Abstract
Axon regeneration has long been studied in vertebrate model organisms and neuronal cultures. Recent development of axon regeneration paradigms in genetic model organisms, such as C. elegans, Drosophila and zebrafish, has opened an exciting field for in vivo functional dissection of regeneration pathways. Studies in these organisms have discovered essential genes and pathways for axon regrowth. The conservation of these genes crossing animal phyla suggests mechanistic relevance to higher organisms. The power of genetic approaches in these organisms makes large-scale genetic and pharmacological screens feasible and can greatly accelerate the mechanistic understanding of axon regeneration.
Keywords: Axon regeneration, genetic models, laser axotomy, DLK MAPKKK, MAPK signaling, mTOR-dependent protein synthesis
Introduction
The limited abilities of mature neurons in adult nervous systems to regenerate or repair damages remain one of the poorly understood phenomena in neuroscience and have been intense subjects of investigation for over a century. Decades of research primarily from vertebrate models, such as rodents and fish, has established that both intrinsic properties of injured neurons and extrinsic environments play intricate roles in the extent and accuracy of regenerating axons. Recent progress in the identification of extrinsic factors, particularly myelin-associated inhibitors, and of intrinsic regeneration-promoting pathways has rapidly moved the field into the molecular era. Studies from in vitro cultured neuronal models and from lower vertebrates and invertebrates such as Aplysia have also greatly enhanced our knowledge of cellular dynamics, particularly in the early phase of injury responses [1]. An exciting development in the last few years is the establishment of axon regeneration models in genetic organisms including the nematode Caenorhabditis elegans, the fruitfly Drosophila melanogaster and the zebrafish Danio rerio. Emerging findings from these model organisms have shown great promises of using forward and reverse genetic manipulations to discover key players of axon regeneration. In this minireview, we will first summarize recently developed axon regeneration paradigms, and then review newly discovered regeneration-regulating pathways by genetic studies. In the end, we will discuss perspectives for future investigations of the genetic basis of axon regeneration.
Newly developed axon regeneration paradigms in genetic organisms
The small size of neurons in C. elegans, Drosophila and lower vertebrates presented technical challenges for nerve injury studies. In 2004, Yanik et al. developed a femtosecond laser surgery protocol to perform acute in vivo axotomy on transgenically labeled fluorescent axons in live C. elegans animals. They found that the GABAergic motor neuron axons are capable of functional regeneration post laser axotomy [2]. Follow-up studies by Wu et al. and Gabel et al. also demonstrated that neurites of various neurons in C. elegans, including mechanosensory neurons, chemosensory neurons and other motoneurons, can regenerate with different degrees of regrowth ability [3–5] (Table 1). More recently, customized microfluidic devices have been developed to facilitate laser axotomy and live imaging of regenerating axons in awake animals, and to enable high-throughput genetic and pharmacological screenings [6]. Similar laser axotomy techniques have also been developed in zebrafish [7–9] and Drosophila [10] (Table 1). Laser-severed trigeminal sensory neurons in zebrafish larvae can regenerate into skin, but their ability to innervate originally denervated skin territory depends on developmental stages [8]. A high-throughput screen method using laser axotomy in zebrafish larvae has also been established very lately [7]. Laser transection of axons or dendrites of Class I dendritic arborization (da) neurons in the fly brain triggers distinct patterns of cytoskeletal rearrangement and neurite regrowth [10]. These efforts have begun to push forward the understanding of genetic pathways regulating axon regeneration (Table 1). They have also prompted laser axotomy as a tool to study regeneration-associated events in mouse hippocampal neurons [11]. Importantly, as described below, the cellular responses and self-repair signalings triggered by laser axotomy exhibit fundamental similarities to those induced by mechanical surgery.
Table 1.
New laser axotomy and regeneration paradigms in C. elegans, Drosophila and zebrafish
| Genetic organism | Neuronal process | Regrowth ability |
|---|---|---|
| C. elegans | Commissures of GABAergic motor neurons | 54% of severed commissures reaches their dorsal distal end within 12–24 hr [2,4,12] |
| C. elegans | Commissures of cholinergic motor neurons | Regrow ~50–60 µm at 24hr towards the dorsal cord [3] |
| C. elegans | Axons of ALM, PLM, and AVM mechanosensory neurons | Regrow ~60–100 µm at 24hr (~1/4 to 1/3 of original axonal length) [2,4,13,28] |
| C. elegans | Axon of HSN motor neuron | Some extent of regrowth [3] |
| C. elegans | Sensory dendrite of AWB chemosensory neuron | Only show slow partial regrowth at 24 hr [4] |
| Drosophila | Class I dendritic arborization (da) neurons* | A dendrite is converted into a regenerating axon [9] |
| Zebrafish | Axons of trigeminal sensory neurons | Regrow robustly but the ability to innervate original skin territory depends on developmental stages [7] |
Note: An Axon or dendrite is severed at a distance from its soma by a two-photon laser, except in Class I da neurons the axon is completely removed by a UV laser.
