Summary
Chordin (Chd) is an abundant protein secreted by Spemann organizer tissue during gastrulation. Chd antagonizes signaling by mature bone morphogenetic proteins (BMPs) by blocking binding to their receptors. Recombinant Xenopus Chd binds to BMP-4 with high affinity (KD 3 × 10−10 M), binding specifically to BMPs but not to activin or TGF-β1. Chd protein is able to dorsalize mesoderm and to neuralize ectoderm in Xenopus gastrula explants at 1 nM. We propose that the noncell-autonomous effects of Spemann’s organizer on dorsoventral patterning are executed in part by diffusible signals that directly bind to and neutralize ventral BMPs during gastrulation.
Introduction
A fundamental experiment for understanding dorsoventral patterning in the vertebrate embryo was that carried out by Spemann and Mangold in 1924. A small region of the embryo, the organizer or dorsal lip, has the ability when grafted to the opposite (ventral) side to cause profound noncell-autonomous effects. Neighboring cells in the ectoderm are induced to form central nervous system and those in the mesoderm to form dorsal structures such as somites. These inductive effects should be mediated by diffusible molecules, and the isolation of the molecular signals involved has been a challenge for generations of embryologists (Hamburger, 1988).
With the advent of molecular cloning, the quest for genes expressed specifically in the organizer has produced many such genes, in particular homeodomain proteins and secreted signaling factors (reviewed by Dawid, 1994; Harland, 1994; De Robertis, 1995). Three secreted factors, noggin, follistatin, and chordin (Chd), have been shown to mimic the two main activities of Spemann’s organizer, neural induction and mesoderm dorsalization, in Xenopus embryo microinjection assays (Smith and Harland, 1992; Smith et al., 1993; Hemmati-Brivanlou et al., 1994; Sasai et al., 1994, 1995). This indicates that the organizer patterns both ectoderm and mesoderm using a common set of signaling molecules and that the differences in the response to this induction should reside in the target tissues (reviewed by De Robertis and Sasai, 1996).
Because dorsal tissue has dominant effects when grafted to the ventral side, attention had been focused on the organizer, and ventral differentiation had been considered a default state. However, recent results have shown that ventral mesodermal development requires its own set of signals. Bone morphogenetic protein-4 (BMP-4), a member of the transforming growth factor (TGF)-β superfamily of growth factors, is a potent ventralizing molecule that is able to overcome the dorsalizing effects of activin and of the organizer itself (Dale et al., 1992; Jones et al., 1992; Fainsod et al., 1994). At the onset of gastrulation, BMP-4 transcripts are distributed relatively uniformly in the animal cap and ventrolateral marginal zone but are excluded from the organizer proper (Fainsod et al., 1994). When BMP signaling is blocked by a dominant-negative BMP receptor, ventral mesoderm develops as dorsal mesoderm, indicating that the ventral state must be actively maintained (Graff et al., 1994; Suzuki et al., 1994). Similarly, inhibition of BMP signaling by dominant-negative ligands or anti-sense BMP-4 RNA results in mesoderm dorsalization (Hawley et al., 1995; Steinbeisser et al., 1995). BMP-7 is expressed widely in the gastrula, heterodimerizes with BMP-4, and is also considered an important component of the ventralizing signal (Hawley et al., 1995; Hazama et al., 1995; Aono et al., 1995).
Ectodermal patterning also requires an intact BMP signaling pathway. Animal cap explants from the early gastrula develop as epidermis (ventral ectoderm), but microinjection of dominant-negative BMP receptor (Sasai et al., 1995; Xu et al., 1995), of dominant-negative ligands (Hawley et al., 1995), or of BMP-4 (but not BMP-2) antisense RNA (Sasai et al., 1995) causes them to develop as neural tissue (dorsal ectoderm). Thus, endogenous BMP-4 present in the animal cap appears to repress neural development. This view is supported by experiments of Wilson and Hemmati-Brivanlou (1995) in which animal cap cells were dissociated, a treatment that leads to neuralization, presumably due to the loss of diffusible factors into the culture medium; addition of BMP-4 to dissociated cells caused differentiation into epidermis and blocked neural development. Further, microinjection of BMP-4 DNA under the control of a promoter expressed at the gastrula stage is able to antagonize neural induction caused by injection of chd, noggin, or follistatin mRNA in animal caps (Sasai et al., 1995).
An apparent paradox is presented by these observations, for neural induction can be caused either by specific protein factors emanating from the organizer or by blocking the BMP pathway in the absence of any organizer signals. An attractive hypothesis that could in principle resolve this paradox is that the diffusible organizer signals might neuralize ectoderm and dorsalize mesoderm by antagonizing the ventral BMP pathway (Graff et al., 1994; Sasai et al., 1995; Wilson and Hemmati-Brivanlou, 1995; Re’em-Kalma et al., 1995). Support for this hypothesis comes from Drosophila, in which the main dorsoventral patterning system has been defined genetically. The short-gastrulation (sog) gene is the structural (François et al., 1994; François and Bier, 1995) and functional (Holley et al., 1995; Schmidt et al., 1995) homolog of Xenopus chd. Gene dosage studies have revealed that sog functions as an antagonist of decapentaplegic (dpp), which acts as a dorsoventral morphogen in the Drosophila embryo (Ferguson and Anderson, 1992a, 1992b; Wharton et al., 1993; Nellen et al., 1996). dpp is functionally homologous to BMP-4 and BMP-2 of vertebrates (Padgett et al., 1993). Thus, it appears that a conserved system for dorsoventral patterning involving two secreted proteins, sog/Chd and dpp/BMP-4, is shared by vertebrates and arthropods (De Robertis and Sasai, 1996). It is clear from genetic studies in Drosophila (Ferguson and Anderson, 1992b; François et al., 1994) and microinjection studies in Xenopus (Sasai et al., 1995; Holley et al., 1995) that the sog/chd and dpp/BMP-4 signals antagonize each other. However, the molecular mechanism by which this antagonism operates remains an open question. In this study, we present evidence that Chd is a relatively abundant soluble protein secreted by organizer cells during gastrulation that specifically binds to mature BMP proteins in the subnanomolar range. This interaction acts extra-cellularly, blocking BMP signaling by preventing binding of mature BMPs to their receptors. Chordin protein acts at the right time and at physiological concentrations in Xenopus development, leading to neuralization and dorsalization of gastrula explants in the low nanomolar range. The results support a molecular mechanism by which soluble Chd secreted by Spemann organizer cells functions by antagonizing the ventral BMP signaling pathway.
Results
Chd Is Secreted as a 120 kDa Protein
To study the biochemical function of Chd, the Xenopus protein was expressed in the baculovirus system, and a peptide antibody was raised against its NH2-terminus. In addition, a myc epitope was introduced at the COOH-terminus (this tag does not affect activity; see Experimental Procedures). The recombinant protein was active in biological assays, even at 1 nM concentration (see Figure 5 below). As shown in Figure 1, Chd was secreted as a soluble protein by infected cells and migrated as a band of 120 kDa. The secreted protein was full-length because it reacted with antibodies directed against both ends of the protein (Figure 1, lanes 4 and 6). Soluble Chd secreted in serum-free conditioned medium was subjected to heparin–agarose chromatography, resulting in a partially purified preparation (about 25% pure; see Experimental Procedures). In all experiments, medium from cells infected with an irrelevant baculovirus lacking Chd was purified in the same way and used as a specificity control. These control proteins were found to be devoid of biochemical and biological activity in all assays.
