Abstract
Introduction
Currently marketed epidermal growth factor receptor inhibitors (egfris) have been associated with high rates of dermatologic toxicity.
Methods
We formally reviewed the literature at medline and embase. Additional searches were conducted using Internet search engines. Studies were eligible if they were randomized controlled clinical trials of egfris, specifically cetuximab and panitumumab, in which at least one arm consisted of a non-egfri treatment and rash safety data were reported. The random effects method was used to pool differences in incident rash rates. Results are summarized as differences in incident rash (egfri therapy rate minus the non-egfri therapy rate) with corresponding 95% confidence intervals (cis) for all severity grades of rash and for grades 3 and 4 rash.
Results
Sixteen studies met the initial inclusion criteria of randomized controlled trials comparing egfri with non-egfri therapy. Seven publications that provided information on all severity grades of rash were found to have an overall difference in incident rash rate of 0.74 (95% ci: 0.68 to 0.81; p < 0.01). Thirteen studies that reported the incidence of grades 3 and 4 rash showed an overall difference in the incident rash rate of 0.12 (95% ci: 0.09 to 0.14; p < 0.01) between egfri and non-egfri therapy. Sensitivity analyses showed that the results were generally robust, but sensitive to small samples.
Conclusions
Results quantify the difference in rash rates between egfri and non-egfri therapy.
Keywords: Epidermal growth factor receptor inhibitors, meta-analysis, rash
1. INTRODUCTION
Overexpression of the epidermal growth factor receptor (egfr) has been associated with the solid tumours of metastatic colorectal cancer (mcrc), head-and-neck cancer, non-small-cell lung cancer (nsclc), and other cancers 1–3. Cetuximab and panitumumab are two marketed monoclonal antibodies that inhibit egfr. Treatment with currently available egfr inhibitors (egfris) can result in a variety of potentially serious toxicities such as dermatologic toxicities, hypomagnesemia, nausea, vomiting, diarrhea, and constipation 4.
In the skin, egfr controls the development and growth of the epidermis, the outermost skin layer 5. Inhibition of egfr in the skin therefore results in toxicity. Dermatologic toxicities are by far the most prevalent side effects seen with the current egfris. Skin toxicities include rash (papulopustular), xerosis, painful cracks and fissures on the palms and soles of the feet, paronychia, pruritus, and abnormal hair and eyelash growth 6. Table i lists the recent definitions and gradings of grades 3 and 4 rash and acneiform rash from the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events, version 4 (ctcae) 7.
TABLE I.
Rash definitions according to the U.S. National Cancer Institute Common Terminology Criteria for Adverse Events, version 4 7
| Maculopapular rash | ||
| Definition | A disorder characterized by the presence of macules (flat) and papules (elevated). Also known as morbilliform rash, it is one of the most common cutaneous adverse events, frequently affecting the upper trunk, spreading centripetally, and associated with pruritus. | |
| Grade 3 | Macules and papules covering more than 30% of the body surface area with or without associated symptoms; limiting self-care activities of daily living | |
| Grade 4 | Not stated | |
| Acneiform rash | ||
| Definition | A disorder characterized by an eruption of papules and pustules, typically appearing on the face, scalp, upper chest, and back. | |
| Grade 3 | Papules or pustules (or both) covering more than 30% of body surface area, which may or may not be associated with symptoms of pruritus or tenderness; limiting instrumental activities of daily living; associated with local superinfection, with oral antibiotics indicated | |
| Grade 4 | Papules or pustules (or both) covering any percentage of body surface area, which may or may not be associated with symptoms of pruritus or tenderness and are associated with extensive superinfection with intravenous antibiotics indicated; life-threatening consequences | |
Rashes can have a significant impact on continuity of therapy, on pain for the patient, and on quality of life. They can cause serious infection, and they are costly to manage. Rashes typically develop within the first 3 weeks of treatment 4,8,9, and radiation dermatitis that can manifest within 5 weeks of treatment is seen when an egfri is combined with radiation 10. Paronychia, xerosis, cracks and fissures, and abnormal hair growth tend to occur later in treatment 9,11. Most skin rashes are considered mild to moderate, but some are severe, leading to infection, dose reduction, dose delay, or discontinuation of the egfri 6,9.
Many studies have reported dermatologic toxicities resulting from treatment with egfris. One study pooled the incident rash rates from a number of single-arm radiotherapy studies 12. However, differences in the rates of rashes reported in studies comparing egfri therapy with treatment not using an egfri (for example, chemotherapy, or best supportive care, or both) have not been systematically quantified. The present analysis used meta-analytic techniques to systematically pool rash event rates so as to determine risk differences between egfris and non-egfri agents.
2. METHODS
We conducted a formal systematic review of medline and embase for the period January 2005 to August 2009. Search criteria included randomized comparative clinical trials, English-language literature, and search terms (mesh, text words) including “egfr inhibitor,” “cetuximab,” “panitumumab,” and “cancer/oncology,” “rash/egfr inhibitor rash.” Additional literature searches using the same search terms were conducted using the Google and Google Scholar Internet search engines. These searches focused on the terms “cetuximab/panitumumab” and “rashes.” The search was not limited to a particular disease site. Abstracts for each article located were reviewed.
Studies were defined as eligible if they were comparative clinical trials of egfris, specifically cetuximab or panitumumab, in which at least one arm of the study did not include a therapy that targeted egfr (for example, chemotherapy or best supportive care) and in which safety data for dermatologic toxicity were reported. Studies involving radiotherapy or non-egfri monoclonal antibody agents (for example, bevacizumab, erlotinib) were excluded from the analysis because of a different mechanism of action. Conference abstracts (for example, those from the American Society of Clinical Oncology) were not included because of the incomplete and preliminary nature of data abstracts. Editorials or commentaries, observational studies, quality-of-life studies, and review articles were also excluded from the analysis.
