Abstract
Amphiphilic triblock copolymer, poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide), Pluronic® P85, is unexpectedly shown to utilize sophisticated cellular trafficking mechanisms and enter brain microvessel endothelial cells and primary neurons that are poorly penetrable. Though caveolae serve as a primary entry site for the copolymer single chains, in cells devoid of caveolae, the copolymer can still exploit caveolae- and clathrin-independent routes. This parallels the copolymer's trafficking itinerary with that of biological pathogens. The similarity is reinforced since both bypass early endosomes/lysosomes and transport to the endoplasmic reticulum. The copolymer finally reaches the mitochondrion that serves as its final destination. Notably, it also succeeds to gain entry in brain microvessel endothelial cells through caveolae and in primary neurons through caveolae- and clathrin-independent pathway. In neurons the copolymer accumulates in the cell body followed by anterograde trafficking towards the axons/dendrites. Overall, dissecting the trafficking of a synthetic polymer in multiple cell types triggers development of novel delivery systems that can selectively target intracellular compartments and provide entry in cells currently considered impenetrable.
Introduction
Millions of years of evolution shaped the ability of viruses and bacteria pathogens to invade mammalian cells. In doing so, these pathogens are employing sophisticated intracellular trafficking mechanisms that allow them to avoid lysosomal degradation. Most recently caveolae/lipid raft-based endocytosis has gained tremendous attention as a portal of entry of many viruses like SV40, as well as bacterial pathogens like Cholera toxin [1]. The high infectiveness of these pathogens to a broad spectrum of cells is believed to be due to the presence of high affinity receptors concentrated in the caveolae/lipid raft structures. Sophisticated structural arrangement is therefore believed to be crucial for the ability of these pathogens to enter cells. Surprisingly in this study we demonstrate that a synthetic polymer with a very simple structure - poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO, also known as Pluronic® or poloxamer), can transport into the cells using exactly the same pathways as the biological pathogens.
Pluronic® block copolymers are very interesting molecules that have attracted attention due to their remarkably broad spectrum of useful pharmacological activities [2]. Examples include the ability of Pluronics to sensitize multidrug-resistant (MDR) tumors [3], activate nuclear import and transcription of DNA [4] and stimulate immunity [5]. These effects are all believed to be related to the interactions of the block copolymer chains with the plasma membrane. An ability of one copolymer, Pluronic® P85 (P85), to localize with caveolae and employ this pathway for cellular entry has been recently shown [6]. In this study we further examine the initial, intermediate and later stages of trafficking of P85, and compare it to Cholera toxin B (CTB), which can employ multiple pathways, such as caveolae as well as caveolae- and clathrin-independent endocytosis, for cellular entry [7]. Along, with the brain microvessel endothelial cells that have functional caveolae we also use cells devoid of caveolae, such as confluent epithelial cells (caveolae absent at the apical side), caveolae-deficient fibroblasts and neurons. The ability of Pluronic® block copolymers to employ multiple pathways and enter the blood brain barrier (BBB) and neuronal cells is of considerable interest for the potential use of these molecules in the development of novel agents for central nervous system (CNS) drug delivery.
Material and Methods
Materials
P85, EO26-PO40-EO26 (lot # WPAU-549B) was kindly provided by BASF Corp. Alexa 488- or 594-labeled CTB, Alexa 488- or 647- labeled Tf, DAPI (4′,6-diamidino-2-phenylindole), ERtracker™, Mitotracker™, Lysotracker™, Lipofectamine™ 2000, Organelle Lights™ Endosome-GFP and Cellular Lights™ Actin-GFP were purchased from Invitrogen Inc (Carlsbad, CA). Methyl-β-cyclodextrin (MBCD), sucrose, tetrarhodamine B isothiocyanate (TRITC) or fluorescein isothiocyanate (FITC) was purchased from Sigma-Aldrich, (St. Louis, MO). Bovine serum albumin (BSA) was purchased from Fisher Scientific (Waltham, MA). DRAQ-5 was purchased from Biostatus Limited (UK). K44A-GFP dynamin mutant was a kind gift from Dr. Steve Caplan lab at the University of Nebraska Medical Center.
Labeling of P85
Anhydrous Pluronic P85 (2 g) was activated with CDI (213 mg) in 10 ml of acetonitrile for 2 h at 37°C and reacted with ethylenediamine (313 mg) in 20 ml ethanol for 12 h at room temperature. The reaction mixture was dialyzed in a 2-kD cutoff membrane against 15 % ethanol for 72 h (change ethanol twice) and lyophilized. TRITC or FITC was dissolved in dimethylformamide (DMF) (2.6 mg/mL) with 0.5 % (v/v) diisoproplethylamine and 520 μL of this solution was added to amino-Pluronic (11.7 mg, 2.54 μM). After 3 days on a rocker at 37 °C the DMF was removed in a stream of air and the residual taken up in 400 μL methanol. Free dye was removed by gel filtration (Sephadex LH20 in methanol) until no band of the free dye was observed. The methanol was removed in a stream of air and the product (8.1 mg) freeze-dried from water.
