Abstract
In mice, BDNF provided by the developing taste epithelium is required for gustatory neuron survival following target innervation. However, we find that expression of BDNF, as detected by BDNF-driven β-galactosidase, begins in the cranial ganglia before its expression in the central (hindbrain) or peripheral (taste papillae) targets of these sensory neurons, and before gustatory ganglion cells innervate either target. To test early BDNF function, we examined the ganglia of bdnf null mice before target innervation, and found that while initial neuron survival is unaltered, early neuron development is disrupted. In addition, fate mapping analysis in mice demonstrates that murine cranial ganglia arise from 2 embryonic populations, i.e., epibranchial placodes and neural crest, as has been described for these ganglia in non-mammalian vertebrates. Only placodal neurons produce BDNF, however, which indicates that prior to innervation, early ganglionic BDNF produced by placode-derived cells promotes gustatory neuron development.
Keywords: BDNF, cranial ganglia, maturation, gustatory, placode, neural crest, neuron, mouse, cre recombinase, fate mapping, ROSA reporter line
Introduction
During nervous system development, initial overproduction of neurons is followed by a natural period of cell death (Clarke, 1985; Oppenheim, 1985), and the final number of surviving sensory neurons is determined by neuronal competition for limited supplies of neurotrophins (Levi-Montalcini and Angeletti, 1968; Buchman and Davies, 1993; Davies, 1997). The details of neurotrophic support are complex. Different populations of neurons have different neurotrophic requirements, neurons of a single type can switch their dependence from one neurotrophin to another, and the same neurotrophin may serve different functions at various stages of a neuron’s development (Davies and Lumsden, 1984; Ernsberger and Rohrer, 1988; Buchman and Davies, 1993; Acheson et al., 1995; Rochlin et al., 2000). In addition to promoting neuron survival, neurotrophins also play important roles in neuronal maturation (Martinez et al., 1998), differentiation (Chan et al., 2008), neurite outgrowth and branching (Bosco and Linden, 1999; Lom and Cohen-Cory, 1999), myelination (Chan et al., 2001; Cosgaya et al., 2002), axon targeting (Ringstedt et al., 1999; Righi et al., 2000; Lopez and Krimm, 2006), synaptogenesis (Martinez et al., 1998; Hu et al., 2005), neuronal life span (Alvarez-Borda et al., 2004), and may even regulate complex behaviors such as depression (Martinowich et al., 2007; Li et al., 2008) and food intake (Kernie et al., 2000; Rios et al., 2001; Lebrun et al., 2006).
Brain derived neurotrophic factor (BDNF) is crucial for gustatory system formation. The absence of BDNF results in loss of taste bud innervation, death of gustatory neurons in the cranial ganglia, and abnormal taste bud development (Liebl et al., 1997; Nosrat et al., 1997; Zhang et al., 1997; Mistretta et al., 1999; Patel and Krimm, 2010). Gustatory neurons that innervate anterior lingual taste buds reside in the geniculate ganglia (gVII), while neurons of the petrosal (gIX) and nodose (gX) ganglia innervate posterior taste buds (Martin and Mason, 1977; Whitehead and Frank, 1983). During embryonic development, BDNF is expressed in the taste bud-bearing papillae of the tongue, and not in surrounding non-gustatory epithelium (Nosrat and Olson, 1995). Expression of BDNF by developing peripheral lingual targets is critical for the guidance of gustatory axons from the geniculate ganglion to developing taste placodes and subsequently for innervation of taste buds (Nosrat et al., 1997; Ringstedt et al., 1999; Krimm et al., 2001; Lopez and Krimm, 2006; Ma et al., 2009). BDNF, and its receptor tyrosine kinase, TrkB, are also expressed in developing geniculate ganglia (Ernfors et al., 1992; Schecterson and Bothwell, 1992; Cho and Farbman, 1999; Farbman et al., 2004), however the role of ganglion-derived BDNF is less clear.
Within gVII, two periods of neuronal cell death occur naturally during embryogenesis; an early period before target innervation and a later period, as peripheral target innervation is established (Carr et al., 2005). Postnatal mice lacking bdnf (bdnf knockouts - KOs) have 50% fewer neurons in the geniculate and petrosal ganglia, compared with wild type (WT) littermates (Jones et al., 1994; Liu et al., 1995; Liebl et al., 1997). By birth, gustatory neurons have long since innervated peripheral taste placodes and central hindbrain nuclei, so the absence of target-derived and ganglionic BDNF could each contribute to neuron loss. BDNF expression in the geniculate ganglia before target innervation and before the earliest reports of cell death in bdnf KO mice (Patel and Krimm, 2010) therefore raises the intriguing possibility of other non-survival roles for early ganglionic BDNF. Additionally, satellite glial cells in gVII may provide local support to gustatory neurons, as do glia in other regions of the central and peripheral nervous systems (Nagtegaal et al., 1998; Sautter et al., 1998; Verderio et al., 2006).
The cranial ganglia in non-mammalian vertebrates arise during development from two neurogenic cell populations: cranial neural crest cells, and epibranchial placodes in the cephalic ectoderm (Stone, 1922; Landacre, 1933; Yntema, 1937; Yntema, 1943; Yntema, 1944; D'Amico-Martel and Noden, 1983; Northcutt and Brändle, 1995; Lwigale, 2001; Gross et al., 2003; Harlow and Barlow, 2007). We have shown previously in amphibian embryos that epibranchial placodes give rise exclusively to taste sensory neurons, while cranial neural crest produces neurons that innervate non-gustatory targets (Harlow and Barlow, 2007). In the current study, we find a similar division of cell fate based on embryonic origin within the geniculate ganglia of mice using an ectoderm-specific Cre transgenic line (Crect; Yang et al., in prep), and the Wnt1Cre line (Danielian et al., 1998) to indelibly mark placode- and neural crest-derived cells, respectively. Specifically, epibranchial placodes give rise exclusively to neurons, while all glial cells within the ganglion derive from neural crest, as do a small proportion of sensory neurons within gVII. Further, we determine that the source of ganglionic BDNF is placode-derived neurons rather than crest-derived glia or neurons. Finally, we find that BDNF expression in the geniculate ganglion is restricted to a subset of placodal neurons, and that this local placodal BDNF is required for early development of gustatory neurons.
Results
BDNF-lacZ is expressed in multiple locations of the embryonic gustatory system
Early in development (E8.5–E9.5 in mice), neural crest cells and neuroblasts from placodal ectoderm migrate to sites of ganglion formation (Kulesa and Fraser, 1998; Kulesa and Fraser, 2000; Begbie and Graham, 2001; Matei et al., 2006; Begbie, 2008). Once consolidated into ganglia, neuroblasts mature and differentiate into sensory neurons, which send out processes to innervate central and peripheral targets. In the developing taste system, peripheral gustatory fibers first enter the base of the developing tongue at E12.0 (Mbiene and Mistretta, 1997; Scott and Atkinson, 1998; Mbiene, 2004), reach the lingual epithelium by E13.5, and invade the epithelium of BDNF-expressing fungiform placodes by E14.5 (Farbman and Mbiene, 1991; Whitehead and Kachele, 1994; Nosrat and Olson, 1995; Nosrat et al., 1996; Mbiene, 2004; Lopez and Krimm, 2006). Centrally directed geniculate ganglion axons also enter the hindbrain between E12–E15 (mouse: Fritzsch et al., 1997; E14–E17 in rats, Altman and Bayer, 1982; Zhang and Ashwell, 2001), before innervating their final target, the nucleus of the solitary tract.
To better understand the role of BDNF in the development of gustatory neurons, we first examined embryos at progressive stages in the context of what is known about the timing of gustatory system formation (Fig. 1). In bdnflacZ/+ mice, one allele of the bdnf promoter drives expression of the lacZ gene in cells actively synthesizing BDNF at the time of collection (Bennett et al., 1999; Yee et al., 2003; Clevenger et al., 2008). As the turnover rate of β-galactosidase protein is presumed to be slower than that of BDNF (Jacobsen and Willumsen, 1995), our data are a temporal approximation of the onset and duration of BDNF expression.
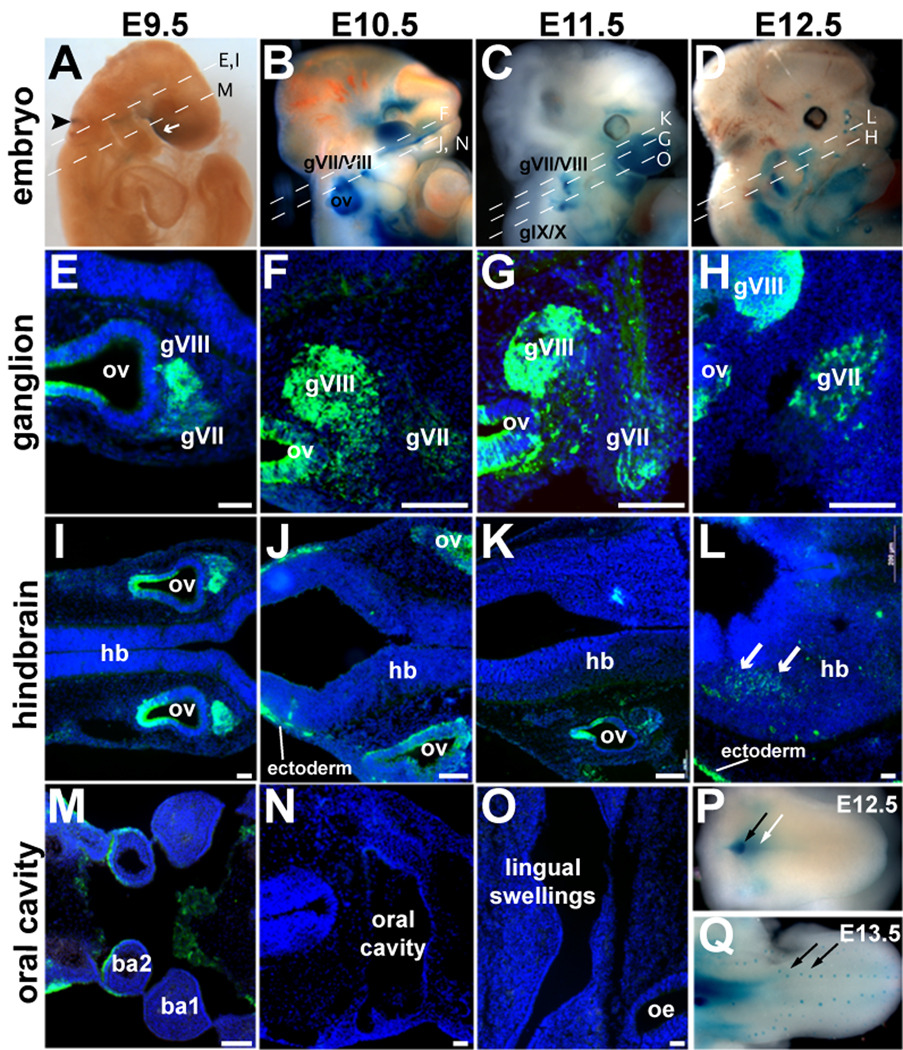
Figure 1. BDNF-driven β-galactosidase expression in the developing gustatory system.
(A–D) Whole-mount bdnflacZ/+ (BDNF-lacZ) embryos at (A) E9.5, (B) E10.5, (C) E11.5, and (D) E12.5 reacted with Xgal have β-galactosidase activity (blue) in the otic vesicle (ov, black arrowhead, A), ventral to the forebrain (white arrow, A) and surrounding ganglia (gVII/VIII, gIX/X, B–D). (E–O). Antisera against β-galactosidase (green) labels BDNF-producing cells in horizontal sections of bdnflacZ/+ embryos. (E–H). BDNF-lacZ is present in the geniculate ganglion (gVII) and acousticovestibular ganglion (gVIII) at (E) E9.5, (F) E10.5, (G) E11.5 and (H) E12.5. (I–K) BDNF-lacZ is not present in the hindbrain (hb) at (I) E9.5, (J) E10.5, or (K) E11.5. (L) BDNF-lacZ is present in the hindbrain at E12.5. (M–O) In the developing oral cavity, BDNF-lacZ cells are not at (M) E9.5, (N) E10.5, or (O) E 11.5. (P) E12.5 tongue reacted with X-gal in whole mount reveals BDNF-lacZ expressing cells (blue) in the posterior lingual mesenchyme (white arrow) and circumvallate papilla (black arrow). (Q) By E13.5, BDNF-lacZ expression focalizes to the developing taste placodes (black arrows). Anterior is to the right in all sections. ba: branchial arches. Scale bars = 50 µm.