Abstracts of studies found through the foregoing search were independently reviewed by two reviewers for appropriateness (namely, original comparative clinical trial of egfri therapy compared with non-egfri treatment, including rash outcomes). Once a study was deemed to be appropriate, study data were extracted.
The primary outcome was the incidence of two classifications of rash: all severity grades, and grades 3 and 4. Because rash has historically been characterized using a number of different terms (acneiform rashes, acne-like rashes, skin toxicity, skin rashes, and rashes in general, for instance), all rash classifications were extracted for analysis. Detailed investigation showed that rash was often labelled using the term “rash” or “skin rash,” but could also be defined according to the standard toxicity severity grading as defined by the ctcae 7. Rash definitions in the individual studies were reviewed in detail. The number of patients with events was used as the numerator. Differences in rash rates were reported for all diseases (for example, nsclc, crc, and other solid tumour types). Data were extracted independently by the two reviewers, and any discordance was discussed and reconciled.
Results are reported as the difference in the rate of rash: specifically, the rate of rash reported for egfris minus the rate of rash reported for non-egfri therapy. Numerators and denominators were extracted. The analysis was performed in the RevMan program (Review Manager, version 5.0: The Nordic Cochrane Centre, Copenhagen, Denmark) using a random-effects model and Mantel–Haenszel methods. Results are summarized as the mean difference in rash rates, with corresponding 95% confidence intervals (cis). Sensitivity analyses were conducted by removing studies from the overall analysis by disease site and subpopulation.
3. RESULTS
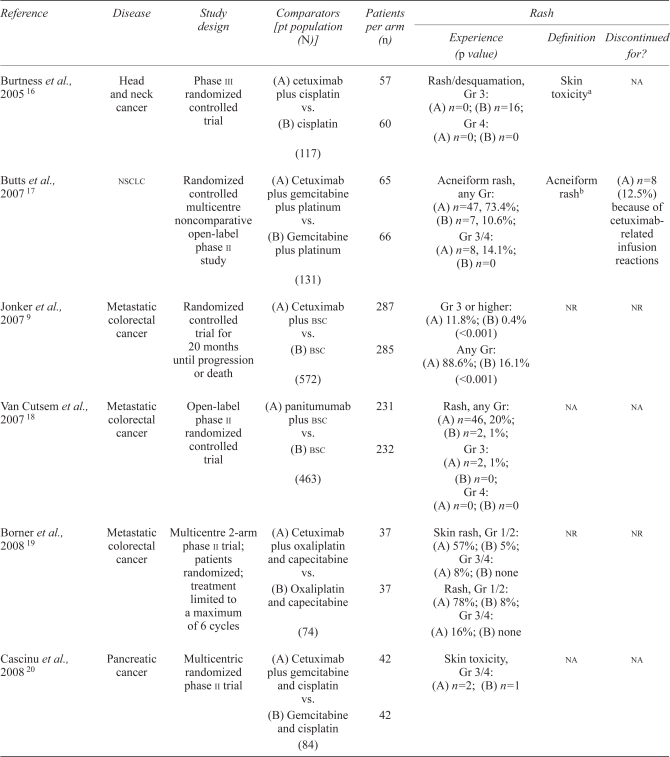
Sixteen studies met the initial inclusion criteria of randomized controlled trials of egfris. Three studies were excluded from the analysis: one study used an egfri in both arms 13, one did not present rash rates according to either grades 3 and 4 criteria or all-grades criteria 14, and one study was a duplicate of another study 15. Thus thirteen studies were eligible for inclusion in the meta-analysis (Table ii).
TABLE II.
Summary of studies included in the systematic review
| Reference | Disease | Study design | Comparators [pt population (N)] | Patients per arm (n) |
Rash |
||
|---|---|---|---|---|---|---|---|
| Experience (p value) | Definition | Discontinued for? | |||||
| Burtness et al., 2005 16 | Head and neck cancer | Phase iii randomized controlled trial | (A) cetuximab plus cisplatin vs. (B) cisplatin (117) |
57 60 |
Rash/desquamation, Gr 3: (A) n=0; (B) n=16; Gr 4: (A) n=0; (B) n=0 |
Skin toxicitya | na |
| Butts et al., 2007 17 | nsclc | Randomized controlled multicentre noncomparative open-label phase ii study | (A) Cetuximab plus gemcitabine plus platinum vs. (B) Gemcitabine plus platinum (131) |
65 66 |
Acneiform rash, any Gr: (A) n=47, 73.4%; (B) n=7, 10.6%; Gr 3/4: (A) n=8, 14.1%; (B) n=0 |
Acneiform rashb | (A) n=8 (12.5%) because of cetuximab-related infusion reactions |
| Jonker et al., 2007 9 | Metastatic colorectal cancer | Randomized controlled trial for 20 months until progression or death | (A) Cetuximab plus bsc vs. (B) bsc (572) |
287 285 |
Gr 3 or higher: (A) 11.8%; (B) 0.4% (<0.001) Any Gr: (A) 88.6%; (B) 16.1% (<0.001) |
