for human behavior is not uncommon; it has already been identified for a variety of different traits, including height, body mass index and obesity (Hebebrand et al., 2000; Silventoinen et al., 2003), adult antisocial behavior (Galbaud du Fort et al., 2002), neuroticism (Lake et al., 2000), major psychiatric diagnoses (Maes et al., 1998), and marijuana use (Hopfer et al., 2003). Additionally, assortative mating for human behavior is an important issue in genetic studies, as it is confounded with shared environment (Neale and Cardon, 1992). Thus failure to model this effect in a twin study will possibly inflate the estimates of shared environmental factors and underestimate genetic influences. The magnitude of spousal concordance for smoking can thus provide some indication of whether assortative mating is present for this trait. Therefore, we aim to evaluate whether assortative mating is relevant for smoking. In addition, it is of special interest to assess the pattern of spousal concordance for smoking over time given the presence of large and opposing changes in the trend for tobacco use in men and women in recent decades.
We used data collected from large scale Australian adult twin panels to estimate tobacco use prevalence across three generations (twins, their parents and grandparents), spanning a period of more than 80 years. The Australian population represents an interesting sample for examining changes in smoking prevalence as tobacco control programs have been in place for several decades. The overall prevalence of smoking in Australia declined from 1980 to 1995, reached a relative plateau during the mid- to late-1990s, and then continued to decline between 1998 and 2001 (White et al., 2003). These two periods of decline in smoking prevalence coincide with concerted tobacco control programs in Australia. The goals of this study were to examine smoking patterns in a number of birth cohorts across generations. We also evaluate the magnitude and trends of spousal concordance of smoking over time.
2. Methods
2.1. Sample
Participants were drawn from the Australian National Health and Medical Research Council volunteer twin registry in two twin panels. The 1981 twin panel (born in 1900–1964) was surveyed by mailed questionnaire in 1980–1981 and was reassessed in 1988–1989 (N=8,315) (Hannah et al., 1985; Heath et al., 1994; Jardine and Martin, 1984). The 1989 young adult twin cohort (born in 1964–1971) was assessed by questionnaire in 1989 and by follow-up interview in 1996–2000 (N=5,034) (Heath et al., 2001). All studies were approved by the Queensland Institute of Medical Research Human Research Ethics Committee. Subjects included in our analyses were from both 1981 and 1989 twin panels that completed questionnaires in the 1989 survey. There were 4,264 same-sex MZ female twins, 2,976 DZ female twins, 2,674 MZ male twins, 2,100 DZ male twins, and 3,995 unlike-sex DZ female/male twins. Eighty twin pairs had missing zygosity data and were not included in analyses. In addition to collecting questionnaire data from twins, relatives of twins, including parents, siblings, spouse/partners, and adult children of twins were invited to participate in the study. A questionnaire was administered to these participants (N=19,411). Among them, there were 2,617 fathers, 3,227 mothers, 3,885 spouses, 6,803 siblings, and 2,862 children of twins participated in assessments. Further details regarding ascertainment and sample characteristics are reported elsewhere (Heath et al., 1995; Lake et al., 2000). In brief, this twin sample represented typical Australian population in many respects including the prevalence of psychiatric symptoms (Kendler et al., 1986), but had slightly more middle class and educated males (Baker et al., 1996).
The mean ages (standard deviation) for twin generation were 33.8 (14.1) for 10263 females and 32.7 (13.3) for 8762 males (overall ranged from 18–90 years). For twin’s parents, the mean ages were 59.3 for mothers and 60.8 for fathers (overall ranged from 27–91 years). Because of the wide range of twins’ age, the magnitude of birth cohort effects in smoking behavior was assessed by grouping twins (and their spouses) into one of seven birth cohorts: 1964–1971 (and all twins in 1989 cohort), 1956–1963, 1948–1955, 1940–1947, 1932–1939, 1924–1931, and pre-1923.
2.2. Measures
Participants completed a series of instruments, including self-reported smoking behavior, lifestyle, personality, drinking habits, attitude, and sociodemographic variables. Smoking related items in questionnaires applied to both twin panels in the 1989 survey were the same and have been described in detail elsewhere (Heath et al., 1995). Smoking status was based on item which operationalized as 1) Never smoked, 2) Ex-smoker, or 3) Current smoker. Participants were asked to classify their smoking habits according to this item, as well as their twin (where relevant), mother, father and partner when they were alive.