The roles of mitogen-activated protein kinase (MAPK) pathway in axon regeneration
Two parallel studies published in 2009 have uncovered a critical role of a conserved MAP kinase in axon regeneration in C. elegans [12,13] (Figure 1A). In a mutant strain that is defective in β-spectrin, a component of the cortical cytoskeleton, axons of GABAergic motor neurons are fragile and undergo progressive breakage caused by locomotion. These broken axons can constantly regenerate toward the dorsal segments in an attempt to re-build their connections [14]. Hammarlund and his colleagues performed a large-scale RNAi screen in this strain in order to isolate mutants showing diminished spontaneous axon regrowth. The DLK-1 (Dual Leucine zipper-bearing Kinase 1), a member of an evolutionarily conserved MAP kinase kinase kinase family, was identified as a candidate with the strongest effect [12]. In a separate study, Yan et al. found that laser-severed PLM mechanosensory neurons also fails to regenerate in dlk-1 loss-of-function (lf) mutant animals [13], whereas overexpressing DLK-1 in these neurons accelerates regrowth following laser axotomy. In both type of neurons, DLK-1 functions cell-autonomously for growth cone formation and initiation of regrowth. It acts through two downstream kinases, the MKK-4 MAP kinase kinase and the PMK-3 p38 MAP kinase [12,13]. This kinase cascade has been previously shown to function in the developing nervous system to regulate synapse formation [15]. Experiments using acutely induced rescue of DLK-1 expression in dlk-1(lf) mutant animals show that DLK-1 is required within a short temporal window around the time of injury, suggesting that its activation needs coincide with injury signals to initiate regrowth [12] (Figure 1A).
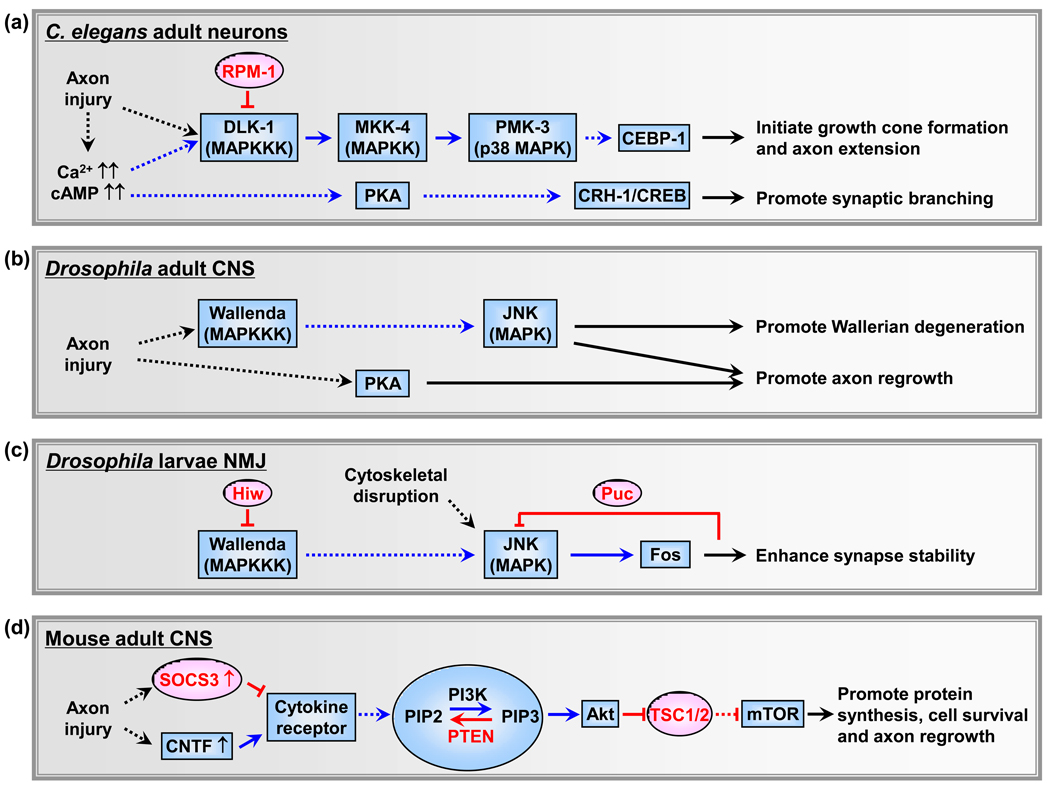
Figure 1.
