Abstract
The tumor suppressor p53 has a crucial role in cellular response to DNA damage caused by ionizing radiation, but it is still unclear whether p53 can modulate radiation-induced bystander effects (RIBE). In the present work, three different hepatoma cell lines, namely HepG2 (wild p53), PLC/PRF/5 (mutation p53) and Hep3B (p53 null), were irradiated with γ-rays and then co-cultured with normal Chang liver cell (wild p53) in order to elucidate the mechanisms of RIBE. Results showed that the radiosensitivity of HepG2 cells was higher than that of PLC/PRF/5 and Hep3B cells. Only irradiated HepG2 cells, rather than irradiated PLC/PRF/5 or Hep3B cells, could induce bystander effect of micronuclei (MN) formation in the neighboring Chang liver cells. When HepG2 cells were treated with 20 μm pifithrin-α, an inhibitor of p53 function, or 5 μm cyclosporin A (CsA), an inhibitor of cytochrome-c release from mitochondria, the MN induction in bystander Chang liver cells was diminished. In fact, it was found that after irradiation, cytochrome-c was released from mitochondria into the cytoplasm only in HepG2 cells in a p53-dependent manner, but not in PLC/PRF/5 and Hep3B cells. Interestingly, when 50 μg/ml exogenous cytochrome-c was added into cell co-culture medium, RIBE was significantly triggered by irradiated PLC/PRF/5 and Hep3B cells, which previously failed to provoke a bystander effect. In addition, this exogenous cytochrome-c also partly recovered the RIBE induced by irradiated HepG2 cells even with CsA treatment. Our results provide new evidence that the RIBE can be modulated by the p53 status of irradiated hepatoma cells and that a p53-dependent release of cytochrome-c may be involved in the RIBE.
Keywords: irradiation, hepatoma cells, bystander effect, p53, cytochrome-c
Introduction
Since Nagasawa and Little (1992) first demonstrated that the induction of sister chromatid exchanges could be increased in the cells that were not directly irradiated but were in the vicinity of the cells directly exposed by α-particles, radiation-induced bystander effects (RIBE) have become a hot topic for study in recent years. It has been known that bystander signaling can lead to a series of cellular responses including cell death (Mothersill and Seymour, 1997), chromosomal damage (Lehnert and Goodwin, 1997), genomic instability (Seymour and Mothersill, 1997; Morgan et al., 2002; Morgan and Sowa, 2007), mutations (Nagasawa and Little, 1999), gene expressions (Azzam et al., 2003a) and carcinogenesis (Mancuso et al., 2008). Meanwhile, many bystander signaling factors have been identified unceasingly, such as reactive oxygen species (Narayanan et al., 1997; Azzam et al., 2003b), transforming growth factors-β1 (Iyer et al., 2000), tumor necrosis factor-α (Emerit et al., 1996), interleukin-8 (Narayanan et al., 1999), nitric oxide (Matsumoto et al., 2001; Shao et al., 2003a), COX-2 (Zhou et al., 2005) and carbon monoxide (Han et al., 2009). These signaling factors can affect the bystander cells through the culture medium and/or the gap junction between directly communicating cells (Shao et al., 2003b; Mitchell et al., 2004). Evidence shows that the nuclear DNA could not be a direct target in the induction of a RIBE. It has been known that microbeam-targeted cytoplasmic irradiation can also induce bystander responses where cell membrane rafts may be involved (Shao et al., 2004). In addition, as a key cell organelle in the cytoplasm, mitochondria has been shown to be a sensor of RIBE (Murphy et al., 2005) and the bystander response cannot be induced in the cells without mitochondria (Tartier et al., 2007; Zhou et al., 2008; Chen et al., 2008b).
Evidence shows that RIBE has an important role in the radiation effect on tumor cells, and a variety of biological bystander effects have been observed with various types of radiation, including radioisotopes (Chen et al., 2008a), low linear energy transfer radiotherapy (Shareef et al., 2007) and heavy particle therapy in vitro (Harada et al., 2009). Our previous studies found that, mediated by nitric oxide and its downstream product of transforming growth factors-β1, a single irradiated glioma cell could induce chromosome damage in hundreds of surrounding glioma cells (Shao et al., 2008a) and also in bystander normal fibroblast cells (Shao et al., 2005, 2008b). The radiosensitivity of breast cancer cells could be enhanced by bystander fibroblast cells irradiated with a low dose through the Akt pathway (Tsai et al., 2009). Hence, the RIBE between tumor cells and normal cells needs to be considered in our understanding of both effectiveness and risk during clinical radiotherapy.
Hepatocellular carcinoma is one of the most prevalent malignant diseases in Asia. On the basis of the rapid technological development, three-dimensional conformal radiotherapy has become an important strategy in the treatment of hepatocellular carcinoma (Ma et al., 2009). It has been known that almost half of hepatoma cells have an abnormal p53 gene (Puisieux et al., 1993), so that these cells are quite different in their sensitivity to radiation and chemical drugs (You et al., 2002; Ng et al., 2006), also show differences in oncogenicity (Ain et al., 1994), malignancy (Bressac et al., 1990) and even in the synthesis and secretion of signal factors (Moses et al., 1983). In fact, the tumor suppressor p53 gene has a major role in cellular response to DNA damage (Vousden and Lane, 2007). But the relationship between p53 and RIBE has not yet been well defined. Our previous study found that the targeted irradiation induced a bystander effect in T98G cells could be modulated by the inhibition of p53 (Shao et al., 2010), nevertheless, others reported that the RIBE for mutation induction was independent of p53 (Zhang et al., 2008). To better understand the mechanism of the bystander effect from irradiated tumor cells to normal cells, the present work investigated the RIBE in Chang liver cells (wp53) neighboring the irradiated cells of three hepatoma cell lines of different p53 status, and is the first report that a p53-dependent release of cytochrome-c was involved in the RIBE.