If there are latent traits/factors influencing assortative mating behavior for smoking, calculation of spousal concordance can be viewed as an assessment of liability to smoking as well as the magnitude of exposure to tobacco use in offspring. Therefore, lifetime smoking was chosen as a more reasonable index than current smoking which may change from time to time. In this study, lifetime smoking was defined dichotomously as yes (ex-smoker/current smoker) or no (never smoked) for each individual.
2.3. Data analysis
Data management and descriptive statistics were conducted using SAS™ (SAS Institute Inc, 2001). As information on smoking status of family members was collected from a number of individuals, measurement models were used to better utilize smoking data from different sources. The estimates of prevalence and spousal correlations for smoking and their 95% confidence intervals in the models were calculated using the Mx program (Neale, 1997). Based on the framework of structural equation modeling, a latent trait in our measurement model represents a “true” smoking status for each individual, which is modeled using one or more indicators (smoking data) gathered from different sources. For instance, to obtain the best-estimate for smoking behavior of one twin, twin’s self-report and spouse-report data were fitted simultaneously into the model.
Assessing parental smoking status was more complicated as it was ascertained via multiple indicators, including both twins reports of parents, parents self-report, and spousal report. Among 5353 twin families, there were less than half of the families (2086) having at least one parent reported their own smoking status. Compared with twins’ reports on their parents’ smoking status, we found parents who were current-smokers tended to not participate in studies (p-value < 0.0001). Due to more missing data from parental self-report and potential attrition bias, we ran measurement models for parental smoking mainly based on data reported by twins as well as parental-report, but allowing missing data where parental reports were not available. Accordingly, parents were assigned birth cohorts based upon those of their twin children. For instance, if twins were in the 1948–1955 cohort, their parents were assigned to the same 1948–1955 cohort category regardless of age differences between father and mother. This was done for reasons mentioned above and to avoid parents whose birth years fall into different cohort categories not being utilized in estimation of spousal concordance.
Information on grandparents’ smoking was only available where parents of twins reported the smoking status of their own parents (grandparents of twins), and consequently limited data were available for this generation. Birth cohorts of the grandparents of twins were then assigned based upon the parents of twins (paternal or maternal) birth cohorts into five cohorts, which were post-1940, 1932–1939, 1924–1931, 1916–1923, and pre-1915.
The measurement models analyzed with Mx dealt well with missing data, and permitted inclusion of unbalanced data reported by various sources. The path coefficients of indicators estimated how well different reports predicted the true (latent) smoking status.1 The correlation between the latent smoking trait of individuals and their spouses was estimated to obtain the magnitude of potential assortative mating for smoking behavior. Smoking status was dichotomized and the underlying liability of smoking was assumed normal distribution. The area under normal distribution that lies above the threshold value represents the prevalence of lifetime smoking. The threshold values for the latent smoking trait were then estimated in the same measurement models. The measurement models were fitted to the different birth cohorts of the three generations.
3. Results
Table I shows the correlation of smoking status reported from various sources of relatives using complete data only. Twin’s self-report agreed with spousal report (r=0.91), and vise versa. For father’s smoking status, father’s self-report agreed with both twins’ report and the agreement was higher with mother’s report (r=0.91). For mother’s smoking status, father’s report also exhibited high agreement. For any other pairwise categories, the agreements were all high (r=0.84–0.90) for parental smoking status.
Table 1.
Correlations of self-report and relatives report of lifetime smoking status for twin, spouse, father, and mother
| Twin | Spouse | Father |
Mother |
||||||
|---|---|---|---|---|---|---|---|---|---|
| twin1 | twin2 | mother | twin1 | twin2 | father | ||||
| twin | 0.90 (2317) | twin2 | 0.86 (3894) | 0.90 (4009) | |||||
| spouse | 0.91 (2326) | father | 0.84 (1363) | 0.85 (1321) | 0.91 (1189) | 0.84 (1342) | 0.85 (1303) | ||
| mother | 0.85 (1797) | 0.84 (1761) | 0.87 (1879) | 0.90 (1845) | 0.90 (1191) | ||||
The numbers in parenthesis are the sample size within each category.