Cell lines
Madin-Darby canine kidney (MDCK) cells were maintained in Dulbecco's modified eagle's medium (DMEM) containing 10% heat inactivated fetal bovine serum (FBS), penicillin/streptomycin as described elsewhere. All tissue material media was obtained from Gibco Life Technologies, Inc. (Grand Island, NY). MDCK cells were used 48 hrs after plating. 3T3 mouse embryonic fibroblasts (MEFs), KO (knock out) cell line (homozygous for a disruption of the caveolin-1 gene Cav-1 -/-, ATCC CRL-2753) and 3T3 MEFs WT (wild type, Cav-1 +/+) cell line (ATCC CRL-2752) were maintained in DMEM, containing 10% heat inactivated FBS and 0.01% penicillin/streptomycin.
Primary culture of cortical neurons and brain microvessel endothelial cells
Primary culture of cortical neurons was prepared as described below. In brief, cortices were isolated from fetal (embryonic 18 days old) rat and then incubated with trypsin for 20 min at 37°C. This enzymatic reaction was stopped by adding 200 μl/ml of fetal bovine serum along with DNAase and incubated at 4°C for 30 minutes. Cells were dissociated by gentle trituration via polished glass pasteur pipettes, washed 3 times with the DMEM media and then plated at a total density of 2×105 cells per well of a 6-well plate having a coverslip coated with poly-D-lysine. Complete neurobasal medium was used for plating the neurons, which consists of Neurobasal/B-27/Dextrose/Pen-strep/Glutamine (Invitrogen Inc Carlsbad, CA). Cells were maintained at 37°C in a humidified 5% CO2 incubator. After 72 hrs, 40% of the media was replaced by fresh medium having 10 μM of Ara-C (Sigma Aldrich, St Louis, MO) so as to prevent glia proliferation. The culture medium was changed every 3 days for the remainder of the experiment. All experiments were performed on cultured cells at days 7-10. Primary culture of brain microvessel endothelial cells isolated from the gray matter of the cerebral cortex of bovine brain was isolated and maintained as previously described [8].
FACS analysis
Brain microvessel endothelial cells (5×104 cell/well in 24 well plates) were exposed to 0.001% FITC-P85 alone in the presence or absence of cells pre-treated for 30 min with inhibitors of endocytosis (5 mM MBCD, or 0.45 M sucrose) and then the same inhibitors were also present during subsequent incubation with the copolymer. In select experiments Cav KO and WT cells were treated with 0.001% TRITC-P85. Cells were washed, trypsinized and resuspended in phosphate-buffered and saline/1% BSA. The mean fluorescence intensity was analyzed using Becton Dickinson FACStarPlus flow cytometery operating under Lysis II (San Jose, CA) equipped with an argon ion laser. Data were acquired in linear mode and visualized in logarithmic mode. Data from 10,000 events were gated using forward and side scatter parameters to exclude debris and dead cells. All experiments were conducted thrice and measurements were conducted in triplicates and data presented as means +/- SEM. Statistical comparisons between groups were made using Student's t-test.
Confocal analysis on live cells
MEF 3T3 KO cells (1×106) were plated in Lab-Tek™ chambered coverglass (Fischer Scientific, Waltham, MA) and after two days (37°C, 5% CO2) were exposed for 30 min to 0.001% TRITC-P85. In select experiments cells were treated in the presence of Alexa 488-CTB (5 μg/ml) or Alexa 488-Tf (5 μg/ml), washed and kept in complete media for imaging using the confocal microscope. Brain microvessel endothelial cells were exposed to 0.001% FITC-P85 for 30 min in the presence or absence of 5 μg/ml Alexa 594-CTB or 5 μg/ml Alexa 647-Tf In another experiment, MDCK cells were treated with Lysotracker™ green (100 nM) or ERTracker™ green (5 μg/ml) or Mitotracker™ green (1μg/ml) for 10 min, cells were washed and incubated with TRITC-P85 for 30 min and imaged under confocal microscope. Cortical neurons grown on coverslips were treated with 0.001% TRITC-P85 for 1 hr. In select experiments the neurons were treated with 0.001% TRITC-P85 in the presence of CTB (5 μg/ml). Cells were washed and coverslips transferred to Bioptech dishes (Fischer Scientific, Waltham, MA) for live cell confocal imaging.
