Abstract
Purpose
This study evaluated the chemosensitizing effects of Pluronic P85 (P85) on the cells expressing multidrug resistance-associated proteins, MRP1 and MRP2.
Methods
Cell models included MRP1- and MRP2-transfected MDCKII cells, as well as doxorubicin-selected COR-L23/R cells overexpressing MRP1. Effects of P85 on cellular accumulation and cytotoxicity of vinblastine and doxorubicin were determined. Mechanistic studies characterized the effects of P85 on ATP and reduced glutathione (GSH) intracellular levels as well as MRPs ATPase and glutathione-S-transferase (GST) activities in these cells.
Results
Considerable increases of vinblastine and doxorubicin accumulation in the cells overexpressing MRP1 and MRP2 in the presence of P85 were observed, while no statistically significant changes in the drug accumulation in the parental cells were found. P85 treatment caused an inhibition of MRPs ATPase activity. Furthermore, P85 induced ATP depletion in these cells similar to that previously reported for Pgp-overexpressing cells. In addition, reduction of GSH intracellular levels and decrease of GST activity following P85 treatment were observed. Finally, significant enhancement of cytotoxicity of vinblastine and doxorubicin by P85 in MRPs -overexpressing cells was demonstrated.
Conclusions
This study suggests that P85 can sensitize cells overexpressing MRP1 and MRP2, which could be useful for chemotherapy of cancers that display these resistant mechanisms.
Keywords: ATP, ATPase, GSH/GST, MRP1, MRP2, Pluronic
INTRODUCTION
Multidrug resistant proteins are part of ATP binding cassette (ABC) superfamily of proteins that play an important role in the defense of cells against a wide range of xenobiotics. This family comprises a broad range of proteins found in organisms from bacteria to humans, which transport structurally diverse substances, such as ions, amino acids, sugars, peptides, and proteins across biological membranes (1). Along with another ABC drug efflux transporter, P-glycoprotein (Pgp), MRPs has been implicated in the development of multidrug resistance in many tumors. The expression of MRPs and/or mRNA has been detected in almost every tumor type examined, including both solid tumors and hematological malignancies (2). Identification of methods for modifying MRPs activity may have a substantial impact in improving treatment of various diseases through altering distribution of therapeutic agents in the body. Therefore, development of safe and effective chemosensitizers that inhibit the MRPs efflux systems are of significant therapeutic interest.
Recently, selected Pluronic block copolymers, poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide), were found to sensitize multidrug resistant (MDR) tumor cells resulting in an increase of the cytotoxic activity of anthracyclines and other cytotoxic drugs by 2 to 3 orders of magnitude (3–5). Furthermore, studies using wild type and knockout mice deficient in mdr1a/1b genes suggest that P85 inhibits Pgp activity in the blood brain barrier (6). The inhibition of Pgp efflux system by Pluronics was attributed to the combined effects resulting in ATP depletion and inhibition of Pgp ATPase activity (7).
Along with the inhibition of Pgp efflux system and enhancement of transport of Pgp substrates P85 was shown to increase accumulation of fluorescein (Flu), in MRPs-overexpressing human pancreatic adenocarcinoma cell line (Panc-1) (8), as well as primary bovine brain microvessel endothelial cells (BBMEC) (9). These studies indicated the possibility of inhibition of MRPs efflux transport by the block copolymer. However, the extent of inhibition and specificity of the effect of Pluronic with respect to MRPs remains unclear.
Studies involving MRPs are complicated by presence of multiple biochemical pathways that can be utilized by the MRPs efflux system to extrude various compounds. In particular, GSH/GST detoxification system is known to act in concert with MRPs, as many MRPs substrates are GSH-dependent (2). These substrates are either co-transported with GSH, or first conjugated with GSH and then transported as the GSH conjugate. Being an ATP depleting agent in MDR cells, Pluronic might also affect the GSH/GST system, which, combined with alterations in MRPs activity, could have complex effects on the accumulation of various MRPs substrates in the cells.
Therefore, using Madin Darby Canine Kidney (MDCKII) cells transfected with MRP1 or MRP2 and human lung large cell carcinoma (COR-L23/R) epithelial cells selected with Dox and overexpressing MRP1 this paper elucidates multiple pathways for Pluronic activities in MRPs overexpressing cells including ATP and GSH depletion, as well as alterations in MRPs ATPase and GST enzymatic activities. The effects of Pluronic in MRPs cells were assessed using MRPs substrates vinblastine (Vin) and doxorubicin (Dox) (10, 11). Following evaluation of the Pluronic effects on drug accumulation, the cytotoxicity studies were conducted to examine whether the block copolymer can sensitize MRPs overexpressing cells with respect to selected anticancer drugs.
MATERIALS AND METHODS
Chemicals and Drugs
Dox and Vin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). [3H]-vinblastine ([3H]-Vin) and [3H]-mannitol ([3H]-Man) were received from Moravek Biochemicals, Inc. (Brea, CA, USA). GF120918 was kindly provided by Dr. Kenneth Brouwer (Glaxo Wellcome, Inc., Research Triangle Park, NC, USA). All reagents were either analytical, or cell culture grade and were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Preparation of Pluronic Solutions
P85 (lot # WPOP-587A) was kindly provided by BASF Corp. (Parispany, NJ, USA). Aqueous solutions of P85 were prepared in assay buffer containing 122 mM sodium chloride, 25 mM sodium bicarbonate, 10 mM glucose, 10 mM HEPES, 3mM potassium chloride, 1.2 mM magnesium sulfate, 1.4 mM calcium chloride and 0.4 mM potassium phosphate dibasic, pH 7.4. P85 solutions were incubated a minimum of 1 hour at 37°C before using. Drug solutions with block copolymer were prepared by addition of the drug to the culture medium containing various concentrations of P85.
Cells and Culture Conditions
The MDCKII cells, MRP1- and MRP2-transfected (MDCKII-MRP1 and MDCKII-MRP2 respectively) and the parental (wild type) cell line (MDCKIIwt) (12) were gifts from Dr. Piet Borst (The Netherlands Cancer Institute, Amsterdam, Netherlands). Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% heat-inactivated fetal bovine serum (FBS), and 50units/ml penicillin/streptomycin. Human lung carcinoma epithelial cell line COR-L23 and MRP1 overexpressing COR-L23/R cell line (13) were purchased from European Collection of Cell Cultures. Cells were maintained in RPMI 1640 medium with 2mM glutamine, 0.2μg/ml doxorubicin (for COR-L23/R), and 10% FBS. Tissue culturem media were obtained from Gibco Life Technologies, Inc. (Grand Island, NY, USA). Serum and medium supplements were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The cells were seeded at a density of 25,000 cells/cm2 into 24-well plates and used for accumulation studies after reaching confluency (typically within 4–5 days).
Western Blot Analysis
Identification of MRP1, MRP2, and Pgp was done using immunoblot technique described previously (14). The monoclonal antibodies to MRP1, MRPm6 (Kamiya Biomedical Corp., Seattle, WA, USA), MRP2, M2III-6 (Alexis Corp., San Diego, CA, USA), and Pgp, C219 (Dako Corp., Carpinteria, CA, USA), were used at 1:100 dilutions. The monoclonal antibodies to β-actin, and anti-β-1-chicken Integrin (Sigma Corp., St. Louis, MO, USA) were used at 1:200 dilution. The secondary horseradish peroxide anti-mouse Ig antibodies (1:1500 dilution) were purchased from Amersham Life Sciences (Cleveland, OH, USA). The specific protein bands were visualized using a chemiluminescence kit (Pierce, Rockford, IL, USA). The levels of MRP1, MRP2 and Pgp expression were quantitated by densitometry (Nucleo Vision, Nucleo Tech, Curitiba-Pr., Brazil). To correct for loading differences, the levels of proteins were normalized to constitutively expressed β-actin.