Because of the ability to deal with missing data in the measurement models, we utilized every data point to estimate path coefficients, or the “true score” regression coefficients. For the twin generation, the coefficients were very high from both twin’s self-report and spousal report (ranged from 0.973–0.9996). For the parental generation, the coefficients were also high (ranged from 0.952–0.997) from every source of the report data including twin’s report, spousal report and self-report. Thus, using spousal report or offspring-report for lifetime smoking behavior was as valid as self-report.
3.1. Prevalence of lifetime smoking in three generations
We report the prevalence of lifetime smoking in the three generations separately. For the twin generation (Table 2), the prevalence of smoking amongst females increased significantly with younger cohorts (z=11.99, p<0.0001 for Cochran-Armitage trend tests). At the time of survey, lifetime smoking prevalence for females was approximately 30% in older birth cohorts, while it was almost 50% in younger cohorts. In contrast, a reverse trend was observed for males in that lifetime smoking prevalence dropped significantly (z= −8.74, p<0.0001) from older to younger cohorts (from 69% to 50%). In Table 3, the prevalence of smoking amongst mothers increased across cohorts, from 9% in the oldest to 42% in the youngest cohort (z=8.78, p<0.0001). However, amongst fathers, smoking prevalence only slightly decreased across cohorts (from 75% to 68%). For the grandparents of twins generation (Table 4), smoking prevalence again significantly increased across birth cohorts (z=10.49, p<0.0001) for grandmothers of twins. In the contrary, a borderline increasing trend (z=1.45, p=0.07) of smoking prevalence exhibited for grandfathers of twins in younger cohorts. The overall smoking prevalence of grandfathers was much higher (64%–77%) than that of grandmothers (6%–33%).
Table 2.
Prevalence and spousal correlation of lifetime smoking for the twin generation, as a function of twin birth cohort
| Twin birth cohort | Nfamilies (%) | Male |
Female |
Spousal correlation |
|||
|---|---|---|---|---|---|---|---|
| Prevalence | 95% CI | Prevalence | 95% CI | Coefficientμ) | 95% CI | ||
| 1964–1971 | 4432 | 0.50 | (0.49–0.50) | 0.49 | (0.49–0.50) | 0.55 | (0.48–0.58) |
| 1956–1963 | 1812 | 0.50 | (0.47–0.51) | 0.47 | (0.44–0.48) | 0.51 | (0.49–0.57) |
| 1948–1955 | 1484 | 0.49 | (0.46–0.51) | 0.41 | (0.38–0.43) | 0.39 | (0.31–0.47) |
| 1940–1947 | 937 | 0.57 | (0.57–0.60) | 0.38 | (0.38–0.40) | 0.45 | (0.44–0.46) |
| 1932–1939 | 500 | 0.58 | (0.54–0.60) | 0.40 | (0.40–0.44) | 0.44 | (0.31–0.56) |
| 1924–1931 | 456 | 0.69 | (0.64–0.70) | 0.28 | (0.26–0.30) | 0.51 | (0.41–0.61) |
| pre 1923 | 381 | 0.64 | (0.59–0.69) | 0.29 | (0.25–0.34) | 0.43 | (0.25–0.59) |
Table 3.
Prevalence and spousal correlation of lifetime smoking for the parental generation, as a function of twin birth cohort
| Twin birth cohort | Nfamilies (%) | Father |
Mother |
Spousal correlation |
|||
|---|---|---|---|---|---|---|---|
| Prevalence | 95% CI | Prevalence | 95% CI | Coefficient(μ) | 95% CI | ||
| 1964–1971 | 2181 | 0.68 | (0.66–0.70) | 0.42 | (0.42–0.44) | 0.41 | (0.37–0.41) |
| 1956–1963 | 1046 | 0.68 | (0.68–0.70) | 0.34 | (0.31–0.37) | 0.46 | (0.45–0.46) |
| 1948–1955 | 838 | 0.71 | (0.71–0.71) | 0.35 | (0.34–0.35) | 0.45 | (0.45–0.47) |
| 1940–1947 | 532 | 0.71 | (0.68–0.74) | 0.32 | (0.32–0.33) | 0.39 | (0.38–0.42) |
| 1932–1939 | 285 | 0.76 | (0.71–0.79) | 0.26 | (0.24–0.27) | 0.27 | (0.24–0.33) |
| 1924–1931 | 258 | 0.75 | (0.71–0.77) | 0.18 | (0.18–0.23) | 0.19 | (−0.05–0.42)NS |
| pre 1923 | 213 | 0.62 | (0.61–0.69) | 0.09 | (0.05–0.11) | 0.34 | (0.10–0.53) |
The path coefficients of offspring report on parental history of smoking were in the range of 0.952–0.993 for fathers and 0.965–0.997 for mothers.