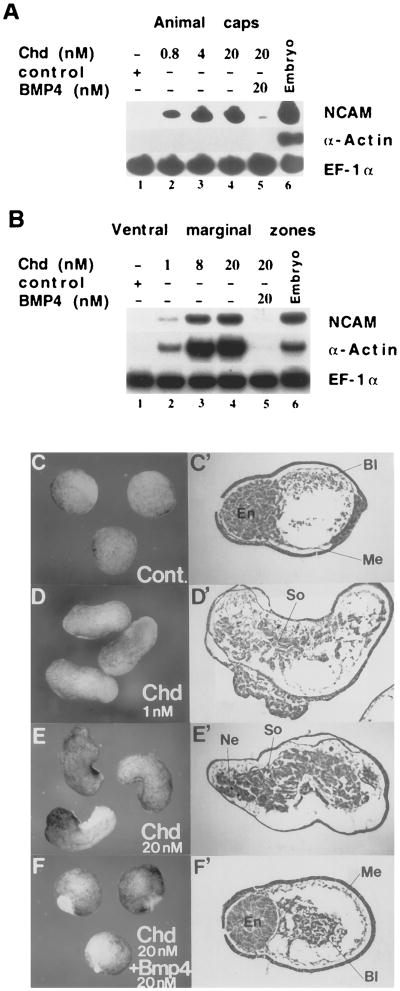
Figure 5. Neural Induction and Mesoderm Dorsalization by Chordin Protein and Their Reversal by Equimolar BMP-4.
(A) Animal cap explants were treated at the gastrula stage with control protein extract prepared from an irrelevant baculovirus infection (lane 1) or with increasing amounts of Chd protein (lanes 2–4). neural cell adhesion molecule induction took place (in the absence of α-actin expression). Addition of 20 nM Chd and 20 nM BMP-4 greatly reduced the levels of neural cell adhesion molecule (lane 5).
(B) VMZ explants (stage 27) treated with increasing concentrations of chordin protein (lanes 2–4). Dorsal mesoderm (α-actin) induction can be observed at 1 nM. The activity of 20 nM chd can be reversed by an equimolar amount of BMP-4 (lane 5). Elongation factor-1α (EF-1α) was used a loading control for the RT–PCR procedure.
(C–F) External views of VMZ explants (stage 27) treated with control protein, 1 nM Chd, 20 nM Chd, and 20 nM Chd plus 20 nM BMP-4, respectively. The corresponding histological sections (stage 33) are shown in C′, D′, E′, and F′. So, somite; Ne, neural; Me, mesothelium and mesenchyme; Bl, blood; En, endoderm.
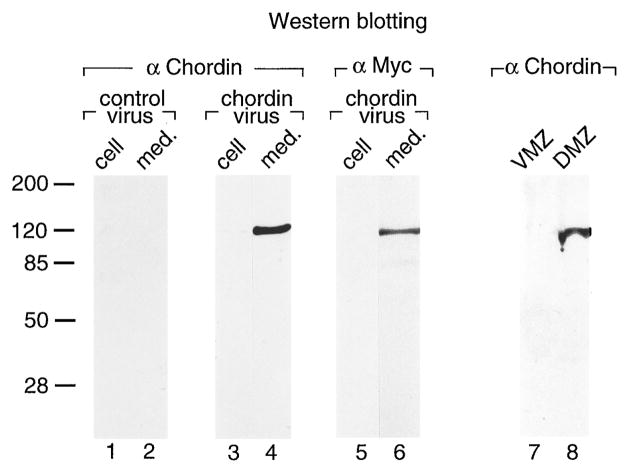
Figure 1. Chordin Protein Is Secreted by Cultured Cells and by Xenopus Organizer Tissue.
Recombinant chordin protein was detected on Western blots with a polyclonal anti–NH2-Chd antibody or with an anti-Myc antibody that recognized, respectively, the NH2 and COOH termini of the recombinant chordin. Endogenous chordin secreted by Xenopus organizer tissue was detected with the anti–NH2-Chd antibody. Lanes 1 and 2: equivalent aliquots of cells or culture medium infected with a control baculovirus lacking Chd do not cross-react with the anti–NH2-Chd antibody. Lanes 3 and 4: cells and medium, respectively, from Chd-baculovirus–infected cells probed with anti–NH2-Chd antibody; note that Chd protein of 120 kDa is secreted into the culture medium. Lanes 5 and 6: cells and medium from Chd-baculovirus–infected cells probed with anti-myc (COOH-terminal) antibody; the full-length protein is secreted. Lanes 7 and 8: culture medium from ventral (VMZ) and dorsal (DMZ) mesoderm, respectively. Chordin secreted by Spemann’s organizer has the same mobility as the recombinant protein loaded on adjacent lanes (data not shown). DMZs or VMZs were explanted at stage 10+, dissociated in calcium and magnesium-free amphibian medium, and cultured for 90 min. Culture medium from 20 explants was analyzed with anti–NH2-Chd antibody in this Western blot. By comparing these samples to a standard curve of recombinant Chd protein, we estimate that each organizer explant secreted 20 pg of chordin. This number permits one to calculate what the concentration range of chordin might be in vivo. (This implies several approximate assumptions: that a stage 10 gastrula has a volume of 1.4 μl, that our explants comprise about one-tenth of it, and that the extracellular space corresponds to 10%–20% of this volume.) This amount (20 pg/organizer) of chordin would yield a concentration of 1.2 nM per total DMZ, and if all of this protein were found in the extracellular space, the concentration would correspond to 6–12 nM Chd. Although these calculations are subject to error and can only be taken as approximations, they suggest that the extracellular space of DMZ explants should contain chordin in the high 10−9 M range in vivo. When explants were homogenized directly without dissociation, the Chd band was similarly detectable in the DMZ but not in the VMZ sample (although some unspecific cross-reacting bands were also seen in both extracts; data not shown).
Endogenous chd is also a soluble protein of 120 kDa and is secreted by dorsal (DMZ) but not by ventral (VMZ) marginal zone explants (Figure 1, lanes 7 and 8). When DMZ (organizer) cells were dissociated at early gastrula and cultured for 90 min, 20 pg per organizer of soluble Chd protein was recovered from the culture medium. While an accurate calculation is difficult (see Figure 1, legend), we estimate that chordin should be present in the extracellular space of DMZ explants well within the 10−9 M range. These results indicate that endogenous Chd is a large and relatively abundant soluble protein secreted by the gastrula stage organizer at the right time and place to mediate neural induction and mesoderm dorsalization in vivo.