Representative axon regeneration-regulating pathways discovered in genetic model organisms. (a) In C. elegans, axon injury triggers elevation of intracellular Ca2+ and cAMP, and activation of the DLK-1 MAPK cascade and PKA kinase. The DLK-1 level is negatively regulated by the E3 ligase RPM-1. The transcriptional factors CEBP-1 and CRH-1/CREB mediates the effects of these kinases respectively and regulate different aspects in axon regeneration. (b) In Drosophila adult CNS, the DLK/Wallenda MAPKKK promotes Wallerian degeneration through the JNK signaling. Together with PKA, JNK also promotes axon regrowth in sLNv neurons in the fly brain. (c) In Drosophila larvae NMJ, the DLK pathway stabilizes synapses after transient cytoskeletal disruption. In the presence of persistent cellular stress, the JNK phosphatase Puckered (Puc) is also activated and leads to synapse disassembly by antagonizing the JNK signaling. (d) In mouse RGCs, the mTOR-dependent protein synthesis is essential for neuron survival and axon regrowth after optic nerve injury. Releasing suppression of SOCS3, PTEN, or TSC1/2 on this pathway can re-activate the mTOR signaling and promote axon regeneration.
In the nervous systems of Drosophila and mouse, the DLK MAPK pathway has been shown to regulate Wallerian-type axon degeneration, a process usually preceding or paralleling axon regeneration in which axons detached from injured neurons undergo self-destruction [16,17]. Miller et al. examined a degeneration paradigm in the olfactory receptor neurons (ORN) in adult flies, and reported that loss of function in DLK (known as Wallenda in Drosophila) slows the Wallerian degeneration [17] (Figure 1B). The degeneration-promoting effect of DLK is also observed in the distal segments of peripherally injured mouse dorsal root ganglion (DRG) neurons [17]. In another parallel study, Massaro et al. showed that in Drosophila larvae disassembly of the neuromuscular junction (NMJ) caused by loss of α-spectrin in presynaptic motor neurons surprisingly shares mechanistic similarities with Wallerian degeneration [16]. However, elevation of the DLK activity, resulted from loss of the DLK -degrading E3 ligase Highwire (Hiw, Drosophila homolog of C. elegans RPM-1), suppresses NMJ retraction in the spectrin mutant [16] (Figure 1C). This finding is consistent with the growth-promoting function of DLK in C. elegans neurons but apparently contrary to Miller et al.’s finding. The differential effects of DLK in these studies could reflect the context-dependent and compartment-specific (proximal vs distal axon fragments) functions of this injury response pathway. In both studies, DLK acts through the Jun N-terminal kinase (JNK) MAP kinase. JNK has also been reported to promote axon regeneration in the small lateral neurons ventral (sLNv) injured by microdissection in the whole explanted Drosophila brain [18], although the role of DLK/Wallenda is yet to be examined (Figure 1B).
Take together, the above findings not only demonstrate the important function of the DLK pathway in adult axon injury responses, but also reveal the complexity underlying the context-dependent action of these conserved kinases. DLK can stabilize injured axons and promote axon regrowth from proximal axon ends, mostly likely by acting as the immediate injury sensor. It can also protect disrupted NMJ synapses from disassembly. Meanwhile, it can accelerate degeneration of distal axon fragments, which may facilitate regrowth by removing biochemical and physical barriers presented by these fragments. It is important to note that in the developing nervous system DLK kinases are critical regulators of synapse formation and growth [15,19]. These studies bring about a surprising convergence between disparate cellular processes in the developing and mature nervous systems. Many questions remain to be addressed regarding the DLK signaling in axon regeneration. For example, how is the DLK MAPK cascade activated by injury signals in the first place? How does the DLK pathway regulate axon regeneration? Microtubule dynamics and global gene transcription have been suggested to mediate the effects of the DLK signaling [20–22]. Moreover, multiple MAPK pathways exist in C. elegans, Drosophila and vertebrates. Do other uncharacterized MAPK pathways play similar or divergent roles in axon regeneration? Further investigations will be directed to fully understand both the general signaling mechanisms and also the neuron- or axon-type specific effects of the DLK cascade in axon regeneration and degeneration.
The role of second messengers and cytoskeletal rearrangement in injury responses
Nerve injury-triggered signals lead to a series of changes, including rearrangement of cytoskeletons, retrograde transport of injury-responding factors, and changes in gene expression, all of which contribute to the repair and regeneration processes [1,21,23,24]. Axonal injury in cultured neurons can cause elevation of intracellular Ca2+ and cAMP, which serve as initial injury messengers [25–27]. However, it was poorly understood whether these initial signals promote axon regeneration in vivo and what their downstream effectors are. Recently, Ghosh-Roy et al. found that in C. elegans PLM mechanosensory neurons, genetic elevation of Ca2+ and cAMP can accelerate formation of regenerating growth cones, facilitate fusion of damaged axons, and promote formation of synaptic branches [28]. Furthermore, the regrowth-promoting effects of Ca2+ and cAMP rely on protein kinase A (PKA) and the DLK-1 MAPK pathway (Figure 1A). Pharmacological inhibition of PKA activity reduces axon regrowth while genetic enhancement of PKA activity causes an opposite effect [28]. A similar regrowth-promoting effect of PKA has also been observed in lateral neurons ventral (LNv) in fruitfly brain [18] (Figure 1B).