Results
Relationship between radiosensitivity and p53
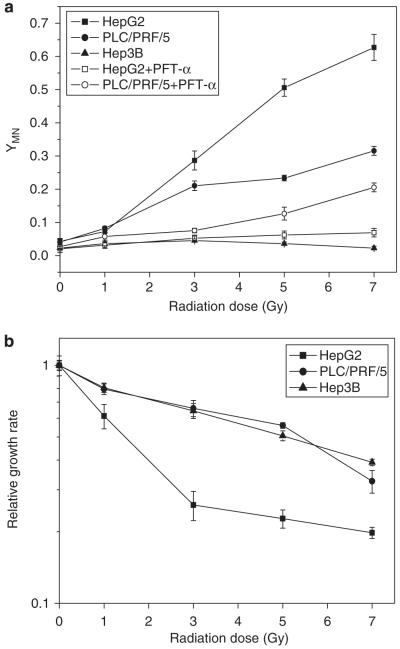
As a result of direct radiation-induced chromosomal damage, MN were detected in the irradiated HepG2 cells (wp53) and PLC/PRF/5 cells (mp53) and their yields increased with dose. In contrast, no significant induction of MN in Hep3B cells (p53 null) was detected even with 7 Gy exposure (Figure 1a). To determine whether p53 was involved in the radiation response of hepatoma cell lines, the HepG2 and PLC/PRF/5 cells were pre-treated with pifithrin-α (PFT-α), a functional inhibitor of p53. Results showed that this treatment produced a marked reduction in the induction of MN, for example, at 7 Gy, the MN yields of irradiated HepG2 cells and PLC/PRF/5 cells were reduced by 88.23 and 34.89%, respectively. Radiation also reduced cell growth and it was found that the relative growth rate of irradiated HepG2 cells was much lower than that of irradiated PLC/PRF/5 and Hep3B cells (Figure 1b). These results meant that the radiosensitivity of HepG2 cells was higher than that of PLC/PRF/5 and Hep3B cells and that radiation-induced DNA damage and cell growth inhibition could be regulated by p53 in hepatoma cells.
Figure 1.
Dose responses of MN formation (a) and relative cell growth rate (b) in three human hepatoma cells (HepG2, PLC/PRF/5, Hep3B) irradiated with γ-rays. In some experiments, HepG2 and PLC/PRF/5 cells were treated with 20 μM PFT-α for 20 h before irradiation and during the subsequent cell culture period. Cell growth rate was measured on the fifth day after irradiation and was normalized to the sham-irradiated (0 Gy) cells.
Relationship between RIBE and p53
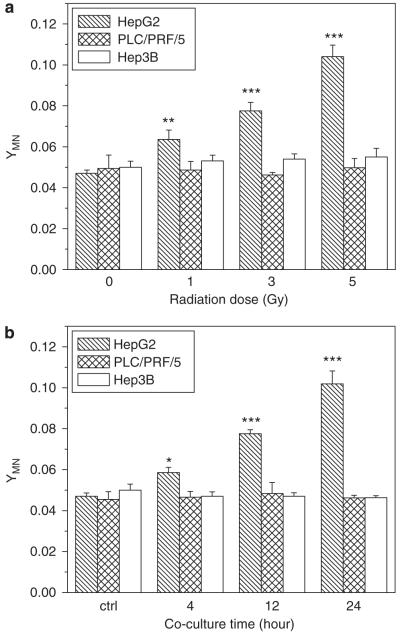
To determine the bystander effect induced by irradiated hepatoma cells, Chang liver cells bearing wp53 were co-cultured with three kinds of hepatoma cells of different p53 status. Figure 2 illustrates that the yield of MN in the bystander Chang liver cells was significantly increased after co-culturing with irradiated HepG2 cells, and it was proportional to both irradiation dose and cell co-culture time. In general, the yield of bystander MN increased with irradiation dose and cell co-culture time, which is different from previous reports that the RIBE is independent of irradiation dose (Mothersill and Seymour, 1997; Shao et al., 2003c). However, the yield of bystander MN was not elevated when the Chang liver cells were co-cultured up to 24 h with either PLC/PRF/5 or Hep3B cells that had been irradiated with different doses. These results indicate that the induction of bystander response may rely on the status of p53 gene. This deduction was further verified by the experiment of treating HepG2 cells with PFT-α. It was found that the bystander response in the Chang liver cells could be fully suppressed by p53 inhibitor and a typical result is shown in Figure 3, where HepG2 cells were irradiated with 3 Gy γ-rays and further co-cultured with non-irradiated Chang liver cells for 12 h, an optimum time condition of bystander response induction. Moreover, Figure 3 also showed that the genotoxic bystander effect on Chang liver cells could be partly reduced when the irradiated HepG2 cells were treated with cyclosporin A (CsA), an inhibitor of cytochrome-c release, which hints that cytochrome-c may have a role of bystander signaling factor in the RIBE.
Figure 2.
MN formation in bystander Chang live cells that had been co-cultured with irradiated hepatoma cells. (a) Dose response of the yield of bystander MN in Chang live cells that were co-cultured with irradiated hepatoma cells for 12 h. **P<0.01, ***P<0.001 compared with the control without irradiation. (b) Time response of bystander MN in Chang live cells that were co-cultured with 3 Gy γ-irradiated hepatoma cells. *P<0.05, ***P<0.001 compared with the control without irradiation. Results correspond to the mean±s.e. of three independent experiments with three replicates in each case.
Figure 3.
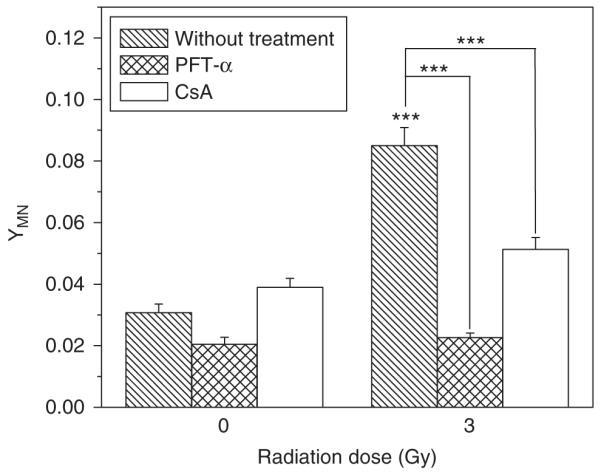
Effect of PFT-α and CsA on bystander MN induction in Chang live cells that had been co-cultured with 3 Gy γ-irradiated HepG2 cells for 12 h. ***P<0.001 compared with the control without irradiation or the indicated group with inhibitor treatment. Results correspond to the mean±s.e. of three independent experiments with three replicates in each case.