Chd Inhibits Signaling by Mature BMPs
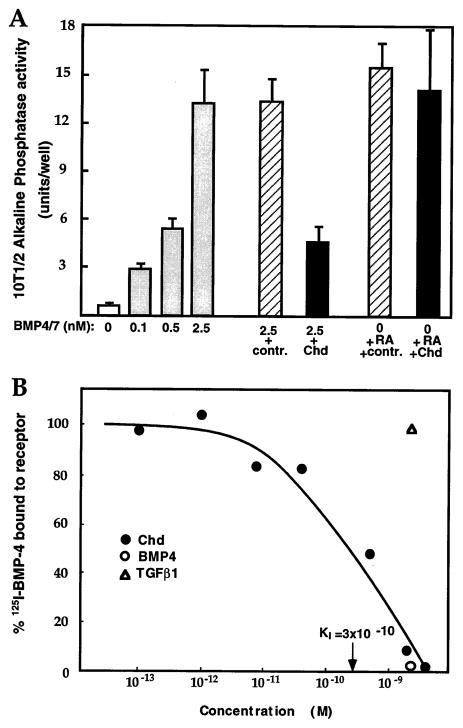
Chd could antagonize BMPs by any of several mechanisms. For example, Chd might, first, interfere with BMP maturation or secretion; second, it could act via a parallel pathway to that of the BMP–receptor interaction; third, it could bind to the BMP receptor; or, fourth, it could bind directly to mature BMPs. To determine whether Chd protein could antagonize BMP proteins after secretion and maturation, we took advantage of an established assay for BMP activity, involving the measurement of alkaline phosphatase levels that reflect osteogenic differentiation in cell culture (Katagiri et al., 1990). In this assay system, a heterodimer of BMP-4 and BMP-7 is the most active BMP (Hazama et al., 1995; Aono et al., 1995; our unpublished data), allowing a dose-dependent response in the 0.1–2.5 nM range. As shown in Figure 2A, alkaline phosphatase induction by BMP-4/7 was inhibited by Chd protein but not by extracts from cells infected with control baculovirus. Interestingly, in this cell line (10t1/2), osteoblastic differentiation can also be induced by retinoic acid (RA) treatment (Katagiri et al., 1990). Unlike BMP treatment, the induction of alkaline phosphatase by 10−7 M RA was not inhibited by Chd. We conclude that Chordin protein can antagonize signaling by fully processed mature BMPs but has no effect on the pathway of osteogenic differentiation triggered by RA.
Figure 2. Chordin Protein Inhibits the Osteogenic Activity of Mature BMP Proteins and Prevents Binding to Their Natural Receptors in 10t1/2 Cells.
(A) Dose-dependent induction of alkaline phosphatase in 10t1/2 cells by BMP-4/7 and its inhibition by Chd. Addition of 10 nM Chd reduces the activity of 2.5 nM BMPs to the level induced by 0.5 nM; proteins purified from control-infected cells lacking Chd have no inhibitory activity. Alkaline phosphatase induced by 10−7 M RA is not affected by Chd. Data indicate the mean value of six alkaline phosphatase determinations plus or minus one standard deviation; significance of Chd inhibition is p < 0.001.
(B) Chordin competes binding of 125I–BMP-4 to its natural receptors on 10t1/2 cells. Live cells were incubated with 25 pM 125I–BMP-4 (Massagué, 1987) and competed with increasing concentrations of chordin (closed circles). A 100-fold excess (2.5 nM) of cold BMP-4 (open circle) competed specific binding (leaving 10%–15% background); TGF-β1 did not compete (open triangle). Each data point is the mean of three determinations.
Chd Blocks Binding of BMP-4 to Its Receptor
To test whether Chd antagonizes BMP signaling upstream of BMP receptor binding, we carried out a radio-receptor binding assay using 10t1/2 cell monolayers (Massagué, 1987; Attisano et al., 1992). 125I–BMP-4 was added at a concentration of 25 pM and background binding levels considered to be those detected in the presence of 100-fold excess of cold BMP-4. As shown in Figure 2B, increasing concentrations of Chd protein displaced binding of 125I–BMP-4 to its natural receptor. Competition was complete at 5 nM, and 50% inhibition was obtained at about 3 × 10−10 M Chd. We conclude that Chd protein can inhibit binding of BMP-4 protein to its cognate receptor at subnanomolar concentrations. Chd should function within a narrow window of the signaling pathway because it acts downstream of BMP maturation and secretion and upstream of BMP–receptor interaction.
Chd Binds Directly to BMPs
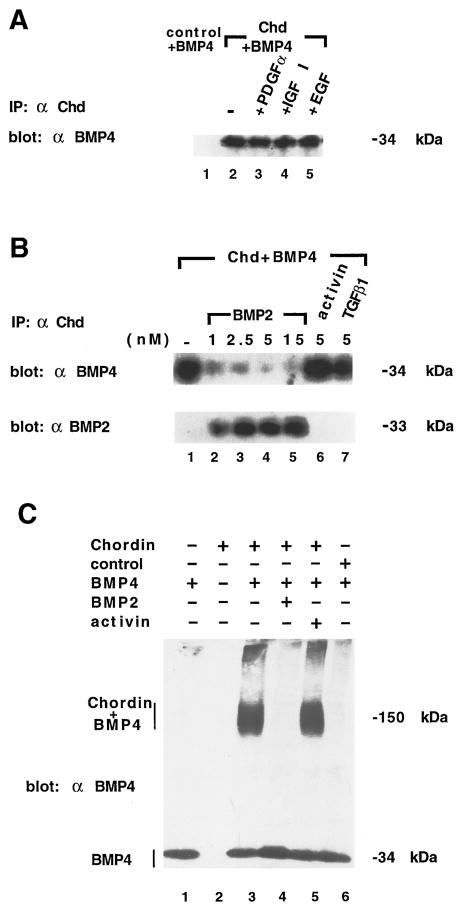
To investigate whether chd can bind BMP-4 directly, we performed immunoprecipitation assays with the anti–NH2-Chd antibody. As shown in Figure 3A, Chd (2.5 × 10−10 M) and cold BMP-4 (at 5 × 10−10 M) were bound at room temperature for 1 hr in 1 ml of phosphate-buffered saline (PBS) binding buffer, immunoprecipitated with anti-Chd antibody, and the amount of BMP-4 bound to Chd detected on Western blots with a monoclonal anti–BMP-4 antibody that detects an epitope entirely specific for BMP-4 (a gift of K. Masuhara et al.). BMP-4 was bound by Chd but not by similarly purified control proteins (Figure 3A, lanes 1 and 2). To address the issue of specificity of this interaction, we competed with a 10-fold molar excess of unrelated growth factors (platelet-derived growth factor, insulin-like growth factor I, and epidermal growth factor; Figure 3A, lanes 3–5), which did not interfere with Chd–BMP-4 binding. As shown in Figure 3B (lanes 1–5), BMP-2, which behaves like BMP-4 but does not cross-react with the anti–BMP-4 monoclonal, competed BMP-4 binding. Increasing concentrations of BMP-2 bound the available Chd protein to saturation, displacing BMP-4; this was visualized by stripping the Western blot with β-mercaptoethanol and SDS and immunostaining with an anti–BMP-2 antibody (Figure 3B). Activin, a TGF-β family member that has opposing biological effects to those of BMP-4 in Xenopus assays (Jones et al., 1992; Dale et al., 1992), as well as TGF-β1, did not compete the binding of BMP-4 to Chd even at 10-fold molar excess (Figure 3B, lanes 6 and 7).
Figure 3. Chordin Binds to BMPs But Not to Activin.
Immunoprecipitation (A and B) and cross-linking (C) results were detected by nonreducing SDS–polyacrylamide gel electrophoresis and Western blot using an anti–BMP-4 monoclonal, as described in Experimental Procedures.
(A) Immunoprecipitation with anti–NH2-Chd antibody of a mixture of 0.25 nM Chd and 0.5 nM BMP-4. Lane 1: control protein purified from an irrelevant viral infection serves as negative control. Lane 2: BMP-4 is bound to chordin and visualized in Western blots probed with anti–BMP-4 monoclonal antibody. Lanes 3–5: BMP-4 binding cannot be competed by 5 nM of the unrelated cytokines platelet-derived growth factor-α, insulin-like growth factor I, and epidermal growth factor.