Axon injury also triggers dynamic rearrangements of actin filaments and microtubules, which contribute to the initial injury signal propagation and subsequent axon extension [29,30]. Reggie-1 and Reggie-2, two conserved homologous proteins originally identified as plasma-membrane associated scaffolding proteins, are up-regulated in retinal ganglion cells (RGCs) after optic nerve section in goldfish and rats [31,32]. Recent studies have revealed that they function in axon regeneration via regulating F-actin dynamics [33,34]. Knocking down Reggies in zebrafish RGCs impedes axon regeneration in vivo, likely by affecting the activation status of Rho GTPases, the N-WASP complex stability, and activation of p38 MAPK and focal adhesion kinase (FAK) [35]. In mice, many environmental inhibitors such as myelin-associated inhibitors act through the actin regulator RhoA and its downstream Rho-associated kinase II (ROCKII) to inhibit axon regrowth [23,36]. Deletion of ROCKII makes neurons less sensitive to inhibition by Nogo or CSPG and improves axonal regrowth after trauma in the adult spinal cord [37].
Isoform-specific regulation of tubulins has long been observed in various injury models. α1 tubulin isoform Tuba1a is up-regulated in zebrafish RGCs after optic nerve injury [38]. Knockdown of Tuba1a, but not the closely related Tuba1b, dramatically hinders RGC axon regeneration [39]. In Drosophila, a global up-regulation of microtubule dynamics occurs when an axon of a neuron is surgically removed. A regenerating axon will be generated from an existing dendrite by the reversal of microtubule polarity in this neurite. Such drastic microtubule rearrangements require the JNK signaling [10]. Similar dendrite-to-axon conversion post axotomy is also observed in cultured mature mouse hippocampal neurons [11]. Conceivably, injury-induced kinase activity can modify the property of cytoskeletal components or the activity of cytoskeleton-binding proteins. Such changes may create new sites of cytoskeleton dynamics and/or facilitate transduction of injury signals mediated by motors and other proteins. Although the regulation of regenerative cytoskeleton dynamics is not fully understood, recent data have suggested that it can be different from that during development. In C. elegans, homologs of Rac, lamellopodin, and Enabled/VASP are required for regenerative growth cone dynamics but not indispensable for initial developmental growth [3]. These studies underscore the importance of further investigation of the mechanisms regulating cytoskeleton dynamics in axon regeneration.
The roles of injury-triggered gene expression in axon regeneration
The intrinsic regenerating ability of injured axons heavily relies on gene transcription and protein translation [24,40,41]. Recent studies have started to reveal key players in these processes. In axon regeneration of worm mechanosensory neurons, a key downstream effector of DLK-1 is a basic leucine zipper (bZip) transcription factor CEBP-1 (the CCAAT/enhancer-binding protein 1) [13]. CEBP-1 mRNAs can be detected in axons, and axotomy induces local translation of axonal CEBP-1 mRNAs in a DLK-1-dependent manner. It remains unknown whether these locally translated CEBP-1 proteins may function retrogradely in the nucleus as a transcription factor or have other roles in the axon [13]. In Drosophila, the JNK signaling stabilizes transiently disrupted NMJ through the bZip transcription factor Fos in the nucleus [16]. In mice, C-Jun, another downstream target of JNK, is involved in facial nerve regeneration [42]. In C. elegans, the Jun homolog JUN-1 is also required for axon regrowth of mechanosensory axons [28]. In contrast, the cAMP response element-binding protein CRH-1/CREB is not required for regrowth, but instead promotes the ventral branching in the same type of neurons [28]. These studies suggest diverse roles of the bZip family of transcriptional factors in axon regeneration and degeneration.
Another family of transcription factors recently emerged from axon regeneration studies is the Zinc finger Kruppel-like transcription factors (KLFs). In zebrafish RGCs, KLF6a and KLF7a and their transcriptional target α1 tubulin isoform Tuba1a are highly induced after injury and promote RGC axon regrowth in explant culture [38,39]. In mouse RGCs, the KLF family is also suggested to play important roles in neurite outgrowth and axon regeneration [43]. KLF4 was identified as a strong suppressor of hippocampal neurite outgrowth. KLF4 knockout mice showed significantly decreased regeneration of adult RGCs in vivo. Moreover, outgrowth-enhancing KLFs (KLF6 and KLF7) are often downregulated while outgrowth-inhibiting KLFs (KLF4 and KLF9) are upregulated in postnatal RGCs [43]. These findings are consistent with the notion that the intrinsic growth capability of neurons is programmed to be attenuated in mammalian adults. It also partially explains why CNS neurons can regenerate robustly in adult Zebrafish in contrast to the poor regenerating performance in mammalian CNS. Additionally, the transcription of some genes, such as GAP-43 and α1 tubulin, can be differentially regulated during development and in regeneration [44–46]. A recent study has discovered that the fish gap43 promoter contains fragments specific for regeneration-associated expression [47]. Whether such regeneration-specific regulation of gene expression is a general strategy used by regenerating neurons remains to be examined.