Immunocytochemistry on Fixed Cells
Brain microvessel endothelial cells were pretreated with DMEM without FBS for 30 min, and then treated with 0.001% FITC-P85 for 1 hr, washed and fixed with 4% paraformaldehyde. Rabbit anti-caveolin-1–Cy3 antibody (Sigma Aldrich, St Louis, MO) (1:100) and mouse anti-clathrin antibody (Affinity Bio-reagents, Golden, CO) (1:10) were incubated in blocking buffer overnight at 4°C. For detection of anti-clathrin antibody, specific IgG antibody (1:250) conjugated to Alexa 568 (Invitrogen Inc, Carlsbad, CA) was added to cells for 1 h at 37°C. Cells were examined under confocal microscope. In a separate experiment primary cortical neuronal cells were pretreated with DMEM without FBS for 30 min, and then treated with 0.001% TRITC-P85 for 1 hr, washed and fixed with 4% paraformaldehyde. Cells were then exposed to mouse anti-MAP-2 antibody (Millipore, Billerica, MA) (1:100) in blocking buffer for overnight at 4°C. For detection of anti-MAP-2, mouse-specific IgG secondary antibody (1:250) conjugated to Alexa 488 (Invitrogen Inc, Carlsbad, CA.) was added to cells and visualized using confocal microscopy.
Cell transfection
MDCK cells were transfected using Organelle Lights™ Endosome-GFP and Organelle Lights™ Actin-GFP kit. This kit contains an Organelle Lights™ reagent which is baculovirus, a efficient delivery system that contains a gene sequence which encodes for Rab5a (targeting sequence, an early endosome marker) or zctin fused to a GFP (fluorescent protein) already incorporated into the viral genome and the kit also has an enhancer solution for increased expression of the chimera. Briefly, cells were plated, allowed to attach, and treated with Organelle Lights reagent at room temperature. in the dark for 2-4 hrs. The reagent was aspirated and cells were incubated in DMEM containing 1X enhancer for 2 hrs followed by washing and addition of complete medium. The transfected cells were treated with TRITC-P85 and exposed for 30 min, washed, and imaged using confocal microscopy. In select experiments MDCK cells were transfected with plasmid DNA (1μg/ml) of K44A dynamin mutant using Lipofectamine™ 2000, with the transfection protocol as previously described [9].
Results
Internalization and intracellular trafficking of Pluronic®
P85 molecules entered cells through vesicular trafficking and remained in distinctive vesicular structures in MDCK cells (Fig 1A, Movie 1). The observed pathways did not depend on the fluorescent label used (TRITC or FITC). Previously, we reported that the initial entry of this copolymer's single chains (unimers) is based on caveolae-mediated endocytosis [6]. However, to our surprise, the copolymer uptake also proceeded in the confluent MDCK cells, which are devoid of functional caveolae endocytosis pathway at the apical side [10]. Therefore, to further tackle the entry mechanism, we transfected MDCK cells with a dynamin mutant K44Adyn, this inhibits both caveolae- and clathrin-dependent endocytosis [11-12]. Interestingly, P85 unimers were able to enter cells expressing K44Adyn, which suggests that they can use a dynamin-independent pathway (Fig. 1B). The internalization of Tf in such cells was completely abolished. We also observed significant colocalization of P85-containing vesicles with the actin filaments (Fig. 1C). This is consistent with our earlier data where P85 entry was reduced by an inhibitor of actin-dependent endocytosis, cytochalasin D [6]. Overall, these data suggest that in addition to caveolae P85 can use alternative endocytic pathway(s), which can be characterized as 1) caveolae- and clathrin-independent, 2) dynamin-independent and 3) actin-dependent. Such pathway(s) even in the absence of caveolae ensure the initial uptake of the P85 unimers in cells. To further validate this conclusion we studied the entry of the copolymer in caveolae KO cells (mouse embryonic fibroblasts, MEF 3T3). Consistent with the above observations KO cells accumulated considerable amount of P85 even though the copolymer uptake was significantly decreased in these cells compared to the wild type (WT) fibroblasts (Fig. 1D). Interestingly, CTB, a marker of caveolae-mediated endocytosis, was also able to enter KO cells. Furthermore, the copolymer colocalized with CTB (Fig. 1E). Although, CTB was shown to enter cells primarily through the caveolae, in the absence of caveolae it was also reported to employ multiple pathways, including caveolae- and clathrin-independent endocytosis [7]. In contrast, as expected there was little if any co-localization of P85 with Tf, a marker of clathrin-mediated endocytosis (CME) (Fig. 1F).
Fig. 1. Initial stage of cellular entry of P85.

In all experiments cells were exposed to 0.001% TRITC-P85 (red) at 37°C for 30 min and analyzed by (A-C, E, F) confocal microscopy or (D) flow cytometry. (A) MDCK cells, (B) MDCK cells transfected with K44Adyn-GFP (dynamin mutant), (C) MDCK cells transfected with cellular Lights™ Actin-GFP, (D) KO and WT MEF 3T3 cells, (E, F) KO cells exposed to the copolymer in the presence of (E) Alexa 488-labeled CTB (5 μg/ml), or (F) Alexa 488-labeled Tf (5 μg/ml). (A) and (B) represent confluent cells. (B, C) Expression of the corresponding proteins is detected by GFP (green). (B) The blue line highlights cells, which express dynamin mutant (green) and show uptake of P85 (red). (D) Data are mean ± SEM, *** p<0.01.