DPH Cells Labeling
DPH was used as a probe to examine the fluidity properties of the hydrocarbon region of the cell membranes (15). Suspensions of MDCKIIwt, MDCKII-MRP1, MDCKII-MRP2, COR-L23 or COR-L23/R cells were washed twice with PBS and incubated with the 2 μM DPH labeling solution for 1 hour at 37°C. Following the initial labeling with DPH cells were washed twice with PBS to remove extracellular DPH and re-suspended in an appropriate volume of PBS. To evaluate the kinetic effects of P85 in the cells, 30 μl of 1% P85 stock solution was added to 3 ml of cell suspension in PBS to obtain 0.01% P85 solution. Changes in the membrane microviscosity were recorded immediately after addition of P85 and up to 60 min following the addition of the copolymer.
Fluorescence Polarization Measurements
Fluorescence polarization measurements were carried out as described earlier (7) with a Hitachi F5000 spectrophotometer equipped with a polarizer set.
Preparation of a Crude Membrane Fraction
Plasma membrane fraction was prepared as described previously (16) with minor modifications. Briefly, 5×108 COR-L23/R cells were harvested, washed twice with phosphate-buffered saline, and centrifuged at 1,200g for 10 min at 4°C. The resulting pellet was diluted 40-fold in hypotonic buffer (1mM NaHCO3, 0.1mM phenylmethylsulfonyl fluoride, 0.1mM EGTA, pH 7.0), and stirred for 16 hours at 4°C. The cell lysate was centrifuged at 100,000g for 30 min at 4°C; remaining pellet was re-suspended in the lysis buffer and homogenized in a glass homogenizer. The homogenate was diluted 2-fold with the buffer A (520mM sucrose, 0.4mM CaCl2, 10mM HEPES/Tris, pH 7.4), and the nuclei were removed by centrifugation at 1,200 g for 10 min at 4°C. Enucleated cell homogenate was diluted 2-fold with buffer B (260mM sucrose, 0.2mM CaCl2, 5mM HEPES/Tris, pH 7.4) and centrifugated at 100,000 g for 45 min at 4°C. The resulted pellet was used as a crude plasma membrane fraction for the MRPs ATPase assay.
MRPs ATPase Assay
Effects of P85 on MRP1 ATPase activity were determined using plasma membrane suspensions from COR-L23/R cells overexpressing MRP1. The COR-L23/R cells have large amounts of MRP1, much smaller quantities of MRP2, and littleif any of Pgp (see Fig.1). MRP1 ATPase activity assay was performed as described earlier (7). Briefly, a mixture of MRP1-overexpressing membranes with or without P85 (0.001% – 1%wt) was added to 4mM MgATP, and incubated for 20 min at 37°C. An identical reaction mixture containing 100 μM sodium orthovanadate was assayed in parallel. Orthovanadate inhibits MRPs ATPase activity by trapping MgADP in the nucleotide-binding site (17). Thus, the ATPase activity measured in the presence of orthovanadate represents non-MRPs ATPase activity that was then subtracted from the ATPase activity measured in the absence of orthovanadate to provide MRPs ATPase activity. The reaction was stopped by the addition of 10% SDS and liberation of inorganic phosphate was detected by colorimetric reaction with ascorbic acid in ammonium molybdate solution according to Druekes et al. (18).
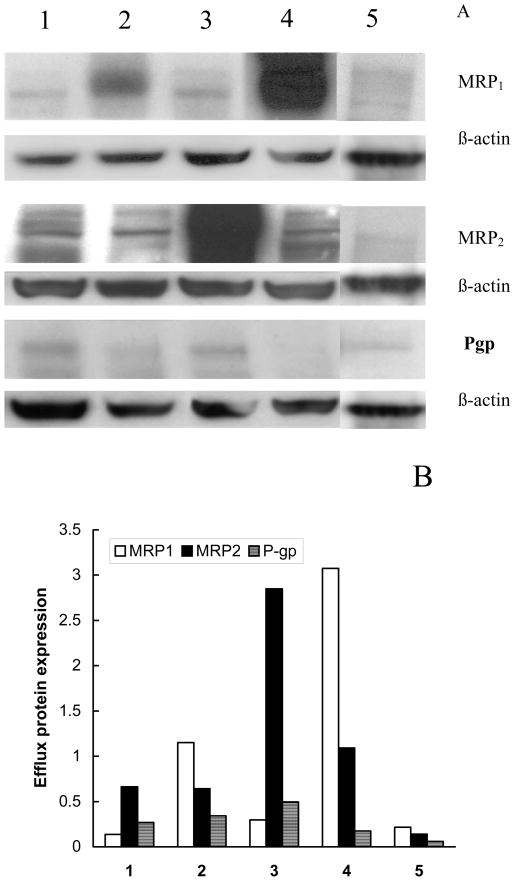
Fig. 1.
A: Western blot analysis of MRP1, MRP2 and Pgp in various cell lines: (1) MDCKIIwt, (2) MDCKII-MRP1, (3) MDCKII-MRP2, (4) COR-L23/R, and (5) COR-L23. A total of 25 μg of protein was loaded onto 7.5% polyacrylamide gels. Primary antibodies for MRP1 (MRPm6), MRP2 (M2III-6), and Pgp (C219) were used at dilutions of 1:100. The monoclonal antibodies to β-actin, anti-β-1-chicken Integrin were used at a dilution of 1:100. Secondary antibody was used at a 1:1500 dilution. The protein was detected using chemiluminescence. B: Levels of expression of efflux proteins in the cells based on the data of the Western blot analysis in panel (A). Expression of each transport protein is normalized to β-actin.
ATP Assay
To examine the effects of P85 on intracellular ATP levels confluent MDCKIIwt, MDCKII-MRP1, MDCKII-MRP2, COR-L23, and COR-L23/R cell monolayers were incubated with various concentrations of P85 for 2 hours. Following treatment the intracellular ATP levels were determined as described previously (14).
GSH Assay
The levels of the intracellular reduced glutathione (GSH) were determined in all cells utilized in this study. Following the cell exposure various concentrations of P85, the cells were lyzed by 4% trichloracetic acid, and precipitated protein was removed by centrifugation (10,000 rpm for 10 min at 4°C). Supernatants were neutralized with NaOH, and the amount of GSH was measured using the GSH-400 method (19).
GST Assay
The effect of P85 on GST activity in MDCKIIwt, MDCKII-MRP1, and MDCKII-MRP2 was determined using 1mM CDNB as a substrate and 1 mM GSH as a co-substrate in one-ml reaction mixture containing 100–400 μg sample protein in 0.1 M potassium phosphate buffer, pH 6.5 (20). Cell aliquots (2×106 cells/sample) were incubated with various concentrations of P85 for 2 hours. Following incubation, cells were washed twice with ice-cold PBS, solubilized in 1.0% Triton X-100, and frozen immediately for subsequent GST activity determination using the GST Detection Module kit (Amersham Pharm. Biotech, Inc., Piscataway, NJ, USA).