nr | nr |
| Van Cutsem et al., 2007 18 | Metastatic colorectal cancer | Open-label phase ii randomized controlled trial | (A) panitumumab plus bsc vs. (B) bsc (463) |
231 232 |
Rash, any Gr: (A) n=46, 20%; (B) n=2, 1%; Gr 3: (A) n=2, 1%; (B) n=0; Gr 4: (A) n=0; (B) n=0 |
na | na |
| Borner et al., 2008 19 | Metastatic colorectal cancer | Multicentre 2-arm phase ii trial; patients randomized; treatment limited to a maximum of 6 cycles | (A) Cetuximab plus oxaliplatin and capecitabine vs. (B) Oxaliplatin and capecitabine (74) |
37 37 |
Skin rash, Gr 1/2: (A) 57%; (B)5%; Gr 3/4: (A) 8%; (B) none Rash, Gr 1/2: (A) 78%; (B) 8%; Gr 3/4: (A) 16%; (B) none |
nr | nr |
| Cascinu et al., 2008 20 | Pancreatic cancer | Multicentric randomized phase ii trial | (A) Cetuximab plus gemcitabine and cisplatin vs. (B) Gemcitabine and cisplatin (84) |
42 42 |
Skin toxicity, Gr 3/4: (A) n=2; (B) n=1 | na | na |
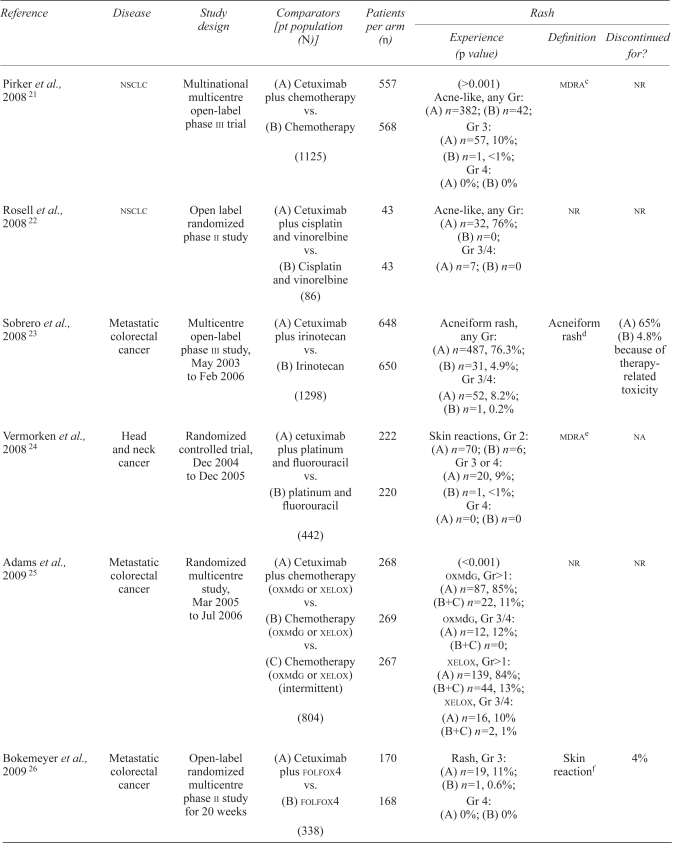
| Pirker et al., 2008 21 | nsclc | Multinational multicentre open-label phase iii trial | (A) Cetuximab plus chemotherapy vs. (B) Chemotherapy (1125) |
557 568 |
(>0.001) Acne-like, any Gr: (A) n=382; (B) n=42; Gr 3: (A) n=57, 10%; (B) n=1, <1%; Gr 4: (A) 0%; (B) 0% |
mdrac | nr |
| Rosell et al., 2008 22 | nsclc | Open label randomized phase ii study | (A) Cetuximab plus cisplatin and vinorelbine vs. (B) Cisplatin and vinorelbine (86) |
43 43 |
Acne-like, any Gr: (A) n=32, 76%; (B) n=0; Gr 3/4: (A) n=7; (B) n=0 |
nr | nr |
| Sobrero et al., 2008 23 | Metastatic colorectal cancer | Multicentre open-label phase iii study, May 2003 to Feb 2006 | (A) Cetuximab plus irinotecan vs. (B) Irinotecan (1298) |
648 650 |
Acneiform rash, any Gr: (A) n=487, 76.3%; (B) n=31, 4.9%; Gr 3/4: (A) n=52, 8.2%; (B) n=1, 0.2% |
Acneiform rashd | (A) 65% (B) 4.8% because of therapy-related toxicity |
| Vermorken et al., 2008 24 | Head and neck cancer | Randomized controlled trial, Dec 2004 to Dec 2005 | (A) cetuximab plus platinum and fluorouracil vs. (B) platinum and fluorouracil (442) |
222 220 |
Skin reactions, Gr 2: (A) n=70; (B) n=6; Gr 3 or 4: (A) n=20, 9%; (B) n=1, <1%; Gr 4: (A) n=0; (B) n=0 |
mdrae | na |
| Adams et al., 2009 25 | Metastatic colorectal cancer | Randomized multicentre study, Mar 2005 to Jul 2006 | (A) Cetuximab plus chemotherapy (oxmdg or xelox) vs. (B) Chemotherapy (oxmdg or xelox) vs. (C) Chemotherapy (oxmdg or xelox) (intermittent) (804) |
268 269 267 |
(<0.001) oxmdg, Gr>1: (A) n=87, 85%; (B+C) n=22, 11%; oxmdg, Gr 3/4: (A) n=12, 12%; (B+C) n=0; xelox, Gr>1: (A) n=139, 84%; (B+C) n=44, 13%; xelox, Gr 3/4: (A) n=16, 10% (B+C) n=2, 1% |
nr | nr |
| Bokemeyer et al., 2009 26 | Metastatic colorectal cancer | Open-label randomized multicentre phase ii study for 20 weeks | (A) Cetuximab plus folfox4 vs. (B) folfox4 (338) |
170 168 |
Rash, Gr 3: (A) n=19, 11%; (B) n=1, 0.6%; Gr 4: (A) 0%; (B) 0% |
Skin reactionf | 4% |
| Van Cutsem et al., 2009 27 | Metastatic colorectal cancer | Randomized open-label multicentre study, Jul 2004 to Nov 2005 | (A) Cetuximab plus folfiri vs. (B) folfiri (1198) |
599 599 |
Any rash, Gr 3/4: (A) n=49, 8.2%; (B) n=0 (<0.001) Acneiform rash, Gr 3: (A) n=97, 16.2% |
nr | nr |
Defined as the presence of one or more incidents of rash or desquamation, dry skin, nail changes, or other skin toxicity of all grades according to the National Cancer Institute’s Common Toxicity Criteria, version 2.0.
