Summary
The Xolloid secreted metalloprotease, a tolloid-related protein, was found to cleave Chordin and Chordin/BMP-4 complexes at two specific sites in biochemical experiments. Xolloid mRNA blocks secondary axes caused by chordin, but not by noggin, follistatin, or dominant-negative BMP receptor, mRNA injection. Xolloid-treated Chordin protein was unable to antagonize BMP activity. Furthermore, Xolloid digestion released biologically active BMPs from Chordin/BMP inactive complexes. Injection of dominant-negative Xolloid mRNA indicated that the in vivo function of Xolloid is to limit the extent of Spemann’s organizer field. We propose that Xolloid regulates organizer function by a novel proteolytic mechanism involving a double inhibition pathway required to pattern the dorsoventral axis:
Introduction
Recent advances on the molecular mechanisms by which the dorsal lip of the gastrula induces neuralization of ectoderm and dorsalization of mesoderm have revealed that the organizer secretes proteins that inhibit ventral signals (reviewed by Graff, 1997; Hemmati-Brivanlou and Melton, 1997; Moon et al., 1997). Three of these proteins, Chordin, Noggin, and Follistatin, bind to ventral BMP signals in the extracellular space, blocking the interaction of BMPs with their receptors (Piccolo et al., 1996; Zimmermann et al., 1996; Fainsod et al., 1997). The role of Chordin in this antagonistic model of organizer function has recently received genetic support with the finding that the ventralizing chordino mutation in zebrafish encodes a null allele of chordin (Hammerschmidt et al., 1996a; Schulte-Merker et al., 1997). In genetic analyses, chordino interacts with the dorsalizing swirl mutation, which is thought to encode a BMP-like activity. Double chordino−/−; swirl−/− mutants display a swirl dorsalized phenotype, suggesting that the sole function of Chordin is to inhibit ventralizing signals (Hammerschmidt et al., 1996b).
Chordin is a homolog of the protein encoded by the Drosophila short-gastrulation (sog) gene, sharing both structural (François and Bier, 1995) and functional (Holley et al., 1995) properties. Because sog is expressed ventrally in Drosophila and chordin dorsally in vertebrates, this finding has lent support to the hypothesis of E. Geoffroy Saint-Hilaire that an inversion of the dorsal-ventral axis occurred during the course of animal evolution (reviewed by De Robertis and Sasai, 1996). In genetic studies, sog functions as an inhibitor of decapentaplegic (dpp) (Ferguson and Anderson, 1992a). DPP is the main zygotic dorsoventral morphogen in Drosophila (Ferguson and Anderson, 1992b; Wharton et al., 1993) and is functionally homologous to vertebrate BMP-2 and BMP-4 (Padgett et al., 1993). As is the case for swirl and chordino, dpp is epistatic to sog in double mutants (Biehs et al., 1996; Holley et al., 1996), suggesting that sog is a dedicated antagonist of dpp.
dpp also interacts genetically with tolloid (tld), but this interaction has opposite effects to those of sog, since tolloid is required to increase the activity of dpp (Ferguson and Anderson, 1992a). Many tld alleles are available, forming an unusually rich allelic complementation series and, interestingly, about a third of them are antimorphs that are thought to act as dominant inhibitors of tld activity (Jürgens et al., 1984; Ferguson and Anderson, 1992a; Childs and O’Connor, 1994; Finelli et al., 1994). tolloid encodes a secreted metalloprotease of the astacin family that contains in its carboxyterminal portion an extended interaction domain. This domain is composed of two EGF repeats and five repeats similar to those found in the protein–protein interaction domains of the serum complement proteins C1r and C1s (Shimell et al., 1991).
The structure of tolloid is related to that of BMP-1, a metalloprotease isolated from demineralized bone extracts that purified together with the TGFβ superfamily members BMP-2 and BMP-3 (Wozney et al., 1988). The copurification suggested that BMP-1 and BMP-2 might physically interact, leading to the idea that Tolloid might increase DPP activity by proteolytically processing DPP precursors (Shimell et al., 1991; Childs and O’Connor, 1994; Finelli et al., 1994). However, the finding that the sog homolog chordin formed an inactive complex with BMPs with high specificity (Piccolo et al., 1996) suggested an alternative possibility. Holley et al. (1996) proposed that the Tolloid protease might cleave a hypothetical complex of SOG and DPP, releasing active DPP from an inactive complex. In other words, the activation of DPP activity by TLD could result from a double inhibition mechanism by which:
This molecular mechanism can be tested biochemically. The availability of active Chordin protein and of recombinant BMPs of Xenopus origin (Hazama et al., 1995; Piccolo et al., 1996) led us to test whether a similar mechanism exists in Xenopus and has been conserved for regulating the activity of Chordin in the Spemann organizer. For this purpose, we used a Xenopus tolloid-like gene called Xolloid (Goodman et al., 1997). Xolloid was chosen because, like BMP-4, it has a strong ventralizing activity in Xenopus animal cap explants, in which it is able to override the dorsalizing effects of activin, leading to the formation of blood and ventral mesoderm, as BMP-4 does (Dale et al., 1992; Jones et al., 1992; Goodman et al., 1997). In the Xenopus embryo, Xolloid mRNA accumulates in oocytes and unfertilized eggs, and levels are maintained through early development until the start of neurulation (Goodman et al., 1997). At these stages, the Xenopus embryo also expresses significant amounts of BMP-2, BMP-4, and BMP-7 (Dale et al., 1992; Nishimatsu et al., 1992; Hemmati-Brivanlou and Thomsen, 1995). Therefore, Xolloid and BMPs are coexpressed during early development. Although Xolloid transcripts appear to be uniformly distributed at these early stages, at the tailbud stage Xolloid transcripts are found in the tail periphery surrounding the chordin-expressing cells of the chordoneural hinge (B. L., unpublished data). Thus, Xolloid is expressed at the right time in development to possibly interact with Chordin and BMPs.
The overall structure of Xolloid is very similar to that of tolloid, with a metalloprotease domain followed by an interaction domain composed of two EGF and five C1r/s repeats. In the mouse, two related genes have been isolated, tolloid-like (mtll) and mBMP-1. mtll has an overall structure similar to that of Xolloid and tolloid (Takahara et al., 1996), although it is unclear at present whether it is a Xolloid homolog. A similar structure is also found in a spliced form of BMP-1, while a shorter form lacks one EGF and two C1r/s repeats. Both forms have been shown to process the C terminus of fibrillar procollagens (Kessler et al., 1996). In contrast to Xolloid, the short-form of Xenopus BMP-1 is unable to mimic the blood-inducing activity of BMP-4 in activin-treated animal caps (Goodman et al., 1997; Maéno et al., 1993), and the long form is reported to dorsalize ventral mesoderm (Lin et al., 1997). Taken together, the biological activity in animal caps, the expression in the early embryo, and the structural similarities suggested that Xolloid was the best candidate to mediate a tolloid-like activity in Xenopus.