Drug Accumulation Experiments
The accumulation of [3H]-Vin, and Dox in the cells was studied as previously described (8). Briefly, confluent cell monolayers were pre-incubated with assay buffer or assay buffer, containing corresponding Pluronic solutions (0.001%–1% wt) for 1 or 24 hours. Following the pre-incubation period, cell monolayers were incubated with [3H]-Vin (2.3 nM), Dox (10 μM) or [3H]-Man (20 nM) in either assay buffer or P85 solutions for 1 hour at 37°C. After incubation, the solutions were removed; cell monolayers were washed three times with ice-cold PBS, and solubilized in Triton X-100 (1.0 %). Aliquots were removed for determination of the cellular dye using a Shimadzu RF5000 fluorescent spectrophotometer. [3H]-Vin and [3H]-Man concentrations were determined using TRI-CARB® 2500 TR liquid scintillation analyzer. [3H]-Man was used to account for possible non-specific effects of P85 on membrane permeability. Samples were taken for protein assay using the Pierce BCA assay. All experiments were carried out in quadruplicate.
Cytotoxicity Assay
To examine the effect of P85 on the cytotoxicity of Dox, or Vin in sensitive and resistant cell lines, cells were seeded in 96 well plates at a density of 5000 cells/well and allowed to reattach overnight. Following treatment with various concentrations of drug either with or without P85 (0.001% – 0.1% wt) for 2 hours at 37°C, the cells were washed and cultured for another three days in complete culture media. Concentrations of P85 were chosen so that if applied without the drug, the copolymer caused little or no toxicity in the cells. The cytotoxic effects of the drug in P85 solutions were determined using a standard MTT assay (21).
Statistical analysis
All statistical tests were performed by Microsoft Excel 2000 SR-1 program using the two-tailed heteroscedastic t-tests. A minimum p value of 0.05 was estimated as the significance level for all tests. SEM values for the MRPs substrates accumulation, GSH levels, microviscosity data, ATPase activity and ATP measurements were less than 10%.
RESULTS
Expression of Efflux Proteins in the Cells
The levels of expression of MRP1, MRP2 and Pgp efflux proteins in the cell lines employed in these studies were determined using Western blot analysis (Figure 1). The expression data for each protein are presented as relative units normalized for the expression of β-actin. As is seen in Figure 1A, the transfected cell lines, MDCKII-MRP1 (line 2) and MDCKII-MRP2 (line 3) showed expression of high levels of either MRP1 or MRP2. In particular, densitometric analysis revealed expression of 1.15 relative units of MRP1 in MDCKII-MRP1 cells and 2.85 relative units of MRP2 in MDCKII-MRP2 cells (Figure 1B). The highest expression of MRP1 (3.08 relative units) was found in Dox-selected COR-L23/R cells (line 4). In contrast, parental MDCKIIwt cells (line 1) and sensitive COR-L23 cells (line 5) displayed much lower levels of endogenous MRP1 and MRP2, although, a considerable amount of MRP2 (0.65 relative units) was detected in MDCKIIwt cells. Various levels of Pgp were also found in all cell lines studied. These levels were the highest in MDCKII-MRP2 (0.45 relative units) and the lowest in COR-L23 cells, but in every case expression of Pgp was less than that of respective MRP homologues. Taking into account that COR-L23/R cells showed very low expression of Pgp, these cells were further used for isolation of MRP-containing membranes in the MRP ATPase activity studies described below.
Effects of P85 on [3H]-Vin Accumulation
Vin is known to be a substrate of both Pgp and MRPs transporters (10). Therefore, all accumulation studies involving MDCKII cell lines that express small levels of endogenous Pgp were carried out in the presence of 1 μM of the acridone carboxamide derivative, GF120918, which completely inhibited Vin efflux in Pgp-expressing cells at this concentration (22). No GF120918 was used with COR-L23 and COR-L23/R as these cells have little, if any, Pgp. As is seen in Table 1, 2-hour exposure of the MRPs-overexpressing cells to P85 significantly enhanced [3H]-Vin accumulation compared to P85-free treatment groups (3.1 times and 3.4 times for MDCKII-MRP2 and COR-L23/R cells respectively). The effects of probenecid on [3H]-Vin accumulation in MDCKII-MRP2 and COR-L23/R cells were found to be considerably less than these of P85 (1.7 to 2.1 times, data not presented). The uptake of [3H]-Vin in the cells treated with high concentrations of P85 (0.1% – 0.5%) first leveled off and then decreased (Table 1). This observation is consistent with the previous reports that Pluronic micelles can inhibit uptake of probes into cells (8, 9). No increases of [3H]-Vin accumulation with P85 were found in MDCKIIwt and COR-L23 cell lines (Table 1). To evaluate a possibility of non-specific alteration of membrane permeability by P85, the accumulation of [3H]-Man was examined in all cell lines. This study found no statistically significant changes in [3H]-Man levels in the presence of P85 (data not shown).
Table 1.
Effects of P85 on the accumulation of [3H]-Vin in MRPs overexpressing and parental cells.
| Cell line | [P85], % wt | Vin accumulation, pmol/mg proteina |
|---|---|---|
| MDCKII-MRP2 | 0 | 57.79 ± 2.8 |
| 0.01 | 158.44 ± 2.0(*) | |
| 0.05 | 180.94 ± 1.2(**) | |
| 0.1 | 151.19 ± 0.6(*) | |
| 0.5 | 159.2 ± 3.1(*) | |
| COR-L23/R | 0 | 131.88 ± 8.4 |
| 0.01 | 446.48 ± 33.64(**) | |
| 0.05 | 388.46 ± 20.47(**) | |
| 0.1 | 384.14 ± 18.78(**) | |
| 0.5 | 140.46 ± 7.44 (n.s.) | |
| MDCKIIwt | 0 | 269.75 ± 12.8 |
| 0.05 | 302.4 ± 12.8 (n.s.) | |
| 0.1 | 207.1 ± 12.2 (n.s.) | |
| 0.5 | 93.4 ± 7.4(*) | |
| COR-L23 | 0 | 190.65 ± 11.1 |
| 0.01 | 194.01 ± 9.3 (n.s.) | |
| 0.05 | 194.49 ± 11.4 (n.s.) | |
| 0.1 | 200.7 ± 5.7 (n.s.) | |
| 0.5 | 89.1 ± 2.4(*) | |
cells were incubated with [3H]-Vin (2.3 nM) with or without P85 as described in Materials and Methods. In the case of MDCKII cells the treatment solutions additionally contained 1μM GF120918 to inhibit endogenous Pgp. In the P85-treatment groups cells were pre-incubated with the copolymer for 60 min before adding the drug. Values represent the mean ± SEM of four monolayers per the treatment group. Statistical significance of the P85 compared to the free-P85 control is shown in the brackets:
(n.s.) non-significant;
p< 0.05;
p<0.005.
Table 2.
Concentration-dependent effects of P85 on the accumulation of Dox in MDCKII and COR-L23 cells
| Cell line | [P85], % wt | Dox accumulation, nmol/mg proteina |
|---|---|---|
| MDCKII-MRP2 | 0 | 207.7 ± 15.5 |
| 0.01 | 216.12 ± 24.9 (n.s.) | |
| 0.05 | 498.8 ± 48.0(*) | |
| 0.1 | 908.14 ± 66.7(**) | |
| 0.5 | 1031.1 ± 80.9(**) | |
| COR-L23/R | 0 | 298.72 ± 7.29 |
| 0.01 | 360.43 ± 18.83(*) | |
| 0.05 | 475.46 ± 26.25(**) | |
| 0.1 | 621.1 ± 48.6(**) | |
| 0.5 | 418.73 ± 38.86(*) | |
| MDCKIIwt | 0 | 339 ± 31.2 |
| 0.01 | 372.6 ± 55.1 (n.s.) | |
| 0.1 | 381.6 ± 15.7 (n.s.) | |
| COR-L23 | 0 | 205.5 ± 19.7 |
| 0.01 | 214.34 ± 18.89 (n.s.) | |
| 0.05 | 202.82 ± 9.45 (n.s.) | |
| 0.1 | 225.41 ± 9.42 (n.s.) | |
| 0.5 | 271.35 ± 49.1 (n.s.) | |
cells were incubated with Dox (10 μM) ) with or without P85 as described in Materials and Methods. In the case of MDCKII cells the treatment solutions additionally contained 1μM GF120918 to inhibit endogenous Pgp. In the P85-treatment groups cells were pre-incubated with the copolymer for 60 min before adding the drug. Values represent the mean ± SEM of four monolayers per the treatment group. Statistical significance of the P85 compared to the free-P85 control is shown in the brackets:
(n.s.) non-significant;
p< 0.05;
p<0.005.