Defined as any event described as rash, rash pustular, rash erythematous, dermatitis acneiform, dermatitis exfoliative, rash papular, rash pruritic, rash generalized, rash macular, rash maculopapular, acne, dry skin, acne pustular, and skin desquamation.
Acne, acne pustular, dermatitis acneiform, dry skin, erythema, folliculitis, pruritus, rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, skin exfoliation, skin hyperpigmentation, telangiectasia, xerosis.
Includes rash, rash pustular, rash erythematous, dermatitis acneiform, dermatitis exfoliative, rash papular, rash pruritic, rash generalized, rash macular, rash maculopapular, acne, acne pustular, skin desquamation, and dry skin.
Acne pustular, acne, celulitis, dermatitis acneiform, dry skin, erysipelas, erythema, face edema, folliculitis, growth of eyelashes, hair growth abnormal, hypertrichosis, nail-bed infection, nail-bed inflammation, nail disorder, nail infection, paronychia, pruritus, rash erythematous, rash follicular, rash generalized, rash macular, rash maculopapular, rash papular, rash pruritic, rash pustular, rash, skin exfoliation, skin hyperpigmentation, skin necrosis, staphylococcal scalded skin syndrome, telangiectasia, wound necrosis, and xerosis.
Included these terms from the Medical Dictionary for Regulatory Activities, version 8.1: acne, acne pustular, celulitis, dermatitis acneiform, dry skin, erysipelas, erythema, face edema, folliculitis, growth of eyelashes, hair growth abnormal, hypertrichosis, nail bed infection, nail bed inflammation, nail disorder, nail infection, paronychia, pruritus, rash, rash erythematous, rash follicular, rash generalized, rash macular, rash maculopapular, rash popular, rash pruritic, rash pustular, skin exfoliation, skin hyperpigmentation, skin necrosis, staphylococcal scalded skin syndrome, telangiectasia, wound necrosis, xerosis.
bsc = best supportive care; Gr = grade; nr = not reported; folfox4 = oxaliplatin–leucovorin–5-fluorouracil; folfiri = folinic acid–5-fluorouracil–irinotecan; oxmdg = oxaliplatin–leucovorin–5-fluorouracil; xelox = capecitabine–oxaliplatin; nsclc = non-small-cell lung cancer; mdra = Medical Dictionary for Regulatory Activities; na = not available.
Most of the studies examined cetuximab in mcrc (n = 7), nsclc (n = 3), head-and-neck cancer (n = 2), and pancreatic cancer (n = 1). One study included in the analysis evaluated the efficacy and safety of panitumumab. Seven publications provided information on the incidence of all grades of rash (Table iii) 9,16,17,19,23,25,27. The difference between egfris and non-egfris in overall incidence rate for all severity grades of rash was 0.74 (95% ci: 0.68 to 0.81; p < 0.01; chi-square: 42.21, p < 0.001). The difference in the rates of rash between egfri and non-egfri therapies ranged between 0.52 and 0.86.
TABLE III.
Risk differences for all severity grades of rash
| Reference | Risk difference | 95% ci |
|---|---|---|
| Burtness et al., 2005 16 | 0.52 | 0.36 to 0.68 |
| Butts et al., 2007 17 | 0.63 | 0.50 to 0.76 |
| Jonker et al., 2007 9 | 0.81 | 0.76 to 0.85 |
| Van Cutsem et al., 2007 18 | 0.84 | 0.79 to 0.89 |
| Borner et al., 2008 19 | 0.86 | 0.75 to 0.98 |
| Cascinu et al., 2008 20 | na | na |
| Pirker et al., 2008 21 | na | na |
| Rosell et al., 2008 22 | na | na |
| Sobrero et al., 2008 23 | 0.70 | 0.67 to 0.74 |
| Vermorken et al., 2008 24 | na | na |
| Adams et al., 2009 25 | 0.72 | 0.67 to 0.77 |
| Bokemeyer et al., 2009 26 | na | na |
| Van Cutsem et al., 2009 27 | na | na |
| POOLED | 0.74 | 0.68 to 0.81 |
ci = confidence interval; na = not available.
Thirteen publications provided information on grades 3 and 4 rashes (Table iv). The overall difference in the rates of grades 3 and 4 rash between egfri and non-egfri therapy was 0.12 (95% ci: 0.09 to 0.14; p < 0.01; chi-square: 48.13, p < 0.001). The risk difference ranged between 0.02 and 0.40. When reporting grades 3 and 4 rates, six of thirteen studies (46%) indicated that no grade 4 rash reactions had occurred.
TABLE IV.