In this study, we present data showing that in mRNA microinjection assays, Xolloid acts upstream of BMP receptor signaling, blocking the effects of chordin but not those of noggin or follistatin. In biochemical studies, soluble Xolloid protein has protease activity, cleaving chordin at two specific sites and inactivating its BMP antagonizing activity. Digestion of an inactive complex of BMPs and Chordin with Xolloid results in the recovery of BMP biological activity. Experiments with a dominant-negative point mutation in Xolloid indicate that the Xolloid protease activity is required in vivo for correct dorsoventral patterning in Xenopus. Taken together, the results suggest a novel mechanism for regulating the activity of Spemann’s organizer mediated by a proteolytic step.
Results
Xolloid mRNA Ventralizes Ectoderm and Mesoderm
To test the hypothesis that Xolloid might inactivate Chordin, we first investigated whether Xolloid microinjection could mimic the effects of chordino loss-of-function in zebrafish. Xolloid mRNA was injected into four blastomeres at the 4- or 8-cell stage (radial injections) of Xenopus development, and the resulting embryos were analyzed by whole-mount in situ hybridization. At the gastrula/neurula stage, a considerable reduction of the neural domain (marked by Sox-2 and Slug) was observed in the ectoderm (Figures 1A and 1B). Markers of dorsal mesoderm were also reduced by injection of Xolloid (sonic hedgehog and MyoD, Figures 1C and 1D), while a ventral mesodermal marker was expanded (Xwnt-8, Figure 1F). Despite the strong ventralization revealed by these early markers, by the swimming tadpole stage (3 days) similarly injected embryos recovered to a considerable extent (Figure 1I). However, these tadpoles still had reduced heads and CNS, the notochord was frequently absent, somites were reduced, and concomitantly, an increased amount of ventral blood islands were observed in histological sections (data not shown).
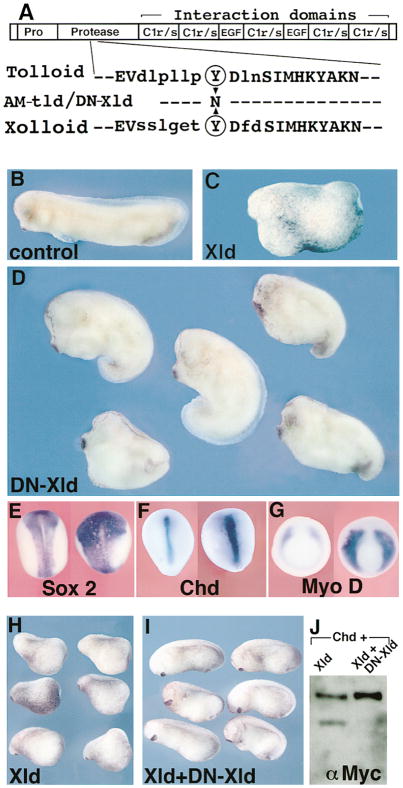
Figure 1. Xolloid (XLD) Ventralizes Ectoderm and Mesoderm.
(A–H) Ventralization of ectoderm and mesoderm revealed by whole-mount in situ hybridization. Uninjected (left) and XLD mRNA–injected embryos (right) were probed for the indicated markers. Injections were done radially with 200 ng of XLD mRNA per blastomere at the 8-cell stage in the animal cap (A and B) or in the marginal zone (C–H). (A–D) Neurula stage embryos stained for (A) the neural marker Sox-2, (B) the neural crest marker slug, (C) the dorsal mesoderm and floor plate marker Shh, and (D) the dorsal mesoderm marker MyoD. Note the reduction of the neural and dorsal mesodermal fields. (E) At midgastrula stages, the ventrolateral marker Xwnt8 is expanded and expressed in the organizer in Xld-injected embryos. (F) At neurula stages, the BMP-4 domain is expanded in Xld-injected embryos, reducing the size of the neural plate. (G and H) Chd at early and late gastrula stages, respectively. Note that the transcription of Chd in Xld-injected embryos starts normally but collapses during the course of gastrulation.
(I) Normal (top) and XLD-injected 3-day tadpoles (bottom). Embryos were injected radially with 200 pg of XLD mRNA in each blastomere at the 4-cell stage. Note the recovery to a relatively mild ventralization (93% affected, DAI = 3.2, n = 311) when compared with the severe reduction in axial and neural markers observed at the early stages.
This recovery of axial structures was reminiscent of the case of the chordino mutation in zebrafish, which has severe reductions of dorsal ectodermal and mesodermal markers at the gastrula stage but undergoes a remarkable recovery by the end of embryogenesis (Hammerschmidt et al., 1996a, 1996b). In addition, in chordino mutants, expression of BMP-4 is increased (Hammerschmidt et al., 1996b) and, although chordino mRNA is initially expressed normally, its transcription collapses in the organizer at mid-gastrula stages (Schulte-Merker et al., 1997). Thus, the loss of Chordin-BMP antagonism in the extracellular space is potentiated by a feedback loop that leads to activation of BMP transcription and repression of chordino transcription (Schulte-Merker et al., 1997). Interestingly, radial Xolloid mRNA injections result in a similar expansion of BMP-4 expression (Figure 1F) and in the lack of maintenance of chordin expression at mid-gastrula stages (compare Figures 1G and 1H).
These results indicate that overexpression of the Xolloid secreted protease results in the loss of dorsal ectodermal and mesodermal tissues at the gastrula stage. The similarities with the zebrafish chordino mutant suggest that the effect of Xolloid overexpression in Xenopus is to partially antagonize the dorsalizing role of organizer signals.
Xolloid Inactivates Chordin
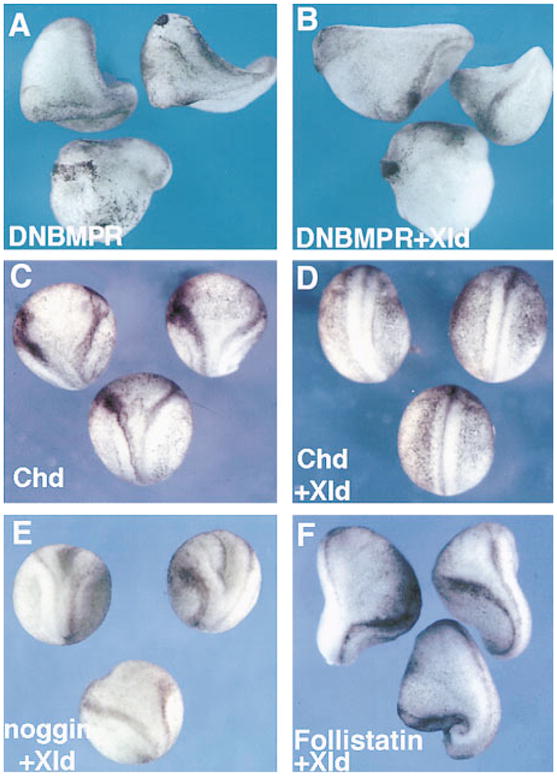
Ventralization by Xolloid mimics the effects of injection of low doses of BMP-4 (Dale et al., 1992; Jones et al., 1992). To test whether Xolloid functions through the BMP pathway, Xolloid mRNA was injected together with a dominant-negative BMP receptor (DNBMPR) that mimics organizer activity, causing the formation of secondary axes after microinjection into ventral blastomeres at the 4-cell stage (Graff et al., 1994; Suzuki et al., 1994). Xolloid mRNA had no effect on these secondary dorsal axes, indicating that Xolloid requires an active BMP pathway to exert its ventralizing activity (Figures 2A and 2B).