BDNF-lacZ is first detected at E9.5; β-galactosidase is limited to the area of the otic vesicle (black arrowhead, Fig. 1A), as well as to small regions near the ventral forebrain (white arrow, Fig. 1A). In sectioned E9.5 embryos, immunostaining for β-galactosidase revealed numerous positive cells in the facio-acoustic (gVII/gVIII) ganglionic complex immediately adjacent to the labeled otic vesicle (Fig. 1E). However, β-galactosidase was not detected in the hindbrain at any level at E9.5 (single section shown; hb, Fig. 1I). At E9.5, the tongue has yet to form from the paired lingual swellings of the branchial arches, and β-galactosidase immunoreactivity (IR) was absent from both oral epithelium and arch mesenchyme (ba1, ba2, Fig. 1M).
At E10.5 (Fig. 1B, F), E11.5 (Fig. 1C, G), and E12.5 (Fig. 1D, H), BDNF-β-galactosidase is present in a subset of geniculate ganglion cells (gVII, Fig. 1F, G, H), albeit at lower levels than in the neighboring acousticovestibular ganglion (gVIII) where BDNF-lacZ expression is particularly robust (Fritzsch et al., 1997b). Upon quantification, we found ~20% of gVII cells were β-galactosidase-IR at E11.5 (Table 1; bdnflacZ/+).
Table 1.
Percentage of geniculate ganglion neurons that express β-galactosidase in E11.5 mice carrying different combinations of Crect, ROSA and bdnf alleles.
| Genotype |
Crect;ROSA (n=4) |
bdnflacZ/+ (n=3) |
Crect;bdnflox/+ (n=3) |
|---|---|---|---|
|
Number of β-gal-IR neurons per gVII (mean ± S.D.) |
164 ± 139 | 377 ± 29 | 131 ± 22 |
|
Total number of neurons per gVII (mean ± S.D.) |
1719 ± 127 | 1825 ± 157 | 1729 ± 278 |
|
Ave. % β-gal-IR neurons per section in gVII (mean ± S.D.) |
10% ± 8% | 21% ± 2% | 8% ± 1% |
In contrast to early ganglion expression, BDNF-lacZ is not expressed in the hindbrain (Fig. 1I–K) or oral cavity (Fig. 1M–O) between E9.5 and E11.5, although low levels of bdnf mRNA have been reported in the hindbrain at these stages (Buchman and Davies, 1993). By E12.5, sparse BDNF-β-galactosidase-IR was detected in the hindbrain for the first time (white arrows, Fig. 1L), as well as in the tongue (Fig. 1P). However, BDNF-β-galactosidase is restricted to the posterior tongue, and is predominantly mesenchymal (white arrow, Fig. 1P). The gustatory circumvallate placode, innervated by the glossopharyngeal (IX) nerve, also expresses BDNF-lacZ (black arrow, Fig. 1P). It is not until E13.5 that robust BDNF-lacZ expression is seen in epithelial cells of the fungiform taste placodes (black arrows, Fig. 1Q), which are the peripheral targets of gVII gustatory neurons. β-galactosidase was absent from non-gustatory lingual epithelium as previously reported for bdnf mRNA (Nosrat and Olson, 1995; Nosrat et al., 1996).
In summary, BDNF is produced in a subset of neurons within the geniculate ganglia several days before gustatory axons reach either their central or peripheral targets, and before either target expresses BDNF (Fig. 1), suggesting that BDNF may function in gustatory neuron development earlier than previously considered.
Neuronal development is disrupted in the absence of BDNF
BDNF null mice display gross changes in ganglion anatomy, i.e. reduced ganglion volume and neuron numbers, when assayed postnatally (0.5–25 days after birth), well after peripheral and central targets are normally innervated by gustatory neurons (Jones et al., 1994; Fritzsch et al., 1997a; Liebl et al., 1997; Nosrat et al., 1997; Mistretta et al., 1999). Here, we examined geniculate ganglia of bdnf KO (bdnflacZ/neo) and WT embryos at E11.5, before target innervation occurs to determine the effects of early deletion of ganglionic BDNF on sensory neuron development. Unlike published reports of older embryos, gVII volume did not differ between BDNF KO and WT embryos at E11.5 (Table 2), suggesting that neither the initial proliferation of neuroblasts nor migration from neural crest or the epibranchial placodes, which both contribute cells to the ganglia, were disrupted by BDNF loss.
Table 2.
Nuclear diameter measurements of immature and differentiated neurons in control and BDNF mutant geniculate ganglia.
| Genotype |
BDNF WT Crect;ROSA (n=4) |
BDNF KO bdnfLacZ/neo (n=4) |
CRECT KO (Placode deleted BDNF) Crect;bdnflox/neo (n=3) |
|---|---|---|---|
|
Ganglion volume (×105 µm3) (mean ± S.D.) |
40 ± 4.1 | 32 ± 5.1 | 35 ± 2.2 |
|
Number of Islet1/2-IR neurons (mean ± S.D.) |
1359 ± 164 | 1026 ± 148 | 1377 ± 213 |
|
Number of NeuN-IR neurons (mean ± S.D.) |
1160 ± 182 | 863 ± 365 | 854 ± 297 |
|
Islet1/2-IR neurons Nuclear diameter (mean ± S.D.) |
6.99 ± 1.26 | 6.42 ± 1.55* | 6.67 ± 1.05 |
|
NeuN-IR neurons Nuclear diameter (mean ± S.D.) |
7.74 ± 1.39 | 6.65 ± 1.29* | 6.85 ± 1.13* |
The ganglia volumes, number of neurons and nuclear diameter size from BDNF WT, BDNF KO, and CRECT KO were compared using one-factor, one-way ANOVA with a Tukey-Kramer post hoc test;
p<0.05, significantly different from WT.
Islet1/2 are expressed by migratory placodal neuroblasts, and by sensory neurons of the cranial and dorsal root ganglia during their transition from neurogenesis to terminal differentiation (Begbie et al., 2002; Davies, 2007; Shiau et al., 2008; Sun et al., 2008; Yang et al., 2008). NeuN, a slightly later marker, is expressed by most post-mitotic neuronal cell types throughout the adult nervous system (Mullen et al., 1992). Thus, the number of Islet1/2-IR (immature) and NeuN-IR (differentiated) neurons was examined in bdnf KO and WT mice at E11.5. Many Islet1/2- and NeuN-IR neurons were seen in the ganglia of embryos of both genotypes, indicating that the neuronal population is transitioning from a neurogenic phase to a mature postmitotic state. However, loss of BDNF had no significant impact on the number of NeuN- or Islet1/2-IR neurons (Table 2), indicating that neuronal survival was unaffected.
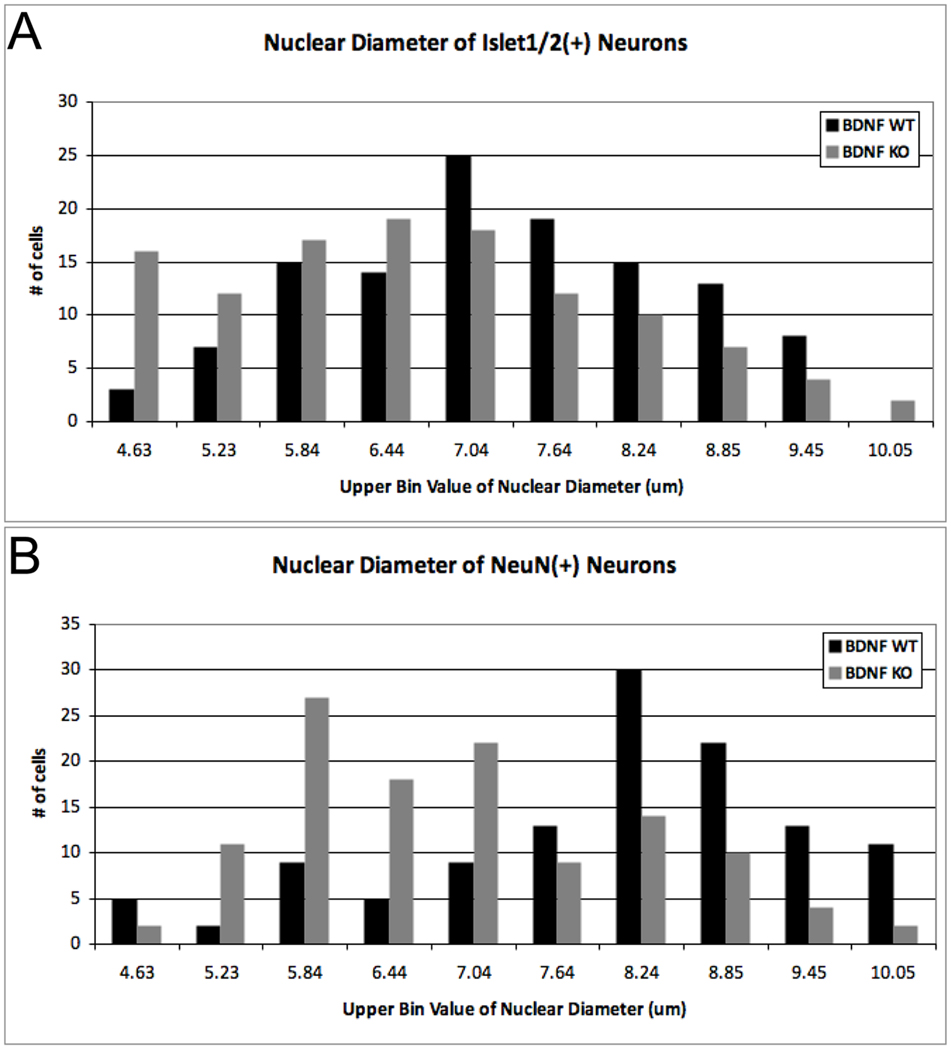
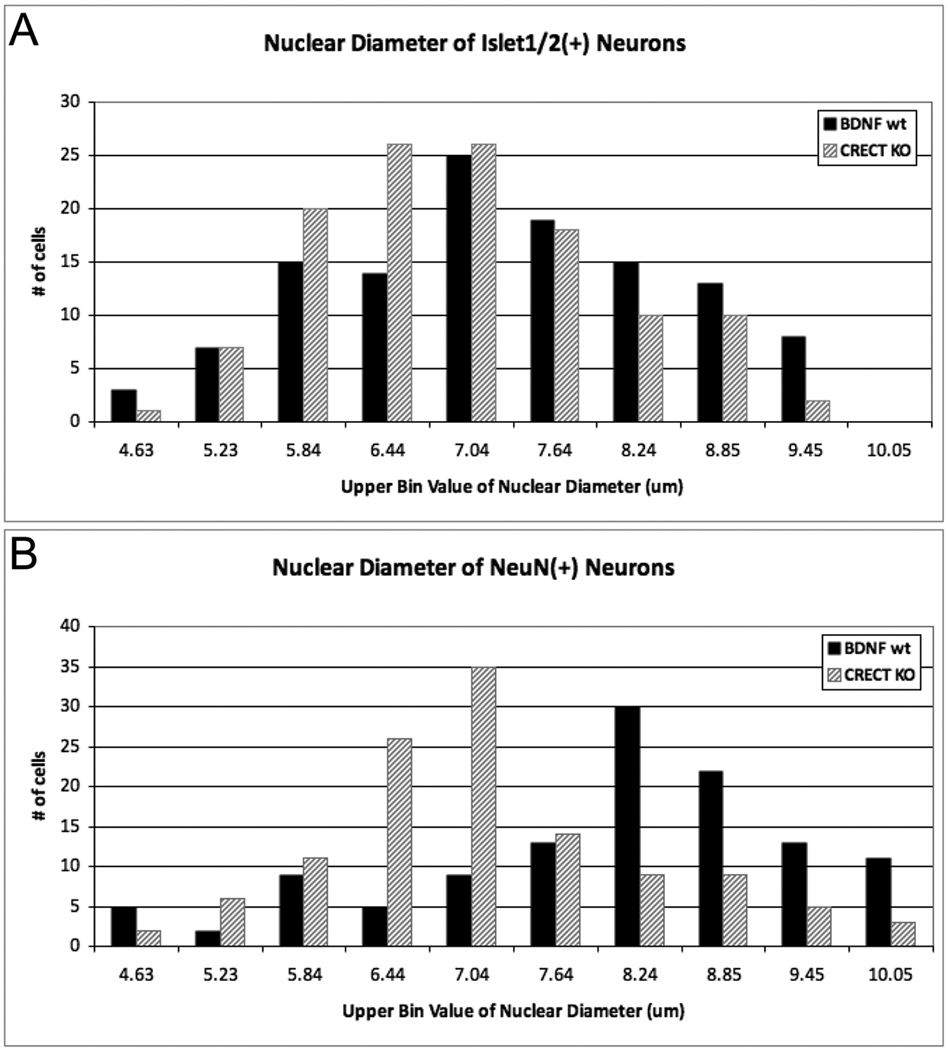
To explore a possible role of early BDNF in maturation rather than neuron survival, we next examined the size distribution of cells within bdnf KO and WT ganglia. Nuclear size positively correlates with neuronal soma size (McConnell and Kaznowski, 1991; Villena et al., 1997), and is an indicator of the state of cellular differentiation: less differentiated precursors have smaller nuclei, and nuclear diameter increases during neuronal maturation (Miller and Peters, 1981; Roberts and Morey, 1985; Roberts et al., 1987; Saucedo and Edgar, 2002). In bdnf KO embryos, the average nuclear size of both Islet1/2-IR and NeuN-IR neurons was significantly decreased compared to those of WT (Table 2; p < 0.05, One-way ANOVA; note, a second placode-specific bdnf mutant was included in this statistical analysis, and is reported below). Moreover, the entire size class distribution for both immature (Fig. 2A) and mature neurons (Fig. 2B) was shifted to smaller diameters in the bdnf KOs (NeuN-IR neurons (p<0.0001); Islet-IR neurons (p<0.001), Mann-Whitney U test). This reduction in nuclear diameter may indicate that development of geniculate ganglion neurons cells is delayed; nuclear shrinkage can also signal impending cell death (Kasischke et al., 2001; Bonde et al., 2002). However, analysis of cell death in the geniculate ganglion of bdnf KO and WT mice at E12.5 via activated caspase-3-IR did not reveal increased cell death in the absence of BDNF (Patel and Krimm, 2010). Thus our data suggest that smaller nuclei in bdnf KOs at E11.5 more likely reflect a delay in maturation.