(B) The immunoprecipitation assay was used to compete the binding of BMP-4 (lane 1) with increasing amounts of BMP-2 (lanes 2–5) and a 10-fold excess of activin or TGF-β1 (lanes 6 and 7). Binding revealed by the anti–BMP-4 monoclonal is shown in the top row; after stripping with β-mercaptoethanol and SDS, the same filter was probed with anti–BMP-2 monoclonal and is shown in the bottom row. Note that BMP-2 (but not activin or TGF-β1) competes with BMP-4 for binding to chd, reaching saturation at 2.5 nM.
(C) Cross-linking analysis of Chd–BMP-4 complexes using dithiobis-(succinimidyl)propionate. Lanes 1 and 2: BMP-4 alone and Chd alone. Chd and BMP-4 form a complex of 150 kDa (lane 3) which is competed by a 10-fold excess of BMP-2 but not of activin (lanes 4 and 5). Control protein extracts from an irrelevant baculovirus infection show no binding (lane 6). We note that BMP-4 may undergo a conformational change when bound to chordin. This is indicated by the observation that the Chd–BMP complex is recognized more intensely than unbound BMP-4 by the monoclonal antibody (compare lanes 1 and 3). The simplest interpretation for this observation is that the monoclonal epitope becomes more accessible in the Chd–BMP-4 complex. This increase in immunostaining was even more evident when BMP-4/7 heterodimer, which per se was barely detected by the monoclonal antibody, was bound by chordin (data not shown).
To confirm these binding results by an independent method, we chemically cross-linked the chordin–BMP-4 complex with dithiobis(succinimidyl)propionate. The products of the reaction were separated in a nonreducing SDS polyacrylamide gel and visualized with anti–BMP-4 monoclonal antibody. As shown in Figure 3C, BMP-4 bound to Chd migrated as a band of about 150 kDa, while the BMP-4 dimer migrated at 34 kDa. This shift is consistent with the cross-linking of one BMP-4 dimer per Chd molecule. The interaction was specific, for it could be competed by BMP-2 but not by activin and did not take place with control proteins (Figure 3C, lanes 3–6).
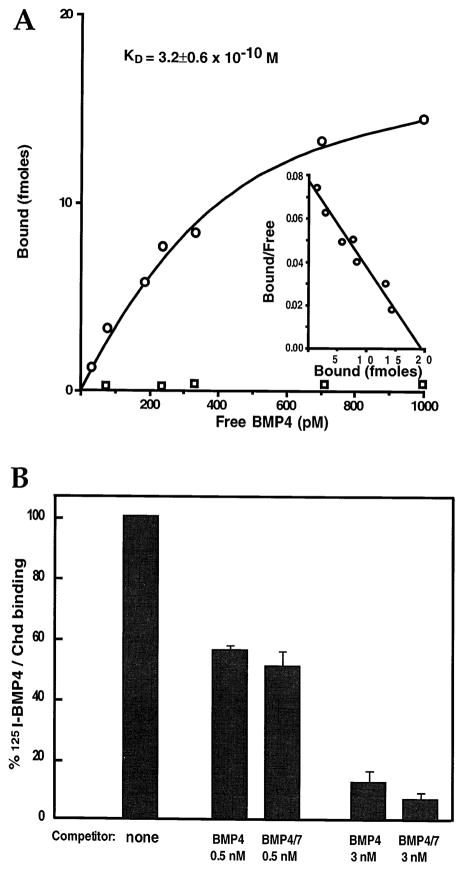
For quantitative studies of binding affinity, we used 125I–BMP-4, which can be counted directly in Chd immunoprecipitates. As shown in Figure 4A, under equilibrium conditions, binding of 125I–BMP-4 to chordin was saturable and Scatchard analysis indicated high affinity. Data from three independent experiments resulted in a dissociation constant (KD) value of 3.2 ± 0.6 × 10−10 M (320 pM), which is within the same range of the KD of BMPs for their receptors (2.5–9 × 10−10 M; Penton et al., 1994; Graff et al., 1994).
Figure 4. Affinity of the Binding of Chordin to BMP-4.
(A) Equilibrium binding of increasing concentrations of 125I–BMP-4 to 250 pM Chd (open circles) or to control medium (open squares). Binding was for 2 hr at room temperature, and the bound and free 125I–BMP-4 was separated by immunoprecipitation. Each data point was in triplicate, and three independent experiments were performed. Scatchard analyses (inset) of these data (using the Cricket Graph computer program) resulted in a KD of 3.2 ± 0.6 × 10−10 M.
(B) Competition by cold BMP-4 or BMP-4/7 (at 0.5 and 3 nM) of the binding of 125I–BMP-4 (60 pM) to chordin (120 pM). Even though BMP-4/7 is more active in biological assays than BMP-4, their binding to chordin protein is not significantly different.
Finally, we tested whether the affinity of BMP-4/7 heterodimers to Chd was comparable to that of BMP-4. From a biological standpoint, it was important to determine this for several reasons: first, Xenopus gastrulae contain BMP-7 mRNA that is coexpressed with BMP-4 (Hawley et al., 1995); second, heterodimerization with BMP-7 greatly increases secretion of BMP-4 subunits (Hazama et al., 1995); and third, recombinant Xenopus BMP-4/7 heterodimers were 15- to 20-fold more active than BMP-4 or BMP-7 homodimers in bone induction assays (Aono et al., 1995). As shown in Figure 4B, unlabeled BMP-4 and BMP-4/7 heterodimers were able to compete with similar affinities in Chd–125I–BMP-4 binding assays. Thus, although BMP-4 and BMP-4/7 may differ in inductive activity, this difference does not reside in their regulation by Chd. We conclude from these binding studies that Chd is able to bind BMP-4, BMP-2, and BMP-4/7 heterodimer, with high affinity and specificity.
Nanomolar Chd Mimics Organizer Inductions
Organizer tissue releases the neural induction and mesoderm dorsalization signals at the gastrula stage (Spemann and Mangold, 1924). Chordin and BMP-4 are expressed at the proper time and place to participate in this process. Expression of chd mRNA is restricted to regions with known organizer activity during gastrulation (Sasai et al., 1994), and Chd protein is secreted into the culture medium by organizer cells during gastrulation (see Figure 1, lane 8). BMP-4 mRNA is expressed in a complementary domain of the gastrula, and the timing of its phenotypic effects suggests that BMP-4 might signal after the onset of gastrulation (Dale et al., 1992; Fainsod et al., 1994; Sasai et al., 1995; Jones et al., 1996). To test whether chordin protein can mimic Spemann’s organizer signals at concentrations close to physiological levels and at the appropriate time in development, we analyzed its effects on Xenopus gastrula animal cap and VMZ explants.
To assay for neural induction activity, we added chordin protein to animal cap explants at stage 10.5 (early gastrula). The experimental protocol and incubation in low calcium magnesium Ringer (LCMR) were as described in previous studies for the noggin protein (Lamb et al., 1993; Lamb and Harland, 1995). Induction of neural cell adhesion molecule was dose-dependent and evident at 0.8 nM Chd protein (Figure 5A, lanes 1–4). This induction took place in the absence of mesoderm formation, as indicated by the lack of expression of α-actin. Addition of equimolar amounts of BMP-4 was able to antagonize neural induction by chordin. When Chd and BMP-4 were added to the gastrula explants at 20 nM each, neural induction was inhibited (Figure 5A, lane 5).