Effects of P85 on Dox Accumulation
To assess the effects of P85 on MRPs-mediated efflux of Dox the accumulation of the drug in MRPs-overexpressing cells was examined. Dox is known also to be a Pgp substrate. Therefore, since MDCKII cell lines have a trace amount of Pgp, all Dox accumulation studies in these cells, similar to the experiments with Vin, were carried out in the presence of 1 μM of GF120918, a potent Pgp inhibitor, to abolish possible contributions of the Pgp efflux pump. As is seen in Table 2, P85 induced significant increases in the intracellular levels of Dox in MRPs overexpressing cells (ca. 5 times for MDCKII-MRP2 and 2 times for CmOR-L23/R). The optimal concentrations of the copolymer causing these effects were ca. 0.1 to 0.5%wt. These doses of P85 did not alter the accumulation of Dox in the parental MDCKIIwt and COR-L23 cells (Table 2). Overall, these data suggest that P85 can selectively increase transport of Dox in MRPs-overexpressing cells. However, the effects of Pluronic on transport of Dox observed in this study, particularly, in COR-L23/R cells, were much less than the effects observed in Pgp-overexpressing cells (4).
Effects of P85 on Cell Membrane Microviscosity
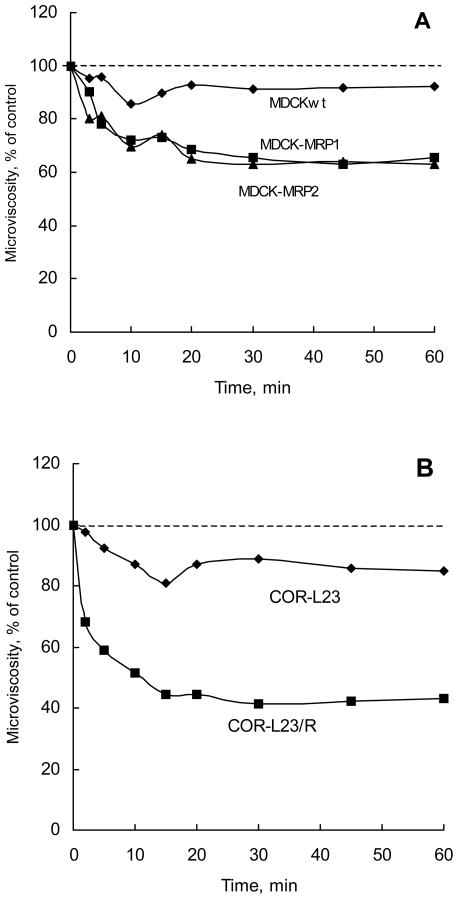
This section describes fluorescence polarization studies using a hydrophobic fluorescent compound, DPH, as a probe to evaluate effects of P85 on the membrane structure. DPH spontaneously incorporates in the hydrocarbon regions of the cell membranes (15). Fluorescence polarization of DPH is strongly dependent on its lipid microenvironment providing information regarding the membrane microviscosity. Present studies examined the time-dependent changes in the fluorescence polarization of DPH following exposure of various cells to 0.01% P85. The microviscosity values were calculated from the polarization measurements as described in Materials and Methods. As is seen in Figure 2, the membrane microviscosity in MRPs-overexpressing cells decreased within 20 minutes following addition of P85 to the cell suspension. After that, it leveled-off and remained constant throughout the entire duration of the experiment. This suggests that P85 molecules rapidly adhered to and incorporated into the cell membranes resulting in structural perturbations of the lipid bilayers. In contrast, membrane microviscosity in MDCKIIwt and drug sensitive COR-L23 cells was affected by P85 to a lesser extent (Figure 2).
Fig. 2.
Time dependent effects of P85 on microviscosity in MDCKII and COR-L23 cells. (A) - MDCKIIwt (filled circles), MDCKII-MRP1 (filled squares), and MDCKII-MRP2 (filled triangles); and (B) - COR-L23 (filled diamonds), and COR-L23/R (filled squares). Cells were treated with 0.01% P85.
Effects of P85 on MRP1 ATPase Activity
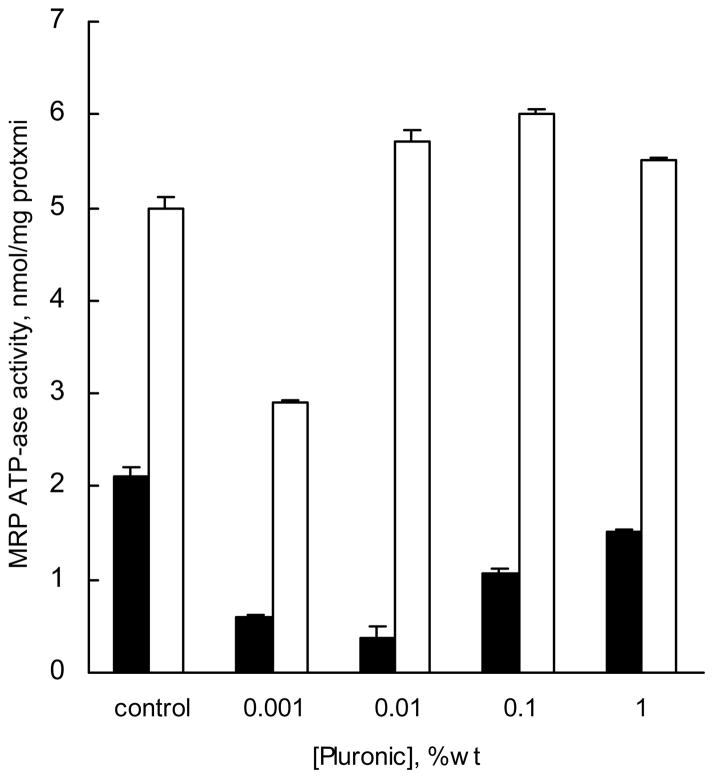
Effects of P85 on MRP1 ATPase activity were examined using isolated crude membrane fraction of COR-L23/R cells, which overexpressed MRP1, and had little if any Pgp. The membranes were exposed to (a) the copolymer-free buffer controls, (b) solutions containing various concentrations of P85, or (c) solutions of Vin with P85. The ATPase activity of MRP1 was assayed by detecting the liberated inorganic phosphate as described elsewhere (18). Decreases in MRP1 ATPase activity were observed with concentrations of P85 as low as 0.001% (Figure 3, filed bars). Maximum inhibition of ATPase activity (over 6-fold) was observed as P85 concentration approximated the CMC (0.03%wt). At higher concentrations of P85 (0.1%–1 %wt) the MRP1 ATPase activity was in part restored, although even at these concentrations activity was lower than that in the absence of the block copolymer (Figure 3). Further, the effect of P85 on MRP1 ATPase activity was studied in the presence of MRPs substrate, Vin (Figure 3, empty bars). It was found that Vin alone caused a significant increase of MRP1 ATPase activity, which was slightly inhibited in the presence of low concentrations (0.001% wt) of P85. At higher concentrations of P85 the MRP1 ATPase activity was similar to that observed with Vin alone. Overall, this study suggests that P85 strongly inhibits MRPs ATPase activity (mainly represented by MRP1), but addition of the MRPs substrate, Vin, abolishes the inhibitory effect of the block copolymer.