In addition to gene transcription, a few recent studies have elegantly demonstrated an essential role of protein translation mediated by the PI3K/mTOR (Phosphoinositide 3-kinase/mammalian target of rapamycin) pathway in regenerating mouse RGCs (Figure 1D). The PI3K/mTOR pathway targets on subunits of the protein translation machinery and is important for cell growth and survival [48]. In neurons, this pathway is necessary for efficient growth cone regeneration [40]. The mTOR pathway is gradually suppressed during development and completely silenced in injured adult RGCs. PTEN (phosphatase and tensin homolog) and TSC complex (tuberous sclerosis complex consisting of TSC1 and TSC2) are negative regulators of the mTOR pathway. It is suggested that removal of PTEN and TSC1 re-activates mTOR-dependent protein translation [49]. In deed, deletion of PTEN or TSC1 from RGCs in mice leads to enhanced cell survival rate and axon regeneration after optic nerve injury in vivo [49]. One of the upstream signaling pathways regulating mTOR in regeneration involves SOCS3 (suppressor of cytokine signaling 3) [50]. SOCS3 deletion releases its suppression on gp130 cytokine receptor which activates the mTOR pathway. Intravitreal application of ciliary neurotrophic factor (CNTF) further enhances axon regeneration in SOCS3-deleted RGCs [50]. It will be intriguing to examine whether the PTEN/mTOR pathway is universally involved in regeneration of PNS and CNS neurons.
The roles of guidance cues and extrinsic inhibitory factors in axon regeneration
Axon guidance cues have long been major interests of regeneration in mature neurons [51]. Correlated expression studies have shown that multiple well-characterized developmental guidance molecules are up- or down- regulated after nerve injury [52,53]. Emerging studies suggest that the effects of the same axon guidance cues may differ during development and in regeneration, possibly through differential use of receptors or activation of redundant pathways. The Slit and Netrin guidance cues are known to primarily act as chemorepellants and chemoattractants respectively to guide the pathfinding of axons during development in various organisms [54]. In adult C elegans, AVM mechanosensory neurons in slt-1/Slit(lf) and unc-6/Netrin(lf) mutants show better regrowth than controls but with lower precision. In contrast, neither the Slit receptor SAX-3/Robo nor the Netrin receptors UNC-40/DCC and UNC-5/Unc5 exhibit a critical requirement as their ligands in AVM regeneration [3]. Similarly, in rodents, Netrin-1 acts as a chemoattractant during development. However, in axon regeneration, it functions as an oligodendrocyte-associated inhibitor and impedes axon regrowth after adult spinal cord injury [55]. The repulsion-mediating netrin-1 receptor UNC5 seems to be involved in this adult axon function. The Ephrin/Eph signaling has also been shown to regulate regrowth and guidance of regrowing axons in C. elegans [4] and in vertebrates [56,57]. The sole Eph receptor VAB-1 in C. elegans can facilitate axon regeneration of mechanosensory neurons [4]. In mice, EphB3 supports adult RGC axon outgrowth [57], while Ephrin-B3 acts as a myelin-based inhibitor of axon regrowth in the spinal cord [56]. These findings illustrate the complexity of axon pathfinding in the mature nervous system. It would be of future interests to define the effective phase in which the axon guidance cues act and the downstream effectors they utilize.
It has long been known that myelin-derived inhibitors represent major obstacles in regenerative growth in mammalian CNS post injury. Among them best characterized are the Nogo, myelinassociated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp) [23,36,58], and readers are referred to the studies of these inhibitors in mouse models summarized in recent papers [59–62]. Another major class of extracellular inhibitors are chondroitin sulfate proteoglycans (CSPGs). Expression of these CSPGs is dramatically upregulated within the extracellular matrix of scar tissue in the brain and spinal cord of mature animals [23,58]. A breakthrough in the understanding of CSPG action is the recent identification of a transmembrane protein tyrosine phosphatase, PTPσ, as the receptor of neural CSPGs in mice [63]. PTPσ-deleted sensory neurons show reduced sensitivity to CSPGs in vitro and can extend further after a spinal cord lesion in vivo [63]. Together with traditional regeneration strategies, genetic approaches will unveil more signaling molecules mediating the environmental guidance and inhibition during axon regeneration in the future.
Perspectives
Genetic model organisms, such as C. elegans and Drosophila, have been rapidly established as tractable models for dissecting the genetic basis of axon regeneration. Encouragingly, new regeneration-associated pathways identified in invertebrates have shown functional conservation and relevance to those in vertebrates, although the effects of a specific pathway on regeneration may display variations depending on cell types and environments. In addition, increased redundancy and complexity of regeneration-regulating pathways in the vertebrate nervous systems present tremendous challenges to current studies. Manipulating one pathway often could not generate notable impacts on functional recovery of injured neurons, while combinatory treatments in mouse spinal cord injury models have led to cheerful results [64,65]. Thus, investigating synergistic effects of multiple genetic mutations can be a future focus of regeneration research in genetic model organisms. Rich sources of genetic mutants and effective methods of manipulating signaling pathways in vivo will no doubt favor such investigations. With elegantly designed large-scale genetic and pharmacological screens, these low-cost genetic model organisms well suit the urgent need of identifying new targets and new chemicals for axon regeneration therapeutics.