To test the effect of Chd on mesoderm dorsalization, VMZs were explanted at stage 10.5 and incubated in LCMR (as described by Lamb et al. [1993]) containing increasing amounts of Chd. Muscle actin mRNA was induced at 1 nM chd and increased at 8 and 20 nM (Figure 5B, lanes 2–4). The neural marker neural cell adhesion molecule was also activated, presumably as a secondary induction caused by mesoderm formation (Figure 5B). The induction of dorsal mesoderm was also evidenced by the elongation of the VMZ explants and the formation of somites (Figures 5D–5E′). Notochord tissue was not formed. Addition of BMP-4 in equimolar amounts (at 1 nM, data not shown; and 20 nM) reversed the dorsalized phenotype caused by Chd. At 20 nM Chd and BMP-4, muscle induction was reversed (Figure 5B, lane 5), and the VMZ explants developed as ventral mesoderm (blood, mesenchyme) with no somites (Figures 5F and 5F′; compare with control protein at similar dilution, Figures 5C and 5C′). The observation that BMP-4 is able to block the neuralizing and dorsalizing activities of chd even at equimolar concentrations is of interest concerning mechanism. While at 1 nM Chd and BMP-4, binding would reduce free Chd to below the signaling threshold, at higher concentrations this is not the case. Indeed, considering that the KD is 3.2 × 10−10 M, a mixture initially containing 20 nM Chd and 20 nM BMP-4 will still contain 2.4 nM free Chd once equilibrium is reached (this can be calculated by solving the chemical mass action equation at equilibrium). If Chd were signaling through its own receptor, 2.4 nM would be sufficient to induce neural and dorsal tissue in explants. Thus, the molar ratios required for inhibition by BMP-4 tend to favor the view that the main inductive activities of Chd are mediated by binding to and inactivating endogenous BMPs present in the embryo.
We conclude that chordin protein can cause neural induction and mesoderm dorsalization in the low nanomolar range. Clear inductive effects by Chd can be observed at physiological concentrations acting within one order of magnitude of its KD for BMP-4. The timing of the inductions by chordin protein and their antagonism by BMP-4 is consistent with that of the noncell-autonomous effects of transplanted organizer tissue.
Discussion
Chd Inactivates BMPs in the Extracellular Space
We have presented data suggesting that the antagonism between Chd and BMP-4 can be mediated by direct binding between these two molecules, as proposed in the molecular mechanism depicted in Figure 6. Dorsoventral patterning in the Xenopus gastrula seems to depend on antagonistic interactions between diffusible molecules in the extracellular space. Chordin mRNA is expressed in the organizer region (i.e., where BMP-4 is not expressed) starting at the gastrula stage (Sasai et al., 1994). Chd protein is secreted in soluble form by dissociated organizer cells and, while an exact calculation is difficult (Figure 1, legend), the concentration of Chd protein in the extracellular space of organizer tissue should be well within the 10−9 M range. In VMZ and animal cap gastrula explants, chordin protein has inductive activities clearly detectable at 1 nM concentration (Figure 5); i.e., within the physiological range. BMP-4 protein can function when added to gastrula explants, overriding the dorsalizing and neuralizing effects of Chd, directly demonstrating that BMP-4 protein can act after the start of gastrulation. The concentration of mature BMP-4 protein in the Xenopus gastrula is not known, but in dissociated animal caps BMP-4 induces epidermis at 40 pM (Wilson and Hemmati-Brivanlou, 1995). If this reflects endogenous concentrations, the amount of Chd present in the extracellular space of the organizer during gastrulation should suffice to block any effects of BMP-4.
Figure 6. Model of the Molecular Mechanism by Which Chordin Antagonizes BMP-4 Signaling.
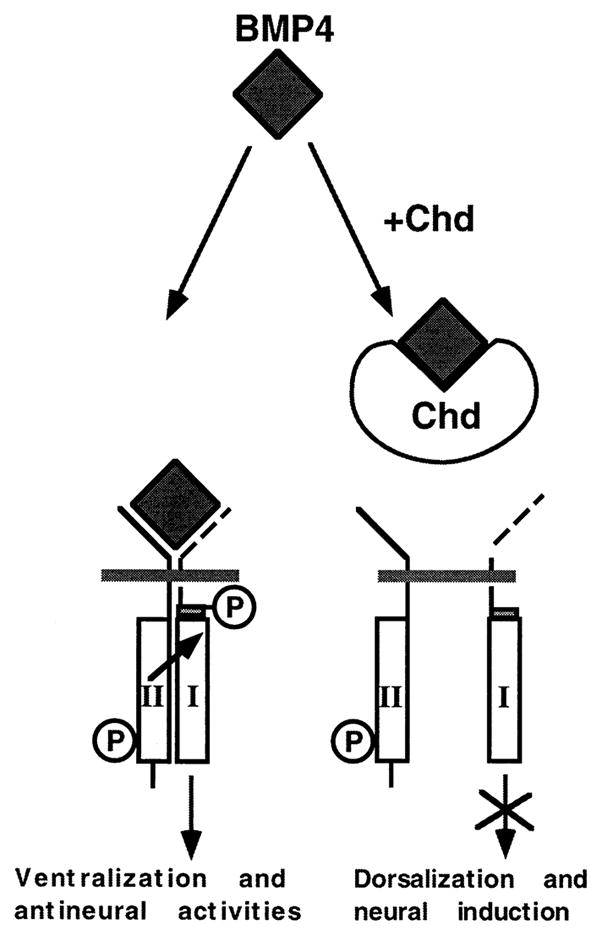
Signaling by members of the TGF-β family is mediated by type I and type II receptors. The BMP-4 dimer would bind to the constitutively active serine–threonine kinase receptor II, recruiting receptor I, which in turn becomes phosphorylated and propagates the signal intracellularly (modified from Wrana et al. [1994]). Chordin binds to BMP-4 and other ventral BMPs in the extracellular space, preventing the BMP–receptor interaction.
The KD for the binding of BMP-4 to Chd is 3 × 10−10 M (Figure 4A). This is within the same physiological range as the affinity of BMPs for their receptors (9 × 10−10 M for the Xenopus BMP-2–4 receptor, Graff et al., 1994; 2.5 × 10−10 M for the thick veins dpp receptor, Penton et al., 1994). To determine that Chd can block binding of BMP-4 to cell-surface receptors, we used a radioreceptor assay, which showed that 50% inhibition was obtained at 3 × 10−10 M (Figure 2B); i.e., at the same concentration as the KD of the Chd–BMP-4 interaction. Thus, the binding of Chd to BMP-4 is sufficient to block BMP signaling through its receptor. The effects of Chd on neural induction and mesoderm dorsalization in Xenopus explants (Figure 5) are presumably mediated by the inhibition of endogenous BMP signals. This is supported by the observation that blocking endogenous BMP signaling by agents such as dominant-negative BMP receptors, antisense RNA, or dominant-negative ligands also results in neuralization and dorsalization in Xenopus assays (Graff et al., 1994; Suzuki et al., 1994; Sasai et al., 1995; Xu et al., 1995; Steinbeisser et al., 1995; Hawley et al., 1995).
Comparison of Chd and noggin
It is worthwhile to compare the activities of Chd and noggin in Xenopus embryos. Both can act as neural inducers (Lamb et al., 1993; Sasai et al., 1995; this study) even when added as purified proteins on the proper substrate, the gastrula animal cap. Follistatin is a neural inducer and a dorsalizing agent when overexpressed as mRNA (Hemmati-Brivanlou et al., 1994; Sasai et al., 1995) but differs from Chd and noggin in that it binds avidly to activin (and with lower affinity also to BMP-7; Yamashita et al., 1995) and is not active at the gastrula stage (Lamb and Harland, 1995). Concerning mesodermal differentiation, until the present study noggin was the only protein known to dorsalize ventral mesoderm at the gastrula stage (Smith et al., 1993). As shown above, Chd protein also has this activity. This is important, because the organizer releases a horizontal signal during gastrulation that is able to dorsalize mesoderm (Dale and Slack, 1987). Both noggin and chordin are expressed at the right time and place to mediate this fundamental organizer property.