Fig. 3.
Effect of P85 alone (filled bars) and P85 in presence of 40 μM vinblastine (empty bars) on MRP ATPase activity in MRP containing membranes. Crude membrane fractions were isolated from COR-L23/R. Cells were incubated with various doses of P85 followed ATPase assay performed as described in Materials and Methods.
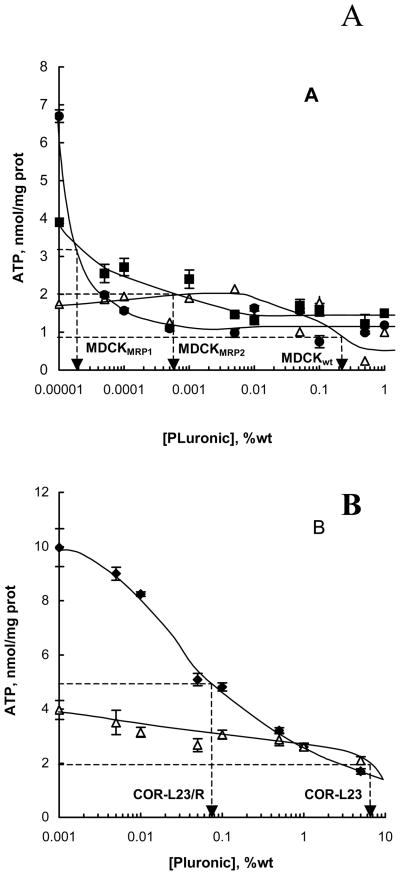
Effects of P85 on Intracellular Levels of ATP
The effects of P85 on intracellular ATP levels in MRPs-overexpressing cells were examined using a luciferin-luciferase assay (23). The ATP levels observed following treatment with various concentrations of P85 are shown in Figure 4. Even very low concentrations of P85 (ca. 0.00005 – 0.005 % wt) induced significant decreases in the ATP levels in the MDCKII-MRP1 and MDCKII-MRP2 cells (Figure 4A). In contrast, ATP levels in the parental MDCKIIwt cells were not significantly affected until relatively high concentrations of the block copolymer (ca. 0.1 % wt.) were used. A similar pattern was observed in the resistant COR-L23/R and parental COR-L23 cell lines (Figure 4B). Further, MDCKII cells appeared to be much more responsive to ATP depletion by the block copolymer than both resistant COR-L23/R and sensitive COR-L23 cells. However, within the cell lines of the same origin the responsiveness to the copolymer appeared to correlate with the expression of MRPs; the higher the level of MRPs expressed in the cells, the less concentrations of P85 are needed to cause ATP depletion in them.
Fig. 4.
Effect of P85 on ATP intracellular levels in (A) MDCKII MRP1 (filed diamonds), MDCKII-MRP2 (filed squares), MDCKIIwt (empty triangles); and (B) COR-L23 (filed diamonds), COR-L23/R (empty triangles) cell lines. Cells were treated with various doses of P85 for 2 hours, and aliquots of cell lysates were collected for ATP quantification, as described in Materials and Methods.
Effect of the ATP Supplementation on MRPs Efflux in the Presence of P85
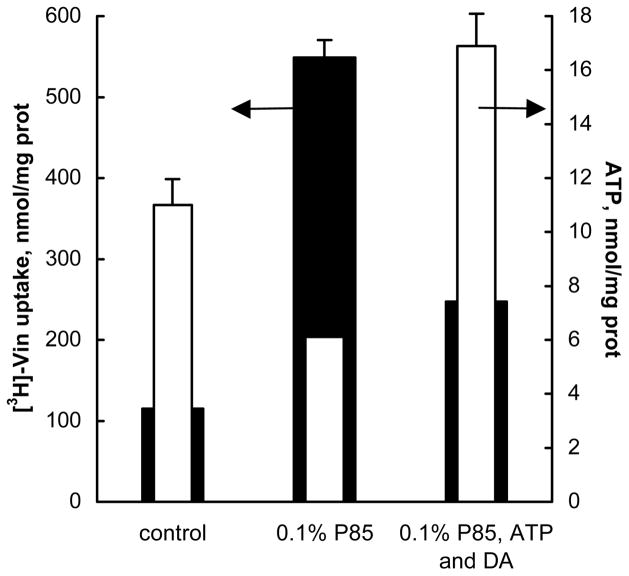
We hypothesized that ATP depletion induced by P85 could result in inhibition of the MRPs drug efflux system and increases in drug accumulation in MRPs-overexpressing cells. To evaluate this hypothesis we examined the relationship between the levels of Vin accumulation in COR-L23/R cells and the levels of intracellular ATP following administration of the block copolymer. For this purpose the cells were exposed for 2 hours to 3H-Vin alone or with 0.1% P85, and the intracellular levels of Vin and ATP were determined as described above. To bypass P85-induced energy depletion, an additional treatment group was also included in this study, in which the 3H-Vin/P85-treatment group was supplemented with 50 μM ATP and 10−5 M dodecylamine, as a permeabilizing agent. As previously reported (24), treatment of the cells with dodecylamine in combination with P85 allows transport of ATP into the cells from the extracellular media. As is seen in Figure 5, there was an inverse correlation between the Vin uptake and ATP intracellular levels. First, in the presence of 0.1% P85 the ATP level was decreased while the drug accumulation was increased compared to the assay buffer control. Second, when the P85 treatment was supplemented by adding extracellular ATP and dodecylamine, Vin uptake was drastically decreased, while the intracellular ATP levels were increased. Exposure the cells to 0.1% P85 and 10−5M dodecylamine alone without extracellular ATP neither increased intracellular concentrations of ATP, nor decreased Vin accumulation (data not shown). This study provides evidence in support of the relationship between the ability of P85 to decrease ATP levels and the increased drug accumulation in MRPs-overexpressing cells.
Fig. 5.
Relationship between ATP intracellular content (filled bars) and Vin accumulation (empty bars) in COR-L23/R cells. Along with the control (first group) and 0.1% P85 solution (second group), cells were treated with energy supplementation system: 0.1% P85, 50μM ATP and 10−5 M dodecylamine (third group).
Effects of P85 on GSH Intracellular Levels
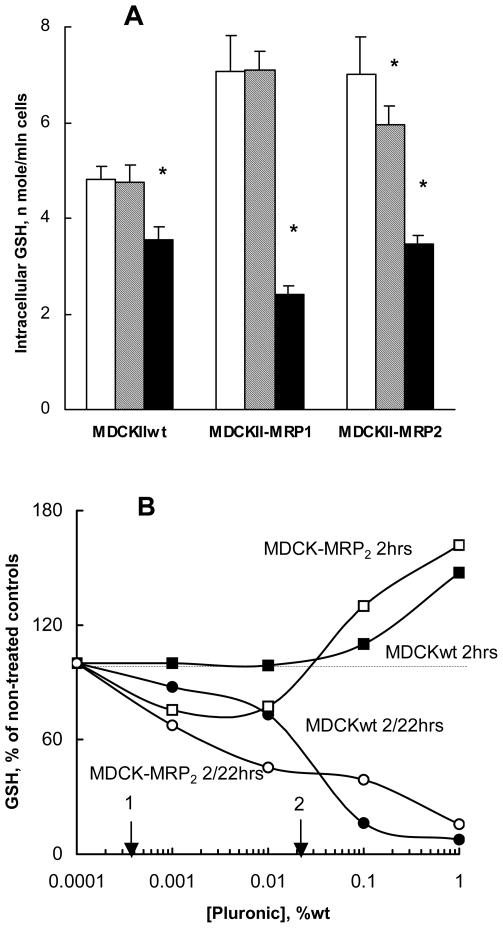
The effects of P85 on GSH levels in MRPs-overexpressing and parental MDCKII cells were examined. As is seen in Figure 6A, there was little or no effect on GSH intracellular levels following 2-hour exposure of MDCKIIwt and MDCKII-MRP1 cells to 0.01% P85. A small (ca. 15%), but statistically significant reduction in GSH levels was observed in MDCKII-MRP2 cells. In contrast, 24-hour exposure to P85 caused drastic reductions in GSH levels in every cell line used. The most pronounced reduction in GSH was observed in MDCKII-MRP1 and MDCKII-MRP2 cells (3- and 2-fold respectively).