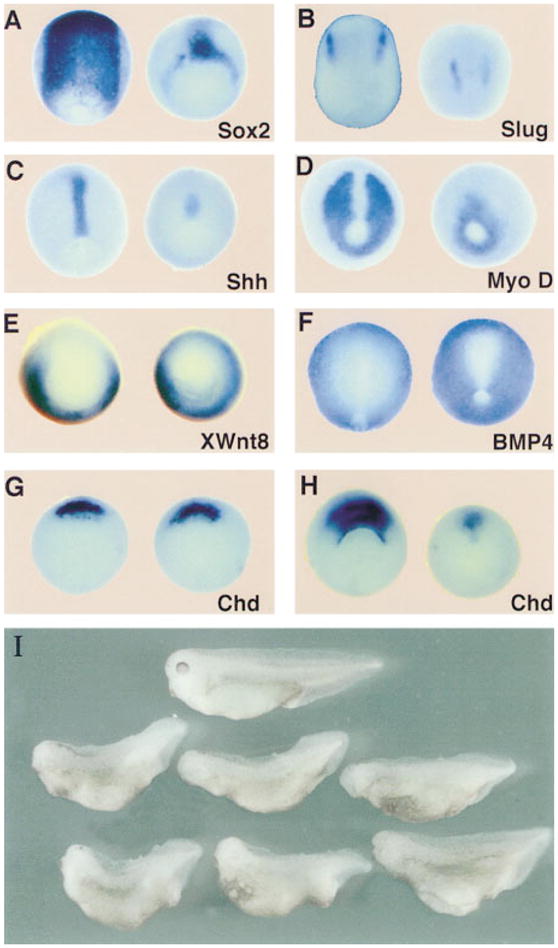
Figure 2. Xolloid Acts Upstream of BMP Receptor and Blocks Secondary Axes Induced by chordin but Not by noggin or follistatin.
(A) Double axes and dorsalized embryos induced by injection of 150 pg of DNBMPR mRNA (67%, n = 57).
(B) Double axes and dorsalized embryos induced by coinjection of 150 pg of DNBMPR with 400 pg of XLD mRNA (62%, n = 53).
(C) Secondary axes induced by injection of 100 pg of chd (69%, n = 71).
(D) XLD mRNA (400 pg) negates the activity of 100 pg of CHD mRNA (2%, n = 128).
(E) Coinjection of 20 pg of noggin mRNA with 400 pg of XLD mRNA results in secondary axis induction (57%, n = 75) at a similar frequency as 20 pg of noggin mRNA alone (48%, n = 55, data not shown).
(F) Coinjection of 150 pg of follistatin mRNA with 400 pg of XLD mRNA results in secondary axis induction (61%, n = 32) at a similar frequency as 150 pg of follistatin mRNA alone (64%, n = 40, data not shown). All embryos were injected in a ventral blastomere at the 4-cell stage. Note that Xolloid specifically blocks the activity of chordin.
Three dorsalizing factors, Chordin, Noggin, and Follistatin, have been shown to inhibit the BMP pathway by sequestering BMPs in the extracellular space, leading to the formation of secondary axes. Xolloid mRNA was found to block secondary axis induction by chordin mRNA (compare Figures 2C and 2D), but had no effect on secondary axes induced by noggin or follistatin (Figures 2E and 2F). The observation that Xolloid is specific for chordin is of particular importance regarding the sequence similarities found between Xolloid and BMP-1. Mammalian BMP-1 is a procollagen processing protease (Kessler et al., 1996; Suzuki et al., 1996), and a case could have been made for the ventralizing effects of Xolloid being mediated by proteolytic modifications of the extracellular matrix. Because noggin, follistatin, or DNBMPR secondary axes were not affected by Xolloid injection, and because a dominant-negative Xolloid construct dorsalized the embryo (see below), the ventralizing effect cannot be due to nonspecific changes in the matrix. Although one cannot exclude the existence of additional, as yet undiscovered, substrates of Xolloid, the present results indicate that the Xolloid protease acts through Chordin.
Xolloid Cleaves Chordin
To test whether Xolloid inactivates Chordin by proteolysis, a biochemical approach was taken. Xolloid enzyme was prepared by transfection of the 293T human kidney cell line with a Xolloid construct tagged at its C terminus with a Flag epitope. A day after transfection, the cells were placed under serum-free conditions (in medium containing Zn) for 48 hr. The conditioned medium contained two bands in Western blots, a weak one of 150 kDa corresponding to the unprocessed proenzyme, and a stronger band at 130 kDa, a size consistent with that of the processed form lacking the aminoterminal proregion (Figure 3A, lane 2). Processing is an absolute requirement for the activity of astacin family zymogens (Stocker et al., 1995). Conditioned medium from cells transfected with DNA vector alone was used as a negative control throughout this study and found to be devoid of activity (Figure 3, lane 1).
Figure 3. Xolloid Is a Secreted Protease That Cleaves Chordin Protein at Two Specific Sites.
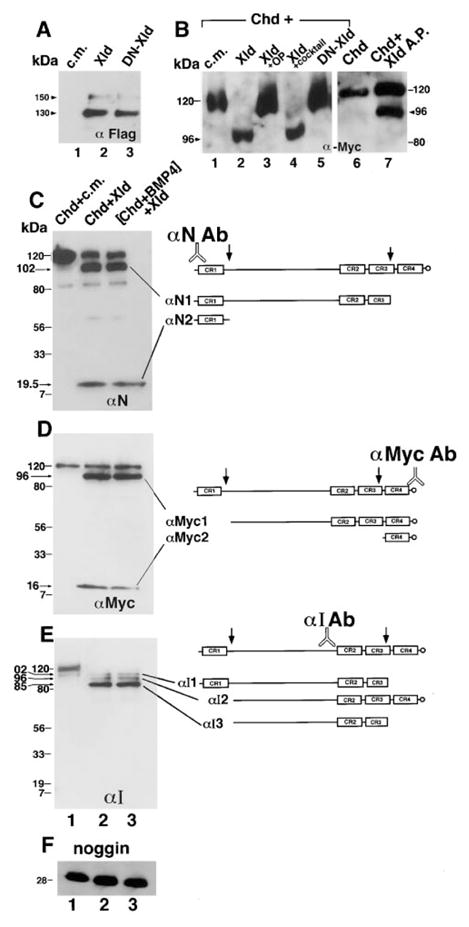
(A) Xolloid is secreted. Recombinant XLD was detected by an anti-Flag monoclonal antibody that recognizes an epitope tag placed at the COOH termini of XLD and of DN-XLD. Lane 1, control conditioned medium (c.m.) from 293T cells transfected with vector DNA only; lanes 2 and 3, equivalent aliquots of conditioned medium from Xld and DN-Xld transfections, respectively. Note that processed Xolloid protein of approximately 130 kDa is secreted into the medium; the faint band at 150 kDa corresponds to the proenzyme.