Radiation-induced expression of phospho-p53
The protein level of phospho-p53 (Ser15) in three hepatoma cell lines was determined by western blotting and the result is shown in Figure 4. In non-irradiated control cells, phospho-p53 was not expressed in Hep3B cells and had a very low expression in HepG2 cells, but it could be accumulated in PLC/PRF/5 cells as this cell has an abnormal p53 gene type. It is a common feature that phospho-p53 can be highly expressed in cells bearing a mutant p53 gene (Bartek et al., 1991). The present study found that, after 12 h of 3 Gy irradiation, the levels of phospho-p53 in HepG2 cells and PLC/PRF/5 cells were increased by 9-fold and 4.7-fold, respectively, but was still not expressed in Hep3B cells. When HepG2 and PLC/PRF/5 cells were treated with PFT-α, the levels of phospho-p53 in these irradiated cells were reduced to 5-fold and 1.6-fold of the control, respectively. So, all these results are quite consistent with the result in Figure 1a showing that the radiation-induced DNA damage depends on the functionality of p53.
Figure 4.
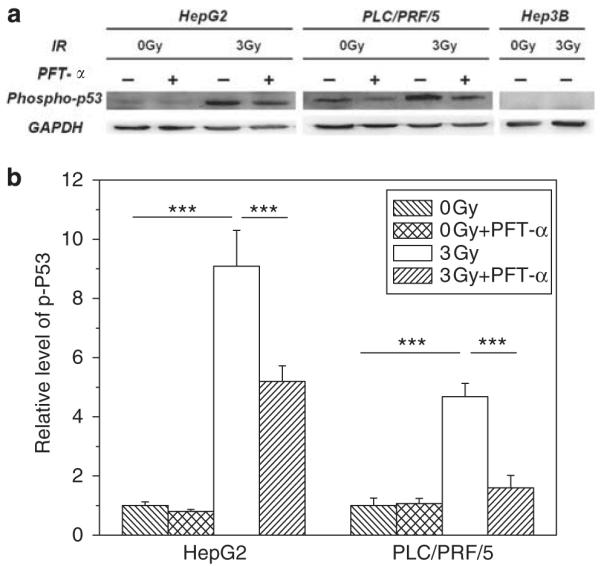
Western blot analysis of the expression of phospho-p53 (Ser15) and GAPDH in human hepatoma cells 12 h after 3 Gy γ-irradiation. In some experiments, HepG2 and PLC/PRF/5 cells were treated with 20 μm PFT-α 20 h before irradiation and during the subsequent cell culture period. (a) Immunoblots of phospho-p53 and GAPDH of the hepatoma cells under different conditions. (b) Relative level of phospho-p53 expression in the hepatoma cells. Values were normalized to GAPDH level in each sample, and then the ratio of each normalized value to its corresponding control (0 Gy) was calculated. ***P<0.001 compared with the control or PFT-α treated cells. All data were presented as the mean±s.e. for three experiments.
Release of cytochrome-c in the irradiated hepatoma cells
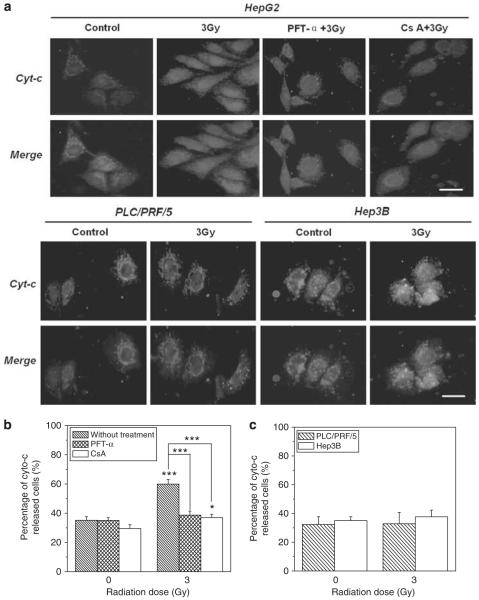
To further understand the role of cytochrome-c in the RIBE, we measured the immunocytochemical distribution of cytochrome-c in the cytoplasm of different hepatoma cell lines. It was found that, under normal condition without irradiation, cytochrome-c was concentrated in the mitochondria surrounding the nuclei of all three cell lines. After irradiation, the cytochrome-c could be released from mitochondria and expressed diffusely in the whole cytosol of HepG2 cells (Figure 5a), so that the percentage of cytochrome-c released was increased from 35% of non-irradiated control cells to 60%. When the HepG2 cells were treated with 20 μm PFT-α or 5 μm CsA, release of cytochrome-c was inhibited and the percentage was significantly decreased to nearly control levels (Figure 5b). But release of cytochrome-c could not be observed for either irradiated PLC/PRF/5 or Hep3B cells where the percentage of cytochrome-c was approximately 33% before and after irradiation (Figure 5c). These results are consistent with the results in Figures 2 and 3 showing that neither irradiated PLC/PRF/5 cells nor Hep3B cells could induce a bystander effect on Chang liver cells and that the bystander effect induced by irradiated HepG2 cells was diminished by either PFT-α or CsA. Accordingly, radiation-induced release of cytochrome-c may be a critical factor of RIBE that can be modulated by p53.
Figure 5.
Release of cytochrome-c from three human hepatoma cells (HepG2, PLC/PRF/5, Hep3B) 12 h after 3 Gy γ-irradiation. In some experiments, HepG2 cells were treated with 20 μm PFT-α before and after irradiation or 1 h with 5 μM CsA before irradiation. (a) Cell image visualized by a fluorescence microscope. Chromatin was stained with 4′,6-diamidino-2-phenylindole and intracellular lipids were stained with Nile red. Immunolocalization of cytochrome-c (green), nuclear (blue) and cell outline morphology (red) were recorded and merged. The size bar in the photo is 20 μm. (b) The percentage of cytochrome-c-released cells in the population of HepG2 cells with and without PFT-α and CsA treatment, respectively. (c) The percentage of cytochrome-c released cells in the population of PLC/PRF/5 and Hep3B cells. *P<0.05 compared with the control without irradiation. ***P<0.001 to the control without irradiation or to the indicated group with inhibitor treatment. Results correspond to the mean±s.e. of three independent experiments with three replicates in each case. A full colour version of this figure is available at the Oncogene journal online.