Fig. 6.
Effect of P85 on GSH intracellular levels in MDCKII cells. (A): GSH levels determined following treatment of cells with P85-free assay buffer for 2 hours (empty bars), or 0.01% P85 for 2 hours (dashed bars) and 24 hours (filled bars). (B): Concentration dependence of P85 effects on GSH levels in MDCKIIwt (filled symbols) and MDCKII-MRP2 (empty symbols). Cells were treated with 0.01% P85 for 2 hours. GSH levels were determined immediately after this treatment (squares), or after 22 hours in copolymer-free media (circles). For comparison the vertical arrows indicate EC50 values measured for ATP depletion by P85 in MDCKII-MRP2 (1) and MDCKIIwt (2). Statistical significance of the P85 compared to the free-P85 control is shown in the brackets: (*) p< 0.05; (**) p<0.005.
In a separate study presented in Figure 6B MDCKIIwt and MDCKII-MRP2 cells were exposed for 2 hours to various concentrations of P85 and the GSH levels were determined immediately after that. As is seen in Figure 6B, the GSH levels remained practically unchanged immediately after exposure to low concentrations (0.0001% to 0.01%) of P85. The high concentrations of the copolymer (0.1%–1%wt) caused increases in GSH levels. In contrast, 22 hours after exposure to the copolymer, after additional incubation of the cells in the copolymer-free media reductions of GSH levels were observed in every treatment group with cell lines examined. In these cases the GSH depleting effect of the copolymer was increased as its concentration was elevated (Figure 6B).
We also conducted the GSH depletion studies in COR-L23/R (data not presented). These cells appeared much less responsive to 24 h exposure to P85, displaying significant GSH depletion (ca. 40 %) only at the relatively high concentration of P85 (0.5 %).
Effect of P85 on GST Activity in MDCKII Cells
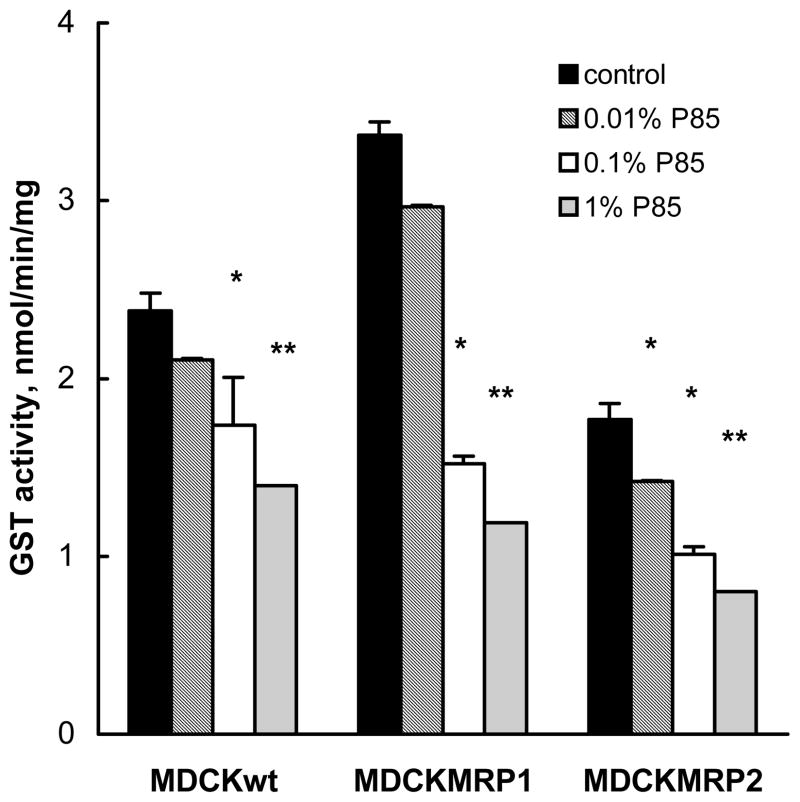
This section evaluated the effects of P85 on GST activity in MDCKIIwt, MDCKII-MRP1 and MDCKII-MRP2 cell lines. As is seen in Figure 7, exposure of the cells to P85 for 2 hours resulted in significant decreases of GST activity in each of these cell lines. These effects of P85 were dose-dependent, as they increased when the concentration of the copolymer was elevated.
Fig.7.
Concentration-dependent effect of P85 on GST activity in MDCKII cells. MDCKIIwt (filled bars), MDCKII-MRP1 (dashed bars), and MDCKII-MRP2 (empty bars) were incubated with P85 for 2 hours, washed with PBS, and GST assay were performed, as described in Materials and Methods. Statistical significance of the P85 effects compared to the P85-free control groups is shown by asterisks: (*) p< 0.05; (**) p<0.005.
Effects of P85 on Drug Cytotoxicity in Resistant and Sensitive Cells
Effects of P85 on cytotoxicity of MRPs substrates, Dox and Vin, were examined using MRPs-overexpressing and non-MRP parental cell lines. Table 3 presents IC50 values for the drugs in the presence and absence of P85. As is seen in the table, P85 caused sensitization of MDCKII-MRP2 cells with respect to Dox, decreasing the IC50 values ca. 125 times. Furthermore, the IC50 of Dox in P85 solutions in resistant cells was similar or even less than that of Dox in P85 or Dox alone in the sensitive cells. In the parental MDCKIIwt cells, alterations in the IC50 values for Dox with P85 were much less pronounced and did not exceed 2 times compared to Dox alone. Exposure of the cells to the same doses of P85 alone did not result in any cytotoxic effects in both cell lines (data not shown). The effect of P85 on Vin cytotoxicity in MDCKII-MRP2 was smaller than the effect obtained with Dox, but still significant (ca. 7 times). In this case, however, similar sensitization was also observed in the parental MDCKIIwt cells, which suggest that the effects involving this drug were not related to overexpression of MRPs.
Table 3.
The IC50 in sensitive and resistant MRPs overexpressing cells#
| Drug | IC50, μM |
|||
|---|---|---|---|---|
| COR-L23 | COR-L23/R | MDCKIIwt | MDCKII-MRP2 | |
| Dox/media | 0.035 | 6.9 | 0.14 | 6.9 |
| Dox/P85 | 0.017 | 6.0 | 0.09 | 0.055 |
| Vin/P85 | 0.025 | 0.01 | 0.01 | 0.5 |
IC50 values were determined at various P85 concentrations ranging from 0.001% to 1% wt. The table presents data for 0.1% wt P85 concentration, at which the changes in IC50 values were the greatest.
The effects of P85 on drug cytotoxicity were also examined in COR-L23/R and COR-L23 cell lines. The results of this study are presented in Table 3. In the case of Dox, P85 did not exhibit the sensitization effect in resistant COR-L23/R cells. Some sensitization was evident in the case of Vin (ca. 10-times respectively). In the sensitive COR-L23 cells the block copolymer had little effect on Vin cytotoxicity resulting in ca. 2 to 2.5 fold changes in IC50 values.