(B) Xolloid cleaves Chordin. Western blot analysis of CHD protein probed with an anti-Myc antibody that recognizes its COOH terminus. CHD protein was incubated for 10 hr as described in the Experimental Procedures with the following solutions: lane 1, control 293T conditioned medium is devoid of cleaving activity; lane 2, Xolloid-containing medium specifically cleaves CHD, removing an NH2-terminal fragment; lane 3, Xolloid is inactivated by incubation with 1–10 ortophenanthroline, an inhibitor of zinc metalloproteases; lane 4, Xolloid retains enzyme activity in the presence of a protease inhibitor cocktail that does not affect metalloproteases but should block serine, aspartic, and cysteine proteases (see Experimental Procedures for details); lane 5, the DN-Xld point mutation is catalytically inactive; lane 6, CHD-Myc protein used as substrate for purified enzyme; lane 7, affinity-purified Xolloid-Flag protein is able to cleave CHD.
(C–E) Mapping the Xolloid cleavage sites on Chordin. Chordin fragments were visualized by (C) anti-NH2-CHD peptide, by (D) anti-COOH-Myc, and by (E) anti-Internal-CHD (αI) antibodies. The resulting proteolytic fragments are indicated. (C–E) Lane 1, CHD is full-length after incubation with control medium. Lane 2, Xolloid cleaves CHD at two sites. Lane 3, before addition of Xolloid, CHD was preincubated for 2 hr at 25°C with a 5 molar excess of recombinant human BMP-4, to cause the formation of CHD/BMP-4 complexes.
(F) Noggin protein is not cleaved by Xolloid. Lanes 1, 2, and 3 are Noggin in control medium, Noggin in Xolloid medium, and Xolloid medium with Noggin-BMP-4 complexes, respectively.
Acrylamide percentages in the SDS–PAGE gels were as follow: (A) and (B) 7%; (C) 6%–18% gradient; (D–F) 7.5%–18% gradient. For samples in gel (E), the incubation time was extended to 16 hr.
When the Xolloid enzyme was incubated with baculovirus-derived Chordin tagged in the carboxyl terminus with Myc epitope (Piccolo et al., 1996), a cleaved product was detected (Figure 3B, lane 2). To determine whether this cleavage was caused by the secreted Xolloid enzyme, a range of protease inhibitors was added, and only the Zinc chelator orthophenantroline was found to be effective (Figure 3B, lanes 3 and 4). Furthermore, we introduced a point mutation in the Xolloid protease domain in a conserved tyrosine affected in the tolloid antimorphic allele tld6P4 (Childs and O’Connor, 1994; Finelli et al., 1994). Structural studies in the Astacin family of metalloproteases (reviewed by Stocker et al., 1995) predict this residue to be dispensable for Zn binding and folding of the protease domain, but crucial for its catalytic activity. This protein, designated as dominant-negative XLD (DN-XLD) for its biological activities (see Figure 6 below), was secreted and processed (Figure 3A, lane 3) but was unable to cleave Chordin (Figure 3B, lane 5). In addition, purified Xolloid enzyme prepared by Flag antibody affinity matrix was found to be active on Chordin substrate (Figure 3B, lanes 6 and 7). These experiments suggest that the cleavage of Chordin is mediated by the Xolloid enzyme and not by other components present in the conditioned medium.
Figure 6. In Vivo Role of Xolloid: Expansion of Organizer Activity in Embryos Injected with Dominant-Negative Xolloid.
(A) Top, schematic structure of Xolloid: EGF and C1r/s are protein–protein interaction domains. Bottom, sequence of the protease domain of Drosophila Tolloid and of Xenopus Xolloid surrounding a conserved tyrosine (circled, position 272 in Tolloid and 296 in Xolloid) mutated in DN-Xld.
(B) Control stage 29 embryo.
(C) Xld gain-of-function: ventralized embryo injected with 200 pg of XLD mRNA in each blastomere at the 4-cell stage.
(D) Dorsalization in embryos similarly injected with 300 pg of DN-XLD mRNA (76%, n = 127). Compare with sibling embryo in (B); the head and cement gland are enlarged, reflecting dorsalization of the embryos.
(E–G) In situ hybridization for the indicated markers in control (left) or DN-XLD mRNA–injected (right) embryos. The neural domain marked by Sox-2 (E) and the dorsal mesoderm domains marked by Chd (F) or MyoD (G) are expanded and increased in intensity in DN-Xld-injected embryos (n = 24, 10, and 15, respectively), indicating increased organizer activity. The injected embryo in (E) is shortened due to dorsalization.
(H) Stage 20 embryos injected in the two dorsal blastomeres at the 8-cell stage with 200 pg of Xolloid mRNA.
(I) Normal development is restored by coexpression of XLD mRNA (200 pg) and DN-XLD mRNA (800 pg). This indicates that DN-Xld can antagonize wild-type Xld.
(J) Western blot showing that DN-Xld protein produced by cotransfection inhibits the enzymatic activity of Xolloid on Chordin substrate.
To map the sites at which Chordin is cleaved by Xolloid, we analyzed the digestion products with three different antibodies. An anti-amino peptide antibody (Piccolo et al., 1996) detected, in addition to the undigested 120 kDa Chordin band, two bands of 19.5 and 102 kDa (Figure 3C), while the carboxyterminal Myc tag identified two bands of 96 and 16 kDa (Figure 3D). These data with antibodies specific for the two termini are consistent with two cleavage sites, one occurring just after the first cysteine-rich repeat (CR1), and another located within CR3 (Figures 3C and 3D). The two cleavages were confirmed by an internal anti-Chordin antibody (αI, newly derived here, see Experimental Procedures), which revealed that under more complete digestion conditions, most of the Chordin protein remained as an 85 kDa fragment lacking the amino- and carboxyterminal fragments (Figure 3E). Importantly, Chordin precomplexed for 2 hr with a 2–10 molar excess of BMP-4, in conditions in which all Chordin biological activity is blocked (Piccolo et al., 1996), was an equally good substrate for the Xolloid enzyme (Figures 3C–3E, lane 3).
The Xolloid protease was completely inactive on a HA-tagged noggin substrate (Figure 3F), in agreement with the mRNA coinjection results (Figure 2E). We conclude that both Chordin and the Chordin/BMP complex are cleaved by the Xolloid metalloprotease at two defined sites.
Cleaved Chordin Protein Is Inactive
Having shown biochemically that Chordin is a substrate for Xolloid, we next tested whether the cleavage inactivated the biological activities of Chordin using ventral marginal zone (VMZ) and animal cap explants. Chordin protein (2 nM final concentration) was digested in vitro with Xolloid conditioned medium to obtain nearly complete cleavage. Untreated VMZ explants rounded up forming ventral mesoderm, but elongated when treated with control Chordin as a consequence of muscle induction (Figures 4A and 4C). When Chordin predigested with Xolloid enzyme was used, the explants did not elongate (Figure 4B) and failed to express the dorsal mesoderm marker α-actin (Figure 4D). This shows that dorsalization by Chordin is inactivated by cleavage with Xolloid.
Figure 4. Cleavage of Chordin Causes Loss of Dorsalizing and BMP Blocking Activity.