Figure 2. Nuclear size distribution of geniculate ganglion neurons at E11.5 is shifted towards smaller sizes in bdnf KO embryos.
The nuclear size of (A) Islet1/2 and (B) NeuN-immunoreactive neurons of bdnflacZ/neo mice (BDNF KO; grey bars) were significantly reduced in the geniculate ganglia compared to Crect;ROSA;bdnf+/+ mice (BDNF WT; black bars). (A) Islet1/2-IR nuclei were significantly smaller in BDNF KO than in BDNF WT (Mann-Whitney U = 5396, n1 = n2 = 120, P<0.001, two-tailed). (B) The nuclear size distribution of NeuN-IR neurons was also significantly smaller in BDNF KO than in BDNF WT (Mann-Whitney U = 3944, n1 = n2 = 120, P<0.0001, two-tailed). Nuclear diameters (40 measurements per ganglion, n=3 ganglia for each genotype) were measured, binned according to size, ranked and compared using a Mann-Whitney U test.
Fate mapping of the geniculate ganglia
Cranial ganglia arise from both neural crest and epibranchial placodes, and we have shown in amphibians that placode-derived neurons are uniquely gustatory (Harlow and Barlow, 2007). Moreover, in mammals, gustatory neurons are known to depend on BDNF for survival (Liebl et al., 1997; Zhang et al., 1997; Patel and Krimm, 2010). Thus BDNF expression in gVII raises the questions of which ganglion cells, placodal and/or neural crest derived, produce BDNF and which cells are capable of responding to BDNF, i.e., express the tyrosine kinase receptor, TrkB.
To identify epibranchial placode-derived gustatory neurons, Cre expression was driven in the ectoderm using a newly developed ectoderm-specific Cre transgenic mouse line where Cre recombinase is expressed under the control of an ectoderm specific enhancer of the AP2alpha promoter (Crect, Yang et al., in prep; and see methods section). While the complete description of this mouse line will be described elsewhere, the efficacy of this Cre driver allele in labeling expected epibranchial placode derivatives was determined here. Crect mice were crossed with ROSALacZ reporter mice (Soriano, 1999) to fate map placodal neurons, while Wnt1Cre;ROSA mice were used to track the fate of neural crest-derived cells (see below, Danielian et al., 1998). β-galactosidase activity was detected in the ectoderm of developing Crect;ROSA embryos at E8.5 (Yang et al., in prep), coincident with the timing of epibranchial placode formation (Fode et al., 1998). The onset of Crect expression varied across individual embryos resulting in mosaic β-galactosidase in the ectoderm between E8.5–9.0 (data not shown, Yang et al., in prep), and therefore also likely in the first placodal neuroblasts, which delaminate from the placodes between E9.0–9.5 (Baker and Bronner-Fraser, 2001). However, by E9.5 Crect-mediated recombination, as detected by β-galactosidase, was uniform throughout the head ectoderm (green, Fig. 3A). In horizontal sections of E9.5 Crect;ROSA embryos, neuroblasts emerging from the epibranchial placodes are β-galactosidase-IR (arrow, inset Fig. 3A), as are placodal and adjacent non-placodal ectoderm (green, Fig. 3A). By E11.5, in addition to robust immunoreactivity in the ectoderm, β-galactosidase is present in placode-derived sensory neurons of the cranial ganglia (trigeminal ganglion, gV, Fig. 3B; gVII, Fig. 3C; distal gIX and gX, Fig. 3E). Importantly, no neural tube or neural crest descendants express β-galactosidase in Crect;ROSA embryos. Although we do observe β-galactosidase-IR trigeminal (gV) afferent fibers in the hindbrain (HB, Fig. 3B), cell bodies in the brain and crest-derived cranial mesenchyme are β-galactosidase-immunonegative (Fig. 3A,B, F), indicating the placodal origin of β-galactosidase-IR cells in the trigeminal ganglion.
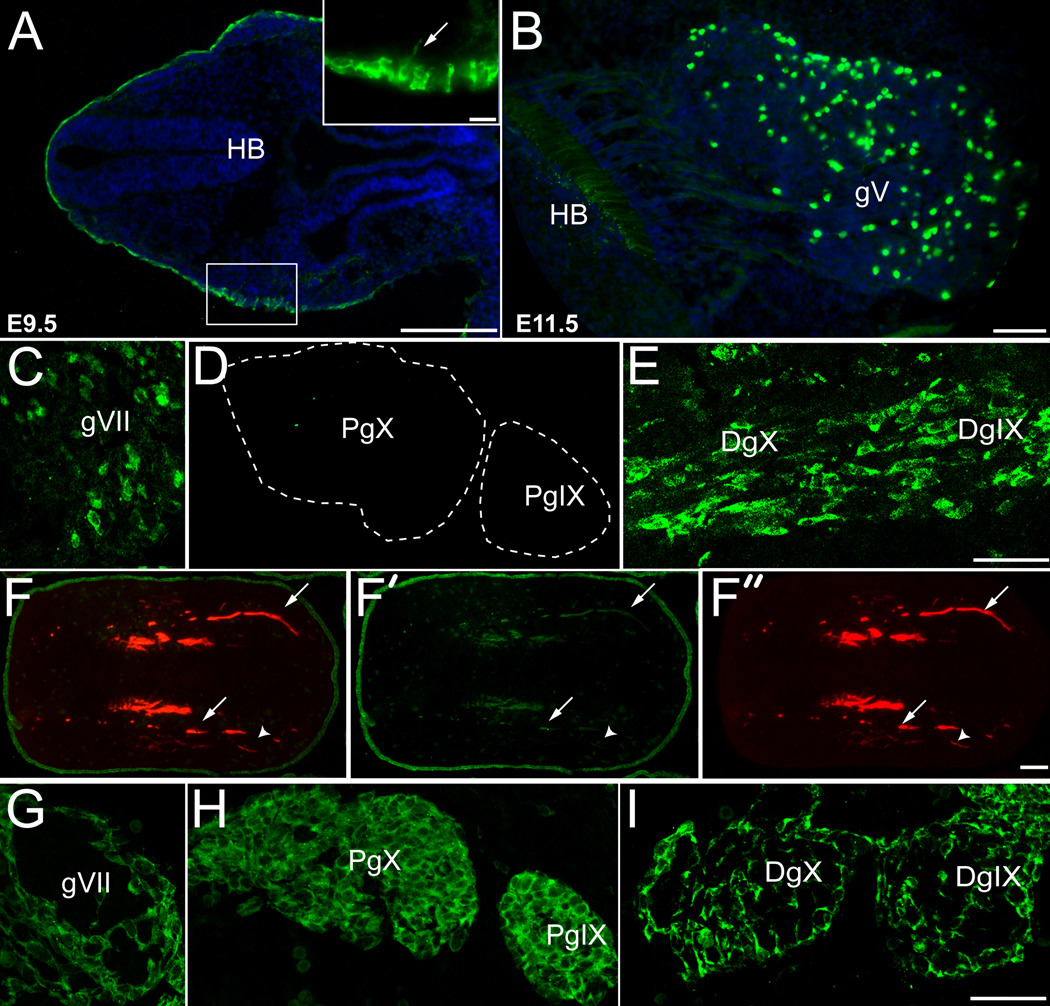
Figure 3. Epibranchial placodes and their derivatives are labeled in Crect;ROSA mice.
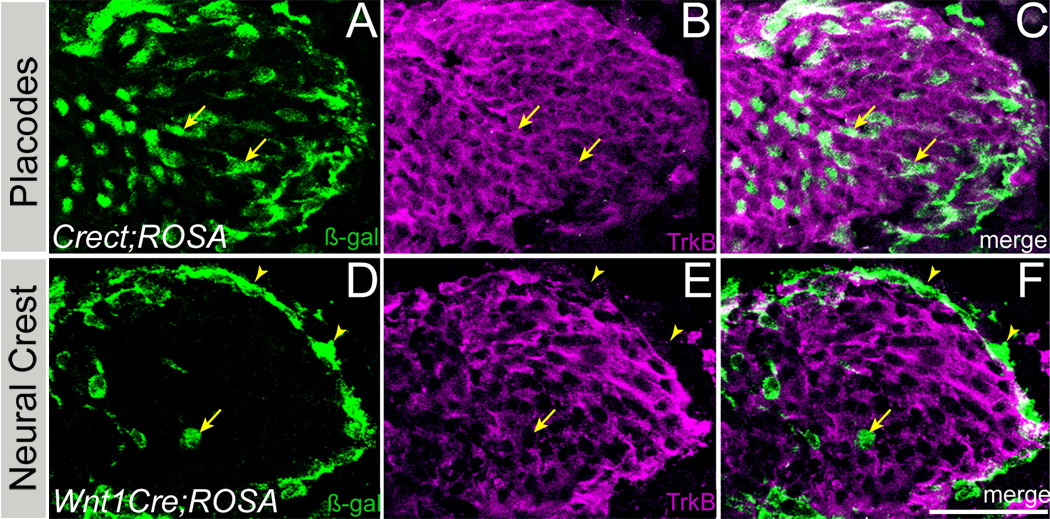
(A–F) Horizontal sections of Crect;ROSA embryos. (A) In E9.5 Crect;ROSA embryos, β-galactosidase (green) is expressed in the surface ectoderm, including the epibranchial placodes (inset). At E11.5, β-galactosidase immunoreactivity is seen in placode-derived neurons of the (B) trigeminal (gV) and (C) geniculate (gVII) ganglia of Crect;ROSA embryos. β-galactosidase-IR cells are not present in the proximal portions of ganglia IX and X (D; PgIX and PgX) in Crect;ROSA embryos, but are present in the distal ganglia of IX and X (E; DgIX and DgX). (F) In the developing tongue of E11.5 Crect;ROSA embryos, most ingrowing neurites (arrows) are positive for neurofilament (red, F, F”), and a subset are immunopositive for β-galactosidase (green, F, F’). Fibers labeled exclusively by anti-neurofilament are indicated with an arrowhead. The tongue epithelium expresses β-galactosidase throughout at this stage (F, F’). (G–I) Horizontal sections of E11.5 Wnt1cre;ROSA embryo have β-galactosidase expression in neural crest-derived cells in the ganglia. (G) Neural crest derived β-galactosidase-IR cells (green) are detected on the surface of the geniculate ganglion (gVII ). Neural crest derived cells (green) are present throughout the proximal ganglia of IX and X (H; PgIX and PgX), and on the surface of the distal ganglia of IX and X (I; DgIX and DgX). Anterior is to the right. Blue: Hoechst stained nuclei. Scale bars are 20 µm in inset of A, 100 µm in A and F, and all others are 50 µm.