DISCUSSION
The objective of the current study was to examine the effects of Pluronic P85 on drug detoxification systems displayed in MRPs-overexpressing cells, and to evaluate whether this block copolymer could be useful in overcoming MRPs-mediated drug resistance. The following questions have been raised in relation to MRPs drug efflux systems. (1) Does Pluronic increase accumulation of MRP substrates in the MRPs-overexpressing cells? (2) Does Pluronic inhibit MRPs ATPase activity? (3) Does Pluronic induce ATP depletion in MRPs-overexpressing cells? Since MRPs are known to act in concert with the GSH/GST detoxification system, the following questions were also considered. (4) Does Pluronic affect intracellular levels of GSH? (5) Does Pluronic inhibit GST activity? Finally, to assess whether these effects combined could result in overcoming drug resistance, this work examined (6) whether Pluronic enhances cytotoxicity of drugs in MRPs-overexpressing cells?
Based on the results of the current study, the answers to each of these questions were affirmative. First of all, P85 increased accumulation of MRPs substrates, Vin and Dox. Second, P85 displayed several affects in MRPs-overexpressing cells, which can play an important role in sensitization of these cells: membrane fluidization, inhibition of MRPs ATPase activity, and ATP depletion. Furthermore, decreases in GSH levels and inhibition of GST activity that can reduce efficacy of the GSH/GST drug detoxification system, were also observed. Finally, this study demonstrated that P85 can sensitize selected MRPs-overexpressing cells with respect to MRPs-dependent drugs.
Evidence of P85 affects on MRPs drug efflux system was observed in the experiments using MRP-dependent drugs, Vin and Dox. Significant enhancements of accumulation of Vin and Dox induced by P85 in MRPs-overexpressing cells were demonstrated, while there was no effect in non-MRPs parental cells.
Exposure of MRP1- and MRP2-overexpressing cells to P85 decreased the microviscosity of the cell membranes. The alterations in the membrane microviscosity in these cells were likely due to incorporation of the propylene oxide chain of the block copolymer into the hydrocarbon inner leaflet of the cell membrane resulting in changes in membrane structure. It was proposed earlier that membrane fluidization by various agents, including nonionic detergents, can contribute to the inhibition of Pgp drug efflux pump (25). We suggest that similar effect could be also responsible for inhibition of MRPs ATPase activity by Pluronic P85, which is crucial for the proper functioning of this transporter. Similar to Pgp, MRP1 and MRP2 are a two-domain proteins with ATP-binding sites located in each domain (2, 26). Proper interaction of these sites is essential for functional activity of the transporter. Such interactions in MRPs might be disturbed as a result of changes in the protein conformation occurring either due to direct contact of the copolymer with the transporter or due to alterations in the membrane structure induced by the copolymer. It is important to note that at high concentration of P85 partial restoration of MRP ATPase activity was observed. This effect might be responsible for appearance of the maximum in the drug accumulation studies as shown in Tables 1 and 2. Similar phenomenon was also observed in the case of Pgp (7). This could be due to the concentration-dependent segregation of the block copolymer molecules in the two-dimensional clusters in the membrane, which diminishes their interactions with the transport proteins. Remarkably, the MRPs substrate, Vin, added at high concentration (40 μM), abolished the inhibitory effect of P85 on MRP1 ATPase. In contrast, it was shown previously that P85 effectively inhibits Pgp ATPase activity even in the presence of the Pgp substrate, verapamil (14). This could be a reason for greater effects of Pluronics on the substrate transport mediated by Pgp compared to that mediated by MRPs, which was also documented earlier (22).
The key new observation made in this work is that P85 can induce ATP depletion in MRP1- and MRP2-overexpressing cells. It was shown earlier, that P85 translocates into the Pgp-overexpressing cells where it interacts with mitochondria and inhibits respiration processes critical for ATP production (7, 27). We suggest that similar to Pgp-overexpressing cells, P85 interacts with the mitochondria membranes of MRPs-overexpressing cells and interferes with ATP synthesis. Because both MRP1 and MRP2 drug efflux systems are ATP-dependent, reduction of intracellular ATP levels by P85 in MRPs overexpressing cells can result in inhibition of MRPs-mediated drug efflux and increase of the drug accumulation in these cells. Involvement of the ATP depletion component in Pluronic effects on drug accumulation was clearly demonstrated in the ATP supplementation study in COR-L23/R cells. This study suggests that MRP1 function in the presence of P85 was restored when addition of the energy supplementation system brought ATP levels back. Since the block copolymer in this experiment was still present and, presumably, bound with the cell membranes, the ATP supplementation study indicates that membrane fluidization alone may not be sufficient for inhibition of the MRPs efflux system in these cells.
Our previous studies have shown that a delicate balance between hydrophilic and lipophilic components in the Pluronic molecule should be accomplished to enable the most significant impact of the block copolymer in the resistant cancer cells (5). The most efficacious block copolymers are those with intermediate lengths of propylene oxide block and relatively hydrophobic structure (HLB < 20), such as P85 or L61. Such block copolymers are efficiently transported into the cells and inhibit respiration resulting in ATP depletion (28). Hydrophilic block copolymers, which have an extended ethylene oxide block, do not incorporate into lipid bilayers, do not transport into the cells, and as a result, have negligible chemosensitizing effect (28). Extremely lipophilic copolymers with long PO blocks anchor in the plasma membranes and do not reach intracellular compartments. As a result, they do not affect intracellular ATP and have little effect on drug efflux transport.
Efflux of Vin and Dox also depends on intracellular levels of GSH. The timeframe of P85 affect on GSH (24 hours) suggests that GSH depletion could not contribute to alterations of drug accumulation in the cells overexpressing MRPs occurring at the relatively short incubation periods. However, GSH depletion can contribute to increases in accumulation of the drugs when the cells are exposed to or pretreated with the copolymer for prolonged time periods (24 hours), since such treatments can result in profound GSH depletion. P85 also inhibited GST activity in MRPs cells. This effect was relatively fast compared to the effect on GSH, and was displayed following 2-hour exposure of these cells to P85. Overall, this work for the first time suggests that Pluronic block copolymers can inhibit GSH/GST detoxification system in drug resistant cells, which potentially can be useful for sensitization of these cells.
P85 enhances cytotoxicity of Vin and Dox in MRP1- and MRP2-overexpressing cells. Nevertheless, the magnitude of the sensitization effects by the copolymer in MRPs overexpressing cells (125-fold decreases in IC50 for Dox in MDCKII- MRP2) appeared to be less than the affects in Pgp overexpressing cells, in which 100- to 1000-fold increases in IC50 were previously reported (4, 5, 14). In this respect it is remarkable that in Dox-selected resistant COR-L23/R cells Pluronic induced no changes in Dox cytotoxicity. One major difference between these cell lines is in their response to Pluronic in ATP depletion studies. COR-L23/R cells appeared to be much less responsive than MDCKII-MRP2 cells. However, previous studies using MDR tumors demonstrated that ATP depletion by Pluronic was a major contributor to sensitization of these tumors with respect to Dox (14). Furthermore, COR-L23/R cells were also less responsive to P85 in GSH depletion experiments. Therefore, it is possible, that the lack of sensitization of COR-L23/R cells with respect to Dox is due to the small extent of Pluronic affect on the metabolic systems in these cells.