Injection of Xenopus noggin mRNA into Drosophila embryos of various mutant backgrounds has provided a heterologous assay system to test the mechanism of action of noggin. It has been found that noggin mRNA blocks dpp signaling upstream of dpp receptor activation (Holley et al., 1996 [this issue of Cell]). This conclusion is in agreement with recent experiments by Zimmerman et al. (1996 [this issue of Cell]) in which direct binding between noggin and BMP-4 is demonstrated. The noggin binding is highly specific, cannot be competed by activin, and has a KD of 2 × 10−11 M. Taken together, these data suggest that chd and noggin function similarly from a mechanistic point of view: both molecules bind to BMPs and prevent signaling by BMP receptors.
Although both noggin and chordin protein can dorsalize and neuralize at the correct stage, there are differences in the concentrations required for these activities. Despite the differences reported in their estimated KD for BMPs (20 pM for noggin and 300 pM for Chd), both proteins are able to dorsalize mesoderm at 1 nM in Xenopus VMZ explants. In the case of noggin, dorsalizing activity can be obtained at 0.8 nM (50 ng/ml of noggin dimer), but the neuralizing activity in intact animal cap explants requires 20-fold higher amounts (15.5 nM or 1 μg/ml; Lamb et al., 1993). In contrast, for chordin, 1 nM is sufficient to obtain detectable amounts of both dorsalizing and neuralizing activity. This suggests that although both molecules act by binding BMPs, differences that are not detected by the biochemical binding assays must exist in their mode of action in vivo, at least for neural induction. In future, it will be interesting to test whether chordin and noggin have additive or synergistic effects.
The organizer secretes two diffusible molecules, noggin and chordin, which are able to bind BMP-4 and block its binding to BMP receptor. The reason for having two different molecules with redundant biochemical activities is unknown, but two possibilities should be discussed. First, chd and noggin are regulated by different mechanisms. The expression of chd is activated by microinjected goosecoid, Xnot-2, and Xlim-1 mRNAs, which encode homeobox genes expressed specifically in the organizer, whereas in the same experiments the expression of noggin is not affected (Sasai et al., 1994, 1995; Taira et al., 1994). In addition, noggin mRNA has an important maternal component (Smith and Harland, 1992), whereas transcription of chd appears to be entirely zygotic (Sasai et al., 1994). Thus, even if both proteins had identical function, their expression patterns would still have important differences. Perhaps the main difference is that chd transcripts are more abundant than those of noggin (Sasai et al., 1994; Bouwmeester et al., 1996). The second possibility that merits discussion is that noggin and chordin might differ in their diffusion rates after they are secreted by organizer tissue. Although there is no direct study of diffusion in vivo as yet, one might expect noggin to diffuse further than Chd due to its smaller molecular mass. This longer range activity is supported by the tendency of ventral injections of noggin mRNA to dorsalize the entire embryo, in conditions in which chd mRNA produces predominantly secondary axes (our unpublished data). Differential diffusion of Chd and noggin would create a complex, perhaps stepwise, gradient of ventral signals. Should the signal be stepwise, it could correlate with the multiple histotypes of the mesoderm (somite, kidney, and lateral plate) and ectoderm (central nervous system, neural crest, and epidermis).
This view of dorsoventral patterning is supported by genetic studies in Drosophila, in which a gradient of dpp activity is set up in part by antagonistic interactions with sog, the chd homolog, and acts as the principal dorsoventral morphogen. We propose that the noncell-autonomous effects of Spemann’s organizer in the vertebrate gastrula may be executed in part by diffusion of the secreted proteins chordin and noggin that directly bind to and antagonize the ventral BMP signal, thus creating pattern in the ectodermal and mesodermal germ layers.
Experimental Procedures
Overexpression and Partial Purification of Chd Protein
An epitope-tagged chd cDNA was constructed by inserting a double-stranded oligonucleotide encoding a human c-Myc epitope (EQKLISEEDL followed by a stop codon) into the NcoI site of Xenopus chd, located one amino acid upstream of the natural stop codon. The resultant tagged chd construct (pSP35–chd–myc) contains the entire cDNA encoding the Chd protein except for the last amino acid. When 150 pg of synthetic mRNA transcribed from pSP35 chd–myc was injected into single blastomeres of eight-cell embryos, strong dorsalizing effects were observed (secondary axis formation in 19/25; hyperdorsalization in 3/25) which were indistinguishable in this assay from chd constructs lacking the myc tag. We concluded that the epitope tag does not affect Chd activity and therefore the coding fragment of pSP35 chd–myc was subcloned into the EcoRI (5′) and NcoI (3′) sites of the baculovirus shuttle vector pVL1393 with corresponding linkers. pVL1393 was converted into baculovirus by using the Baculogold system (Pharmingen) and Sf9 cells (Invitrogen). Single-virus isolation was performed by endpoint dilution. After amplification of the virus stock in Sf9 cells, High-Five cells (Invitrogen) were infected with baculovirus encoding either chd–myc or an irrelevant protein (the nonsecreted adenoviral E1B 55K protein, provided by Dr. A. Berk, University of California, Los Angeles) at a multiplicity of infection of ~2.5 and cultured in serum-free medium (EX-CELL, JRH Biosciences). Proteins secreted by cells infected with the latter virus were used as a negative control throughout this study. Optimal expression levels were observed 4 days after infection, and conditioned medium and cells were collected separately and stored at −80°. For further purification, 50 ml of conditioned media from insect cell cultures were incubated for 1 hr at room temperature with 1 ml of heparin–agarose beads (Sigma) pre-treated with 1% bovine serum albumin (BSA) for 1 hr. After rinsing in PBS twice, the beads were subjected to column chromatography. The bound proteins were eluted in 5 ml of 1 M NaCl, 5 mM HEPES (pH 7.4), 2 mM KCl. The eluant was dialyzed twice against 1 l of 5 mM HEPES (pH 7.4), 100 mM NaCl, 2 mM KCl, and 2 mM NaHCO3 for 2 hr at room temperature. Quantitation of Chd protein was performed both by immunoblotting with anti-myc antibody, using as a standard a pure protein containing the myc epitope (a gift from Dr. A. Berk), and by Coomassie Blue staining of SDS–polyacrylamide gel electrophoresis gels using BSA as a standard. Both measurements gave congruent results. In SDS–polyacrylamide gel electrophoresis gels stained with Coomassie, the 120 kDa Chd protein band was the strongest one, comprising roughly 25% of the total protein. Starting with 50 ml of the Chd-conditioned medium, we typically obtained 25 μg of recombinant Chordin protein.
Peptide Growth Factors and Antibodies
Purified recombinant BMP-4/7 heterodimer overproduced in baculovirus was a gift of Dr. M. Hazama (Takeda Pharmaceutical Co.). Recombinant human BMP-2 and human BMP-4 were supplied by Genetics Institute and human β-A activin by Genentech. TGF-β, platelet-derived growth factor, insulin-like growth factor I, and epidermal growth factor were purchased from Genzyme. Monoclonal antibodies against human BMP-4 and against human BMP-2 were gifts of Dr. K. Masuhara (Osaka University School of Medicine) and of Genetics Institute, respectively. Anti-myc monoclonal antibody was purchased from Santa Cruz.