At E11.5, Crect-mediated β-galactosidase expression within the cranial ganglia is evident in locations consistent with epibranchial placodal lineages mapped in non-mammalian vertebrates, including neurons in the geniculate ganglion (Fig. 3C), and in the distal postotic petrosal (DgIX) and nodose (DgX) ganglia of the IXth and Xth cranial nerves (Fig. 3E). Cells labeled with β-galactosidase were not seen in the proximal crest-derived jugular and superior ganglia of IX and X in Crect;ROSA embryos (pIX and PX in Fig. 3D; D’Amico-Matel and Noden, 1983). β-galactosidase expression in placode-derived ganglia varied across individual embryos. In some Crect;ROSA litters less than 3% of neurons within gVII were β-galactosidase-IR, while in other litters upwards of 18% of gVII cells were β-galactosidase-IR (Table 1; Crect:ROSA). This likely represents inherent variability between the onset of Crect expression and the timing of placodal cell delaminations from the ectoderm. Nonetheless, the β-galactosidase labeling pattern of cranial ganglia in Crect;ROSA embryos was consistent across litters in terms of selectively labeling predicted placodal derivatives. Thus, our ectoderm specific Crect line is efficacious for following the fate of cells derived from the epibranchial placodes, as well as driving Cre activity specifically within this cell population during taste system development.
In the tongues of E11.5 Crect;ROSA embryos, the lingual epithelium is β-galactosidase-IR, as are nerve fibers within large neurofilament-positive nerve bundles (arrows, Fig. 3F-F”). However, not all neurofilament-positive fibers express β-galactosidase, (arrowhead, Fig. 3F-F”); we speculate that these neurites are the non-gustatory lingual innervation supplied by neural crest-derived neurons of the trigeminal ganglion (Chan and Byers, 1985; Farbman and Mbiene, 1991; Arvidsson et al., 1995).
We next mapped the neural crest contribution to developing cranial ganglia using the Wnt1Cre line. In Wnt1Cre;ROSA embryos, β-galactosidase-IR was detected in cells along the margins of gVII (Fig. 3G), and of the distal ganglia of IX and X (DgIX and DgX, Fig. 3I). By contrast, Wnt1Cre driven β-galactosidase was evident in neural crest derived cells throughout the proximal ganglia of IX and X (PgIX and PgX, Fig. 3H). Thus, the locations of neural crest cells within the cranial ganglia of mice are identical to those described previously for chick (D'Amico-Martel and Noden, 1983) and axolotl (Barlow and Northcutt, 1995; Harlow and Barlow, 2007).
In mouse geniculate ganglia, epibranchial placodes produce only neurons, while neural crest predominantly contributes glia
To determine the cell type contributions of epibranchial placodes and neural crest to the geniculate ganglia of mice, we examined β-galactosidase expression in E11.5 Crect;ROSA and Wnt1Cre;ROSA mice in combination with neuronal and glial immunomarkers (Fig. 4). Islet1/2 is evident in immature neurons (Begbie et al., 2002; Davies, 2007; Shiau et al., 2008; Yang et al., 2008), NeuN is expressed by post-mitotic neurons (Mullen et al., 1992), and Sox10 is a transcription factor expressed by migrating neural crest-derived precursors, and then restricted to neural crest-derived glia (Britsch et al., 2001). In the geniculate ganglia of E11.5 Crect;ROSA embryos (Fig. 4A–C), β-galactosidase-IR (placodal cells: green) co-localizes with both Islet1/2-(arrows, Fig. 4A) and NeuN-IR (arrows, Fig. 4B), confirming the neuronal identity of placodal cells. However, not all NeuN or Islet labeled cells were immunoreactive for β-galactosidase in Crect;ROSA ganglia. As mentioned above, when the first neuroblasts migrate from the placodes, Cre activity is mosaic in the ectoderm, and the epibranchial placodes are similarly mosaic for lacZ (Yang et al., in prep; Table 1). In addition, not all placodal cells were NeuN-IR or Islet-IR, possibly because they are immature neuroblasts cells that do not yet express either marker.
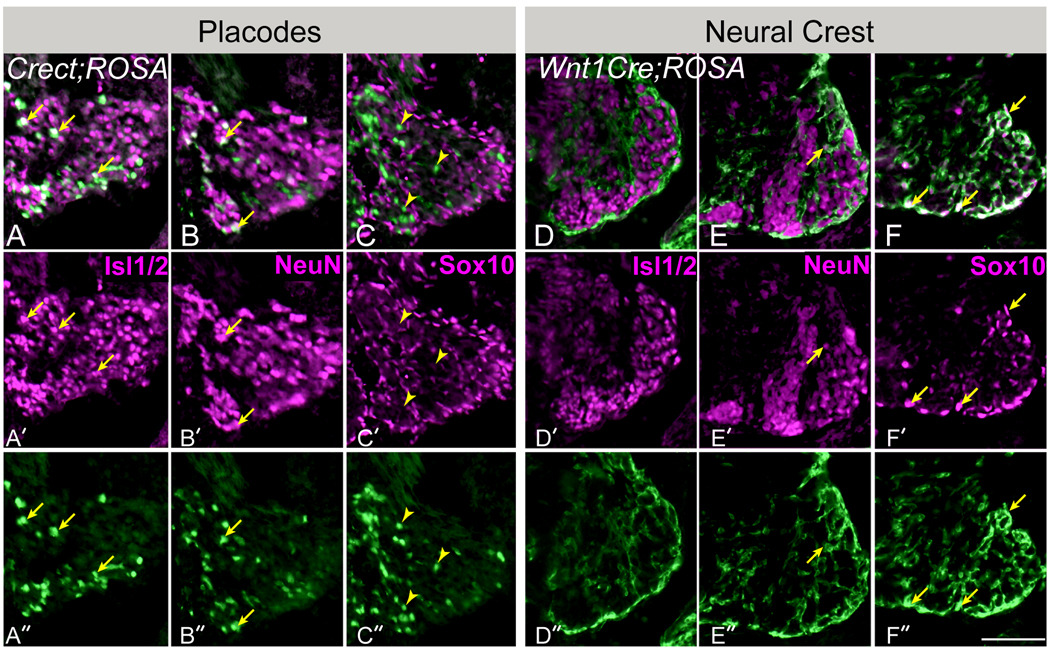
Figure 4. Epibranchial placodes contribute only neurons to the geniculate ganglia, while glial cells arise exclusively from neural crest.
(A–C). Horizontal sections of the geniculate ganglia in E11.5 Crect;ROSA embryos. (A) The early neuronal immunomarker Islet1/2 (purple, A, A’) labels many β–galactosidase labeled placode-derived cells (green) in Crect;ROSA embryos (arrows in A-A”). (B) An immunomarker of post-mitotic neurons, NeuN (purple, B, B’) also labels β–galactosidase –IR (green) placode-derived cells (arrows in B-B”). (C) Sox10, a glial cell immunomarker (purple, C, C’) does not co-localize with placodal cells labeled with β-galactosidase via Crect;ROSA (arrowheads in C-C” indicate examples of β-galactosidase immunoreactive cells that are not immunopositive for Sox10). (D–F) Horizontal sections of the geniculate ganglia in E11.5 Wnt1cre;ROSA embryos. (D,E) Most β-galactosidase labeled neural crest-derived cells (green) are negative for both neuronal markers (D; Islet1/2, and E; NeuN), although a few small-diameter neural crest cells are double immunopositive (arrow in E-E”). (F). Almost all Sox10-positive glial cells (purple, F-F”) were crest-derived, i.e., labeled with β-galactosidase (green, F-F”). Arrows in F-F” point out examples of double immunolabeled cells. Anterior is to the bottom right. Scale bar=25µm.
We next assessed if epibranchial placodes contribute glia to gVII, using Sox10 as a marker of both intraganglionic satellite and ensheathing glial cells (Ayer-Le Lievre and Le Douarin, 1982; Kelsh et al., 2000; Hanani, 2005). Placodally derived cells expressing the β-galactosidase were never double labeled by Sox10 antiserum (Fig. 4C, arrowheads indicate examples of β-galactosidase immunopositive and Sox10-immunonegative cells). Although the percentage of labeled placodal cells within gVII was somewhat low (on average ~10%), if placodal cells had given rise ganglionic glia, we would expect to have encountered at least some β-galactosidase positive cells that also express the glial marker Sox10 in one or more of the Crect;ROSA embryos, given the large number of Sox10-IR cells (Fig. 4C-C”, F-F”) found throughout in ganglion. However, we never encountered a single ganglion cell exhibiting this staining combination. Thus we infer in mammals, as in other vertebrates, that epibranchial placodes provide neurons but not glia to the cranial ganglia.
By contrast, in Wnt1Cre;ROSA embryos, most Sox10-IR ganglionic glial cells were also β-galactosidase-IR, indicating their neural crest origin (arrows, Fig. 4F). Moreover, crest-derived ganglion cells have a distinctly glial morphology, i.e., cells are large with thick processes. In addition, the majority of β-galactosidase-IR neural crest-derived cells were negative for both Islet1/2 (Fig. 4D) and NeuN (Fig. 4E), although infrequent double-labeled neural crest descendent neurons were evident (arrow, Fig. 4E), which likely are somatosensory neurons of the posterior auricular nerve of gVII (Boudreau et al., 1971; Semba et al., 1984; Harlow and Barlow, 2007). In sum, our results indicate that the neural crest cell type contribution to gVII in mice is primarily glial (arrows, Fig. 4F), as has been shown for geniculate ganglia in non-mammalian vertebrates.
Placode-derived neurons express TrkB and produce BDNF
Using antiserum specific for full-length TrkB (Rage et al., 2007), most cells within the geniculate ganglion at E11.5 express the BDNF receptor (purple, Fig. 5). At E11.5, placode-derived cells in Crect;ROSA ganglia (β-galactosidase-IR, green, Fig. 5A,C) are TrkB-IR (arrows indicate examples; Fig. 5A–C). By contrast, neural crest-derived cells within the body of Wnt1cre;ROSA ganglia (arrow, β-galactosidase-IR green, Fig. 5D–F) and neural crest derived glia at the ganglion periphery did not express TrkB (arrowheads, Fig. 5D–F). Thus, at the early stages of ganglion development, placode derived neurons, but not neural crest derived cells, express the active form of TrkB, and are poised to respond to BDNF.
Figure 5. TrkB is expressed by neurons derived from epibranchial placodes but not by neural crest-derived cells.