Subsequent stages of internalization of P85 were studied in MDCK cells. In cells transfected with Rab5-GFP, a marker of early endosomes, no colocalization with the copolymer was observed during the first 3 min. (Fig. 2A, Movies 2) or any time after that. However, after 15 min. P85 partially colocalized with ERTracker™, a marker for endoplasmic reticulum (ER) (Fig. 2B). Yet this colocalization was transient and after 30 min completely disappeared. At this time point (and later) P85 exhibited strong colocalization with the mitochondria (MitoTracker™) (Fig. 2C), but no colocalization with the lysosomes (MitoTracker™) (Fig. 2D). Altogether, these data suggest that following its initial entry into cells P85 bypassed early endosomes or lysosomes but appeared to first transport to endoplasmic reticulum (ER) and then to mitochondria, which serve as the final destination.
Fig.2. Intermediate and late stages of cellular entry of P85.
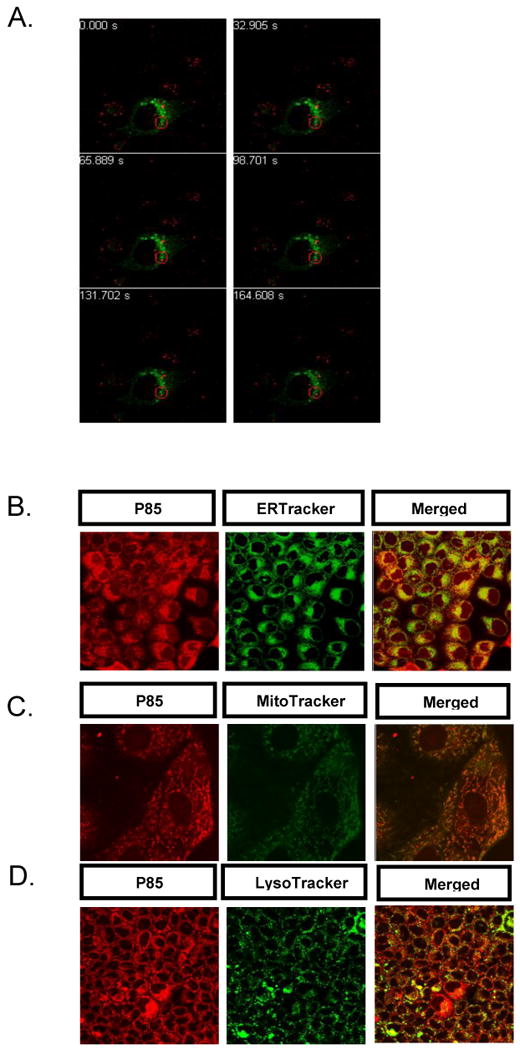
In all experiments cells were exposed to 0.001% w/v TRITC-P85 (red) at 37°C for 30 min. (A) MDCK cells transfected with Rab5-GFP (green). (B-D) MDCK cells stained with (B) ERTracker™ (green) (5 μg/ml) (C) MitoTracker™ (green) (5 μg/ml) (D) LysoTracker™ (100 nM) (green). (A) The red circle highlights distinct localization of the copolymer and early endosomes. See Movie S2 for complete time lapse experiment with Rab-5 GFP transfected MDCK cells.
Caveolae-mediated trafficking of P85 in brain cells
Our next goal was to determine whether P85 can employ selected endocytic pathways to enter brain cells, which are commonly considered impenetrable. Since, P85 was shown to serve as a transducer to target proteins across the BBB [13] our initial studies focused on the primary bovine brain microvessel endothelial cells. Flow cytometery suggested that P85 uptake in brain microvessel endothelial cells was significantly reduced by inhibition of caveolae with methyl-β-cyclodextrin but remained unchanged in the presence of hypertonic sucrose, which inhibits CME (Fig. 3A). A selective interaction of the copolymer with caveolae was further reinforced by immunocytochemistry, which revealed strong colocalization of P85 with caveolin-1 but not with clathrin-1 antibodies (Fig. 3B, C). This was also consistent with the live cell imaging, which suggested colocalization of P85 with CTB but not with Tf (Fig. 3D). Taken together these data suggest that P85 can enter brain microvessel cells using caveolae-mediated endocytosis as a primary entry route into the BBB.
Fig.3. Entry of P85 in primary bovine brain microvessel endothelial cells.