Risk differences for grades 3 or 4 rashes
| Reference | Risk difference | 95% ci |
|---|---|---|
| Burtness et al., 2005 16 | 0.28 | 0.16 to 0.39 |
| Butts et al., 2007 17 | 0.14 | 0.05 to 0.23 |
| Jonker et al., 2007 9 | 0.11 | 0.08 to 0.15 |
| Van Cutsem et al., 2007 18 | 0.14 | 0.09 to 0.19 |
| Borner et al., 2008 19 | 0.16 | 0.04 to 0.29 |
| Cascinu et al., 2008 20 | 0.02 | –0.06 to 0.10 |
| Pirker et al., 2009 21 | 0.10 | 0.08 to 0.13 |
| Rosell et al., 2008 22 | 0.40 | 0.25 to 0.55 |
| Sobrero et al., 2008 23 | 0.08 | 0.06 to 0.10 |
| Vermorken et al., 2008 24 | 0.09 | 0.05 to 0.13 |
| Adams et al., 2009 25 | 0.10 | 0.06 to 0.14 |
| Bokemeyer et al., 2009 26 | 0.17 | 0.11 to 0.23 |
| Van Cutsem et al., 2009 27 | 0.08 | 0.06 to 0.10 |
| POOLED | 0.12 | 0.09 to 0.14 |
ci = confidence interval.
For the sensitivity analysis related to rashes overall, we examined rash rate differences in mcrc patients (five studies 9,18,19,23,25), in studies with active comparators (five studies 16,17,19,23,25), and in studies with best supportive care as the comparator (two studies 9,18, Table v).
TABLE V.
Sensitivity analysis for rash severity
| Variable | Studies (n) | Risk difference | 95% ci | Chi-square | p Value |
|---|---|---|---|---|---|
| All grades of rash | |||||
| Pooled | 7 | 0.74 | 0.68 to 0.81 | 42.2 | <0.001 |
| mcrc only | 5 | 0.78 | 0.72 to 0.84 | 28.6 | <0.01 |
| Active comparator only | 5 | 0.70 | 0.63 to 0.77 | 14.7 | <0.01 |
| bsc only | 2 | 0.82 | 0.79 to 0.86 | 1.0 | 0.33 |
| Grade 3/4 rashes | |||||
| Pooled | 13 | 0.12 | 0.09 to 0.14 | 48.1 | <0.01 |
| mcrc only | 7 | 0.11 | 0.08 to 0.13 | 16.5 | 0.01 |
| Active comparator only | 11 | 0.12 | 0.09 to 0.14 | 43.8 | <0.001 |
| bsc only | 2 | 0.12 | 0.10 to 0.15 | 0.7 | 0.4 |
| Grade 3 rash only | 7 | 0.09 | 0.07 to 0.11 | 8.3 | 0.22 |
| nsclc only | 3 | 0.20 | 0.06 to 0.03 | 16.5 | <0.01 |
ci = confidence interval; mcrc = metastatic colorectal cancer; bsc = best supportive care; nsclc = non-small-cell lung cancer.
For the sensitivity analysis related to grades 3 and 4 rashes, we examined the rash differences in studies reporting grade 4 reactions (seven studies 9,17,19,20,23–25), in studies with active comparators (eleven studies 16,17,19–27), in studies with best supportive care as the comparator (two studies 9,18), in studies evaluating crc patients only (seven studies 9,18,19,23,25–27), in studies evaluating nsclc patients only (three studies 17,21,22), and in studies with best supportive care as a comparator (two studies 9,18, Table v).
4. DISCUSSION
The results from this systematic review provide a pooled value for the difference in the rates of rash associated with therapies using egfris and not using egfris in patients diagnosed with a number of solid tumours. Studies were heterogeneous, with variation in rash definition, sample size, disease site, and non-egfri comparator.
Sensitivity analyses were conducted by removing studies from the overall pooled analyses. The sensitivity analyses were limited by the number of studies available for subgroup analyses and by the availability of rash rates. Examination of subpopulations in the included studies showed generally similar rash rate differences for grades 3 and 4 reactions when studies with only crc patients or with only active comparators were included. By contrast, studies examining patients with nsclc showed a rate difference with respect to grades 3 and 4 rash between egfri and non-egfri therapies that was higher than the rate difference overall (0.20 vs. 0.12 overall). This difference may be related to the small number of combinable studies or to a disease site that may be associated with a risk of developing rash. Results also showed that the difference in rates of rash was slightly lower in studies in which no grade 4 rashes were reported during the trial (0.09 vs. 0.12 overall).
Examination of all severity grades of rash showed that differences were relatively similar to those for the overall study sample, with rash rates ranging between 0.70 and 0.78. Studies in which a non-egfri comparator was considered best supportive care reported higher differences in rash rates (0.82 vs. 0.74 overall). With active comparators, that difference declined (0.74 overall vs. 0.70). More studies had active comparators than inactive comparators, but the sample sizes in the studies with inactive comparators were large and thus contributed to the overall value of the pooled estimate.
It is important to note that, of the thirteen clinical trials included in the analysis, twelve were based on the use of cetuximab; only one trial reported on the use of panitumumab. Despite a similar mechanism of action, the results as presented here should arguably be considered to be applicable only to cetuximab.
We pooled rash rates for cetuximab and panitumumab because those egfris met the inclusion requirements and because those two agents have the same mechanism of action in that they target the extracellular domain of egfr. Erlotinib and gefitinib target tyrosine kinase and have a different anatomical therapeutic chemical classification. Bevacizumab, although in the same class as cetuximab and panitumumab, is an inhibitor of vascular endothelial growth factor.