In conclusion, the present work demonstrates sensitization effects of P85 displayed in MRP1- and MRP2-overexpressing cells. These effects included enhanced accumulation of typical MRP substrates and increased cytotoxicity of selected drugs in these cells. Traditional MRPs inhibitors, such as indomethacin or probenecid, were found to be much less efficacious than Pluronic in both, drug accumulation and cytotoxicity studies. This work suggests that Pluronic inhibited MRP ATPase activity, induced ATP and GSH depletion, and inhibited GST activity in MRPs overexpressing cells. Each of these effects or their combination could contribute to the sensitization of MRPs phenotype cells in various ways and to different extents. Further understanding of the mechanisms of the Pluronic effects will be crucial for the future successful development and use of Pluronic formulations to improve chemotherapy of resistant tumors.
Acknowledgments
This study was supported by National Institutes of Health grant CA89225. We would like to thank Piet Borst (Netherlands Cancer Institute, Amsterdam, Netherlands) for kindly providing MRP-transfected cell lines and Dr. Kenneth Brouwer (Glaxo Wellcome, Inc., Research Triangle Park, NC, USA) for kindly providing GF120918.
ABBREVIATIONS
- BBMEC
bovine brain microvessel endothelial cells
- CDNB
1-chloro-2,4-dinitrobenzene
- CMC
critical micelle concentration
- Dox
doxorubicin
- DPH
1,6-diphenyl-1,3,5-hexatriene
- GSH
reduced glutathione
- GST
glutathione S-transferase
- HLB
hydrophilic-lipophilic balance
- Man
mannitol
- MDCKII
Madin Darby Canine Kidney cells
- MDR
multidrug resistance
- MRPs
multidrug resistance-associated proteins
- Pgp
P-glycoprotein
- P85
Pluronic P85
References
- 1.Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 2.Hipfner DR, Cole SP. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta. 1999;1461:359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 3.Alakhov VYu, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by Pluronic P85 block copolymer. Bioconjug Chem. 1996;7:209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 4.Venne A, Mandeville R, Kabanov AV, Alakhov VYu. Hypersensitizing effect of Pluronic L61 on cytotoxic activity, transport, and subcellular distribution of doxorubicin in multiple drug-resistant cells. Cancer Res. 1996;56:3626–3629. [PubMed] [Google Scholar]
- 5.Batrakova E, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm Res. 1999;16:1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- 6.Batrakova EV, Li S, Alakhov VY, Kabanov AV, Elmquist WF. Pluronic P85 enhances the delivery of digoxin to the brain: in vitro and in vivo studies. J Pharmacol Exp Ther. 2001;296:551–557. [PubMed] [Google Scholar]
- 7.Batrakova EV, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- 8.Miller DW, Kabanov AV. Inhibition of multidrug resistance-associated protein (MRP) functional activity with pluronic block copolymers. Pharm Res. 1999;16:396–401. doi: 10.1023/a:1018873702411. [DOI] [PubMed] [Google Scholar]
- 9.Batrakova EV, Miller DW, Kabanov AV. Pluronic P85 increases permeability of a broad spectrum of drugs in polarized BBMEC and Caco-2 cell monolayers. Pharm Res. 1999;16:1366–1372. doi: 10.1023/a:1018990706838. [DOI] [PubMed] [Google Scholar]
- 10.Evers R, Sparidans R, Beijnen J, Wielinga PR, Lankelma J, Borst P. Vinblastine and sulfinpyrazone export by the multidrug resistance protein MRP2 is associated with glutathione export. Br J Cancer. 2000;83:375–383. doi: 10.1054/bjoc.2000.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbottle A, Atherton K, Campbell FC. Role of glutathione S-transferase P1, P-glycoprotein and multidrug resistance-associated protein 1 in acquired doxorubicin resistance. Int J Cancer. 2001;92:777–783. doi: 10.1002/ijc.1283. [DOI] [PubMed] [Google Scholar]
- 12.Evers R, van Deemter L, Janssen H, Calafat J, Oomen LC, Paulusma CC, Oude Elferink RP, Baas F, Schinkel AH, Borst P. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest. 1998;101:1310–1319. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas GA, Stewart S, Rabbitts PH, Williams ED, Twentyman PR. Expression of the multidrug resistance-associated protein (MRP) gene in human lung tumours and normal tissue as determined by in situ hybridisation. Eur J Cancer. 1994;30A:1705–1709. doi: 10.1016/0959-8049(94)00290-l. [DOI] [PubMed] [Google Scholar]
- 14.Batrakova EV, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluronic block copolymers: Selective energy depletion. Br J Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Laat SW, Shinitzky M. Microviscosity modulation during the cell cycle of neuroblastoma cells. Proc Natl Acad Sci U S A. 1977;74:4458–4461. doi: 10.1073/pnas.74.10.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada H. Characterization of the ATPase activity of the Mr 170,000 to 180,000 membrane glycoprotein (P-glycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res. 1988;48:4926–4932. [PubMed] [Google Scholar]
- 17.Taguchi Y, Takada Y, Komano T, Ueda K. Anti-cancer drugs and glutathione stimulate vanadate-induced trapping of nucleotide in multidrug resistance-associated protein (MRP) FEBS Lett. 1997;401:11–14. doi: 10.1016/s0014-5793(96)01421-4. [DOI] [PubMed] [Google Scholar]
- 18.Druekes P, Palm D. Photometric microtiter assay of inorganic phosphate in the presence of acid-labile organic phosphates. Analytical Biochem. 1995;230:173–177. doi: 10.1006/abio.1995.1453. [DOI] [PubMed] [Google Scholar]
- 19.Draper MP, Levy SB. Active efflux of the free acid form of the fluorescent dye 2′, 7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein in multidrug-resistance-protein-overexpressing murine and human leukemia cells. Eur J Biochem. 1997;243:219–224. doi: 10.1111/j.1432-1033.1997.0219a.x. [DOI] [PubMed] [Google Scholar]
- 20.Habig WH, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 21.Ferrari M, Isetta AM. MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods. 1990;131:165–172. doi: 10.1016/0022-1759(90)90187-z. [DOI] [PubMed] [Google Scholar]
- 22.Evers R, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. Br J Cancer. 2000;83:366–374. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garewal HS, Schifman RB, Celniker A. ATP assay: ability to distinguish cytostatic from cytocidal anticancer drug effects. J Natl Cancer Inst. 1986;77:1039–1045. [PubMed] [Google Scholar]
- 24.Slepnev VI, Gubin AN, Batrakova EV, Alakhov VYu, Kabanov AV. Micelles of poly(oxyethylene)-poly(oxypropylene) block copolymer (pluronic) as a tool for low-molecular compound delivery into a cell: phosphorylation of intracellular proteins with micelle incorporated [gamma-32P]ATP. Biochem Int. 1992;26:587–595. [PubMed] [Google Scholar]
- 25.Regev R, Eytan GD. Membrane fluidization by ether, other anesthetics, and certain agents abolishes P-glycoprotein ATPase activity and modulates efflux from multidrug-resistant cells. Eur J Biochem. 1999;259:18–24. doi: 10.1046/j.1432-1327.1999.00037.x. [DOI] [PubMed] [Google Scholar]
- 26.Hagmann W, Konig J, Frey M, Zentgraf H, Keppler D. Purification of the human apical conjugate export pump MRP2 reconstitution and functional characterization as substrate-stimulated ATPase. Eur J Biochem. 1999;265:281–289. doi: 10.1046/j.1432-1327.1999.00735.x. [DOI] [PubMed] [Google Scholar]
- 27.Rapoport N, Timoshin AA. Effect of a polymeric surfactant on electron transport in HL-60 cells. Arch Biochem Biophys. 2000;384:1000–1008. doi: 10.1006/abbi.2000.2104. [DOI] [PubMed] [Google Scholar]
- 28.Batrakova EV, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain microvessel endothelial cells. J Pharmacol Exp Ther. 2003;304:845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]