Table 4.
Prevalence and spousal correlation of lifetime smoking for the grandparental generation, as a function of parental birth cohort
| Twin birth cohort | Nfamilies (%) | Grandfather |
Grandmother |
Spousal correlation |
|||
|---|---|---|---|---|---|---|---|
| Prevalence | 95% CI | Prevalence | 95% CI | Coefficient (μ) | 95% CI | ||
| 1940- | 580 | 0.77 | (0.73–0.80) | 0.33 | (0.29–0.37) | 0.37 | (0.22–0.50) |
| 1932–1939 | 980 | 0.76 | (0.73–0.78) | 0.25 | (0.22–0.28) | 0.38 | (0.26–0.49) |
| 1924–1931 | 975 | 0.76 | (0.73–0.78) | 0.17 | (0.15–0.20) | 0.48 | (0.43–0.59) |
| 1916–1923 | 581 | 0.72 | (0.68–0.75) | 0.08 | (0.06–0.11) | 0.19 | (−0.02–0.38)NS |
| pre 1915 | 239 | 0.64 | (0.57–0.70) | 0.06 | (0.03–0.09) | 0.47 | (0.10–0.75) |
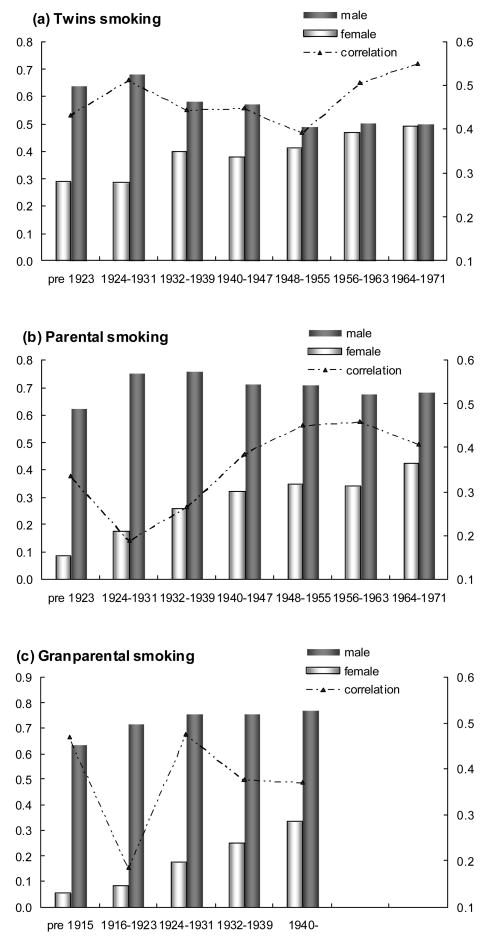
Comparing the prevalence of lifetime smoking across the three generations, we found that females in the same birth cohort had similar prevalence of lifetime smoking across generations. For instance, the prevalence of grandmaternal smoking in cohort 1940–1947 (Table 4) was very similar to that of maternal smoking in cohort 1940–1947, and so on for the following older cohorts. Results were similar for the comparison of female twin and maternal generations. The increasing trends of female smoking over time were very consistent across the three generations. For males, there was no such consistent trend. In both male twin and paternal generations, smoking prevalence had a decreasing trend in younger cohorts, but demonstrated an increasing trend in younger cohorts in grandpaternal generation. Figure 1 clearly demonstrated the change in smoking prevalence for both genders. In recent birth cohorts, especially in the twin generation, the differences in smoking prevalence between males and females appeared to decrease dramatically.
Figure 1.