Acknowledgements
Z. Wang is supported by the Jane Coffin Childs Memorial Fund. Y. J. is an investigator of the Howard Hughes Medical Institute. The work in our lab is supported by HHMI and NIH. We thank members of our lab for discussions and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erez H, Malkinson G, Prager-Khoutorsky M, De Zeeuw CI, Hoogenraad CC, Spira ME. Formation of microtubule-based traps controls the sorting and concentration of vesicles to restricted sites of regenerating neurons after axotomy. J Cell Biol. 2007;176:497–507. doi: 10.1083/jcb.200607098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- 3.Gabel CV, Antoine F, Chuang CF, Samuel AD, Chang C. Distinct cellular and molecular mechanisms mediate initial axon development and adult-stage axon regeneration in C. elegans. Development. 2008;135:1129–1136. doi: 10.1242/dev.013995. [DOI] [PubMed] [Google Scholar]
- 4.Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc Natl Acad Sci U S A. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh-Roy A, Chisholm AD. Caenorhabditis elegans: a new model organism for studies of axon regeneration. Dev Dyn. 239:1460–1464. doi: 10.1002/dvdy.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo SX, Bourgeois F, Chokshi T, Durr NJ, Hilliard MA, Chronis N, Ben-Yakar A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat Methods. 2008;5:531–533. doi: 10.1038/nmeth.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardo-Martin C, Chang TY, Koo BK, Gilleland CL, Wasserman SC, Yanik MF. High-throughput in vivo vertebrate screening. Nat Methods. doi: 10.1038/nmeth.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Brien GS, Martin SM, Sollner C, Wright GJ, Becker CG, Portera-Cailliau C, Sagasti A. Developmentally regulated impediments to skin reinnervation by injured peripheral sensory axon terminals. Curr Biol. 2009;19:2086–2090. doi: 10.1016/j.cub.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien GS, Rieger S, Martin SM, Cavanaugh AM, Portera-Cailliau C, Sagasti A. Two-photon axotomy and time-lapse confocal imaging in live zebrafish embryos. J Vis Exp. 2009 doi: 10.3791/1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM. Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol Biol Cell. 21:767–777. doi: 10.1091/mbc.E09-11-0967. * This study shows that Drosophila class I da neuron can convert a dendrite to a regenerating axons after the complete removal of the original axon. This process involves drastic microtubule rearrangements as revealed by EB1-GFP live imaging in vivo. The authors also found that JNK signaling is essential for the up-regulation of microtubule dynamics and the following morphology change.
- 11.Gomis-Ruth S, Wierenga CJ, Bradke F. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr Biol. 2008;18:992–1000. doi: 10.1016/j.cub.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 12. Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. ** This C. elegans study elegantly used a spontaneously regenerating model to screen for regrowth-associated essential genes. The authors identified the DLK-1 MAP3K, alongside with its downstream MKK-4 MAP3K and PMK-3 MAK, as key factors initiating axon regrowth in regenerating GABAergic motor neurons.
- 13. Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. ** These authors used an impressive combination of C. elegans genetics, biochemistry, laser axotomy and fluorescent imaging in live animals to reveal that the DLK-1 MAPK cascade, alongside with its downstream MAK-2 (MAPK activated protein kinase 2) and CEBP-1, regulates synapse formation and axon morphology during development and axon regeneration in adult. It also convincingly demonstrates that axotomy in mechanosensory neurons induces MAPK-dependent local translation of axonal CEBP-1 mRNAs involving their 3’UTR.
- 14.Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking beta-spectrin. J Cell Biol. 2007;176:269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell. 2005;120:407–420. doi: 10.1016/j.cell.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 16. Massaro CM, Pielage J, Davis GW. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. J Cell Biol. 2009;187:101–117. doi: 10.1083/jcb.200903166. ** This study demonstrates that the JNK MAPK and its downstream c-Fos stabilize Drosophila NMJ destabilized by cytoskeleton disruption. An intriguing model is presented in which the JNK signaling acts as a stress response system that is either activated to protect the stability of transiently disrupted NMJ or turned off by the JNK phosphatase to allow NMJ disassembly during persistent cytoskeleton disruption.
- 17. Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12:387–389. doi: 10.1038/nn.2290. ** The molecular mechanisms of Wallerian axon regeneration in neurons are poorly understood. This study reveals the DLK-JNK MAPK signaling as a novel component of the intrinsic axon degeneration program.
- 18. Ayaz D, Leyssen M, Koch M, Yan J, Srahna M, Sheeba V, Fogle KJ, Holmes TC, Hassan BA. Axonal injury and regeneration in the adult brain of Drosophila. J Neurosci. 2008;28:6010–6021. doi: 10.1523/JNEUROSCI.0101-08.2008. ** This study developed a novel Drosophila model for CNS axonal injury and regeneration studies and found that JNK and PKA promote axon regrowth of axotomized sLNv neurons in fly brain explant culture.