At E11.5, full-length TrkB receptor immunoreactivity is present on most cells within the geniculate ganglion of Crect;ROSA (A–C) and Wnt1Cre;ROSA (D–F) mice. (A–C). Placodal cells express β-galactosidase (green, A, C) in Crect;ROSA embryos, and these cells are also TrkB immunoreactive (purple, B, C). Arrows indicate examples of double-labeled cells. (D–F). In Wnt1Cre;ROSA embryos, neural crest descendent cells are β-galactosidase immunoreactive (green), and comprise predominantly glia (arrowheads) with a small number of neurons (arrow) which do not express TrkB (purple, E, F). Anterior is up. Scale bar = 25µm.
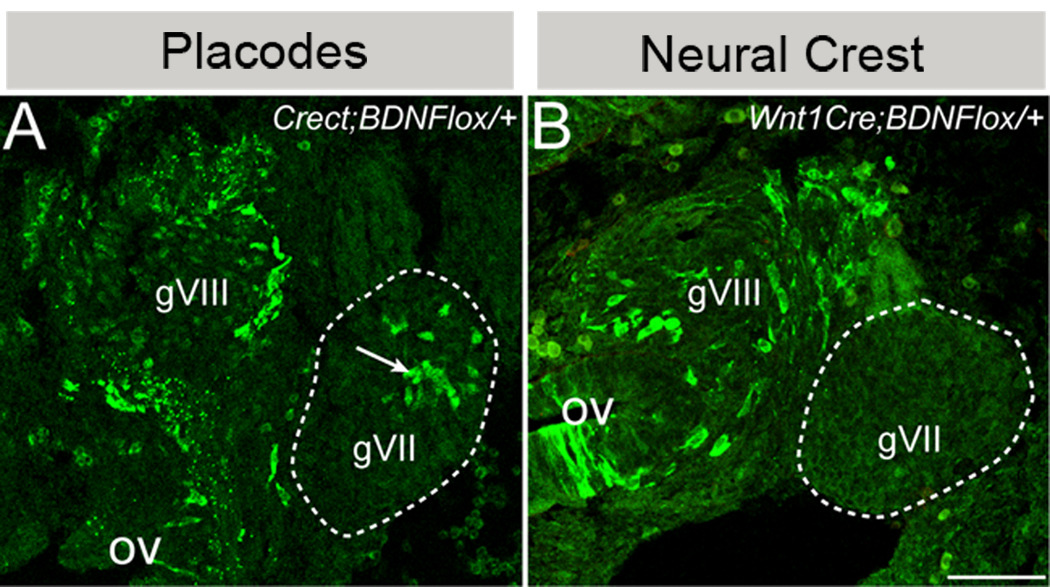
To determine which ganglion cells produce BDNF, those derived from placodes and/or neural crest, each Cre line was crossed with a floxed bdnf deletion/reporter allele (bdnflox/+, Gorski et al., 2003a; Gorski et al., 2003b; Baquet et al., 2005), which results in deletion of bdnf, as well as in lacZ expression, driven by the BDNF promoter in cells that have undergone Cre recombination. Unlike the ROSA reporter allele, which indelibly marks cells that have undergone Cre recombination as well as their daughters, β-galactosidase expression in Crect;bdnflox/+ mice is restricted to placodally derived cells actively producing BDNF (Baquet et al., 2005). Examination of Crect;bdnflox/+ embryos at E11.5 revealed that placodal cells within gVII produce BDNF (Fig. 6A). The expected frequency of β-galactosidase-IR neurons in Crect;bdnflox/+ ganglia should reflect the product of the frequencies of labeled placodal neurons in Crect;ROSA ( ~10%; Table 1) and BDNF expressing neurons (bdnflacZ, ~20%; Table 1), i.e., 2% if BDNF expression is not biased with respect to when neuroblasts emerge from the epibranchial placodes, i.e., before or after Crect is activated in placodal ectoderm. However, the proportion of BDNF-expressing placode derived cells was ~8% of neurons (Table 1, Crect;bdnflox/+), significantly greater than the expected labeling frequency (Chi squared 2-tailed, p<0.0001), suggesting that BDNF expression is more likely in later generated neurons after Cre activity has turned on in placodes of Crect embryos.
Figure 6. A subset of placode-derived geniculate ganglion cells produce BDNF, while neural crest-derived cells do not.
(A). In the geniculate ganglion of E11.5 Crect;bdnflox/+ embryos, BDNF-producing placodal cells express BDNF-lacZ and are labeled with anti-β-galalactosidase (green, arrow). (B). In E11.5 Wnt1cre;bdnflox/+ embryos, neural crest-derived cells in the geniculate ganglion (dashed outline) do not produce BDNF-lacZ and are not labeled via anti-β-galactosidase (green). However both gVIII and cells in the otic vesicle are β-galactosidase immunopositive. See text for details. Anterior is to the right. gVIII: acousticovestibular ganglion, ov: otic vesicle. gVII: geniculate ganglion is encircled with the dashed line. Anterior is to the right. Scale bar is 25µm.
Unlike placodal cells, cells in the geniculate ganglia of Wnt1Cre;bdnflox/+ embryos are not β-galactosidase-IR (Fig. 6B), indicating that neural crest-derived neurons and glial cells do not produce BDNF at this stage. Wnt1Cre-mediated BDNF-β-galactosidase is evident in the neighboring acousticovestibular ganglion and otic vesicle (gVIII and ov, Fig. 6B), which are not derived from neural crest, but express both Wnt1 (Echelard et al., 1994) and BDNF (Bianchi et al., 1996). Therefore, lack of β-galactosidase in gVII of Wnt1Cre embryos reflects a biologic phenomenon rather than a technical artifact of the genetic system.
Results of these genetic experiments exclude neural crest-derived cells as an early ganglionic source of BDNF. Further, neural crest-derived glia are not TrkB-IR (Fig. 5), indicating that ganglionic glial cells do not require BDNF at this stage of development. Consistent with this lack of TrkB expression, the distribution and number of Sox10-positive glial cells are not affected in bdnf KO embryos at E11.5 (data not shown). In contrast, placodal cells express TrkB and a subset of placodal neurons produce BDNF, suggesting that BDNF functions in an autocrine and/or paracrine manner to support maturation of ganglionic neurons.
Deletion of BDNF from placodal cells results in smaller nuclei of both immature and differentiating neurons
We next determined if loss of placode-derived BDNF within the ganglion accounted for the delayed maturation phenotype seen in bdnf KO mice at E11.5 (Fig. 2). Using the Crect line, we selectively deleted bdnf from placodal neuroblasts (Crect;bdnflox/neo; see methods section for the genetics of this cross), and assessed the ganglia from these placodally-deleted bdnf KO (Crect KO) embryos at E11.5. As expected, ganglion anatomy and volumes of Crect KO embryos were no different than those of bdnf KO or WT mice (Table 2); the number of immature and differentiated neurons in Crect KO ganglia also did not differ from bdnf KOs or WTs (Table 2).
While in bdnf KO embryos both immature (Islet1/2) neurons and mature (NeuN) neurons were smaller than wild type embryos, we found that in Crect KO embryos only the NeuN-IR population had a significantly smaller mean nuclear size (Table 2; Crect;bdnflox/neo). However, the nuclear size distributions of both neuronal populations of in Crect KO ganglia were shifted significantly to smaller sizes compared to WT (Fig. 7; NeuN-IR neurons (p<0.001), Islet-IR neurons (p<0.05), Mann-Whitney U test). Thus placodal deletion of BDNF closely resembles the global BDNF KO effect on neuronal maturation, despite early mosaicism of Cre activity in the Crect line (Table 1, Fig. 3). These results suggest that within gVII, placodal neurons provide local BDNF for early gustatory neuronal maturation, prior to their contact of peripheral targets.
Figure 7. Placode specific deletion of BDNF replicates the shift in neuronal size of the global bdnf KO.
The nuclear size of (A) Islet1/2 and (B) NeuN-immunoreactive neurons are significantly reduced in the geniculate ganglia of Crect;bdnflox/neo (CRECT KO) mice as compared to Crect;ROSA:bdnf+/+ (BDNF wt). (A) The nuclear size distribution of Islet1/2-IR neurons in placodally BDNF-deleted ganglia differed significantly from that of WT ganglia (Mann-Whitney U = 6039.5, n1 = n2 =120, P<0.05, two-tailed). (B) Nuclear diameters of NeuN-IR neurons were significantly smaller in the ganglia of placodally BDNF-deleted ganglia compared to that of WT (Mann-Whitney U = 4147.5, n1 = n2 =120, P<0.0001, two-tailed). Nuclear diameters (40 measurements per ganglion, n=3 ganglia for each genotype) were measured compared using a Mann-Whitney U test.
Discussion
In the taste system, BDNF is a key neurotrophin for proper development. To date, however, BDNF has been implicated primarily as a target derived survival and peripheral guidance factor. Here we show that BDNF is expressed in gustatory ganglia well before it is expressed in the targets of gustatory neurons, i.e., taste epithelia and gustatory hindbrain nuclei, and before taste afferents arrive at either target, suggesting an early, intraganglionic function for BDNF. We go on to show that this early function likely is local support of neuron maturation prior to target innervation. We hypothesize that when ganglionic BDNF is lost, the resulting maturational delay presages the subsequent death of these neurons (Liebl et al., 1997; Zhang et al., 1997; Patel and Krimm, 2010), as they fail to develop sufficiently by the time their axons reach their targets. We also elucidate here for the first time the precise cellular contributions of neural crest and epibranchial placodes to the VIIth cranial ganglion of mice: epibranchial placodes produce exclusively neurons, while cranial neural crest gives rise to all ganglionic glia, and a small population of sensory neurons, as occurs in cranial ganglia of non-mammalian vertebrates. (Landacre, 1910; 1912; 1921; 1931; 1933; Yntema, 1937; 1943; 1944; D'Amico-Martel and Noden, 1983; Le Douarin, 1984; Harlow and Barlow, 2007). Importantly, neurons that produce BDNF in gVII of mice are derived from the epibranchial placodes. As we have shown in amphibians that placodal neurons are exclusively gustatory in function (Harlow and Barlow 2007), these early BDNF-expressing taste sensory neurons in mouse embryos are precisely the population assumed to be dependent upon target-derived BDNF for survival at later stages (ElShamy and Ernfors, 1997; Ringstedt et al., 1999; Patel and Krimm, 2010).
Early BDNF supports maturation of gustatory neurons
Loss of BDNF results in significant sensory neuron death, when assessed well after these neurons have contacted their targets during embryogenesis (Jones et al., 1994; ElShamy and Ernfors, 1997; Liebl et al., 1997; Patel and Krimm, 2010). Given that the earliest expression of BDNF within the developing taste system occurs in the geniculate ganglia, we hypothesized that deletion of ganglionic BDNF would trigger cell death, and result in decreased neuron number and ganglion volume. However, at E11.5, neither ganglion size nor neuron number was reduced in bdnf KO embryos compared to WTs. Rather, gustatory neurons survive initially without ganglionic BDNF, suggesting that as development proceeds, additional sources of BDNF in the hindbrain and periphery are required for continued survival, or that ganglionic BDNF must be absent for a longer period of time before neuronal death occurs. Regardless, BDNF loss results in smaller neurons at E11.5, which may in turn set the stage for the widespread cell death (Deppmann et al., 2008) that occurs in gVII at E13.5 in bdnf KO mice (Patel and Krimm, 2010).
Specification of neuronal subtypes occurs as multipotent neuroblasts become fate-restricted in response to extracellular signals, including neurotrophins (Vicario-Abejón et al., 1995; Baker and Bronner-Fraser, 2000). Signaling by neurotrophins promotes the differentiation of specific subsets of neurons (Averbuch-Heller et al., 1994; Ghosh and Greenberg, 1995), and also affects sensory neuron maturation prior to the earliest detectable survival function (Ernsberger and Rohrer, 1988; Vogel and Davies, 1991; Wright et al., 1992; Deppmann et al., 2008). For example, exposure of cultured nodose (gX) ganglion neurons to BDNF causes an increase in cell size that precedes BDNF’s effect on neuron survival (Vogel, 1991). Similarly, our finding in gVII of mice of significantly smaller nuclei for both NeuN- and Islet1/2-labeled geniculate neurons in bdnf KOs is consistent with an early requirement of BDNF for neuron maturation (Miller and Peters, 1981; Roberts and Morey, 1985; Roberts et al., 1987).