RIBE triggered by exogenous cytochrome-c
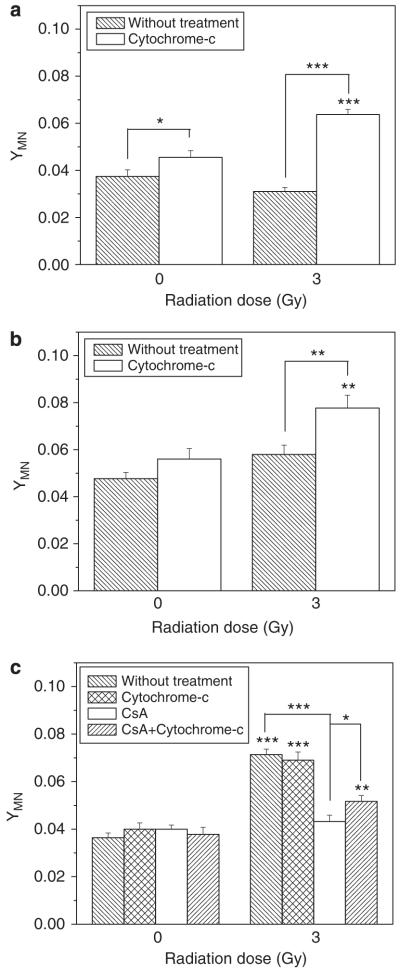
To further verify the critical role of cytochrome-c in the RIBE, we added exogenous cytochrome-c into the co-culture medium of irradiated hepatoma cells and bystander Chang liver cells, and measured the yield of bystander MN. Figure 6 illustrates that the exogenous cytochrome-c had a limited toxic effect on non-irradiated Chang liver cells and slightly increased the cellular MN level, but this exogenous chemical enhanced obviously the yield of MN in the bystander Chang liver cells that were co-cultured with either irradiated PLC/PRF/5 or irradiated Hep3B cells, indicating that the exogenous cytochrome-c stimulated the hepatoma cells bearing abnormal p53 to generate some unknown signaling factors, which further induced bystander damage in their vicinal cells. An unexpected result was that, with respect to HepG2 cells, exogenous cytochrome-c failed to increase the MN yield in the bystander Chang liver cells. This may be because of the bystander signaling factors released from irradiated HepG2 cells, having reached a plateau that saturates the bystander response so that additional exogenous cytochrome-c could not further enhance the bystander effect. However, when the HepG2 cells were treated with CsA, the exogenous cytochrome-c partly recovered the decreased yield of MN in the bystander Chang liver cells, indicating again that some unknown bystander signaling factors may be triggered by the exogenous cytochrome-c, although the release of intrinsic cytochrome-c was blocked.
Figure 6.
Effect of exogenous cytochrome-c on bystander MN induction in the Chang live cells that had been co-cultured for 12 h with 3 Gy γ-irradiated PLC/PRF/5 cells (a), Hep3B cells (b) and HepG2 cells (c). **P<0.01, ***P<0.001 compared with the control without irradiation, *P<0.05, **P<0.01, ***P<0.001 compared with CsA-treated cells. Results correspond to the mean±s.e. of three independent experiments with three replicates in each case.
Discussion
The present study found that loss or inhibition of p53 function produced cellular radioresistance and growth arrest, which is in accord with other studies showing that p53 is a key molecule involved in the cellular response to irradiation and regulates the radiosensitivity of mammalian cells (Komarov et al., 1999; Concin et al., 2000; Komarova et al., 2003). p53 not only promotes the repair of minor DNA damage induced by radiation but also has a complementary role in suppressing the repair of severe DNA damage and then switching on programmed cell death (Zhang et al., 2009). Failure of this p53-dependent apoptosis system may result in cancer development and genomic instability after irradiation (Viani et al., 2003).
We then studied the function of p53 in the RIBE and observed a clear genotoxic bystander effect in Chang liver cells induced by neighboring irradiated HepG2 cells, but not by irradiated PLC/PRF/5 or Hep3B cells. It was noted that exogenous cytochrome-c could heighten the bystander MN in Chang liver cells co-cultured with irradiated PLC/PRF/5, Hep3B cells and CsA-treated HepG2 cells. Our results indicate that cytochrome-c is a key signaling factor involved in the RIBE in cells with different p53 status. Accumulating evidence demonstrates that the release of mitochondrial cytochrome-c could be triggered via a p53-induced activation of pro-apoptotic Bcl2 family genes, including BAX (Schuler et al., 2000), Noxa (Wang et al., 2000) and PUMA (Harada et al., 2009), in a p53-dependent manner. Therefore, p53 activation and cytochrome-c release can be detected in the irradiated HepG2 cells (wp53), but not in Hep3B cells (p53 null). However, the situation in PLC/PRF/5 (mp53) cells was quite complicated. Following irradiation, phospho-p53 could be activated in PLC/PRF/5 cells despite having mutated p53. Interestingly, this activation could also be efficiently inhibited by PFT-α. Taken together with the results in Figure 1 that the MN yield in the irradiated PLC/PRF/5 cells could be reduced by PFT-α, it can be deduced that the mutated p53 in PLC/PRF/5 cells may also have the ability to participate in DNA damage response, but with an abnormal function so that no p53-dependent cytochrome-c can be released from these cells.
Several reports have suggested that the p53 function is important for RIBE by using a retroviral wild-type p53-expression vector (Ryan et al., 2008), and p53 could modulate the export of growth-suppressive stimuli from damaged cells to neighboring cells (Komarova et al., 1998). in vivo research found that an ongoing activation of p53 pathway response was involved in persistent activation of inflammatory-type responses in irradiated tissues (Coates et al., 2008), which may contribute to the mechanism of RIBE. However, there have been some contradictory reports in the literature suggesting that p53 function was not necessary for the RIBE. Using cells of three human lymphoblastoid cell lines, Zhang et al. (2008) reported that p53 status did not affect either the production of radiation bystander mutagenic signals or the response to these signals. Our previous studies showed that T98G cells (mp53) could also induce a genotoxic bystander response similar to AG1522 cells (wp53) although their mechanisms were quite different; nitric oxide was involved in the irradiated T98G-induced bystander response, whereas reactive oxygen species contributed to the irradiated AG1522-induced bystander effect (Shao et al., 2003c, 2005). Further study showed that reactive oxygen species might cause the release of cytochrome-c from mitochondria in the cells with wp53 (Atlante et al., 2000). Consequently, the different pattern of p53 mutation may be the reason why some but not all p53-mutated cells can induce RIBE, and the RIBE may depend on the function but not the phenotype of p53 gene. The incompatible cascade in the activation of p53 and the release of cytochrome-c could be the key reason of the phenomenon that PLC/PRF/5 cells with specific mp53 failed to invoke an RIBE.