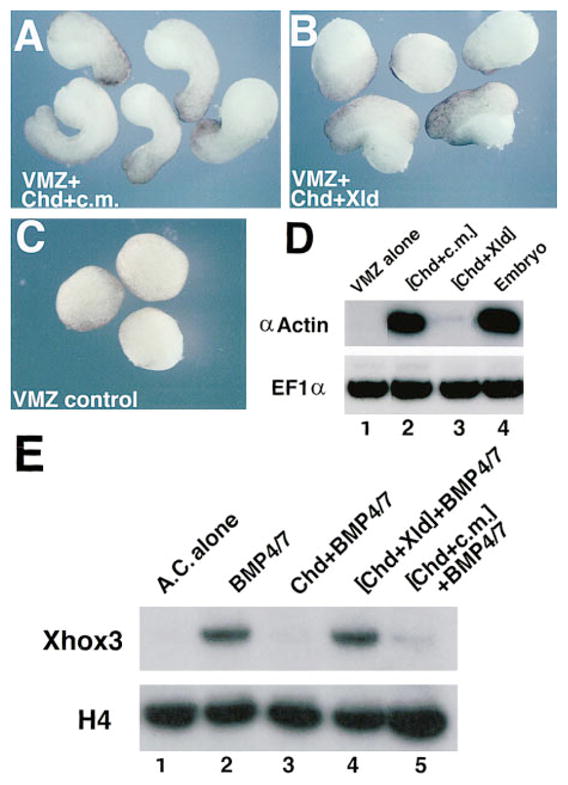
(A–C) External views of ventral marginal zones (VMZs): (A) treated with full-length CHD protein in control medium, (B) treated with XLD-cleaved Chordin protein, and (C) untreated VMZs. Note the lack of elongation in (B).
(D) Dorsal mesoderm is not induced in VMZs treated with digested CHD. RT–PCR analysis was used to score for expression of the muscle-specific marker α-actin in VMZs (n = 20 per lane). EF1α (elongation factor 1α) provides a loading control. Note that digestion with Xolloid inactivates dorsalization by Chordin.
(E) Cleaved CHD is unable to block the activity of BMP-4/7. Animal caps were dissected at blastula stage 8 and incubated with the indicated proteins until siblings reached stage 10̂. Lane 1, untreated animal caps (AC). Lane 2, incubation in 0.7 nM BMP-4/7 induces the ventral mesodermal marker Xhox3. Lanes 3–5, animal caps plus 0.7 nM BMP-4/7 preincubated for 2 hr at 25°C with, respectively, CHD, CHD cleaved by XLD, and CHD incubated in control medium. Note that Chordin preincubated with Xolloid is unable to block BMP signaling.
Since Chordin should act by blocking BMP signaling, we next tested whether cleaved Chordin lost its ability to antagonize BMPs. Animal caps prepared at stage 8 were incubated until early gastrula (2–3 hr) with a Xenopus BMP-4/7 heterodimer that has an order of magnitude more biological activity than BMP-4 homodimers (Hazama et al., 1995; Suzuki et al., 1997). Xolloid conditioned medium alone was devoid of activity in this assay (data not shown). Addition of 0.7 nM BMP-4/7 activated the ventral marker Xhox3, and 1 nM Chordin prevented this activation (Figure 4E, lanes 2 and 3). However, the same concentration of cleaved Chordin had lost its ability to antagonize BMP-4/7 (Figure 4E, lane 4). The results indicate that the cleavage by Xolloid inactivates the anti-BMP activity of Chordin.
Xolloid Releases Active BMP from Chordin/BMP Complexes
To test whether Xolloid digestion is able to release active BMPs from inactive Chordin/BMP complexes, we used an established assay for BMP activity. This assay is very sensitive, permitting detection of BMP activity in the subnanomolar range, at levels lower than those of any current biochemical method. Wilson and Hemmati-Brivanlou (1995) found that BMP-4 is able to prevent the autoneuralization caused by dispersal of the inner layer of the animal cap. Furthermore, BMP-4 acts as an epidermal (keratin) inducer in this assay. In our experiments, addition of 0.7 nM BMP-4/7 was sufficient to prevent autoneuralization and to induce keratin in dispersed animal cap cells (Figure 5, lane 4). These activities were blocked by preincubating BMP-4/7 with 1 nM Chordin (Figure 5, lane 5). However, when the animal caps were isolated from embryos injected into the animal pole with Xolloid mRNA, BMP activity was restored from the previously inactive Chordin/BMP complex, repressing neural tissue and inducing keratin (Figure 5, lane 6). Thus, Xolloid is able to release active BMP by proteolytic digestion of the Chordin substrate.
Figure 5. Xolloid Digestion Causes the Release of Biologically Active BMP-4/7 from Inactive CHD/BMP Complexes.
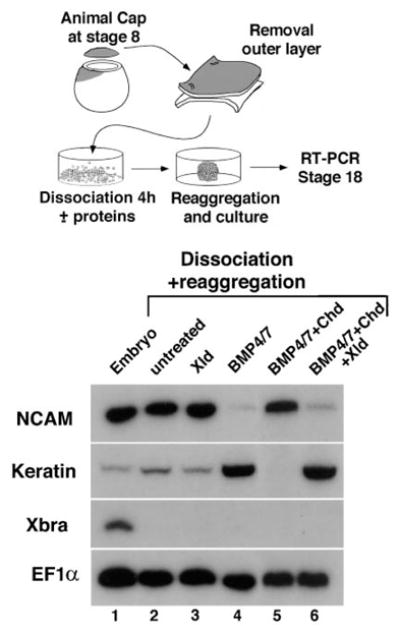
Top: experimental design. Bottom: RT–PCR of dissociated-reaggregated animal cap cells. N-CAM is used as a pan neural marker, keratin as an epidermal-specific marker, XBRA as a mesodermal marker, and EF1α as a loading control. Lane 1, stage 18 whole embryos. Lanes 2 and 3, autoneuralization, denoted by the high NCAM and low keratin band intensities, caused by cell dispersal in, respectively, uninjected and Xld-injected animal caps. Lane 4, BMP-4/7 protein (0.7 nM) induces epidermis and suppresses neural induction. Lane 5, BMP signaling is blocked by preincubation of BMP-4/7 with Chordin (1 nM). Lane 6, injection of XLD mRNA into animal cap cells at the 8-cell stage causes release of active BMP-4/7 from the previously inactive complex (compare to lane 5).
Dominant-Negative Xolloid Dorsalizes Ectoderm and Mesoderm
To test the in vivo function of Xolloid protein in Xenopus, we constructed a dominant-negative enzyme. A strong tolloid antimorphic allele, called tld6P4, mapped to tyrosine 272 (Childs and O’Connor, 1994; Finelli et al., 1994), a residue that is conserved in the Xolloid protein (Figure 6A). The same Tyr-to-Asn point mutation was introduced in Xolloid. The mutant protein was processed and secreted normally (Figure 3A, lane 3) but was enzymatically inactive on its Chordin substrate (Figure 3B, lane 5). Radial injection at the 4-cell stage of mRNA encoding this point mutation (DN-Xld) resulted in dorsalized embryos with enlarged heads and cement glands (Figure 6D), whereas wild-type Xld had ventralizing activity (Figure 6C). The dorsalization caused by DN-Xld affected patterning of the ectodermal and mesodermal germ layers, expanding and increasing the intensity of expression of Sox-2 (neural plate), chordin (organizer and notochord), and MyoD (somitic muscle) markers (Figures 6E–6G), as expected from increased Spemann organizer activity.