In addition to serving as a measure of maturation, however, reduced nuclear size can be an early indicator of cell death (McConnell and Kaznowski, 1991; Villena et al., 1997), which includes chromatin condensation, followed by DNA fragmentation (Kroemer et al., 1995). Although we did not observe pycnotic or apoptotic nuclei in bdnf KO ganglia (data not shown), some of the smaller sensory neuron nuclei we tallied in bdnf KO embryos may have been in early stages of nuclear condensation ahead of cell death. However, previous studies of bdnf KO mice did not detect cell death within the ganglion at E12.5; significant cell death was first detected at E13.5 using activated casapse-3-IR as a marker (Patel and Krimm, 2010), suggesting that apoptosis occurs only at later stages despite the early absence of BDNF. Nonetheless the reduction in nuclear size at E11.5 may presage this death as cells fail to mature without proper neurotrophin supply, and for this reason we can not disentangle a requirement for BDNF in maturation from an early survival requirement.
That deletion of BDNF did not result in a significant reduction in ganglion cell number at E11.5 (Table 2) also suggests that other neurotrophin(s) may support sensory neuron survival early on. For example, gVII contains both NT4- and NT3- dependent neuronal populations, although the precise timing of the requirement for these neurotrophins is not known (Ernfors et al., 1994; Jones et al., 1994; Conover et al., 1995; Liu et al., 1995; Liebl et al., 1997). Hence, NT4 and NT3 may provide early support to specific cell populations in gVII, and these neurons would thus be unaffected by loss of BDNF early on; only later would BDNF become required for neuronal survival. There is evidence for this scenario in the petrosal-nodose ganglia, where survival of sensory neurons is initially supported by NT3, then NT4 and then BDNF (ElShamy and Ernfors, 1997). A similar sequence in trophic support remains to be demonstrated for the geniculate ganglion.
Neural crest and epibranchial placodes contributions to cranial sensory ganglia are evolutionarily conserved
Both cranial neural crest and epibranchial placodes contribute to the cranial sensory ganglia of chick and amphibia (axolotl: Stone, 1922; Stone, 1924; Landacre, 1931; Landacre, 1933; Barlow and Northcutt, 1995; Northcutt and Brändle, 1995; Harlow and Barlow, 2007); (chick: Yntema, 1944; Hamburger, 1961; Ayer-Le Lievre and Le Douarin, 1982; D'Amico-Martel and Noden, 1983; Lwigale, 2001). Thus, our detection of BDNF expression in early cranial ganglia raised the question of which of these embryonic cell population(s) express BDNF and which were able to respond to it, i.e., express TrkB. Previous studies of rodents have tracked neural crest and placodal cells to the ganglia and revealed early distribution patterns similar to those of birds and amphibians (Adelmann, 1925; Halley, 1955; Hatini et al., 1999). However, initial cytological differences between the two embryonic cell types become indistinguishable once neuroblasts coalesce within the ganglia (Adelmann, 1925; Halley, 1955). Similarly, the genetic marker BF-1 (Foxg1) used to drive lacZ expression in epibranchial placodes no longer reports in the ganglia after E10.5, preventing the later study of placode-derived cells (Hatini et al., 1999). Moreover, Foxg1-lacZ is a reporter of regulated gene expression, i.e., Foxg1, which while informative, does not allow for concise lineage tracing.
Using conditional genetic tools, we now have mapped the derivatives of epibranchial placodes and neural crest in the cranial ganglia of mice. We employed a newly developed ectoderm specific Cre line, Crect (Yang et al., in prep) to show that placode-derived cells within the geniculate ganglia of mice give rise exclusively to neurons. In contrast, fate mapping in Wnt1Cre;ROSA embryos revealed that a small number of neurons, but all glia, of the mouse geniculate ganglion arise from neural crest, as in non-mammalian vertebrates.
Despite mosaicism with respect to the onset of ectodermal Cre activity, the Crect line proved, nonetheless, to be the best genetic tool for fate mapping epibranchial placodes. In pilot experiments with other Cre lines, we found that none were as efficacious in activating Cre in placodal cells as Crect. For example, cytokeratin 14 (K14) lines (Dassule et al., 2000; Andl et al., 2004) initiate Cre expression in cranial ectoderm well after genesis of neuroblasts from placodes, while variable activation of Cre in early head ectoderm in the Foxg1-Cre line (Hebert and McConnell, 2000) resulted in extremely inconsistent labeling of cells in gVII (data not shown). Other lines, such as AP2alphaIRESCre or Emx1Cre (Abel et al., 2001; Bluher et al., 2002; Gorski et al., 2002) drive Cre activity in epibranchial placodes, as well as in neural crest and/or the central nervous system, which would have confounded our analysis.
Early support of geniculate neurons is via BDNF produced by placodal neurons
Using Crect and BDNF reporter lines, we ascertained that only a subset of epibranchial placode-derived sensory neurons produce BDNF in the early geniculate ganglion. However, all gVII neurons appear to express TrkB, so that BDNF produced by a subpopulation of ganglion cells could support development of all neurons via paracrine and perhaps autocrine mechanisms, or that some of these TrkB-expressing cells are supported by NT4, which also binds TrkB. Studies of intraganglionic neurotrophic support have focused on the dorsal root ganglia (DRG, Ernsberger and Rohrer, 1988; Wright et al., 1992; Robinson et al., 1996), where maturation of DRG neurons, which are derived entirely from neural crest, depends upon BDNF at a stage prior to axon outgrowth or target contact, and before DRG neurons become dependent on target-derived BDNF for survival (Wright et al., 1992). In our studies, we find that ganglionic BDNF is likewise required for early maturation of gVII sensory neurons. Interestingly, we find that BDNF expression is biased to neurons that emerge from the placode somewhat later, once Cre is active in the placodes in Crect mice. These findings raise the intriguing question as to potential differences in developmental outcomes of neurons that receive paracrine BDNF signals, versus those that produce and thus receive autocrine BDNF.
Experimental Procedures
Fate mapping of cells in cranial sensory ganglia
All animal procedures were conducted with the approval of the University of Colorado Institutional Animal Care and Use Committee. Mice used in this study had a mixed 129/J and C57Bl/6 background unless otherwise stated. BdnflacZ/+ (Bennett et al., 1999) and ROSA26R (Soriano, 1999) transgenic lines have been described previously. Wnt1Cre mice exhibit Cre-mediated loxP recombination in neural crest cells (Danielian et al., 1998) and were bred with ROSA mice to label all neural crest cells. To label placodal cells, Crect mice (FVB background) were bred with ROSA mice. The Crect allele comprises an ectodermal enhancer of Tcfap2a to drive Cre expression specifically in the ectoderm, and will be described elsewhere (Yang, Melvin and Williams, In prep). To determine which cells produce BDNF within the ganglia, Crect or Wnt1Cre mice were crossed with mice carrying a floxed allele of bdnf (Gorski et al., 2003a; 2003b; Baquet et al., 2005) in which Cre in bdnf-expressing cells results in bdnf deletion and β-galactosidase production.
Generation of global, placode-restricted or neural crest-restricted BDNF null mice
To generate bdnf null mice, bdnfneo/+ mice carrying a null allele with a neomycin cassette in place of the BDNF coding exon (Jones et al., 1994) were bred with bdnflacZ/+ knock-in mice resulting in bdnflacZ/neo mice which have two null alleles of bdnf, and produce β-galactosidase from one allele. To selectively delete bdnf from placode-derived cells, Crect mice were bred with bdnfneo/+ mice to generate Crect;bdnfneo/+ mice, which were then bred with bdnflox/+ mice. Offspring with all three transgenes (Crect;bdnflox/neo) as determined by PCR are null for BDNF in placode-derived cells expressing Crect. Similarly, Wnt1Cre;bdnfneo/+ mice were bred with bdnflox/+ mice to selectively delete BDNF from neural crest cells (Wnt1Cre;bdnflox/neo). All comparisons were made between mutant littermates and wild type controls (n=3, unless noted).
Embryo and tissue collection
The morning of a vaginal plug was considered embryonic day (E) 0.5. Timed pregnant mice were killed by cervical dislocation, and at E9.0–14.5 embryos were dissected out in PBS and heads fixed in 0.2% paraformaldehyde (PFA) in 0.1M phosphate buffer (PB) overnight at 4°C or 2% PFA for 2 hours at 4°C. Unfixed torsos were used for DNA extraction and PCR genotyping. Heads were sunk in 20% sucrose in PB, embedded in OCT (TissueTek), and cryosectioned at 14µm from rostral to caudal in the horizontal plane. Sections were processed for immunofluorescence as described below. In some cases, tongues were dissected prior to fixation and reacted with Xgal (Thirumangalathu et al., 2009).
Immunohistochemistry
Tissue sections were blocked in 5% normal goat serum in 0.1M phosphate-buffed saline (PBS) for 2 hours at room temperature. Primary antisera were diluted in 0.1M PBS+1% BSA+0.3% TritonX-100: mouse anti-neurofilament (1:1000, 2H3, Developmental Studies Hybridoma Bank, DSHB, University of Iowa), guinea pig anti-β-galactosidase (1:1000, gift from T. Finger, University of Colorado, School of Medicine; Yee et al, 2003) or rabbit anti-β-galactosidase (1:1000, MP Biomedicals), mouse anti-NeuN (1:1000, Chemicon), mouse anti-Islet1/2 (1:200, 39.4.D5, DSHB), goat anti-Sox10 (1:200, Santa Cruz Biotech) and rabbit anti-TrkB (1:400, Santa Cruz Biotech) overnight at 4°C. The M.O.M. kit (Vector Labs) was used with NeuN and Islet antibodies. Sections were rinsed in 0.1M PBS, and the appropriate fluorescently labeled secondary antiserum (conjugated with Alexa-546 or Alexa-488, Molecular Probes) was used at 1:600 in 0.1M PBS for 2 hours at room temperature. Sections were nuclear counterstained with Hoechst 33258 (Molecular Probes) diluted 1:10,000 in 0.1M phosphate buffer (PB), rinsed in PB and mounted with Fluormount G (Southern Biotech). For Xgal staining, tongues were collected from embryos at E12.5–14.5, stained with filtered Xgal solution (5µg/ml) at 37°C. Tongues were then post-fixed in 4% PFA for 2 hours at RT.
Image acquisition
Multichannel fluorescent images were acquired sequentially via single channel excitation using an Axioplan fluorescence microscope with an Axiocam CCD camera and Axiovision software (Zeiss). Z-stack confocal images were acquired at 0.7 or 1.0µm intervals through 14µm cryosections on an Olympus Fluoview confocal laser scanning microscope and Fluoview version 4.0, or Olympus IX81 inverted microscope with spinning disk attachment and Slidebook (Intelligent Imaging, Colorado, USA). Images were saved as TIFFs, contrast adjusted and cropped, and figures compiled with Adobe Photoshop CS for Macintosh.
Ganglion cell counts
Sequential images of each ganglion were opened in ImageJ v1.41 (http://rsb.info.nih.gov), and tiled to reconstruct each ganglion from rostral to caudal. Ganglion VII volume was calculated by measuring the cross sectional area of the ganglion in each section, multiplying by the section thickness and summing the total sectional volumes. Neuronal numbers were determined with the Cell Counter plug-in for ImageJ by counting in every fourth section all NeuN- or Islet1/2-IR cell profiles possessing Hoechst-positive nuclei with diameters >4µm. Total neuron number per ganglion was estimated by multiplying the number of cells per section by 4. This number (n) was then multiplied by the Abercrombie correction factor [T/(T+D)] so that total number of neurons (N) = n × [T/(T+D)], where T is section thickness and D is average nuclear diameter (Abercrombie, 1946). The average nuclear diameter for each cell type was based on the nuclear measurements of 40 randomly selected NeuN-, Islet1/2- or β-galactosidase-IR neurons per ganglion using ImageJ. For nuclear measurements of neurons in each section, the most dorsomedial (top left) neuron was assigned the number 1 and nuclei were then enumerated in rows, left to right, and top to bottom. Forty neurons were selected by a random number generator, measured and used to calculate the average nuclear diameter. The Abercrombie correction factor was calculated for each ganglion and total numbers and mean diameters of each cell population were compared across genotypes.