Recent evidence has shown that, as a medium-derived soluble bystander factor, cytochrome-c may transfer between cells through gap junction and mediate RIBE and induce apoptosis in bystander cells (Peixoto et al., 2009). Another interesting report showed that cytochrome-c or mitochondrial function was required for non-targeted cells to respond to bystander signals (Yang et al., 2009). The present study showed that RIBE could be triggered by exogenous cytochrome-c from irradiated PLC/PRF/5 and Hep3B cells and even CsA pre-treated HepG2 cells. These findings indicate that cytochrome-c has a role as the sensor of RIBE. In addition, when the irradiated HepG2 cells were pre-treated with CsA, the MN induction in bystander Chang liver cells was not fully reduced to background levels as seen with PFT-α treatment, thus some p53-related bystander signal factors besides cytochrome-c, such as reactive oxygen species (Preta et al., 2009), transforming growth factors-β1 (Iyer et al., 2000) and Fas (Chhipa and Bhat, 2007; Luce et al., 2009), could also be involved in the RIBE.
On the basis of the present and other findings, we propose that the p53, as a center of the radiation response, domains the genetic damage and survival of hepatoma cells; meanwhile, it also alters the function of mitochondria and then subsequently mediates the release of cytochrome-c in irradiated cells and thus may have an important role in the regulation of the RIBE. This study may provide a new insight into the combined clinical trials of p53 gene therapy and radiation therapy to hepatocellular carcinoma of abnormal p53 status, that is, we should evaluate properly the merits and demerits of the antitumor efficacy and the concomitant adverse reaction to surrounding normal tissue in this combined therapy.
Materials and methods
Cell culture
Three hepatoma cell lines (HepG2, PLC/PRF/5 and Hep3B) and a normal liver cell line (Chang liver) were obtained from the Shanghai Cell Bank of China. Each has a different p53 status. HepG2 cells (wp53), Chang liver cells (wp53, verified by p53 reporter gene assay and western blotting assay (Yuan et al., 2010)) and PLC/PRF/5 cells (mp53) were maintained in the Dulbecco’s modified Eagle medium (HyClone, Beijing, China) containing glucose (4.5 g/l) and supplemented with penicillin (100 U/ml), streptomycin (100 U/ml), glutamate (2 mm) and 10% fetal bovine serum (Gibco Invitrogen, Grand Island, NY, USA). Hep3B cells (p53 null) were grown in the Minimum Essential Medium Alpha Medium (HyClone) supplemented with penicillin, streptomycin and 15% fetal bovine serum. All the cell lines were cultured in a humidified atmosphere of 5% CO2 in air at 37 °C.
Cell irradiation and co-culture
Three hepatoma cell lines and Chang liver cells were seeded on a coverslip (1 × 105 cells) and allowed to grow for 1 day before irradiation. The hepatoma cells were irradiated with γ-rays at adose rate of 0.83Gy/min using a 137Cs Gammacell-40 irradiator (Nordion International Inc., Kanata, Ontario, Canada). After irradiation, two coverslips with irradiated hepatoma cells and non-irradiated Chang liver cells were placed face-to-face with a 3 mm gap and co-cultured within a 35 mm dish. After 4, 12 or 24 h of co-culture, the cells were washed with phosphate-buffered saline (PBS) and fixed in situ for the MN assay.
Cell treatments
In some experiments, hepatoma cells were treated with 20 μm PFT-α (Sigma Co., St Louis, MO, USA) for 20 h or 5 μm CsA (Sigma Co.) for 1 h before irradiation. PFT-α is a reversible inhibitor of the function of p53. CsA, a mitochondrial permeability transition pore inhibitor, can irreversibly inhibit the release of cytochrome-c from mitochondria. After irradiation, cells treated with CsA were immediately washed three times with PBS, but 20 μm PFT-α was still added to the medium during cell co-culture. In addition, to elucidate the effect of cytochrome-c on the bystander response, exogenous cytochrome-c (Sigma Co.) with a final concentration of 50 μg/ml was added to the cell co-culture medium until the MN assay.
Cell growth assay
Radiosensitivity of the three hepatoma cell lines was measured using the cell growth assay. After irradiation with different doses of γ-rays, the cells were immediately treated with 0.25% trypsin solution containing 0.53 mm EDTA. A total of 5 × 104 cells were reseeded and cultured in a 60 mm dish. At 1–7 days post irradiation, cells were suspended and counted by a cell coulter (Beckman counter Z2, Hialeah, FL, USA). The relative cell growth rate was calculated by normalizing the number of irradiated cells to the number of non-irradiated control cells.
MN assay
MN were measured using the cytokinesis-block technique. Briefly, the cells were treated with 1 μg/ml cytochalasin B (Sigma Co.) for 28–29 h and then fixed in situ with methanol/acetic acid (9:1 v/v) for 20 min. Air-dried cells were stained with 0.01% acridine orange (Sigma Co.) and then observed under a fluorescence microscope (Olympus, Tokyo, Japan). MN were scored in at least 500 binucleated cells and the MN yield, YMN, was calculated as the ratio of the number of MN to the scored number of binucleated cells.