- 19.Collins CA, Wairkar YP, Johnson SL, DiAntonio A. Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron. 2006;51:57–69. doi: 10.1016/j.neuron.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Lewcock JW, Genoud N, Lettieri K, Pfaff SL. The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron. 2007;56:604–620. doi: 10.1016/j.neuron.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Erez H, Spira ME. Local self-assembly mechanisms underlie the differential transformation of the proximal and distal cut axonal ends into functional and aberrant growth cones. J Comp Neurol. 2008;507:1019–1030. doi: 10.1002/cne.21522. [DOI] [PubMed] [Google Scholar]
- 22.Zrouri H, Le Goascogne C, Li WW, Pierre M, Courtin F. The role of MAP kinases in rapid gene induction after lesioning of the rat sciatic nerve. Eur J Neurosci. 2004;20:1811–1818. doi: 10.1111/j.1460-9568.2004.03641.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 24.Sun F, He Z. Neuronal intrinsic barriers for axon regeneration in the adult CNS. Curr Opin Neurobiol. doi: 10.1016/j.conb.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ziv NE, Spira ME. Axotomy induces a transient and localized elevation of the free intracellular calcium concentration to the millimolar range. J Neurophysiol. 1995;74:2625–2637. doi: 10.1152/jn.1995.74.6.2625. [DOI] [PubMed] [Google Scholar]
- 26.Appenzeller O, Palmer G. The cyclic AMP (adenosine 3',5'-phosphate) content of sciatic nerve: changes after nerve crush. Brain Res. 1972;42:521–524. doi: 10.1016/0006-8993(72)90553-7. [DOI] [PubMed] [Google Scholar]
- 27.Carlsen RC. Axonal transport of adenylate cyclase activity in normal and axotomized frog sciatic nerve. Brain Res. 1982;232:413–424. doi: 10.1016/0006-8993(82)90284-0. [DOI] [PubMed] [Google Scholar]
- 28. Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci. 30:3175–3183. doi: 10.1523/JNEUROSCI.5464-09.2010. ** A comprehensive set of experiments from this C. elegans study show that Ca2+ and cAMP promote axon regrowth, fusion and synaptic branch formation in axotomized PLM mechanosensory neurons. It also dissects the differential roles of bZip family transcription factors in axon regeneration.
- 29.Pak CW, Flynn KC, Bamburg JR. Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci. 2008;9:136–147. doi: 10.1038/nrn2236. [DOI] [PubMed] [Google Scholar]
- 30.Gonzalez-Billault C, Jimenez-Mateos EM, Caceres A, Diaz-Nido J, Wandosell F, Avila J. Microtubule-associated protein 1B function during normal development, regeneration, and pathological conditions in the nervous system. J Neurobiol. 2004;58:48–59. doi: 10.1002/neu.10283. [DOI] [PubMed] [Google Scholar]
- 31.Schulte T, Paschke KA, Laessing U, Lottspeich F, Stuermer CA. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development. 1997;124:577–587. doi: 10.1242/dev.124.2.577. [DOI] [PubMed] [Google Scholar]
- 32.Lang DM, Lommel S, Jung M, Ankerhold R, Petrausch B, Laessing U, Wiechers MF, Plattner H, Stuermer CA. Identification of reggie-1 and reggie-2 as plasmamembrane-associated proteins which cocluster with activated GPI-anchored cell adhesion molecules in non-caveolar micropatches in neurons. J Neurobiol. 1998;37:502–523. doi: 10.1002/(sici)1097-4695(199812)37:4<502::aid-neu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 33.Langhorst MF, Reuter A, Stuermer CA. Scaffolding microdomains and beyond: the function of reggie/flotillin proteins. Cell Mol Life Sci. 2005;62:2228–2240. doi: 10.1007/s00018-005-5166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuermer CA, Plattner H. The 'lipid raft' microdomain proteins reggie-1 and reggie-2 (flotillins) are scaffolds for protein interaction and signalling. Biochem Soc Symp. 2005:109–118. doi: 10.1042/bss0720109. [DOI] [PubMed] [Google Scholar]
- 35. Munderloh C, Solis GP, Bodrikov V, Jaeger FA, Wiechers M, Malaga-Trillo E, Stuermer CA. Reggies/flotillins regulate retinal axon regeneration in the zebrafish optic nerve and differentiation of hippocampal and N2a neurons. J Neurosci. 2009;29:6607–6615. doi: 10.1523/JNEUROSCI.0870-09.2009. ** The authors found that knocking down reggies in Zebrafish RGC led to dramatically reduced axon regeneration post optic nerve section in vivo. Biochemical experiments indicated that reggie knockdown strongly affects the activation status of Rho GTPases, the N-WASP complex stability, and activation of p38 MAPK and focal adhesion kinase (FAK) in mouse neuroblastoma N2a cells.