(a)–(c): Estimated prevalence and spousal correlation of lifetime smoking, as a function of birth cohorts in the (a) twin generation; (b) parental generation; and (c) grandparental generation. The prevalence estimates follow the primary Y-axis, and the spousal correlation estimates follow the secondary Y-axis.
3.2. Spousal concordance in three generations
Estimates of spousal correlations are also shown in Tables and Figure 1. There were moderate but significant spousal correlations across the three generations, except for two older birth cohorts in the parental and grandparental generations. Spousal correlations were higher (ranged from 0.39–0.55) in the twin generation, and lower in the parental (0.19–0.46) and grandparental (0.19–0.48) generations. Due to fewer participants among these two generations, confidence intervals for spousal correlations were wider than those for twin generation. As shown in Figure 1, the smoking prevalence in females consistently increased from the oldest (6%) to the youngest (49%) cohorts, whereas the patterns of smoking concordance only demonstrated a weak increase following the prevalence change in women, this maybe partly due to the presence of large and opposing changes in the trend for tobacco use in men and women. Nevertheless, there was still a trend with higher smoking concordance in younger cohorts in both twins and parental generations.
4. Discussion
Consonant with other data, this study demonstrated a steadily increasing trend of lifetime smoking for females, from less than 10% in the oldest cohort to 49% in the 1964–1971 cohort. In contrast, a significant decreasing trend was found only for the male twin generation, from around 70% in older cohorts to 45% in the 1964–1971 cohort, and an increasing trend in the grandparental generation. This is consistent with Lopez and colleagues cigarette epidemic model, in which from stage II to stage III, male smoking prevalence first increases then declines, but female smoking prevalence keeps rising until reaching a plateau. Similar trends were found in population-based epidemiological surveys of smoking in Finland and Australia (Laaksonen et al., 1999; White et al., 2003). The Smoking and Health Survey in Australia reported that smoking prevalence in males was 40% in 1980 and only 25% in 2001, while female smoking prevalence was 30% in 1980 and 21% in 2001 (Laaksonen et al., 1999; White et al., 2003). If stratified by age, this secular trend still holds. Meanwhile, younger adults have higher smoking prevalence than older groups. Although our prevalence estimates were largely similar to those found in epidemiological studies of different birth cohorts, it is still necessary to bear in mind that the identification of risk factors associated with tobacco use may be quite different across generational or panel data. In the future, extended twin models which include data from different generations can be expanded to evaluate the continuity of possible predictors and mediators for the risk of smoking within families.
Males have a higher average prevalence of smoking than females; however, this gap is smaller in younger cohorts and this trend has been found worldwide. The Global Youth Tobacco Survey report based on 76 countries found more than half of the countries showed no difference by sex for cigarette smoking and other tobacco products (Global Youth Tobacco Survey Collaborating, 2003). This pattern is also similar for data from the US adults that the difference of smoking prevalence between males and females progressively diminished across years. In 1955, males had higher prevalence than women of 28.5% and reducing to 4.5% in 2001.
In the present study, tobacco use was operationalized as lifetime history of smoking rather than current smoking. Interestingly, we notice that compared to the results of current smoking prevalence obtained from the national cross-sectional survey in Australia by age groups, our lifetime smoking prevalence was roughly 5–10% higher than their rates of current smoking for female twins in all birth cohorts and for male twins in only younger cohorts. In male older birth cohorts (twins born before 1931), however, prevalence of current smoking in White et al. (2003) was much less than lifetime smoking in the present study (20–40% vs. over 60%). One reason to explain this may be the differences of smoking cessation rates across genders and cohorts. We would speculate that older men tend to stop smoking more frequently than younger men, but females appear to have similar smoking cessation rates across different age groups. This observation is partly supported by studies which found that men are more likely to quit smoking than women, and that older people are more successful at smoking cessation (Franks et al., 2002; Lopez et al., 1994; Monso et al., 2001). It indicates that the female population would have lower cessation rate, especially among younger women. Although female smoking cessation rates were not assessed in this study, based on our findings that nearly 30% of females in older cohorts smoked and the fact that young females in Australia have the highest smoking prevalence, tobacco use in women merits increased research scrutiny. Effective intervention programs need to not only to prevent smoking among young women, but should also target females for smoking cessation programs.