Western blotting assay
The expression of phospho-p53 was measured with western blotting. Briefly, 12 h after irradiation, the cells (1.5 × 106 cells pre-seeded in 60 mm petridish) were washed three times with ice-cold Tris-buffered saline (TBS) and treated with RIPA lysis buffer (Beyotime Biotechnology, Jiangsu, China). The cells were then scraped off the dish with a cold plastic scraper, collected into a pre-cooled Eppendorf tube and centrifuged at 14 000 r.p.m. for 5 min at 4 °C. The protein concentration in the supernatant fraction was measured with the bicinchoninic acid protein assay kit (Beyotime Biotechnology) using bovine serum albumin as a standard. Protein sample with 1 × loading buffer (0.25 m Tris–HCl (pH 6.8), 2.2% (w/v) SDS, 10% (v/v) glycerol, 1% (v/v), β-mercaptoethanol and bromophenol blue in deionized water) was denatured at 100 °C for 10 min to denature, then electrophoresed by 12% SDS–polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% non-fat dry milk powder in TBST (TBS containing 0.05% Tween 20) for 1 h to reduce non-specificity binding, then incubated overnight at 4 °C with the primary antibody against phospho-p53 (Ser15; Cell Signalling Technology, Danvers, MA, USA; 1:1000 dilution in blocking buffer) and GADPH (Kangchen Bio-tech, Shanghai, China; 1:5000 dilution in blocking buffer). After washing with TBST (four times, 10 min), the membrane was further incubated with the horseradish peroxidase-labeled secondary antibody (Pro-teintech Group Inc., Chicago, IL, USA; 1:4000 dilution in TBST) for 1 h at room temperature. After several washes, the protein bands were visualized using the ChemiDoc XRS system (Bio-Rad Laboratories, Hercules, CA, USA) after incubation with ECL Plus (Beyotime Biotechnology). The protein level was measured using Quantity One software (Bio-Rad). The results were expressed as a relative gray density of the bands. Equal loading of protein samples in each lane was indicated by the equal intensity of loading control protein, GAPDH.
Immunocytochemical assay of cytochrome-c
Cytoplasmic cytochrome-c was immunocytochemically detected in situ. Briefly, 12 h after irradiation, the irradiated cells (5 × 104 cells grown on coverslip) were rinsed once with PBS and then fixed with 4% paraformaldehyde for 30 min at room temperature. Cells were then rinsed with PBS (three times, 5 min), air dried, blocked with blocking buffer (0.5% Triton X-100, 3% bovine serum albumin in PBS) for 1 h, then incubated with cytochrome-c antibody (Abcam, Cambridge, MA, USA; 1:100 in blocking buffer) overnight at 4 °C in a humidified chamber, and the excess antibody was removed by washing the cells with PBS (three times, 5 min). The cells were incubated, in the dark, with an fluorescein isothiocyanate-labeled secondary antibody (Invitrogen, Carlsbad, CA, USA; 1:200 in blocking buffer) for 2 h at room temperature, rinsed with PBS for 1 min, incubated with 100 ng/ml of 4′,6-diamidino-2-phenylindole (Sigma Co.) and 200 ng/ml Nile red (Genmed Scientifics Inc., Arlingion, CA, USA) for 2min, and washed with PBS (three times, 5min). Finally, the coverslip was mounted onto a slide using mounting medium (Vector Laboratories, Burlingame, CA, USA) and the cells were observed with a fluorescence microscope (Olympus). Cells that had released cytochrome-c (relatively diffusely expressed in cytosol) and cytochrome-c normal cells (cytochrome-c was relatively concentrated in mitochondria) were counted in 10 randomly selected fields. Triplicate results from each of three experiments were averaged to obtain the percentages of cytochrome-c-released cells per sample. Images were constructed using Image-Pro Plus software (Media Cybernetics Inc., Silver Spring, MD, USA).
Statistical analysis
All results are presented as the means±s.e. of the data obtained from three independent experiments with three replicates in each case. Significant differences were determined by the unpaired Student’s test or the one-way analysis of variance at P<0.05 using the SPSS 11.5 software (SPSS Inc., Chicago, IL, USA).
Acknowledgements
We thank the National Nature Science Foundation of China Grant numbers 30770644 and 31070758, the Shanghai Health Bureau (08GWD09, 08GWZX0602) for funding this work.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- Ain JF, Gouillat C, Bertrand S, Fourel I, Guillaud M, Saguier G, et al. Human hepatocellular carcinoma transplanted in nude mice: a relevant experimental model to assess tumoral destruction by alcoholization. J Surg Res. 1994;57:366–372. doi: 10.1006/jsre.1994.1156. [DOI] [PubMed] [Google Scholar]
- Atlante A, Calissano P, Bobba A, Azzariti A, Marra E, Passarella S. Cytochrome c is released from mitochondria in a reactive oxygen species (ROS)-dependent fashion and can operate as a ROS scavenger and as a respiratory substrate in cerebellar neurons undergoing excitotoxic death. J Biol Chem. 2000;275:37159–37166. doi: 10.1074/jbc.M002361200. [DOI] [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little JB. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res. 2003a;63:7128–7135. [PubMed] [Google Scholar]
- Azzam EI, de Toledo SM, Little B. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene. 2003b;22:7050–7057. doi: 10.1038/sj.onc.1206961. [DOI] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Vojtesek B, Staskova Z, Lukas J, Rejthar A, et al. Aberrant expression of the p53 oncoprotein is a common feature of a wide spectrum of human malignancies. Oncogene. 1991;6:1699–1703. [PubMed] [Google Scholar]