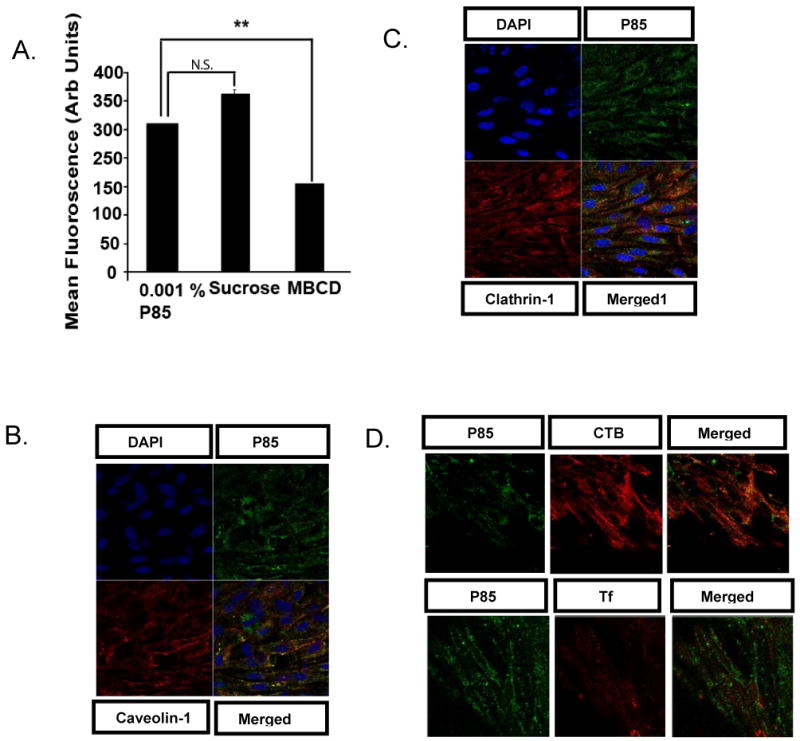
(A) Flow cytometery was performed on cells exposed to the copolymer (FITC-P85, 0.001% w/v) for 60 min. in the presence or absence of different inhibitors of endocytosis (sucrose-0.45 M, MBCD (methyl-β-cyclodextrin)- 5mM). (B-D) Confocal microscopy was performed on cells exposed to FITC-P85 (green, 0.001% w/v) for 30 min. (B-C) Cells were fixed and exposed to primary antibody (4° C overnight) against (B) Cy-3 labeled antibody against Caveolin-1 (5 μg/ml) (C) Clathrin-1 (5 μg/ml), and detected by exposing cells with Alexa 594 labeled secondary antibody. (D) Live cell imaging was performed in presence of Alexa 594- labeled CTB (5 μg/ml) or Alexa 647-labeled Tf (5 μg/ml)). (A) Data are mean +/- S.E.M. (n = 3). Statistical comparisons between the control group (no inhibitor) and the corresponding inhibitor group are shown as follows: **p = 0.01, n.s-not significant. (B-C) Cells were stained with DAPI (10nM) for detection of cell nucleus.
Even more surprisingly, we demonstrated that the copolymer is capable to transport into primary cortical neurons which are devoid of caveolae [7]. P85 is shown to localize within the vesicular structures of the cell body as well as axons/dendrites in neuronal cells (Fig. 4A). Confocal microscopy in fixed neurons revealed that P85 colocalized with MAP-2 (Microtubule Associate Protein-2) antibody, a specific marker for neurons (Fig. 4B). Furthermore, the live cell imaging of neurons revealed that P85 initially entered the cell body of primary neurons (Fig 4C). Interestingly, its entry was separate from that of CTB since many cells stained by P85 did not appear to carry CTB at the same time. However, in a few cells strong colocalization of CTB and P85 was observed in the cell body. Subsequently, both the P85 and CTB transported from the cell body towards the axons/dendrites where they also colocalized (Fig 4C).
Fig. 4. Entry of P85 in primary rat cortical neurons visualized by confocal microscopy.