A modest but significant spousal concordance for smoking was found across most birth cohorts; dropping the spouse correlation causes significant lost of model fit. It indicates the presence of assortative mating for smoking in this population, i.e. increasing the probability that individuals will marry someone with a similar underlying liability for smoking. Even among older cohorts with relatively low female smoking prevalence, the spousal resemblance was still modest and significant. A number of different explanations could put forward to explain this finding. It could be due to direct phenotypic correlations between spouses for smoking behavior, and therefore may affect the genetic risk for smoking in offspring. Alternatively, spousal concordance could be the result of social homogamy in which people prefer mates from a similar culture, social class, or other factor related to but distinct from smoking. Regardless of whether this similarity is mediated by genetic or environmental factors, the increasing spousal concordance for smoking raises the risk of smoking in the offspring generation.
The link between spousal concordance and female smoking prevalence was not remarkable. This may be because the different patterns of smoking prevalence change by sex over time. However, while not significant, there were still patterns shown in the younger cohorts (See Figure 1) that spousal correlations for smoking were higher with increasing prevalence of female smoking. If the prediction of Lopez and colleagues model is correct, after female smoking rate reaches a plateau in stage III, both male and female smoking rate would decline in stage IV and end up with a smoking prevalence around 30%. Thus, by reducing the prevalence of female smoking and maintaining the declining trends in male smoking, we might reduce the spousal correlation and lower the risk of smoking in offspring. On the other hand, if the concordance of spouses for smoking status is due to shared environmental factors experienced after marriage, we would expect that spousal concordance would increase as a function of years of marriage. However, the degree of exposure to shared smoking environment during marriage was not assessed in this study. As parental assortative mating for lifetime smoking may be composed of both genetic and non-genetic mechanisms, the present study highlights the need for subsequent behavior genetic studies of parent-offspring data that can more clearly decompose the relative magnitude of environmental and genetic components underlying the concordance rates observed here.
There were two limitations in the present study. First, the lifetime smoking measurement was based on subjective report of participants’ smoking habit and not using quantified measures. We dichotomized the one-item smoking assessment, which apparently does not include the pattern and amount of lifetime tobacco use. Nevertheless, the average daily cigarette consumption of ex-smokers was not significant different from current-smokers (data not shown). Second, although we applied measurement models which incorporated multiple indicators to increase the precision of predicting the “true” smoking status for each individual, we were not able to gain the same power for grandparental smoking status due to single resource of parent-report only (Note the path coefficients estimated in the measurement models for twin report on parental smoking status were all above 0.95). To test the robustness of our results, we reduced the precision of path coefficients to 0.9 in the grandparental model (results not shown). Lower precision inflated the smoking correlation among grandparents but did not change the thresholds estimates. Thus, our estimates for smoking prevalence in grandparental generation were not influenced by report precision, but the spousal concordance might be inflated in this generation.
In summary, using large scale national twin samples, the present study demonstrated the changes of smoking patterns across a wide range of birth cohorts in both males and females. Significant assortative mating for smoking behavior was also observed in this population and the correlation exceeds 0.5 in younger cohorts.
Acknowledgments
We acknowledge the funding sources that supported this project, and earlier studies that collected the data used: NIH (USA) grants (DA00272, DA12854, DA12540, CA75581, AA007535, AA013320, AA013326, AA014041, AA07728, AA10249, AA11998) and NHMRC (Australia) grants (941177, 951023, 950998, 981339, 241916 and 941944). K.I.M. is supported by an Ian Scott Fellowship from the Australian Rotary Health Research Fund. The authors also thank Nathan Gillespie for helpful data management.