- Bressac B, Galvin KM, Liang TJ, Isselbacher KJ, Wands JR, Ozturk M. Abnormal structure and expression of p53 gene in human hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:1973–1977. doi: 10.1073/pnas.87.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Jia RF, Yu L, Zhao MJ, Shao CL, Cheng WY. Bystander effects induced by continuous low-dose-rate 125I seeds potentiate the killing action of irradiation on human lung cancer cells in vitro. Int J Radiat Oncol Biol Phys. 2008a;72:1560–1566. doi: 10.1016/j.ijrobp.2008.07.038. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhao Y, Han W, Zhao G, Zhu L, Wang J, et al. Mitochondria-dependent signalling pathway are involved in the early process of radiation-induced bystander effects. Br J Cancer. 2008b;98:1839–1844. doi: 10.1038/sj.bjc.6604358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhipa RR, Bhat MK. Bystander killing of breast cancer MCF-7 cells by MDA-MB-231 cells exposed to 5-fluorouracil is mediated via Fas. J Cell Biochem. 2007;101:68–79. doi: 10.1002/jcb.21153. [DOI] [PubMed] [Google Scholar]
- Coates P, Robinson J, Lorimore S, Wright E. Ongoing activation of p53 pathway responses is a long-term consequence of radiation exposure in vivo and associates with altered macrophage activities. J Pathol. 2008;214:610–616. doi: 10.1002/path.2321. [DOI] [PubMed] [Google Scholar]
- Concin N, Zeillinger C, Stimpfel M, Schiebel I, Tong D, Wolff U, et al. p53-dependent radioresistance in ovarian carcinoma cell lines. Cancer Lett. 2000;150:191–199. doi: 10.1016/s0304-3835(99)00393-6. [DOI] [PubMed] [Google Scholar]
- Emerit I, Garban F, Vassy J, Levy A, Filipe P, Freitas J. Superoxide-mediated clastogenesis and anticlastogenic effects of exogenous superoxide dismutase. Proc Natl Acad Sci USA. 1996;93:12799–12804. doi: 10.1073/pnas.93.23.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Wu L, Chen S, Yu KN. Exogenous carbon monoxide protects the bystander Chinese hamster ovary cells in mixed co-culture system after alpha-particle irradiation. Carcinogenesis. 2009;31:275–280. doi: 10.1093/carcin/bgp301. [DOI] [PubMed] [Google Scholar]
- Harada K, Nonaka T, Hamada N, Sakurai H, Hasegawa M, Funayama T, et al. Heavy-ion-induced bystander killing of human lung cancer cells: role of gap junctional intercellular communication. Cancer Sci. 2009;100:684–688. doi: 10.1111/j.1349-7006.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Lehnert BE, Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Diatchenko L, Rokhlin OW, Hill JE, Wang ZJ, Krivokrysenko VI, et al. Stress-induced secretion of growth inhibitors: a novel tumor suppressor function of p53. Oncogene. 1998;17:1089–1096. doi: 10.1038/sj.onc.1202303. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Neznanov N, Komarov PG, Chernov MV, Wang K, Gudkov AV. p53 inhibitor pifithrin alpha can suppress heat shock and glucocorticoid signaling pathways. J Biol Chem. 2003;278:15465–15468. doi: 10.1074/jbc.C300011200. [DOI] [PubMed] [Google Scholar]
- Lehnert BE, Goodwin EH. A new mechanism for DNA alterations induced by alpha particles such as those emitted by radon and radon progeny. Environ Health Perspect. 1997;105(Suppl 5):1095–1101. doi: 10.1289/ehp.97105s51095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce A, Courtin A, Levalois C, Altmeyer-Morel S, Romeo PH, Chevillard S, et al. Death receptor pathways mediate targeted and non-targeted effects of ionizing radiations in breast cancer cells. Carcinogenesis. 2009;30:432–439. doi: 10.1093/carcin/bgp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Jiao B, Liu X, Yi H, Kong D, Gao L, et al. Approach to radiation therapy in hepatocellular carcinoma. Cancer Treat Rev. 2009;36:157–163. doi: 10.1016/j.ctrv.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, Di Majo V, et al. Oncogenic bystander radiation effects in patched heterozygous mouse cerebellum. Proc Natl Acad Sci USA. 2008;105:12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Hayashi S, Hatashita M, Ohnishi K, Shioura H, Ohtsubo T, et al. Induction of radioresistance by a nitric oxide-mediated bystander effect. Radiat Res. 2001;155:387–396. doi: 10.1667/0033-7587(2001)155[0387:iorban]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mitchell SA, Marino SA, Brenner DJ, Hall EJ. Bystander effect and adaptive response in C3H 10T(1/2) cells. Int J Radiat Biol. 2004;80:465–472. doi: 10.1080/09553000410001725116. [DOI] [PubMed] [Google Scholar]
- Morgan WF, Hartmann A, Limoli CL, Nagar S, Ponnaiya B. Bystander effects in radiation-induced genomic instability. Mutat Res. 2002;504:91–100. doi: 10.1016/s0027-5107(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Morgan WF, Sowa MB. Non-targeted bystander effects induced by ionizing radiation. Mutat Res. 2007;616:159–164. doi: 10.1016/j.mrfmmm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Moses AC, Freinkel AJ, Knowles BB, Aden DP. Demonstration that a human hepatoma cell line produces a specific insulin-like growth factor carrier protein. J Clin Endocrinol Metab. 1983;56:1003–1008. doi: 10.1210/jcem-56-5-1003. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- Murphy JE, Nugent S, Seymour C, Mothersill C. Mitochondrial DNA point mutations and a novel deletion induced by direct low-LET radiation and by medium from irradiated cells. Mutat Res. 2005;585:127–136. doi: 10.1016/j.mrgentox.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- Nagasawa H, Little JB. Unexpected sensitivity to the induction of mutations by very low doses of alpha-particle radiation: evidence for a bystander effect. Radiat Res. 1999;152:552–557. [PubMed] [Google Scholar]
- Narayanan PK, Goodwin EH, Lehnert BE. Alpha particles initiate biological production of superoxide anions and hydrogen peroxide in human cells. Cancer Res. 1997;57:3963–3971. [PubMed] [Google Scholar]
- Narayanan PK, LaRue KE, Goodwin EH, Lehnert BE. Alpha particles induce the production of interleukin-8 by human cells. Radiat Res. 1999;152:57–63. [PubMed] [Google Scholar]
- Ng LT, Chiang LC, Lin YT, Lin CC. Antiproliferative and apoptotic effects of tetrandrine on different human hepatoma cell lines. Am J Chin Med. 2006;34:125–135. doi: 10.1142/S0192415X06003692. [DOI] [PubMed] [Google Scholar]