Embryonic Manipulations
Artificial fertilization and mRNA injection were performed as described (Sasai et al., 1994). For the neural induction assay, we followed the procedure previously used for noggin (Lamb et al., 1993; Lamb and Harland, 1995). Briefly, animal caps devoid of marginal zone cells were excised in LCMR (Lamb et al., 1993) at stage 91/2 and aged until stage 10. It is known that in the animal cap assay, soluble factors can act only on the inner (sensorial) layer and that they are not effective after the caps have rounded up and healed. To keep animal cap explants open until chordin protein was applied, the animal caps were incubated in very low Ca++, Mg++ Ringer (Lamb et al., 1993; except that in our case for the Ca++, Mg++-free Ringer solution, CMFR, 10 mM HEPES buffer [pH 7.35] was used instead of Tris buffer). The explants were incubated overnight with either Chd protein or similarly purified control virus medium in LCMR supplemented with 0.2% BSA. For this incubation, 96 well tissue-culture plates precoated with 5% BSA were used. The animal caps were cultured further in tissue-culture dishes in 0.4 × MMR supplemented with penicillin and streptomycin until the indicated stage.
VMZ explants (comprising 60° of the VMZ) from stage 101/4 embryos were prepared in LCMR, opened with an eyebrow knife, and immediately transferred to LCMR containing Chd protein or control-conditioned medium. 96 well polymerase chain reaction (PCR) plates (Costar) precoated with 5% BSA were used, and a single explant was put in each well containing 20 μl of Chd solution. After overnight incubation, the explants were transferred to 0.4 × MMR.
Recovery of soluble endogenous Chd protein was performed by dissociating 10–20 DMZ or VMZ explants and culturing in 80 μl of CMFR using PCR plate wells saturated in BSA. After incubating 1.5 hr with slow rotary agitation, the cells were allowed to settle, and the supernatant was collected, centrifuged at 10,000 g for 2 min, and stored at −80°C in presiliconized tubes (Intermountain Corp.).
Antibody Production and Immunoblotting
An oligopeptide corresponding to the amino-terminal 19 residues of Xenopus Chd following the signal sequence was synthesized and conjugated to KLH carrier. Antisera against the NH2-terminal peptide conjugate were raised in rabbits. After three boosts, the antiserum was subjected to immunoaffinity purification using immobilized NH2-peptide. Protein samples were resolved by SDS–polyacrylamide gel electrophoresis and electroblotted onto nitrocellulose membranes. The filter was blocked with TBST (10 mM Tris [pH 7.5], 100 mM NaCl, 0.1% Tween 20) containing 15% goat serum and incubated in the same buffer with the primary Ab for 2 hr at room temperature. The dilutions of the primary antibodies were 1000-fold for anti–NH2-chordin polyclonal, 1 μg/ml for anti-myc monoclonal antibody, and 10-fold and 200-fold dilution for culture supernatants containing anti–BMP-4 monoclonal antibody and anti–BMP-2 monoclonal antibody, respectively. The antibodies bound to Western blots were developed with the chemiluminescent ECL kit (Amersham) using anti-rabbit or anti-mouse secondary antibodies, as described by the manufacturer.
Osteogenic Differentiation Assay
We chose to use the multipotent mesodermal cell line 10t1/2, instead of the commonly usedW20 or MC3T3 cells, because the background alkaline phosphatase levels were found to be minimal (Katagiri et al., 1990; our unpublished data). 10t1/2 cells were cultured in αMEM supplemented with 10% fetal calf serum (Gibco). The osteogenic differentiation assay was performed according to Katagiri et al. (1990). 10t1/2 cells (American Type Culture Collection) were plated in 24 well plates (Corning) at 50% confluency. After 24 hr incubation, the medium was changed to αMEM with 5% NuSerum (Collaborative Research) containing the indicated concentrations of BMP-4/7, Chd, and/or all-trans RA (Sigma). After 2 days of incubation, alkaline phosphatase activity of the cells was assayed using a colorimetric kit (Sigma). Cells were washed in PBS and lysed by sonication in 300 μl 2 mM MgCl2, 0.2% NP-40. Substrate solution (200 μl) was added and incubated at 37°C for 30 min. After stopping with 0.1 N NaOH, absorbance at 405 nM was measured and compared to a standard curve of p-nitrophenol (Katagiri et al., 1990).
Radioreceptor Binding Assay
BMP-4 protein was labeled with 125I using the chloramine T method (Frolik et al., 1984), and a specific activity of 70 Ci/g was obtained. The cell-surface receptor binding assay was performed using 10t1/2 cells, basically as described (Massagué, 1987). 10t1/2 cells were placed in 24 well plates at 75% confluency in αMEM/fetal calf serum and reached 100% confluency the next day. The cells were rinsed twice with Hank’s buffered salt solution and incubated with 25 pM 125I–labeled BMP-4 in 0.2 ml Hank’s buffered salt solution containing increasing concentrations of Chd protein at room temperature for 1 hr. Then the plates were chilled on ice and each well quickly rinsed three times with 1.5 ml ice-cold Hank’s buffered salt solution. The bound 125I–BMP-4 was solubilized with 0.5 ml solubilization buffer and counted with a γ counter (Massagué, 1987).
Immunoprecipitation and Cross-Linking Assay
Chd and cold BMP-4 were incubated for 1 hr at room temperature in 1 ml of PBS containing 1 mM CaCl2, 3 mM MgCl2, 0.2% NP-40, and 1 mg/ml BSA. For 125I–BMP-4, 0.7 ml were used. Anti–NH2-Chd antibody (2 μl of serum) was bound to protein A sepharose (Pharmacia) and 20 μl of beads added to each reaction. After binding at 4°C for 3 hr in an Eppendorph mixer, beads were pelleted for 1 min, resuspended in 1 ml PBS as above with the addition of 0.2% Tween-20, and washed three times. The entire procedure took about 5 min. For cross-linking, Chd and BMP-4 in 80 μl PBS were incubated for 1 hr at room temperature. Dithiobis(succinimidyl)propionate (Pierce) was added to a final concentration of 0.15 mM, incubated for 15 min at room temperature, and stopped by adding Tris–HCl (pH 7.4) to a final concentration of 30 mM. Samples were electrophoresed in SDS gels under nonreducing conditions.
RT–PCR Analysis and Histology
Expression of marker genes at stage 27 was assayed by reverse transcription–polymerase chain reaction (RT–PCR). The conditions and primer sequences were as described in Sasai et al. (1995). Histological analysis was performed using 10 μM paraffin section and hematoxylin–eosin staining.
Acknowledgments
We thank Dr. K. Masuhara and Genetics Institute for monoclonal antibodies against BMP-4 and BMP-2, respectively; Takeda Pharmaceutical Co. and Genetics Institute for BMP proteins; Drs. L. Rome and W. Stanford (UCLA) for kind instruction on iodination and osteogenic differentiation assays, respectively; and Dr. A. Berk for the c-myc standard protein and control baculovirus. We thank Dr. Richard Harland (UC, Berkeley) for communicating results prior to publication and Drs. J. Fessler and L. Leyns for critical comments on the manuscript. S. P. is an awardee of a Comitato Promotore Telethon postdoctoral fellowship. E. M. D. R. is an investigator of the Howard Hughes Medical Institute. This work received support from National Institutes of Health grant HD21502-11 and the Norman Sprague Endowment.