To demonstrate that DN-Xld can indeed antagonize the activity of wild-type Xld, coinjection and biochemical experiments were carried out. Injection of XLD mRNA into dorsal blastomeres caused ventralization with reduction of head structures (Figure 6H), which was rescued by coinjection with a 4-fold excess of DN-Xld (Figure 6I). For biochemical tests, DN-Xld was cotransfected with Xld into 293T cells and the conditioned medium tested for Chordin cleaving activity. The sample cotransfected with DN-Xld had much reduced enzyme activity when compared to Xolloid alone, although similar amounts of wild-type Xolloid, marked by a HA-epitope tag, were present in both samples (Figure 6J and data not shown). These studies with DN-Xld suggest that the endogenous Xolloid product is required to limit the extent of the Chordin field of activity in vivo.
Discussion
Chordin Is a Substrate for Xolloid
Xolloid is a gene expressed uniformly in unfertilized eggs and during early embryogenesis through late gastrula stages, and its mRNA is a ventralizing agent that mimics the effects of injection of low doses of BMP-4 (Goodman et al., 1997 and Figure 1). Microinjection of DNBMPR showed that an active BMP pathway is required for ventralization by Xolloid mRNA. The known dorsalizing factors that work by inhibiting BMPs, Chordin, Noggin, and Follistatin, were tested in coinjection experiments, and Xolloid was found to specifically block the activity of Chordin. Biochemical analyses using Xenopus proteins showed that Chordin, but not Noggin, was a substrate for the Xolloid zinc metalloprotease. Xolloid cleaved Chordin at two sites, one located just after CR1 and the other within CR3. These cleavages inactivate the biological activity of Chordin, indicating that Xolloid acts through Chordin. It should be noted, however, that our data do not exclude that Xolloid could have additional, as yet undiscovered, substrates.
In a companion study, Marqués et al. (1997 [this issue of Cell, 417–426]) show that Drosophila tolloid mRNA inhibits chordin, but not noggin, activity in Xenopus embryos. In transient transfection experiments, Drosophila SOG was cleaved at three specific sites by Tolloid, and two of these sites mapped to regions similar to those described for Xenopus Chordin and Xolloid in this study. Cleavage of SOG by Tolloid was stimulated by DPP (Marqués et al., 1997), whereas Chordin digestion by Xolloid was equally active when Chordin alone or Chordin/BMP-4 complex was offered as substrate. The cause of the BMP stimulation difference is unknown, but could be due to biochemical assay conditions, to the properties of the protease, to the folding of Sog and Chordin substrates when complexed with BMPs, or to additional protein factors present in Drosophila cultured cells. However, it is clear that Xolloid can cleave Chordin/BMP complexes effectively.
By using a sensitive dissociation/reaggregation animal cap assay, we were able to show that cleavage by Xolloid of an inactive Chordin/BMP-4/7 complex results in the release of BMP antineuralizing and epidermis-inducing activity (Figure 5). The digestion of Chordin/BMP complexes by a metalloprotease provides a novel mechanism by which a latent growth factor, rendered inactive by binding to an extracellular antagonist, can be reactivated at particular locations. If the Chordin/BMP complex were diffusible, this mechanism could facilitate the transport and redistribution of BMPs as has been proposed for SOG/DPP (Holley et al., 1996). In Drosophila, it is clear that the sog product must be diffusible over long distances, because mosaic studies have shown that sog is required in ventral ectoderm for the formation of the dorsal-most tissue (Zusman et al., 1988). The biochemical and biological data show that Xolloid can proteolytically inactivate Chordin; thus, the ventralization caused by Xolloid seems to act through a double inhibition mechanism involving Chordin:
Xolloid Is Required for D-V Patterning
A dominant-negative Xolloid (DN-Xld) was generated by introducing a point mutation previously identified in a Drosophila tolloid antimorphic allele (Childs and O’Connor, 1994; Finelli et al., 1994), and overexpression throughout the embryo resulted in expanded dorsal structures in both ectoderm and mesoderm. This reversal of the Xolloid mRNA ventralizing phenotype by a dominant-negative construct suggests that the normal function of Xolloid in Xenopus development is to limit the expansion of Spemann’s organizer field. DN-Xolloid retains a wild-type interaction domain but its protease domain is catalytically inactive on Chordin substrate. In view of the present results, it appears that inactivating the catalytic activity is sufficient to block the activity of the wild-type enzyme. In principle, mutations similar to the one introduced here could be used as a general method to block the function of most members of the growing family of zinc metalloproteases (Stocker et al., 1995) in vivo.
An inversion of the dorsoventral axis has occurred during evolution, but the molecular mechanisms of patterning by the DPP/BMP and SOG/CHD extracellular proteins has been conserved (Holley et al., 1995; De Robertis and Sasai, 1996). Our results suggest that an additional component of this regulatory cascade, a zinc metalloprotease, has also been conserved. A point mutation identified in the original Heidelberg screen for zygotic mutations in the third chromosome of Drosophila (Jürgens et al., 1984) permitted the construction of the dominant-negative form of Xolloid used in this study. It is remarkable that the mechanisms of dorsoventral development have been conserved to such an extent that one can design dominant-negative mutations based on information from Drosophila, an organism that diverged from a common Urbilaterian ancestor 500 million years ago.
Dorsoventral patterning of the embryo is mediated by the graded activity of DPP in Drosophila and of BMP-4/2 in vertebrates (Ferguson and Anderson, 1992b; Dosch et al., 1997). However, the transcripts of these genes are not expressed in graded fashion (Ferguson and Anderson, 1992b; Wharton et al., 1993; Fainsod et al., 1994; Hemmati-Brivanlou and Thomsen, 1995). Work from Drosophila suggests that the gradient of DPP activity results in part from the diffusion of SOG (Biehs et al., 1996; Holley et al., 1996). It is not known whether Chordin is diffusible in Xenopus, but in view of the patterning defects observed in the chordino mutation in zebrafish (Hammerschmidt et al., 1996b; Schulte-Merker et al., 1997), this remains a distinct possibility. The present study suggests the existence of an additional layer of regulation of Chordin function. We propose that the Xolloid protease may act as a clearing system for Chordin. Whereas BMP-4 can be constantly removed by interactions with its receptor, such a mechanism is not available for Chordin, which lacks a receptor. In this view, the function of the Xolloid protease might be to provide a molecular sink required to maintain a gradient of Chordin dorsalizing activity emanating from Spemann’s organizer.