- Peixoto PM, Ryu SY, Pruzansky DP, Kuriakose M, Gilmore A, Kinnally KW. Mitochondrial apoptosis is amplified through gap junctions. Biochem Biophys Res Commun. 2009;390:38–43. doi: 10.1016/j.bbrc.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preta G, de Klark R, Glas R. A role for nuclear translocation of tripeptidyl-peptidase II in reactive oxygen species-dependent DNA damage responses. Biochem Biophys Res Commun. 2009;389:575–579. doi: 10.1016/j.bbrc.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Puisieux A, Galvin K, Troalen F, Bressac B, Marcais C, Galun E, et al. Retinoblastoma and p53 tumor suppressor genes in human hepatoma cell lines. Faseb J. 1993;7:1407–1413. doi: 10.1096/fasebj.7.14.8224613. [DOI] [PubMed] [Google Scholar]
- Ryan LA, Smith RW, Seymour CB, Mothersill CE. Dilution of irradiated cell conditioned medium and the bystander effect. Radiat Res. 2008;169:188–196. doi: 10.1667/RR1141.1. [DOI] [PubMed] [Google Scholar]
- Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J Biol Chem. 2000;275:7337–7342. doi: 10.1074/jbc.275.10.7337. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Delayed expression of lethal mutations and genomic instability in the progeny of human epithelial cells that survived in a bystander-killing environment. Radiat Oncol Investig. 1997;5:106–110. doi: 10.1002/(SICI)1520-6823(1997)5:3<106::AID-ROI4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Shao C, Aoki M, Furusawa Y. Bystander effect on cell growth stimulation in neoplastic HSGc cells induced by heavy-ion irradiation. Radiat Environ Biophys. 2003a;42:183–187. doi: 10.1007/s00411-003-0202-y. [DOI] [PubMed] [Google Scholar]
- Shao C, Folkard M, Michael BD, Prise KM. Targeted cytoplasmic irradiation induces bystander responses. Proc Natl Acad Sci USA. 2004;101:13495–13500. doi: 10.1073/pnas.0404930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Folkard M, Michael BD, Prise KM. Bystander signaling between glioma cells and fibroblasts targeted with counted particles. Int J Cancer. 2005;116:45–51. doi: 10.1002/ijc.21003. [DOI] [PubMed] [Google Scholar]
- Shao C, Folkard M, Prise KM. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2008a;27:434–440. doi: 10.1038/sj.onc.1210653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C, Furusawa Y, Aoki M, Ando K. Role of gap junctional intercellular communication in radiation-induced bystander effects in human fibroblasts. Radiat Res. 2003b;160:318–323. doi: 10.1667/rr3044. [DOI] [PubMed] [Google Scholar]
- Shao C, Furusawa Y, Kobayashi Y, Funayama T, Wada S. Bystander effect induced by counted high-LET particles in confluent human fibroblasts: a mechanistic study. Faseb J. 2003c;17:1422–1427. doi: 10.1096/fj.02-1115com. [DOI] [PubMed] [Google Scholar]
- Shao C, Prise KM, Folkard M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mutat Res. 2008b;638:139–145. doi: 10.1016/j.mrfmmm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Shao CL, Zhang JH, Prise KM. Differential modulation of a radiation-induced bystander effect in glioblastoma cells by pifithrin-alpha and wortmannin. Nucl Instrum Methods Phys Res B. 2010;268:627–631. [Google Scholar]
- Shareef MM, Cui N, Burikhanov R, Gupta S, Satishkumar S, Shajahan S, et al. Role of tumor necrosis factor-alpha and TRAIL in high-dose radiation-induced bystander signaling in lung adenocarcinoma. Cancer Res. 2007;67:11811–11820. doi: 10.1158/0008-5472.CAN-07-0722. [DOI] [PubMed] [Google Scholar]
- Tartier L, Gilchrist S, Burdak-Rothkamm S, Folkard M, Prise KM. Cytoplasmic irradiation induces mitochondrial-dependent 53BP1 protein relocalization in irradiated and bystander cells. Cancer Res. 2007;67:5872–5879. doi: 10.1158/0008-5472.CAN-07-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KK, Stuart J, Chuang YY, Little JB, Yuan ZM. Low-dose radiation-induced senescent stromal fibroblasts render nearby breast cancer cells radioresistant. Radiat Res. 2009;172:306–313. doi: 10.1667/RR1764.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viani P, Giussani P, Brioschi L, Bassi R, Anelli V, Tettamanti G, et al. Ceramide in nitric oxide inhibition of glioma cell growth. Evidence for the involvement of ceramide traffic. J Biol Chem. 2003;278:9592–9601. doi: 10.1074/jbc.M207729200. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Wang B, Ohyama H, Haginoya K, Odaka T, Itsukaichi H, Yukawa O, et al. Adaptive response in embryogenesis. III. Relationship to radiation-induced apoptosis and Trp53 gene status. Radiat Res. 2000;154:277–282. doi: 10.1667/0033-7587(2000)154[0277:arieir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yang G, Wu L, Chen S, Zhu L, Huang P, Tong L, et al. Mitochondrial dysfunction resulting from loss of cytochrome c impairs radiation-induced bystander effect. Br J Cancer. 2009;100:1912–1916. doi: 10.1038/sj.bjc.6605087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You KR, Wen J, Lee ST, Kim DG. Cytochrome c oxidase subunit III: a molecular marker for N-(4-hydroxyphenyl)retinamise-induced oxidative stress in hepatoma cells. J Biol Chem. 2002;277:3870–3877. doi: 10.1074/jbc.M109284200. [DOI] [PubMed] [Google Scholar]
- Yuan D, Pan Y, Zhang J, Shao C. Role of nuclear factor-kappaB and P53 in radioadaptive response in Chang live cells. Mutat Res. 2010;688:66–71. doi: 10.1016/j.mrfmmm.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Zhang XP, Liu F, Cheng Z, Wang W. Cell fate decision mediated by p53 pulses. Proc Natl Acad Sci USA. 2009;106:12245–12250. doi: 10.1073/pnas.0813088106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhou J, Held KD, Redmond RW, Prise KM, Liber HL. Deficiencies of double-strand break repair factors and effects on mutagenesis in directly gamma-irradiated and medium-mediated bystander human lymphoblastoid cells. Radiat Res. 2008;169:197–206. doi: 10.1667/RR1189.1. [DOI] [PubMed] [Google Scholar]
- Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, et al. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci USA. 2005;102:14641–14646. doi: 10.1073/pnas.0505473102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ivanov VN, Lien YC, Davidson M, Hei TK. Mitochondrial function and nuclear factor-kappaB-mediated signaling in radiation-induced bystander effects. Cancer Res. 2008;68:2233–2240. doi: 10.1158/0008-5472.CAN-07-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]