Discontinuation rates because of adverse events or rashes were not reported consistently in the included studies. Only one study provided a rate of discontinuation because of rash (4%) 26; others reported discontinuations related to egfri reactions—for example, infusion-related or general toxicity 13,17,23. Consequently, a systemic review of discontinuation rates could not be conducted, and the pooled difference in rates of discontinuation because of rash could not be determined. Discontinuation may be an important outcome to provide, because it may act as a surrogate outcome for the effect and importance of rash for the patient. Indeed, in one survey of oncology practices, 76% of physicians reported having to temporarily withhold treatment, 60% reported dose reductions, and 32% reported having to discontinue treatment because of rash 6.
Post-radiotherapy radiation dermatitis was not included in this analysis of chemotherapy agents. One trial comparing radiation alone with radiation plus cetuximab in squamous cell carcinoma of the head and neck was found 28, but it was excluded from the meta-analysis because the comparator group did not receive chemotherapy. The rate of grades 3–5 radiation dermatitis was higher in the radiation plus cetuximab arm (23% vs. 18%), but the difference was found to be statistically nonsignificant. Other studies (case series) have suggested that the combination of cetuximab and radiotherapy in actual practice resulted in even higher rates of moderate-to-severe skin toxicity, with estimates as high as 49% for the occurrence of grades 3 and 4 rashes in head-and-neck patients receiving cetuximab plus radiotherapy 29–32. A recent pooled analysis of mostly noncomparative studies reported incident high-grade radiation dermatitis rates of 31.3% in patients receiving egfris and radiation 12. However, a larger retrospective analysis of 115 patients with locally advanced head-and-neck cancer receiving radiation and cetuximab showed grades 3 and 4 radiation dermatitis occurring in 23% of patients 10. That rate was similar to the rate reported by Bonner et al. 28. The mechanism of action for skin toxicity with radiotherapy and egfris is unknown 30.
Prophylaxis was not routinely discussed in the studies and was therefore not pooled. The search identified two studies that used an egfri in combination with bevacizumab for mcrc 33,34. Bevacizumab is a monoclonal antibody targeting the vascular endothelial growth factor 35. All other studies used chemotherapy or best supportive care. The bevacizumab studies were excluded from the pooling because skin toxicities that would confound the difference in rash rates with egfris have also been associated with bevacizumab 35.
It is important to emphasize that the term “rash” was reported in an inconsistent manner—namely, different labels were used for rash in the included studies. Some studies used the label “acneiform rash” when rash was actually a composite of various dermatologic toxicities 17; other studies provided many different labels for rash (for example, skin rash, rash, acneiform 19,26). We believe that these labelling differences are attributable to inconsistent reporting of rash by investigators, given that there is no common definition for egfri-induced rash. Specifically, the term “acneiform” should be avoided, because it describes rashes related to acne, which is a type of rash different from the rashes arising from egfris 36. Given the terms used in each study, careful dissection and consultation with a dermatologist were required to identify event rates for all rashes and for grades 3 and 4 rashes for appropriate pooling. Challenges were associated with definitions (for example, “rash,” “acneiform”—ctcae definitions, but which version?) and with grades (for example, typical ctcae gradations, but which version?). The potential for “misclassification” of a rash definition may have influenced the overall rates of rash. The potential for “misclassification” of a grade definition may have influenced the difference reported for severe rashes (that is, grades 3 and 4), but should not have affected overall rash rates.
In clinical trials and post-marketing studies, careful recording and standardized labelling of rashes would ensure that rash rates can be clearly identified. Location of rash and total body surface area affected by rash were not reported in the included studies, but would provide important information on the scope of rashes. The ctcae grading of rash does not take into account the localization of rash on the body; it focuses strongly on body surface area coverage. Because of the relatively localized nature of egfri-induced rashes, the ctcae grading system may not be appropriate for this type of rash and may underreport rash severity. Adherence to standard terminology or definitions—and adoption of their systematic use—is recommended, as is proper recording.
With the increased use of egfris, skin rashes and their incidence and management have recently received attention 6,37–39. Current anti-egfr therapies can adversely inhibit non-tumour egfr in the skin, which may lead to skin toxicities. It has been reported that rashes typically resolve without treatment after discontinuation of anti-egfr therapy 4 and that they are seldom fatal 40. Despite the seriousness of rash, a number of studies have found a positive association between severity of rash and increased survival 4,9,38, indicating that rash associated with egfris can be a predictor of survival 38.
Publications examining rashes associated with egfris have commented on the effect of a rash on a patient’s quality of life 12,41. It is hypothesized that, if toxicity becomes severe, there is a chance that patients would not continue treatment and thus jeopardize the possibility of improved survival outcome. However, there is some conflicting evidence. Tejwani and colleagues 12 hypothesized that patients were willing to tolerate rashes because, with some agents, rashes were associated with improved survival and considered a necessary part of treatment in a situation with no other options. Osio and colleagues 42 conducted a retrospective analysis of the chronic cutaneous effects of these egfris and impact of rash on quality of life. In a small sample of patients (n = 16), investigators found that all patients reported cutaneous adverse events, with 38% being of severity grades 1 and 2. Dose reductions or discontinuation of egfris were required in 6 patients (38%). In 6 patients (38%), rash was reported to be associated with an impact on quality of life 42. Most of the instruments used to determine quality of life in these studies are considered to be general quality-of-life instruments for oncology, including the Functional Assessment of Cancer Therapy (fact) and the European Organization for Research and Treatment of Cancer qlq-C30, although specific dermatology instruments (the Dermatology Life Quality Index, for instance) have been used 42. General instruments measuring quality of life may not be sensitive to changes associated with dermatologic conditions. A precedent for creating quality-of-life tools with a focus on an adverse events has been set with the creation of the fact-Neutropenia 43. A quality-of-life instrument specific to egfri-related rash may be useful.