Experimental Procedures
Expression Constructs and Synthetic mRNA Preparation
Full-length Xld DNA (GenBank accession number Y09660) was excised with EcoRI–DraIII and subcloned in the EcoRI–StuI sites of pCS2 to generate pCS2-Xld. A Flag-tagged version of Xld was derived by PCR by inserting the sequence DYKDDDDK-Stop after the last amino acid of Xolloid. mRNA prepared from the resulting plasmid (pCS2-Xld-Flag) had indistinguishable activity from Xld mRNA lacking the tag (ventralization 88%, n = 52). Xld-HA was derived by a similar procedure. Dominant-negative Xld contains a point mutation (TAT into AAT) of Tyr-296 into Asn, introduced by a PCR-based approach. After checking the presence of the mutation and the fidelity of the amplified DNA by sequencing, a point-mutated 5′ EcoRI–SphI fragment was ligated to a wild-type SphI–XbaI Xld-Flag fragment into pCS2 to generate pCS2-DN-Xld. For transfection studies, Xld-Flag and DN-Xld-Flag sequences were subcloned in the EcoRI–XbaI sites of pcDNA3.1. HA-tagged noggin (pcDNA3.1 noggin) was generated by PCR, incorporating the sequence YPYDVP-DYA-Stop after the last amino acid of noggin. All of these PCR reactions were done with Pfu polymerase (Stratagene) that contains proofreading activity.
Synthetic mRNA was prepared using the Message Machine in vitro transcription kit (Ambion). For sense mRNAs, pCS2-Xld and pCS2-DN-Xld were linearized with NotI and transcribed with SP6. DNBMPR, noggin, and follistatin mRNA were synthesized as described in Sasai et al. (1995).
In Situ Hybridization and RT–PCR
Whole-mount in situ hybridizations were carried out according to Harland’s method (Harland, 1991) with minor modifications. The activities of CHD and BMP proteins (Figures 4 and 5) were assayed by RT–PCR, using conditions and primers previously described in Sasai et al. (1995) for testing CHD activity, in Suzuki et al. (1997) for Xhox3, and in Wilson and Hemmati-Brivanlou (1995) for the dissociation-reaggregation experiments.
Protein Expression and Purification
293T cells (70% confluency) were transfected by CaPO4 with Xld, DN-Xld, and noggin cDNAs in pcDNA 3.1 (15 μg per 10 cm Petri dish) in DMEM-10% fetal calf serum. 24 hr after transfection, a subconfluent cell monolayer was washed three times with Hank’s balanced salt solution and cultured in serum-free medium for 48 hr. Optimal expression was observed using a mixture of DMEM, H12, and Iscoves media (33% each). H12 was included because it contains zinc. Proteins secreted by 293T cells transfected with pcDNA3.1 only and cultured in the same way were used as negative control throughout this study. The conditioned media were clarified by low- and high-speed centrifugation and, after addition of 2.5 mM CaCl2, concentrated to 10% its original volume with Centriplus (Amicon). Concentrated media were then dialyzed against 10 mM HEPES (pH 7.6), 150 mM NaCl, 2.5 mM CaCl2, 1 mM MgCl2, and 1 μM ZnCl2 (buffer A). Aliquots were snap-frozen and stored at −80°C. During this study, we used different preparations of Xolloid (n = 4), control (n = 3), and DN-Xld (n = 2) proteins with similar results.
For affinity purification of Xolloid-Flag (Figure 3B, lane 7), 10 ml of Xld-Flag conditioned medium received NaCl to 0.5 M and of Brij35 to 0.2% final concentrations and was then subjected to affinity purification using a Flag affinity matrix (IBI). Binding was for 2 hr in batch; washings were in a column with 10 mM HEPES (pH 7.6), 0.5 M NaCl, and 0.2% Brij35 followed by buffer A. Elution was with Flag peptide (IBI) in buffer A. Fractions were identified by Western blot and silver staining and frozen.
CHD protein was obtained from baculovirus as described (Piccolo et al., 1996). For digestion, we incubated 5 ng of CHD-Myc protein with 7.5 μl of Xolloid-Flag (about 1 ng), DN-Xld-Flag, or control medium for 10 hr at 30°C. When a more complete digestion was required in experiments assaying the activity of cleaved CHD (Figure 4), 15 μl of enzyme and 16 hr of incubation were used. Some of our preparations of baculovirus CHD-Myc had degraded C termini lacking C3 and C4, giving low molecular weight fragments positive for the anti-Myc antibody. To overcome this background problem, for the anti-Myc Western blotting of Figure 3C we used a full-length CHD protein purified by cation exchange chromatography. Briefly, cleared baculovirus medium was loaded in a High S column (Bio-Rad), washed with 10 mM HEPES (pH 7.6), NaCl 80 mM, and proteins eluted with a linear gradient of 80–1200 mM NaCl in 10 mM HEPES (pH 7.6). Full-length undegraded Chordin eluted around 0.8 M NaCl.
For protease inhibition studies, we used 1 mM 1–10 orthophenanthroline (a Zn metalloprotease inhibitor) and a cocktail of 1 mM PMSF, 10 μM Pepstatin, and 1 mM E64 that inhibit, respectively, serine, aspartic, and cysteine proteases.
Antibodies and Western Blotting
For Western blotting, protein samples were resolved under reducing conditions by SDS–PAGE and electroblotted onto Immobilon P (Millipore) PVDF membranes. The filters were blocked with 5% powdered Carnation milk in TBST and incubated in the same buffer with a 1:500 dilution of all antibodies except for the anti-Internal-CHD antibody, for which a 1:3000 dilution was used. The ECL luminescence detection kit was from Amersham. Detection of the various epitope tags was carried out with the following mouse monoclonal antibodies: anti-myc (Babco), anti-Flag M2 (IBI), and anti-HA (Babco). Anti-NH2 CHD peptide antibody was produced as described (Piccolo et al., 1996). For obtaining an anti-Internal-CHD antibody, a CHD peptide (coding from amino acid 592 to 692) was expressed in bacteria as a GST fusion protein, purified in a glutathione column (Pharmacia), and injected into rabbits (Babco).
Embryological Manipulations
Ventral marginal zone explants (comprising 60° of the VMZ) from stage 10 embryos were prepared in LCMR, opened with an eyebrow knife, and immediately transferred to LCMR containing Xolloid or control digested CHD protein. 96-well PCR plates (Costar) treated with 5% PolyHEMA (Polysciences) were used, and a single explant was put in each well containing 20 μl of solution. After overnight incubation at 20°C, the explants were transferred to 0.4 × MMR. In experiments testing the activity of BMP-4/7, intact caps were excised at stage 8 and kept open using VLCMR (Piccolo et al., 1996). The explants were incubated in BMP-4/7 protein or CHD/BMP-4/7 complexes until siblings reached stage 10 1/2. To test whether Xolloid proteolytic activity releases active BMP-4/7 from inactive complexes with CHD, stage 8 animal caps were dissociated as described in Wilson and Hemmati-Brivanlou (1995). For each point, cells from 15 animal caps from wild-type or from embryos injected with 250 pg/blastomere XLD mRNA in the animal pole were used in a final volume of 70 μl. After 4 hr, cells were reaggregated by addition of 70 μl of 1.4 × MMR and cultured to stage 18. All explant experiments were performed 2–3 times with similar results.