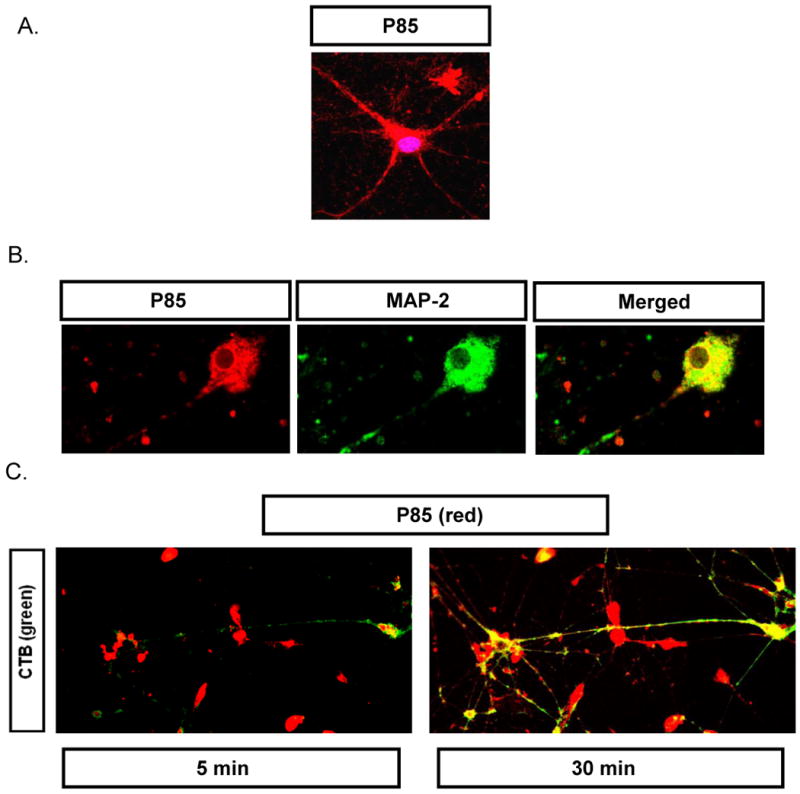
(A) Cells exposed to TRITC-P85 (red, 0.001% w/v) for 1 hr and then stained by DRAQ-5 (20 nM, 30 min.) to visualize nucleus. (B) Cells exposed to TRITC-P85 as in panel (A) and then fixed and stained by anti-MAP-2 antibody (10 μg/ml, 4° C overnight) and Alexa 488- labeled secondary antibody. (C) Cells exposed to TRITC-P85 (0.001% w/v) in the presence of Alexa 488-labeled CTB (5 μg/ml) for 60 min, washed and subjected to time lapse live cell imaging after 5 and 30 min.
Discussion
There are multiple endocytic pathways involved in the delivery of macromolecules to distinct intracellular compartments within the cells [14]. Among these portals of cellular entry, CME ranks as the most well characterized pathway and is considered the classical route of endocytosis. This pathway requires polymerization of clathrin that binds to the plasma membrane, recruits a variety of adaptor and accessory proteins and forms membrane invaginations, which then pinch off through scission by the GTPase dynamin. The clathrin-coated vesicles are either destined for the lysosomes (which is preceded by shedding off the clathrin coat and successive fusion of the vesicles to early and late endosomes), or undergo recycling towards the plasma membrane [14]. Nanomaterials like Pluronic® micelles [6] or poly(lactic-co-glycolic acid (PLGA) nanoparticles [15], have been shown to use this pathway for cellular entry. This pathway is, however, predicted to be a caveat for delivery of many drugs, proteins and polynucleotides, as they can be degraded in the acidic environment of the lysosomes [16]. Caveolae-mediated endocytosis is an alternative route based on pre-formed cholesterol-rich “flask-shaped” invaginations in the cell membrane, termed caveolae. This pathway also requires dynamin for scission, but its destination varies from that of CME [17]. Most of the caveolae cargo transports to neutral caveosomes and enters the ER, bypassing the lysosomes. We demonstrate that this is the case of single chains of P85. Furthermore, we report that the eventual destination of Pluronic® is mitochondria, which is consistent with its previously reported metabolic activities [2].
In addition to caveolae, Pluronic® is demonstrated here to enter cells through a distinct pathway, which is 1) caveolae- and clathrin-independent, 2) dynamin-independent and 3) actin-dependent. Therefore, caveolae seem to be sufficient but not necessary for cellular entry of the block copolymer chains. Perhaps because of its ability to use multiple cellular entry pathways, Pluronic® exhibits high permeability with respect to many cells including primary brain endothelial cells and neurons, which are considered nearly impenetrable. In this regard this block copolymer is unique for synthetic macromolecules and similar to proteins that are responsible for cellular entry of pathogens such as Cholera toxin, SV40 and Shiga toxin. These pathogens have been shown to utilize both caveolae-mediated endocytosis as a primary entry route as well as caveolae- and clathrin-independent endocytosis in caveolae-deficient cells [7, 18]. Such similarity between the trafficking of P85 and CTB is striking in our study. In particular, in caveolae-deficient cells P85 did not use CME, since it did not colocalize with Tf, but did colocalize with CTB. Current evidence suggests that caveolae- and clathrin-independent route is also dynamin-independent [7]. It employs Rab5 negative endosomes and a newly characterized clathrin-independent carrier (CLIC)/GPI-AP-enriched early endosomal compartment (GEEC) that allows it to bypass the early endosomes/lysosomes and reach the ER [7]. Consistent with these findings P85 cellular entry is dynamin-independent since it readily enters cells transfected with dynamin mutant (K44A).