As long as efficacy is maintained, agents that do not cause skin toxicity would be preferable over agents that do. There is some evidence that newer egfris with intermediate binding affinity are on the horizon; such agents may be able to reduce skin-related toxicities while maintaining efficacy 44,45.
Substantial resources and costs are associated with the outpatient and inpatient management of egfri-induced rashes 6,40,41,46,47. Once risk differences are determined, the costs of rash can be calculated. Here, the costs of rash-associated treatment and hospitalization were determined using resource utilization from the published literature and costs based on 2009 U.S. Medicaid and Medicare numbers. We determined that the cost of treatment may range from US$500 (grade 3) to US$15,000 (grade 4) per rash episode, including the cost of hospitalization. The result could be a budget impact of approximately US$20 million per annum for patients treated with an egfri in mcrc, based on a difference of 12% in grades 3 and 4 rash as derived from the present study (Chan B. Personal communication) 48.
5. CONCLUSIONS
Skin toxicities are a common adverse drug reaction seen with egfris such as cetuximab and panitumumab. Compared with non-egfri therapy, egfri therapy carries an overall risk difference of 74% for rashes of all severities and of 12% for grades 3 and 4 rashes. Standardization of rash definitions is recommended to facilitate appropriate pooling of data.
Footnotes
6. CONFLICT OF INTEREST DISCLOSURES
The authors received unrestricted funding from YM BioSciences. YM Biosciences had the opportunity to review and comment on the manuscript.
7. REFERENCES
- 1.Adenis A, Aranda Aguilar E, Robin YM, et al. Expression of the epidermal growth factor receptor (egfr or her1) and human epidermal growth factor receptor 2 (her2) in a large scale metastatic colorectal cancer (mcrc) trial [abstract 3630] J Clin Oncol. 2005;23 [Available online at: www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=34&abstractID=31696; cited January 31. 2011] [Google Scholar]
- 2.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–6. [PubMed] [Google Scholar]
- 3.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer. 2002;94:1593–611. doi: 10.1002/cncr.10372. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase ii trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 5.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–85. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72:152–9. doi: 10.1159/000112795. [DOI] [PubMed] [Google Scholar]
- 7.United States Department of Health and Human Services, National Institutes of Health, National Cancer Institute(nci) Common Terminology Criteria for Adverse Events (CTCAE) Bethesda, MD: nci; 2010. Ver. 4. [Available online at: evs.nci.nih.gov/ftp1/CTCAE/About.html; cited January 31, 2011] [Google Scholar]
- 8.Molinari E, De Quatrebarbes J, Andre T, Aractingi S. Cetuximab-induced acne. Dermatology. 2005;211:330–3. doi: 10.1159/000088502. [DOI] [PubMed] [Google Scholar]
- 9.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 10.Koutcher LD, Wolden S, Lee N. Severe radiation dermatitis in patients with locally advanced head and neck cancer treated with concurrent radiation and cetuximab. Am J Clin Oncol. 2009;32:472–6. doi: 10.1097/COC.0b013e318193125c. [DOI] [PubMed] [Google Scholar]
- 11.Lacouture ME, Lai SE. The pride (papulopustules and/or paronychia, regulatory abnormalities of hair growth, itching, and dryness due to epidermal growth factor receptor inhibitors) syndrome. Br J Dermatol. 2006;155:852–4. doi: 10.1111/j.1365-2133.2006.07452.x. [DOI] [PubMed] [Google Scholar]
- 12.Tejwani A, Wu S, Jia Y, Agulnik M, Millender L, Lacouture ME. Increased risk of high-grade dermatologic toxicities with radiation plus epidermal growth factor receptor therapy. Cancer. 2009;115:1286–99. doi: 10.1002/cncr.24120. [DOI] [PubMed] [Google Scholar]
- 13.Bourhis J, Rivera F, Mesia R, et al. Phase i/ii study of cetuximab in combination with cisplatin or carboplatin and fluorouracil in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. J Clin Oncol. 2006;24:2866–72. doi: 10.1200/JCO.2005.04.3547. [DOI] [PubMed] [Google Scholar]
- 14.Peeters M, Siena S, Van Custem E, et al. Association of progression-free survival, overall survival, and patient-reported outcomes by skin toxicity and KRAS status in patients receiving panitumumab monotherapy. Cancer. 2009;115:1544–54. doi: 10.1002/cncr.24088. [DOI] [PubMed] [Google Scholar]
- 15.Giusti RM, Shastri K, Pilaro AM, et al. U.S. Food and Drug Administration approval: panitumumab for epidermal growth factor receptor-expressing metastatic colorectal carcinoma with progression following fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens. Clin Cancer Res. 2008;14:1296–302. doi: 10.1158/1078-0432.CCR-07-1354. [DOI] [PubMed] [Google Scholar]
- 16.Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase iii randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–54. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 17.Butts CA, Bodkin D, Middleman EL, et al. Randomized phase ii study of gemcitabine plus cisplatin or carboplatin [corrected], with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J Clin Oncol. 2007;25:5777–84. doi: 10.1200/JCO.2007.13.0856. [Erratum in: J Clin Oncol 2008;26:3295] [DOI] [PubMed] [Google Scholar]
- 18.Van Custem E, Peeters M, Siena S, et al. Open-label phase iii trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 19.Borner M, Koeberle D, Von Moos R, et al. Adding cetuximab to capecitabine plus oxaliplatin (xelox) in first-line treatment of metastatic colorectal cancer: a randomized phase ii trial of the Swiss Group for Clinical Cancer Research sakk. Ann Oncol. 2008;19:1288–92. doi: 10.1093/annonc/mdn058. [DOI] [PubMed] [Google Scholar]