References
- Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y. Potent ectopic bone-inducing activity of bone morphogenetic protein 4/7 heterodimer. Biochem Biophys Res Commun. 1995;210:670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Cheifetz S, Massagué J. Novel actin receptors: distinct genes and alternative mRNA splicing generate a repertoire of serine/threoninekinase receptors. Cell. 1992;68:97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S-H, Sasai Y, Lu B, De Robertis EM. Cerberus, a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature. 1996 doi: 10.1038/382595a0. in press. [DOI] [PubMed] [Google Scholar]
- Dale L, Slack JMW. Regional specification within the mesoderm of early embryos of Xenopus laevis. Development. 1987;100:279–295. doi: 10.1242/dev.100.2.279. [DOI] [PubMed] [Google Scholar]
- Dale L, Howes G, Price BMJ, Smith JC. Bone morphogenetic protein-4: a ventralizing factor in early Xenopus development. Development. 1992;115:573–585. doi: 10.1242/dev.115.2.573. [DOI] [PubMed] [Google Scholar]
- Dawid IB. Intercellular signaling and gene regulation during early embryogenesis of Xenopus laevis. J Biol Chem. 1994;269:6259–6262. [PubMed] [Google Scholar]
- De Robertis EM. Dismantling the organizer. Nature. 1995;374:407–408. doi: 10.1038/374407a0. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Sasai Y. A common plan for dorso-ventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. decapentaplegic acts as a morphogen to organize dorsal–ventral pattern in the Drosophila embryo. Cell. 1992a;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. Localized enhancement and repression of the activity of the TGF-β family member, decapentaplegic, is necessary for dorsal–ventral pattern formation in the Drosophila embryo. Development. 1992b;114:583–597. doi: 10.1242/dev.114.3.583. [DOI] [PubMed] [Google Scholar]
- François V, Bier E. Xenopus chordin and Drosophila sog genes encode homologous proteins functioning in dorsal–ventral axis formation. Cell. 1995;80:19–20. doi: 10.1016/0092-8674(95)90446-8. [DOI] [PubMed] [Google Scholar]
- François V, Solloway M, O’Neill JW, Emery J, Bier E. Dorsal–ventral patterning of the Drosophila embryo depends on a putative negative growth factor encoded by the short gastrulation gene. Genes Dev. 1994;8:2602–2616. doi: 10.1101/gad.8.21.2602. [DOI] [PubMed] [Google Scholar]
- Frolik CA, Wakefield LM, Smith DM, Sporn MB. Characterization of a membrane receptor for transforming growth factor-β in normal rat kidney fibroblasts. J Biol Chem. 1984;259:10995–11000. [PubMed] [Google Scholar]
- Graff JM, Scott Thies R, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm–inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Hamburger V. The Heritage of Experimental Embryology: Hans Spemann and the Organizer. Oxford: Oxford University Press; 1988. [Google Scholar]
- Harland RM. The transforming growth factor-β family and induction of the vertebrate mesoderm: bone morphogenetic proteins are ventral inducers. Proc Natl Acad Sci. 1994;91:10243–10246. doi: 10.1073/pnas.91.22.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama M, Aono A, Ueno N, Fujisawa Y. Efficient expression of a heterodimer of bone morphogenetic protein subunits using a baculovirus expression system. Biochem Biophys Res Commun. 1995;209:859–866. doi: 10.1006/bbrc.1995.1578. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hawley SHB, Wünnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KWY. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffman FM, Ferguson EL. A conserved system for dorsal–ventral patterning in insects and vertebrates involving short gastrulation and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O’Connor MB, De Robertis EM, Ferguson EL. The Xenopus Dorsalizing Factor NOGGIN Ventralizes Drosophila Embryos by Preventing DPP from Activating Its Receptor. Cell. 1996;86 doi: 10.1016/s0092-8674(00)80134-8. this issue. [DOI] [PubMed] [Google Scholar]
- Jones CM, Lyons KM, Lapan PM, Wright CVE, Hogan BLM. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115:639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Jones CM, Dale L, Hogan BLM, Wright CVE, Smith JC. Bone morphogenetic protein-4 (BMP-4) acts during gastrula stages to cause ventralization of Xenopus embryos. Development. 1996;122:1545–1554. doi: 10.1242/dev.122.5.1545. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA, Tanaka H, Omura S, Suda T. The nonosteogenic mouse pluripotent cell line C3H10t1/2 is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochem Biophys Res Commun. 1990;172:295–299. doi: 10.1016/s0006-291x(05)80208-6. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior–posterior neural pattern. Development. 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopoulos GD, Harland RM. Neural induction by secreted polypeptide noggin. Science. 1993;262:713–178. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Massagué J. Identification of receptors for type-β transforming growth factor. Meth Enzymol. 1987;46:174–195. doi: 10.1016/s0076-6879(87)46020-5. [DOI] [PubMed] [Google Scholar]
- Masuhara K, Nakase T, Suzuki S, Takaoka K, Matsui M, Anderson HC. Use of monoclonal antibody to detect bone morphogenetic protein-4 (BMP-4) Bone. 1995;16:91–96. doi: 10.1016/s8756-3282(94)00014-x. [DOI] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. Direct and long range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- Padgett RW, Wozney JM, Gelbart WM. Human BMP sequences can confer normal dorsal–ventral patterning in the Drosophila embryo. Proc Natl Acad Sci USA. 1993;90:2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penton A, Chen Y, Staehling-Hampton K, Wrana JL, Attisano L, Szidonya J, Cassill JA, Massagué J, Hoffman FM. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell. 1994;78:239–250. doi: 10.1016/0092-8674(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Re’em-Kalma Y, Lamb T, Frank D. Competition between noggin and bone morphogenetic protein-4 activities may regulate dorsalization during Xenopus development. Proc Natl Acad Sci USA. 1995;92:12141–12145. doi: 10.1073/pnas.92.26.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the chd and BMP-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Schmidt JE, François V, Bier E, Kimelman D. Drosophila short gastrulation induces an ectopic axis in Xenopus: evidence for conserved mechanisms of dorsal–ventral patterning. Development. 1995;121:4319–4328. doi: 10.1242/dev.121.12.4319. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Uber induktion von embryona-lanlagen durch implantation artfremder organisatoren. Roux’s Arch Entw Mech. 1924;100:599–638. [Google Scholar]
- Steinbeisser H, Fainsod A, Niehrs C, Sasai Y, De Robertis EM. The role of gsc and BMP-4 in dorsal–ventral patterning of the marginal zone in Xenopus: a loss-of-function study using antisense RNA. EMBO J. 1995;14:5230–5243. doi: 10.1002/j.1460-2075.1995.tb00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Scott Thies R, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal–ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci USA. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M, Otani H, Saint-Jeannet JP, Dawid I. Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature. 1994;372:677–679. doi: 10.1038/372677a0. [DOI] [PubMed] [Google Scholar]
- Wharton KA, Ray RP, Gelbart WM. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development. 1993;117:807–822. doi: 10.1242/dev.117.2.807. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by BMP-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-β receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Xu RJ, Kim J, Taira M, Zhan S, Sredni D, Kung H. A dominant-negative bone morphogenetic protein-4 receptor causes neuralization in Xenopus ectoderm. Biochem Biophys Res Commun. 1995;212:212–219. doi: 10.1006/bbrc.1995.1958. [DOI] [PubMed] [Google Scholar]
- Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein-4. Cell. 1996 doi: 10.1016/s0092-8674(00)80133-6. this issue. [DOI] [PubMed] [Google Scholar]