Statistics
For statistical comparisons of ganglion volume, neuron number, and nuclear size across and within groups, a one-tailed, one factor ANOVA was used with a post hoc Tukey-Kramer test. For comparison of the size distribution of nuclei of Islet1/2 and NeuN neurons, which did not fit a normal distribution, the non-parametric Mann-Whitney U test was used. In all cases, significance was taken as p<0.05. A two-tailed Chi squared test was used to determine the difference between expected versus observed β-galactosidase labeling frequency of neurons in Crect;bdnflox/+ ganglia (Table 1).
Acknowledgements
We thank Kevin Jones (University of Colorado Boulder) for BDNF transgenic lines, David Clouthier (University of Colorado School of Dental Medicine) for Wnt1Cre mice, and Brooke Baxter for genotyping. In addition, we thank Tom Finger (University of Colorado School of Medicine) and Kristin Artinger (University of Colorado School of Dental Medicine) for helpful discussions and editorial critique of the manuscript. The anti-neurofilament antibody (2H3) developed by J. Dodd and T.M. Jessell and the 39.4D5 antibody to Islet proteins developed by S. Brenner-Morton and T.M. Jessell were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. This work was supported by the Rocky Mountain Taste and Smell Center Core Facilities (NIDCD P30 DC004657) as well as an NRSA DC007796 to DEH, R01 DE12728 to TW, and R01s DC003947 & DC008373 to LAB.
References
- Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;95:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- Adelmann HB. The development of the neural folds and cranial ganglia of the rat. J Comp Neurol. 1925;39:19–171. [Google Scholar]
- Altman J, Bayer SA. Development of the cranial nerve ganglia and related nuclei in the rat. Advances in anatomy, embryology, and cell biology. 1982;74:1–90. doi: 10.1007/978-3-642-68479-1. [DOI] [PubMed] [Google Scholar]
- Alvarez-Borda B, Haripal B, Nottebohm F. Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons. Proc Natl Acad Sci USA. 2004;101:3957–3961. doi: 10.1073/pnas.0308118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, 3rd, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Arvidsson J, Fundin BT, Pfaller K. Innervation of the hard palate in the rat studied by anterograde transport of horseradish peroxidase conjugates. J Comp Neurol. 1995;351:489–498. doi: 10.1002/cne.903510402. [DOI] [PubMed] [Google Scholar]
- Averbuch-Heller L, Pruginin M, Kahane N, Tsoulfas P, Parada L, Rosenthal A, Kalcheim C. Neurotrophin 3 stimulates the differentiation of motoneurons from avian neural tube progenitor cells. Proc Natl Acad Sci USA. 1994;91:3247–3251. doi: 10.1073/pnas.91.8.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer-Le Lievre CS, Le Douarin NM. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Developmental Biology. 1982;94:291–310. doi: 10.1016/0012-1606(82)90349-9. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Establishing neuronal identity in vertebrate neurogenic placodes. Development. 2000;127:3045–3056. doi: 10.1242/dev.127.14.3045. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Developmental Biology. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Bickford PC, Jones KR. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow LA, Northcutt RG. Embryonic origin of amphibian taste buds. Developmental Biology. 1995;169:273–285. doi: 10.1006/dbio.1995.1143. [DOI] [PubMed] [Google Scholar]
- Begbie J. Migration of neuroblasts from neurogenic placodes. Dev Neurosci. 2008;30:33–35. doi: 10.1159/000109849. [DOI] [PubMed] [Google Scholar]
- Begbie J, Ballivet M, Graham A. Early steps in the production of sensory neurons by the neurogenic placodes. Mol Cell Neurosci. 2002;21:502–511. doi: 10.1006/mcne.2002.1197. [DOI] [PubMed] [Google Scholar]
- Begbie J, Graham A. Integration between the epibranchial placodes and the hindbrain. Science. 2001;294:595–598. doi: 10.1126/science.1062028. [DOI] [PubMed] [Google Scholar]
- Bennett JL, Zeiler SR, Jones KR. Patterned expression of BDNF and NT-3 in the retina and anterior segment of the developing mammalian eye. Invest Ophthalmol Vis Sci. 1999;40:2996–3005. [PubMed] [Google Scholar]
- Bianchi L, Conover J, Fritzsch B, DeChiara T, Lindsay R, Yancopoulos G. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122:1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- Bonde C, Noraberg J, Zimmer J. Nuclear shrinkage and other markers of neuronal cell death after oxygen-glucose deprivation in rat hippocampal slice cultures. Neurosci Lett. 2002;327:49–52. doi: 10.1016/s0304-3940(02)00382-8. [DOI] [PubMed] [Google Scholar]
- Bosco A, Linden R. BDNF and NT-4 Differentially Modulate Neurite Outgrowth in Developing Retinal Ganglion Cells. J Neurosci Res. 1999;57:759–769. [PubMed] [Google Scholar]
- Boudreau JC, Bradley BE, Bierer PR, Kruger S, Tsuchitani C. Single unit recordings from the geniculate ganglion of the facial nerve of the cat. Exp Brain Res. 1971;13:461–488. doi: 10.1007/BF00234278. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes & Development. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- Carr VM, Sollars SI, Farbman AI. Neuronal cell death and population dynamics in the developing rat geniculate ganglion. Neuroscience. 2005;134:1301–1308. doi: 10.1016/j.neuroscience.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Chan JP, Cordeira J, Calderon GA, Iyer LK, Rios M. Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol Cell Neurosci. 2008;39:373–383. doi: 10.1016/j.mcn.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc Natl Acad Sci USA. 2001;98:14661–14668. doi: 10.1073/pnas.251543398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Byers MR. Sensory nerve endings of the incisive papilla of rat hard palate studied by peroxidase cytochemical methods. J Comp Neurol. 1985;234:192–200. doi: 10.1002/cne.902340206. [DOI] [PubMed] [Google Scholar]
- Cho TT, Farbman AI. Neurotrophin receptors in the geniculate ganglion. Brain Res Mol Brain Res. 1999;68:1–13. doi: 10.1016/s0169-328x(99)00006-6. [DOI] [PubMed] [Google Scholar]
- Clarke PGH. Neuronal death in the development of the vertebrate nervous system. Trends Neurosci. 1985;8:345–349. [Google Scholar]
- Clevenger AC, Salcedo E, Jones KR, Restrepo D. BDNF promoter-mediated beta-galactosidase expression in the olfactory epithelium and bulb. Chem Senses. 2008;33:531–539. doi: 10.1093/chemse/bjn021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- D'Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian cranial peripheral ganglia. American Journal of Anatomy. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Davies AM. Neurotrophin switching: where does it stand? Curr Opin Neurobiol. 1997;7:110–118. doi: 10.1016/s0959-4388(97)80128-6. [DOI] [PubMed] [Google Scholar]
- Davies AM, Lumsden A. Relation of target encounter and neuronal death to nerve growth factor responsiveness in the developing mouse trigeminal ganglion. J Comp Neurol. 1984;223:124–137. doi: 10.1002/cne.902230110. [DOI] [PubMed] [Google Scholar]
- Davies D. Temporal and spatial regulation of alpha6 integrin expression during the development of the cochlear-vestibular ganglion. J Comp Neurol. 2007;502:673–682. doi: 10.1002/cne.21302. [DOI] [PubMed] [Google Scholar]
- Deppmann CD, Mihalas S, Sharma N, Lonze BE, Niebur E, Ginty DD. A model for neuronal competition during development. Science. 2008;320:369–373. doi: 10.1126/science.1152677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y, Vassileva G, McMahon AP. Cis-acting regulatory sequences governing Wnt-1 expression in the developing mouse CNS. Development. 1994;120:2213–2224. doi: 10.1242/dev.120.8.2213. [DOI] [PubMed] [Google Scholar]
- ElShamy WM, Ernfors P. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 complement and cooperate with each other sequentially during visceral neuron development. J Neurosci. 1997;17:8667–8675. doi: 10.1523/JNEUROSCI.17-22-08667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells Expressing mRNA for Neurotrophins and their Receptors During Embryonic Rat Development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Rohrer H. Neuronal precursor cells in chick dorsal root ganglia: differentiation and survival in vitro. Developmental Biology. 1988;126:420–432. doi: 10.1016/0012-1606(88)90151-0. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Brann JH, Rozenblat A, Rochlin MW, Weiler E, Bhattacharyya M. Developmental expression of neurotrophin receptor genes in rat geniculate ganglion neurons. J Neurocytol. 2004;33:331–343. doi: 10.1023/B:NEUR.0000044194.71426.ee. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Mbiene JP. Early development and innervation of taste bud-bearing papillae on the rat tongue. J Comp Neurol. 1991;304:172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Sarai PA, Barbacid M, Silos-Santiago I. Mice with a targeted disruption of the neurotrophin receptor trkB lose their gustatory ganglion cells early but do develop taste buds. Int J Dev Biol. 1997a;15:563–576. doi: 10.1016/s0736-5748(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997b;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003a;121:341–354. doi: 10.1016/s0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003b;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JB, Gottlieb AA, Barlow LA. Gustatory neurons derived from epibranchial placodes are attracted to, and trophically supported by, taste bud-bearing endoderm in vitro. Developmental Biology. 2003;264:467–481. doi: 10.1016/j.ydbio.2003.08.024. [DOI] [PubMed] [Google Scholar]
- Halley G. The placodal relations of the neural crest in the domestic cat. J Anat. 1955;89:133–152. [PMC free article] [PubMed] [Google Scholar]
- Hamburger V. Experimental analysis of the dual origin of the trigeminal ganglion in the chick embryo. J Exp Zool. 1961;148:91–123. doi: 10.1002/jez.1401480202. [DOI] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Research Reviews. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Harlow DE, Barlow LA. Embryonic origin of gustatory cranial sensory neurons. Developmental Biology. 2007;310:317–328. doi: 10.1016/j.ydbio.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatini V, Ye X, Balas G, Lai E. Dynamics of placodal lineage development revealed by targeted transgene expression. Dev Dyn. 1999;215:332–343. doi: 10.1002/(SICI)1097-0177(199908)215:4<332::AID-AJA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Developmental Biology. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Hu B, Nikolakopoulou AM, Cohen-Cory S. BDNF stabilizes synapses and maintains the structural complexity of optic axons in vivo. Development. 2005;132:4285–4298. doi: 10.1242/dev.02017. [DOI] [PubMed] [Google Scholar]
- Jacobsen KD, Willumsen BM. Kinetics of expression of inducible beta-galactosidase in murine fibroblasts: high initial rate compared to steady-state expression. J Mol Biol. 1995;252:289–295. doi: 10.1006/jmbi.1995.0496. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke K, Buchner M, Ludolph AC, Riepe MW. Nuclear shrinkage in live mouse hippocampal slices. Acta Neuropathol. 2001;101:483–490. doi: 10.1007/s004010000317. [DOI] [PubMed] [Google Scholar]
- Kelsh RN, Dutton K, Medlin J, Eisen JS. Expression of zebrafish fkd6 in neural crest-derived glia. Mech Dev. 2000;93:161–164. doi: 10.1016/s0925-4773(00)00250-1. [DOI] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm RF, Miller KK, Kitzman PH, Davis BM, Albers KM. Epithelial overexpression of BDNF or NT4 disrupts targeting of taste neurons that innervate the anterior tongue. Developmental Biology. 2001;232:508–521. doi: 10.1006/dbio.2001.0190. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Petit P, Zamzami N, Vayssiere JL, Mignotte B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. Neural crest cell dynamics revealed by time-lapse video microscopy of whole embryo chick explant cultures. Developmental Biology. 1998;204:327–344. doi: 10.1006/dbio.1998.9082. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127:1161–1172. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- Landacre FL. The origin of the cranial ganglia in Ameiurus. J Comp Neurol. 1910;20:309–411. [Google Scholar]