- 36.Nash M, Pribiag H, Fournier AE, Jacobson C. Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol. 2009;40:224–235. doi: 10.1007/s12035-009-8083-y. [DOI] [PubMed] [Google Scholar]
- 37.Duffy P, Schmandke A, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM. Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci. 2009;29:15266–15276. doi: 10.1523/JNEUROSCI.4650-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312:596–612. doi: 10.1016/j.ydbio.2007.09.019. ** The authors used laser capture microdissection to isolate adult RGCs post optic nerve injury and performed microarray analysis. They identified 347 induced and 29 repressed regeneration associated genes which demonstrate an overlapping yet differential pattern from genes involved in RGC development. Among highly-induced genes, KLF6 and KLF7 are necessary for robust RGC axon regrowth in explant culture.
- 39.Veldman MB, Bemben MA, Goldman D. Tuba1a gene expression is regulated by KLF6/7 and is necessary for CNS development and regeneration in zebrafish. Mol Cell Neurosci. 43:370–383. doi: 10.1016/j.mcn.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raivich G, Makwana M. The making of successful axonal regeneration: genes, molecules and signal transduction pathways. Brain Res Rev. 2007;53:287–311. doi: 10.1016/j.brainresrev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 42.Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 43. Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. ** The authors screened >100 genes whose expression is significantly upregulated postnatally mouse RGCs and identified KLF-4 as a strong suppressor of both developmental and regenerating growth. Among 17 KLF family members assessed in this study, KLF4 and KLF9 are growth suppressors while KLF6 and KLF7 are growth enhancers during development.
- 44.Goldman D, Ding J. Different regulatory elements are necessary for alpha1 tubulin induction during CNS development and regeneration. Neuroreport. 2000;11:3859–3863. doi: 10.1097/00001756-200011270-00051. [DOI] [PubMed] [Google Scholar]
- 45.Senut MC, Gulati-Leekha A, Goldman D. An element in the alpha1-tubulin promoter is necessary for retinal expression during optic nerve regeneration but not after eye injury in the adult zebrafish. J Neurosci. 2004;24:7663–7673. doi: 10.1523/JNEUROSCI.2281-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Udvadia AJ, Koster RW, Skene JH. GAP-43 promoter elements in transgenic zebrafish reveal a difference in signals for axon growth during CNS development and regeneration. Development. 2001;128:1175–1182. doi: 10.1242/dev.128.7.1175. [DOI] [PubMed] [Google Scholar]
- 47.Kusik BW, Hammond DR, Udvadia AJ. Transcriptional regulatory regions of gap43 needed in developing and regenerating retinal ganglion cells. Dev Dyn. 239:482–495. doi: 10.1002/dvdy.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 49. Park KK, Liu K, Hu Y, Smith D, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. ** This exciting study aimed to identify intrinsic pathways controlling axon regrowth in adult mouse RGCs in vivo. The authors have uncovered a center role of PTEN/mTOR-dependent protein synthesis in adult CNS regeneration using an elegant RGC-specific gene deletion strategy.
- 50. Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. ** This study found that re-activating mTOR-dependent protein synthesis by releasing the suppression of SOCS3 on gp130 cytokine receptor can significantly improve RGC regeneration after optic nerve injury. Together with [49], these findings suggest that aiming on improving intrinsic regenerating ability of injured neurons should be a main focus of future studies.
- 51.Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2:a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tessier-Lavigne M. Wiring the brain: the logic and molecular mechanisms of axon guidance and regeneration. Harvey Lect. 2002;98:103–143. [PubMed] [Google Scholar]
- 53.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Low K, Culbertson M, Bradke F, Tessier-Lavigne M, Tuszynski MH. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci U S A. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu X, Hawkes E, Ishimaru T, Tran T, Sretavan DW. EphB3: an endogenous mediator of adult axonal plasticity and regrowth after CNS injury. J Neurosci. 2006;26:3087–3101. doi: 10.1523/JNEUROSCI.4797-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Lee JK, Geoffroy CG, Chan AF, Tolentino KE, Crawford MJ, Leal MA, Kang B, Zheng B. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 66:663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cafferty WB, Duffy P, Huebner E, Strittmatter SM. MAG and OMgp synergize with Nogo-A to restrict axonal growth and neurological recovery after spinal cord trauma. J Neurosci. 30:6825–6837. doi: 10.1523/JNEUROSCI.6239-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ji B, Case LC, Liu K, Shao Z, Lee X, Yang Z, Wang J, Tian T, Shulga-Morskaya S, Scott M, et al. Assessment of functional recovery and axonal sprouting in oligodendrocyte-myelin glycoprotein (OMgp) null mice after spinal cord injury. Mol Cell Neurosci. 2008;39:258–267. doi: 10.1016/j.mcn.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 63.Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alto LT, Havton LA, Conner JM, Hollis Ii ER, Blesch A, Tuszynski MH. Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]