- 20.Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase ii trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]
- 21.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (flex): an open-label randomised phase iii trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 22.Rosell R, Robinet G, Szczesna A, et al. Randomized phase ii study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in egfr-expressing advanced non-small-cell lung cancer. Ann Oncol. 2008;19:362–9. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 23.Sobrero AF, Maurel J, Fehrenbacher L, et al. epic: phase iii trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–19. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 24.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 25.Adams RA, Meade AM, Madi A, et al. Toxicity associated with combination oxaliplatin plus fluoropyrimidine with or without cetuximab in the mrc coin trial experience. Br J Cancer. 2009;100:251–8. doi: 10.1038/sj.bjc.6604877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 27.Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 28.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 29.Budach W, Bolke E, Homey B. Severe cutaneous reaction during radiation therapy with concurrent cetuximab. N Engl J Med. 2007;357:514–15. doi: 10.1056/NEJMc071075. [DOI] [PubMed] [Google Scholar]
- 30.Giro C, Berger B, Bolke E, et al. High rate of severe radiation dermatitis during radiation therapy with concurrent cetuximab in head and neck cancer: results of a survey in eortc institutes. Radiother Oncol. 2009;90:166–71. doi: 10.1016/j.radonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Lord HK, Junor E, Ironside J. Cetuximab is effective, but more toxic than reported in the Bonner trial. Clin Oncol (R Coll Radiol) 2008;20:96. doi: 10.1016/j.clon.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Pryor DI, Porceddu SV, Burmeister BH, et al. Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother Oncol. 2009;90:172–6. doi: 10.1016/j.radonc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 33.Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase iiib trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–80. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 34.Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–72. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 35.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–9. [PubMed] [Google Scholar]
- 36.International Federation of Pharmaceutical Manufacturers and Associations (ifpma) Geneva, Switzerland: ifpma; Medical Dictionary for Regulatory Activities (MedDra) [Web resource] Ver. 12.0. n.d. [Available online at: www.meddramsso.com; cited January 23, 2011] [Google Scholar]
- 37.Li T, Perez–Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009;4:107–19. doi: 10.1007/s11523-009-0114-0. [DOI] [PubMed] [Google Scholar]
- 38.Perez–Soler R. Can rash associated with her1/egfr inhibition be used as a marker of treatment outcome? Oncology (Williston Park) 2003;17(suppl 12):23–8. [PubMed] [Google Scholar]
- 39.Jatoi A, Nguyen DL. Do patients die from rashes from epidermal growth factor inhibitors? A systematic review to help counsel patients about holding therapy. Oncologist. 2008;13:1201–4. doi: 10.1634/theoncologist.2008-0149. [DOI] [PubMed] [Google Scholar]
- 40.Jatoi A, Rowland K, Sloan JA, et al. Tetracycline to prevent epidermal growth factor receptor inhibitor–induced skin rashes: results of a placebo-controlled trial from the North Central Cancer Treatment Group (N03CB) Cancer. 2008;113:847–53. doi: 10.1002/cncr.23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner LI, Lacouture ME. Dermatologic toxicities associated with egfr inhibitors: a clinical psychologist’s perspective. Oncology (Williston Park) 2007;21(suppl 5):34–6. [PubMed] [Google Scholar]
- 42.Osio A, Matues C, Soria JC, et al. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol. 2009;161:515–21. doi: 10.1111/j.1365-2133.2009.09214.x. [DOI] [PubMed] [Google Scholar]
- 43.Wagner LI, Beaumont JL, Ding B, et al. Measuring health-related quality of life and neutropenia-specific concerns among older adults undergoing chemotherapy: validation of the Functional Assessment of Cancer Therapy-Neutropenia (fact-N) Support Care Cancer. 2008;16:47–56. doi: 10.1007/s00520-007-0270-7. [DOI] [PubMed] [Google Scholar]
- 44.Boland WK, Bebb G. Nimotuzumab: a novel anti-egfr monoclonal antibody that retains anti-egfr activity while minimizing skin toxicity. Expert Opin Biol Ther. 2009;9:1199–206. doi: 10.1517/14712590903110709. [DOI] [PubMed] [Google Scholar]
- 45.Lammerts van Bueren JJ, Bleeker WK, Brannstrome A, et al. The antibody zalutumumab inhibits epidermal growth factor receptor signaling by limiting intra- and intermolecular flexibility. Proc Natl Acad Sci U S A. 2008;105:6109–14. doi: 10.1073/pnas.0709477105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Melosky B, Burkes R, Rayson D, Alcindor T, Shear N, Lacouture M. Management of skin rash during egfr-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol. 2009;16:16–26. doi: 10.3747/co.v16i1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sipples R. Common side effects of anti-egfr therapy: acneform rash. Semin Oncol Nurs. 2006;22(suppl 1):28–34. doi: 10.1016/j.soncn.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Decision Resources . Colorectal Cancer (Stage IV): Therapies Must Increase Overall Survival over IFL Plus Bevacizumab to Reach Blockbuster Status. Burlington, MA: Decision Resources; 2008. pp. 70–3. DecisionBase Report. [Google Scholar]