Footnotes
For the grandparents of twins generation, information on lifetime smoking was obtained from only one source, as each parent (mother or father) reported on their own parents. Therefore, the path coefficients were set as 1.0. To test for the robustness of model results, we reduced the path coefficients to 0.9 (details in Limitation section). Results showed that the threshold estimates were fairly stable but the spousal correlations might be inflated.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker LA, Treloar SA, Reynolds CA, Heath AC, Martin NG. Genetics of educational attainment in Australian twins: sex differences and secular changes. Behav Genet. 1996;26:89–102. doi: 10.1007/BF02359887. [DOI] [PubMed] [Google Scholar]
- Birkett NJ. Trends in smoking by birth cohort for births between 1940 and 1975: a reconstructed cohort analysis of the 1990 Ontario Health Survey. Preventive Medicine. 1997;26:534–541. doi: 10.1006/pmed.1997.0169. [DOI] [PubMed] [Google Scholar]
- Bray F, Tyczynski JE, Parkin DM. Going up or coming down? The changing phases of the lung cancer epidemic from 1976 to 1999 in the 15 European Union countries. Eur J Cancer. 2004;40:96–125. doi: 10.1016/j.ejca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson C, Rose J, Sherman SJ, Prost J. Parental smoking cessation and adolescent smoking. Journal of Pediatric Psychology. 2002;27:485–496. doi: 10.1093/jpepsy/27.6.485. [DOI] [PubMed] [Google Scholar]
- Corrao MA, Guindon GE, Cokkinides V, Sharma N. Building the evidence base for global tobacco control. Bulletin of the World Health Organization. 2000;78:884–890. [PMC free article] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003;362:847–852. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Hoorn SV, Murray CJL. Selected major risk factors and global and regional burden of disease. The Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Schiaffino A, Borras JM, Shafey O, Villalbi JR, La Vecchia C. Prevalence of cigarette smoking by birth cohort among males and females in Spain, 1910–1990. European Journal of Cancer Prevention. 2003;12:57–62. doi: 10.1097/00008469-200302000-00009. [DOI] [PubMed] [Google Scholar]
- Franks MM, Pienta AM, Wary LA. It takes two: Marriage and smoking cessation in the middle years. Journal of Aging and Health. 2002;14:336–354. doi: 10.1177/08964302014003002. [DOI] [PubMed] [Google Scholar]
- Galbaud du Fort G, Boothroyd LJ, Bland RC, Newman SC, Kakuma R. Spouse similarity for antisocial behaviour in the general population. Psychological Medicine. 2002;32:1407–1416. doi: 10.1017/s0033291702006530. [DOI] [PubMed] [Google Scholar]
- Global Youth Tobacco Survey Collaborating, G. Differences in worldwide tobacco use by gender: findings from the Global Youth Tobacco Survey. Journal of School Health. 2003;73:207–215. doi: 10.1111/j.1746-1561.2003.tb06562.x. [DOI] [PubMed] [Google Scholar]
- Hannah MC, Hopper JL, Mathews JD. Twin concordance for a binary trait. II. Nested analysis of ever-smoking and ex-smoking traits and unnested analysis of a “committed-smoking” trait. American Journal of Human Genetics. 1985;37:153–165. [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Cloninger CR, Martin NG. Testing a model for the genetic structure of personality: A comparison of the personality systems of Cloninger and Eysenck. J Personal Soc Psychol. 1994;66:762–775. doi: 10.1037//0022-3514.66.4.762. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Kirk KM, Madden PA, Bucholz KK, Nelson EC, Slutske WS, Statham DJ, Martin NG. Predictors of non-response to a questionnaire survey of a volunteer twin panel: findings from the Australian 1989 twin cohort. Twin Research. 2001;4:73–80. doi: 10.1375/1369052012182. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Slutske WS, Martin NG. Personality and the inheritance of smoking behavior: A genetic perspective. Behavior Genetics. 1995;25:103–117. doi: 10.1007/BF02196921. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Wulftange H, Goerg T, Ziegler A, Hinney A, Barth N, Mayer H, Remschmidt H. Epidemic obesity: are genetic factors involved via increased rates of assortative mating? International Journal of Obesity. 2000;24:345–353. doi: 10.1038/sj.ijo.0801135. [DOI] [PubMed] [Google Scholar]
- Hopfer CJ, Stallings MC, Hewitt JK, Crowley TJ. Family transmission of marijuana use, abuse, and dependence. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:834–841. doi: 10.1097/01.CHI.0000046874.56865.85. [DOI] [PubMed] [Google Scholar]