Of interest is the ability of P85 to reach mitochondria. The trafficking to mitochondria is not fully understood. However, recent studies suggest that viral proteins are transported from the ER to mitochondria through mitochondria-associated membrane (MAM) compartment [19]. The MAM sites responsible for ER-mitochondria contact enable lipid transfer and generation of mitochondrial signaling and response to stress. Different tethering proteins in eukaryotes have been discovered which may serve as a channel between the ER and mitochondria [20]. One might speculate that after reaching ER, Pluronic® might be able to associate with these domains through its hydrophobic chains, resulting in mitochondrion targeting. However, existence of a direct pathway towards mitochondria cannot be excluded. The observation of the delivery of Pluronic® to mitochondria is of paramount significance for the studies of the pharmacological effects of this block copolymer in MDR cells. Being one of the most potent known synthetic chemosensitizers of MDR, Pluronic® was shown to exhibit its effect P-glycoprotein (Pgp) ATPase pump through inhibition of mitochondrial respiration and ATP depletion [2]. These effects of Pluronic® are selective to the MDR cell phenotype, and it remains to be seen whether the amounts of Pluronic® delivered to mitochondria in MDR and non-MDR cells are also different.
Finally, improved delivery of drugs, biomacromolecules and imaging agents to neuronal cells is essential for treatment and diagnosis of many CNS diseases. The brain microvessel endothelial cells form tight junctions, have relatively low endocytic activity, and express Pgp [21]. As a result BBB represents a formidable challenge for delivery to the brain. Strategies employed to cross the BBB include the use of nanomaterials targeted to receptors or Pluronic® to inhibit Pgp at the lumenal side of brain capillaries [22-23].
The present finding that P85 enters brain microvessel endothelial cells through caveolae is significant in view of previously demonstrated ability of this copolymer to carry protein molecules across BBB [13], [24] and into neurons [25]. This insight is important since caveolae-mediated transport is considered to be a major player in the transcytosis across endothelial cells [26]. Moreover, P85 utilizes distinctive punctuate structures within the neuronal cell body and like CTB can traffic toward the dendrites and axons. Anterograde trafficking of different cargoes towards the synapses is an essential part of neuronal physiological function, including interneuron communication and plasticity of excitatory synaptic transmission [27-28]. The ability of a simple synthetic polymer to reach these secluded nanoscopic areas in neurons holds significance since different diseases impede this communication.
Conclusions
We demonstrate that Pluronic® block copolymers exploit highly specific and regulated endocytic machinery reminiscent of biological pathogens. Similarly to such pathogens they can enter nearly impenetrable cells and reach precisely located intracellular organelles, such as mitochondria and neuronal dendrites. In this study we demonstrate that P85, similarly to CTB, can employ multiple pathways, such as caveolae as well as caveolae- and clathrin-independent endocytosis, for cellular entry. This multiplicity of pathways allows the copolymer transport in a variety of cells including cells with and without caveolae. Following the initial entry into cells, P85 is shown to bypass early endosomes and lysosomes and transport first to the ER and then to the mitochondria, which serves the copolymer's final destination. These intriguing findings for the first time show that single chains of synthetic polymers can employ the same initial and final stages of trafficking as much more complex biological structures. We hope that future understanding of the portals of entry exploited by synthetic polymers in cells will allow designing highly selective and efficacious polymer therapeutics.
Supplementary Material
The movie shows that the fluorescently labeled copolymer is associated with the vesicle-like structures, which exhibit characteristic oscillating behavior and periodic translocation reminiscent of vesicular trafficking in cells. Cells were exposed to TRITC-P85 for 15 min. and time lapse imaging was performed for 5 min., each image was taken every 30 s. The duration of the movie is 2 s. Temperature, humidity and CO2 were maintained using a confocal chamber.
MDCK transfected with Rab5-GFP prior to the experiment cells were exposed to TRITC-P85 for 30 min. and time lapse imaging was performed for 3 min., each image was taken every 10 s. Temperature, humidity and CO2 was maintained using a confocal chamber.
Acknowledgments
This study was supported National Institutes of Health R01CA089225 and R01NS051334 to AVK and UNMC Bukey fellowship to GS. We would also like to thank the Confocal Microscopy and Flow Cytometery Core Facilities at UNMC.
Footnotes
Authors Contribution: AVK designed research; AVK and GS analyzed data and wrote the paper; GS performed all experiments, VG isolated and cultured primary cortical neurons and RL synthesized TRITC labeled Pluronic® P85.