- Landacre FL. The epibranchial placodes of Lepidosteus osseus and their relation to the cerebral ganglia. J Comp Neurol. 1912;22:1–69. [Google Scholar]
- Landacre FL. The fate of the neural crest in the head of urodeles. J Comp Neurol. 1921;33:1–44. [Google Scholar]
- Landacre FL. Data on the relative time of formation of the cerebral ganglia of Amblystoma jeffersonianum. J Comp Neurol. 1931;53:205–224. [Google Scholar]
- Landacre FL. The epibranchial placode of the facial nerve in Amblystroma jeffersonianum. J Comp Neurol. 1933;58:289–309. [Google Scholar]
- Le Douarin NM. Ontogeny of the peripheral nervous system from the neural crest and the placodes. A developmental model studied on the basis of the quail-chick chimaera system. Harvey Lect. 1984;80:137–186. [PubMed] [Google Scholar]
- Lebrun B, Bariohay B, Moyse E, Jean A. Brain-derived neurotrophic factor (BDNF) and food intake regulation: A minireview. Autonomic Neuroscience: Basic and Clinical. 2006 doi: 10.1016/j.autneu.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375:238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez GF, Krimm RF. Epithelial overexpression of BDNF and NT4 produces distinct gustatory axon morphologies that disrupt initial targeting. Developmental Biology. 2006;292:457–468. doi: 10.1016/j.ydbio.2006.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lwigale PY. Embryonic origin of avian corneal sensory nerves. Developmental Biology. 2001;239:323–337. doi: 10.1006/dbio.2001.0450. [DOI] [PubMed] [Google Scholar]
- Ma L, Lopez GF, Krimm RF. Epithelial-derived brain-derived neurotrophic factor is required for gustatory neuron targeting during a critical developmental period. J Neurosci. 2009;29:3354–3364. doi: 10.1523/JNEUROSCI.3970-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MR, Mason CA. The seventh cranial nerve of the rat. Visualization of efferent and afferent pathways by cobalt precipitation. Brain Res. 1977;121:21–41. doi: 10.1016/0006-8993(77)90436-x. [DOI] [PubMed] [Google Scholar]
- Martinez A, Alcantara S, Borrell V, Del Rio JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I, Soriano E. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature Neuroscience. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Matei VA, Feng F, Pauley S, Beisel KW, Nichols MG, Fritzsch B. Near-infrared laser illumination transforms the fluorescence absorbing X-Gal reaction product BCI into a transparent, yet brightly fluorescent substance. Brain Res Bull. 2006;70:33–43. doi: 10.1016/j.brainresbull.2005.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbiene J-P. Taste placodes are primary targets of geniculate but not trigeminal sensory axons in mouse developing tongue. J Neurocytol. 2004;33:617–629. doi: 10.1007/s11068-005-3331-1. [DOI] [PubMed] [Google Scholar]
- Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 1997;160:139–158. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- Miller M, Peters A. Maturation of rat visual cortex. II. A combined Golgi-electron microscope study of pyramidal neurons. J Comp Neurol. 1981;203:555–573. doi: 10.1002/cne.902030402. [DOI] [PubMed] [Google Scholar]
- Mistretta CM, Goosens KA, Farinas I, Reichardt LF. Alterations in size, number, and morphology of gustatory papillae and taste buds in BDNF null mutant mice demonstrate neural dependence of developing taste organs. J Comp Neurol. 1999;409:13–24. [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nagtegaal ID, Lakke EA, Marani E. Trophic and tropic factors in the development of the central nervous system. Arch Physiol Biochem. 1998;106:161–202. doi: 10.1076/apab.106.3.161.4380. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Brändle K. Development of branchiomeric and lateral line nerves in the axolotl. J Comp Neurol. 1995;355:427–454. doi: 10.1002/cne.903550309. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Blomlöf J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbances, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Ebendal T, Olson L. Differential expression of brain-derived neurotrophic factor and neurotrophin 3 mRNA in lingual papillae and taste buds indicates roles in gustatory and somatosensory innervation. J Comp Neurol. 1996;376:587–602. doi: 10.1002/(SICI)1096-9861(19961223)376:4<587::AID-CNE7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Olson L. Brain-derived neurotrophic factor mRNA is expressed in the developing taste bud-bearing tongue papillae of rat. J Comp Neurol. 1995;360:698–704. doi: 10.1002/cne.903600413. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Naturally occurring cell death during neural development. Trends Neurosci. 1985;8:487–493. [Google Scholar]
- Patel AV, Krimm RF. BDNF is required for the survival of differentiated geniculate ganglion neurons. Developmental Biology. 2010;340:419–429. doi: 10.1016/j.ydbio.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rage F, Silhol M, Biname F, Arancibia S, Tapia-Arancibia L. Effect of aging on the expression of BDNF and TrkB isoforms in rat pituitary. Neurobiol Aging. 2007;28:1088–1098. doi: 10.1016/j.neurobiolaging.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Righi M, Tongiorgi E, Cattaneo A. Brain-derived neurotrophic factor (BDNF) induces dendritic targeting of BDNF and tyrosine kinase B mRNAs in hippocampal neurons through a phosphatidylinositol-3 kinase-dependent pathway. J Neurosci. 2000;20:3165–3174. doi: 10.1523/JNEUROSCI.20-09-03165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringstedt T, Ibáñez CF, Nosrat CA. Role of brain-derived neurotrophic factor in target invasion in the gustatory system. J Neurosci. 1999;19:3507–3518. doi: 10.1523/JNEUROSCI.19-09-03507.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- Roberts WE, Fielder PJ, Rosenoer LM, Maese AC, Gonsalves MR, Morey ER. Nuclear morphometric analysis of osteoblast precursor cells in periodontal ligament, SL-3 rats. Am J Physiol. 1987;252:R247–R251. doi: 10.1152/ajpregu.1987.252.2.R247. [DOI] [PubMed] [Google Scholar]
- Roberts WE, Morey ER. Proliferation and differentiation sequence of osteoblast histogenesis under physiological conditions in rat periodontal ligament. Am J Anat. 1985;174:105–118. doi: 10.1002/aja.1001740202. [DOI] [PubMed] [Google Scholar]
- Robinson M, Buj-Bello A, Davies AM. Paracrine interactions of BDNF involving NGF-dependent embryonic sensory neurons. Mol Cell Neurosci. 1996;7:143–151. doi: 10.1006/mcne.1996.0011. [DOI] [PubMed] [Google Scholar]
- Rochlin MW, O'Connor R, Giger RJ, Verhaagen J, Farbman AI. Comparison of neurotrophin and repellent sensitivities of early embryonic geniculate and trigeminal axons. J Comp Neurol. 2000;422:579–593. [PubMed] [Google Scholar]
- Saucedo LJ, Edgar BA. Why size matters: altering cell size. Curr Opin Genet Dev. 2002;12:565–571. doi: 10.1016/s0959-437x(02)00341-6. [DOI] [PubMed] [Google Scholar]
- Sautter J, Sabel M, Sommer C, Strecker S, Weidner N, Oertel WH, Kiessling M. BDNF and TrkB expression in intrastriatal ventral mesencephalic grafts in a rat model of Parkinson's disease. J Neural Transm. 1998;105:253–263. doi: 10.1007/s007020050054. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Scott L, Atkinson ME. Target pioneering and early morphology of the murine chorda tympani. J Anat. 1998;192(Pt 1):91–98. doi: 10.1046/j.1469-7580.1998.19210091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba K, Sood V, Shu NY, Nagele RG, Egger MD. Examination of geniculate ganglion cells contributing sensory fibers to the rat facial 'motor' nerve. Brain Res. 1984;308:354–359. doi: 10.1016/0006-8993(84)91077-1. [DOI] [PubMed] [Google Scholar]
- Shiau CE, Lwigale PY, Das RM, Wilson SA, Bronner-Fraser M. Robo2-Slit1 dependent cell-cell interactions mediate assembly of the trigeminal ganglion. Nature Neuroscience. 2008;11:269–276. doi: 10.1038/nn2051. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stone L. Experiments on the Development of the Cranial Ganglia and the Lateral Line Sense Organs in Amblystoma Punctatum. Journal Experimental Zoology. 1922;35:421–496. [Google Scholar]
- Stone LM. Experiments on the Transplantation of Placodes of the Cranial Ganglia in the Amphibian Embryo. I. Heterotopic transplantations of the ophthalmic placode upon the head of Amblystoma punctatum. J Comp Neurol. 1924;38:73–105. [Google Scholar]
- Sun Y, Dykes IM, Liang X, Eng SR, Evans SM, Turner EE. A central role for Islet1 in sensory neuron development linking sensory and spinal gene regulatory programs. Nature Neuroscience. 2008;11:1283–1293. doi: 10.1038/nn.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Bianco F, Blanchard MP, Bergami M, Canossa M, Scarfone E, Matteoli M. Cross talk between vestibular neurons and Schwann cells mediates BDNF release and neuronal regeneration. Brain Cell Biol. 2006;35:187–201. doi: 10.1007/s11068-007-9011-6. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejón C, Johe KK, Hazel TG, Collazo D, McKay RD. Functions of basic fibroblast growth factor and neurotrophins in the differentiation of hippocampal neurons. Neuron. 1995;15:105–114. doi: 10.1016/0896-6273(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Villena A, Diaz F, Requena V, Chavarria I, Rius F, Perez de Vargas I. Quantitative morphological changes in neurons from the dorsal lateral geniculate nucleus of young and old rats. Anat Rec. 1997;248:137–141. doi: 10.1002/(SICI)1097-0185(199705)248:1<137::AID-AR16>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Vogel KS, Davies AM. The duration of neurotrophic factor independence in early sensory neurons is matched to the time course of target field innervation. Neuron. 1991;7:819–830. doi: 10.1016/0896-6273(91)90284-7. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Frank ME. Anatomy of the gustatory system in the hamster: central projections of the chorda tympani and the lingual nerve. J Comp Neurol. 1983;220:378–395. doi: 10.1002/cne.902200403. [DOI] [PubMed] [Google Scholar]
- Whitehead MC, Kachele DL. Development of fungiform papillae, taste buds, and their innervation in the hamster. J Comp Neurol. 1994;340:515–530. doi: 10.1002/cne.903400405. [DOI] [PubMed] [Google Scholar]
- Wright EM, Vogel KS, Davies AM. Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron. 1992;9:139–150. doi: 10.1016/0896-6273(92)90229-7. [DOI] [PubMed] [Google Scholar]
- Yang X, Zhou Y, Barcarse EA, O'Gorman S. Altered neuronal lineages in the facial ganglia of Hoxa2 mutant mice. Developmental Biology. 2008;314:171–188. doi: 10.1016/j.ydbio.2007.11.032. [DOI] [PubMed] [Google Scholar]
- Yee CL, Jones KR, Finger TE. Brain-derived neurotrophic factor is present in adult mouse taste cells with synapses. J Comp Neurol. 2003;459:15–24. doi: 10.1002/cne.10589. [DOI] [PubMed] [Google Scholar]
- Yntema CL. An experimental study of the origin of the cells which constitute the VIIth and VIIIth cranial ganglia and nerves in the embryo of Amblystoma punctatum. J Exp Zool. 1937;75:75–101. [Google Scholar]
- Yntema CL. An experimental study of the origin of sensory neurons and sheath cells of the IXth and Xth cranial nerves in Amblystoma punctatum. J Exp Zool. 1943;92:93–119. [Google Scholar]
- Yntema CL. Experiments on the origin of the sensory ganglia of the facial nerve in the chick. J Comp Neurol. 1944;81:147–164. [Google Scholar]
- Zhang C, Brandemihl A, Lau D, Lawton A, Oakley B. BDNF is required for the normal development of taste neurons in vivo. Neuroreport. 1997;8:1013–1017. doi: 10.1097/00001756-199703030-00039. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Ashwell KW. The development of cranial nerve and visceral afferents to the nucleus of the solitary tract in the rat. Anat Embryol. 2001;204:135–151. doi: 10.1007/s004290100185. [DOI] [PubMed] [Google Scholar]