- Jardine R, Martin NG. Causes of variation in drinking habits in a large twin sample. Acta Geneticae Medicae et Gemellologiae. 1984;33:435–450. doi: 10.1017/s0001566000005882. [DOI] [PubMed] [Google Scholar]
- Kearsey MJ, Pooni HS. The genetical analysis of quantitative traits. Chapman and Hall; London: 1996. [Google Scholar]
- Kemm JR. A birth cohort analysis of smoking by adults in Great Britain 1974–1998. Journal of Public Health Medicine. 2001;23:306–311. doi: 10.1093/pubmed/23.4.306. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath A, Martin NG, Eaves LJ. Symptoms of anxiety and depression in a volunteer twin population. The etiologic role of genetic and environmental factors. Arch Gen Psychiatry. 1986;43:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- Komro KA, McCarty MC, Forster JL, Blaine TM, Chen V. Parental, family, and home characteristics associated with cigarette smoking among adolescents. American Journal of Health Promotion. 2003;17:291–299. doi: 10.4278/0890-1171-17.5.291. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, Heath AC, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behavior Genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- Laaksonen M, Uutela A, Vartiainen E, Jousilahti P, Helakorpi S, Puska P. Development of smoking by birth cohort in the adult population in eastern Finland 1972–97. Tobacco Control. 1999;8:161–168. doi: 10.1136/tc.8.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake RI, Eaves LJ, Maes HH, Heath AC, Martin NG. Further evidence against the environmental transmission of individual differences in neuroticism from a collaborative study of 45,850 twins and relatives on two continents. Behavior Genetics. 2000;30:223–233. doi: 10.1023/a:1001918408984. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Collishaw NE, Piha T. A descriptive model of the cigarette epidemic in developed countries. Tob Control. 1994;3:242–247. [Google Scholar]
- Madden PA, Heath AC, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The genetics of smoking persistence in men and women: a multicultural study. Behavior Genetics. 1999;29:423–431. doi: 10.1023/a:1021674804714. [DOI] [PubMed] [Google Scholar]
- Maes HHM, Neale MC, Kendler KS, Hewitt JK, Silventoinen K, Foley DL, Meyer JM, Rutter M, Simonoff E, Pickles A, Eaves LJ. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychological Medicine. 1998;28:1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- Monso E, Campbell J, Tonnesen P, Gustavsson G, Morera J. Sociodemographic predictors of success in smoking intervention. Tobacco Control. 2001;10:165–169. doi: 10.1136/tc.10.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC. Genetics and Human Development Technical Report, Box 98012. Medical College of Virginia; Richmond, Va: 1997. Mx: Statistical Modeling. [Google Scholar]
- Neale MC, Cardon LR, editors. Methodology for Genetic Studies of Twins and Families. Kluwer Academic; New York: 1992. [Google Scholar]
- Osler M, Prescott E, Gottschau A, Bjerg A, Hein HO, Sjol A, Schnohr P. Trends in smoking prevalence in Danish adults, 1964–1994. The influence of gender, age, and education. Scandinavian Journal of Social Medicine. 1998;26:293–298. doi: 10.1177/14034948980260041101. [DOI] [PubMed] [Google Scholar]
- Proctor RN. Tobacco and the global lung cancer epidemic. Nature Reviews Cancer. 2001;1:82–86. doi: 10.1038/35094091. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT User’s Guide: Release Version 8.02. SAS Institute Inc; Cary, NC: 2001. [Google Scholar]
- Silventoinen K, Kaprio J, Lahelma E, Viken RJ. Assortative mating by body height and BMI: Finnish twins and their spouses. American Journal of Human Biology. 2003;15:620–627. doi: 10.1002/ajhb.10183. [DOI] [PubMed] [Google Scholar]
- Twombly R. Tobacco Use a Leading Global Cancer Risk, Report Says. J Natl Cancer Inst. 2003;95:11–12. doi: 10.1093/jnci/95.1.11. [DOI] [PubMed] [Google Scholar]
- Tyas SL, Pederson LL. Psychosocial factors related to adolescent smoking: a critical review of the literature. Tobacco Control. 1998;7:409–420. doi: 10.1136/tc.7.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White V, Hill D, Siahpush M, Bobevski I. How has the prevalence of cigarette smoking changed among Australian adults? Trends in smoking prevalence between 1980 and 2001. Tob Control Suppl. 2003;2:ii67–74. doi: 10.1136/tc.12.suppl_2.ii67. [DOI] [PMC free article] [PubMed] [Google Scholar]