Disclosure: AVK is a co-founder, shareholder and chair of scientific advisory board of Supratek Pharma Inc. (Montreal, Canada), which developed Pluronic-based SP1049C and SP1017 formulations for chemotherapy and gene delivery respectively.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Medina-Kauwe LK. “Alternative” endocytic mechanisms exploited by pathogens: New avenues for therapeutic delivery? Adv Drug Deliv Rev. 2007;59(8):798–809. doi: 10.1016/j.addr.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130(2):98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minko T, Batrakova EV, Li S, Li Y, Pakunlu RI, Alakhov VY, et al. Pluronic block copolymers alter apoptotic signal transduction of doxorubicin in drug-resistant cancer cells. J Control Release. 2005;105(3):269–278. doi: 10.1016/j.jconrel.2005.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Z, Sahay G, Sriadibhatla S, Kabanov AV. Amphiphilic block copolymers enhance cellular uptake and nuclear entry of polyplex-delivered DNA. Bioconjug Chem. 2008;19(10):1987–1994. doi: 10.1021/bc800144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaymalov ZZ, Yang Z, Pisarev VM, Alakhov VY, Kabanov AV. The effect of the nonionic block copolymer pluronic P85 on gene expression in mouse muscle and antigen-presenting cells. Biomaterials. 2009;30(6):1232–1245. doi: 10.1016/j.biomaterials.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahay G, Batrakova EV, Kabanov AV. Different internalization pathways of polymeric micelles and unimers and their effects on vesicular transport. Bioconjug Chem. 2008;19(10):2023–2029. doi: 10.1021/bc8002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doherty GJ, McMahon HT. Mechanisms of Endocytosis. Annu Rev Biochem. 2009;78(1):857. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 8.Miller DW, Audus KL, Borchardt RT. Application of cultured endothelial cells of the brain microvasculature in the study of the blood-brain barrier. Methods Cell Sci. 1992;14(4):217–224. [Google Scholar]
- 9.Gebhart CL, Kabanov AV. Evaluation of polyplexes as gene transfer agents. Journal Control Release. 2001;73(2-3):401–416. doi: 10.1016/s0168-3659(01)00357-1. [DOI] [PubMed] [Google Scholar]
- 10.Vogel U, Sandvig K, van Deurs B. Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci. 1998;111(6):825–832. doi: 10.1242/jcs.111.6.825. [DOI] [PubMed] [Google Scholar]
- 11.Damke H, Baba T, Warnock D, Schmid S. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127(4):915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141(1):101–114. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi X, Batrakova E, Banks WA, Vinogradov S, Kabanov AV. Protein conjugation with amphiphilic block copolymers for enhanced cellular delivery. Bioconjug Chem. 2008;19(5):1071–1077. doi: 10.1021/bc700443k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 15.Vasir JK, Labhasetwar V. Quantification of the force of nanoparticle-cell membrane interactions and its influence on intracellular trafficking of nanoparticles. Biomaterials. 2008;29(31):4244–4252. doi: 10.1016/j.biomaterials.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harush-Frenkel O, Altschuler Y, Benita S. Nanoparticle-Cell Interactions: Drug Delivery Implications. Crit Rev Ther Drug Carrier Syst. 2008;25(6):485–544. doi: 10.1615/critrevtherdrugcarriersyst.v25.i6.10. [DOI] [PubMed] [Google Scholar]
- 17.Carver LA, Schnitzer JE. Caveolae: mining little caves for new cancer targets. Nat Rev Cancer. 2003;3(8):571–581. doi: 10.1038/nrc1146. [DOI] [PubMed] [Google Scholar]
- 18.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson Chad D, Colberg-Poley Anamaris M. Access of viral proteins to mitochondria via mitochondria-associated membranes. Rev Med Virol. 2009;19(3):147–164. doi: 10.1002/rmv.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, et al. An ER-Mitochondria Tethering Complex Revealed by a Synthetic Biology Screen. Science. 2009;325(5939):477–481. doi: 10.1126/science.1175088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 22.Pardridge W. Drug Targeting to the Brain. Pharm Res. 2007;24(9):1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 23.Kabanov AV, Gendelman HE. Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog Polym Sci. 2008;32(8-9):1054–1082. doi: 10.1016/j.progpolymsci.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batrakova EV, Vinogradov SV, Robinson SM, Niehoff ML, Banks WA, Kabanov AV. Polypeptide point modifications with fatty acid and amphiphilic block copolymers for enhanced brain delivery. Bioconjug Chem. 2005;16(4):793–802. doi: 10.1021/bc049730c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi X, Z M, Vinogradov S, Kabanov A. Pluronic Modified Superoxide Dismutase 1 (SOD1) attenuates Angiotensin II induced increase in intracellular superoxide in neurons. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2010.04.039. under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25(3):327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cognet L, Groc L, Lounis B, Choquet D. Multiple Routes for Glutamate Receptor Trafficking: Surface Diffusion and Membrane Traffic Cooperate to Bring Receptors to Synapses. Sci STKE. 2006;2006(327):pe13. doi: 10.1126/stke.3272006pe13. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29(1):325. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The movie shows that the fluorescently labeled copolymer is associated with the vesicle-like structures, which exhibit characteristic oscillating behavior and periodic translocation reminiscent of vesicular trafficking in cells. Cells were exposed to TRITC-P85 for 15 min. and time lapse imaging was performed for 5 min., each image was taken every 30 s. The duration of the movie is 2 s. Temperature, humidity and CO2 were maintained using a confocal chamber.
MDCK transfected with Rab5-GFP prior to the experiment cells were exposed to TRITC-P85 for 30 min. and time lapse imaging was performed for 3 min., each image was taken every 10 s. Temperature, humidity and CO2 was maintained using a confocal chamber.