Acknowledgments
We thank Dr. Michael O’Connor (UC Irvine) for communicating results prior to publication; Drs. J. Fessler, P. Garrity, H. Herschman, J. A. Belo, L. Leyns, and O. Wessely for comments on the manuscript; and Y. Kim for technical assistance. We thank Takeda Pharmaceutical Co. and Genetics Institute for BMP proteins and Drs. R. Harland, R. Moon, R. Rupp, and M. Sargent for plasmids. S. P. was supported by a Comitato Promotore Telethon postdoctoral fellowship, and E. A. by the Association pour la Recherche sur le Cancer (France). E. D. R. is an Investigator of the Howard Hughes Medical Institute. This work received support from the National Institutes of Health (R37 HD21502-11) and the British Medical Research Council.
References
- Biehs B, François V, Bier E. The Drosophila short gastrulation gene prevents Dpp from autoactivating and suppressing neurogenesis in the neuroectoderm. Genes Dev. 1996;10:2922–2934. doi: 10.1101/gad.10.22.2922. [DOI] [PubMed] [Google Scholar]
- Childs SR, O’Connor MB. Two domains of the tolloid protein contribute to its unusual genetic interaction with decapentaplegic. Dev Biol. 1994;162:209–220. doi: 10.1006/dbio.1994.1079. [DOI] [PubMed] [Google Scholar]
- Dale L, Howes G, Price BMJ, Smith JC. Bone morphogenetic protein 4: a ventralizing factor in early Xenopus development. Development. 1992;115:573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- Dosch R, Gawantka V, Delius H, Blumestock C, Niehrs C. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997;124:2325–2334. doi: 10.1242/dev.124.12.2325. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. Localized enhancement and repression of the activity of the TGF-β family member, decapentaplegic, is necessary for dorsal-ventral pattern formation in the Drosophila embryo. Development. 1992a;114:583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992b;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Finelli AL, Bossie CA, Xie T, Padgett RW. Mutational analysis of the Drosophila tolloid gene, a human BMP-1 homolog. Development. 1994;120:861–870. doi: 10.1242/dev.120.4.861. [DOI] [PubMed] [Google Scholar]
- François V, Bier E. Xenopus chordin and Drosophila short gastrulation genes encode homologous proteins functioning in dorsal-ventral axis formation. Cell. 1995;80:19–20. doi: 10.1016/0092-8674(95)90446-8. [DOI] [PubMed] [Google Scholar]
- Goodman S, Albano R, Wardle F, Matthews G, Tannahill D, Dale L. BMP1-related proteins promote the development of ventral mesoderm in early Xenopus embryos. Dev Biol. 1997 doi: 10.1006/dbio.1997.8840. in press. [DOI] [PubMed] [Google Scholar]
- Graff JM. Embryonic patterning: to BMP or not to BMP, that is the question. Cell. 1997;89:171–174. doi: 10.1016/s0092-8674(00)80196-8. [DOI] [PubMed] [Google Scholar]
- Graff JM, Scott Thies R, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJM, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, et al. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996a;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Serbedzija GN, McMahon AP. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev. 1996b;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Meth Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Hazama M, Aono A, Ueno N, Fujisawa Y. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Commun. 1995;209:859–866. doi: 10.1006/bbrc.1995.1578. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88:13–17. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Thomsen GH. Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev Genet. 1995;17:78–89. doi: 10.1002/dvg.1020170109. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffman FM, Ferguson EL. A conserved system for dorsal-ventral patterning in insects and vertebrates involving short gastrulation and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Jones CM, Lyons KM, Lapan PM, Wright CVE, Hogan BLM. DVR-4(Bone Morphogenetic Protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115:639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Jürgens G, Wieschaus E, Nüsslein-Volhard C, Kluding H. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux’s Arch Dev Biol. 1984;193:283–293. doi: 10.1007/BF00848157. [DOI] [PubMed] [Google Scholar]
- Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Maeda R, Ong RC, Kim J, Lee LM, Kung HF, Maéno M. XBMP-1B (Xtld), a Xenopus homolog of the dorsoventral polarity gene in Drosophila, modifies tissue phenotypes of ventral explants. Dev Growth Diff. 1997;39:43–51. doi: 10.1046/j.1440-169x.1997.00006.x. [DOI] [PubMed] [Google Scholar]
- Maéno M, Xue Y, Wood TI, Ong RC, Kung HF. Cloning and expression of cDNA encoding Xenopus laevis bone morphogenetic protein-1 during early embryonic development. Gene. 1993;134:257–261. doi: 10.1016/0378-1119(93)90103-a. [DOI] [PubMed] [Google Scholar]
- Marqués G, Musacchio M, Shimell MJ, Wünnenburg-Stapleton K, Cho KWY, O’Connor MB. Production of a Dpp activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell. 1997;91:417–426. doi: 10.1016/s0092-8674(00)80425-0. this issue. [DOI] [PubMed] [Google Scholar]
- Moon RT, Brown JD, Yang-Snyder JA, Miller JR. Structurally related receptors and antagonists compete for secreted Wnt ligands. Cell. 1997;88:725–728. doi: 10.1016/s0092-8674(00)81915-7. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S, Suzuki A, Shoda A, Murakami K, Ueno N. Genes for bone morphogenetic proteins are differentially transcribed in early amphibian embryos. Biochem Biophys Res Commun. 1992;184:1487–1495. doi: 10.1016/s0006-291x(05)81574-8. [DOI] [PubMed] [Google Scholar]
- Padgett RW, Wozney JM, Gelbart WM. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc Natl Acad Sci USA. 1993;90:2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the chd and BMP-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Shimell MJ, Ferguson EL, Childs SR, O’Connor MB. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell. 1991;67:469–481. doi: 10.1016/0092-8674(91)90522-z. [DOI] [PubMed] [Google Scholar]
- Stocker W, Grams F, Baumann U, Reinemer P, Gomis-Ruth FX, McKay DB, Bode W. The metzincinstopological and sequential relations between the astacins, adamalysins, serralysins, and matrixins (collagenases) define a superfamily of zinc-peptidases. Prot Sci. 1995;4:823–840. doi: 10.1002/pro.5560040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Scott Thies R, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci USA. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kaneko E, Maeda J, Ueno N. Mesoderm induction by BMP-4 and −7 heterodimers. Biochem Biophys Res Commun. 1997;232:153–156. doi: 10.1006/bbrc.1997.6219. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, Hogan BL. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122:3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- Takahara K, Brevard R, Hoffman GG, Suzuki N, Greenspan DS. Characterization of a novel gene product (mammalian tolloid-like) with high sequence similarity to mammalian tolloid/bone morphogenetic protein-1. Genomics. 1996;34:157–165. doi: 10.1006/geno.1996.0260. [DOI] [PubMed] [Google Scholar]
- Wharton KA, Ray RP, Gelbart WM. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development. 1993;117:807–822. doi: 10.1242/dev.117.2.807. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesús-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- Zusman SB, Sweeton D, Wieschaus EF. short gastrulation, a mutation causing delays in stage-specific cell shape changes during gastrulation in Drosophila melanogaster. Dev Biol. 1988;129:417–427. doi: 10.1016/0012-1606(88)90389-2. [DOI] [PubMed] [Google Scholar]