Abstract
Several homo-, random and block copolymers based on poly(2-oxazoline)s (POx) were synthesized and conjugated to horseradish peroxidase (HRP) using biodegradable and non-biodegradable linkers. These conjugates were characterized by amino group titration, polyacrylamide gel electrophoresis (PAGE), isoelectric focusing, enzymatic activity assay and conformation analysis. The conjugates contained on average from about one to two polymer chains per enzyme. From 70% to 90% of enzymatic activity was retained in most cases. Circular dichroism (CD) analysis revealed that HRP modification affected the secondary structure of the apoprotein but did not affect the tertiary structure and heme environment. Enhanced cellular uptake was found in the conjugates of two block copolymers using both MDCK cells and Caco-2 cells, but not in the conjugates of random copolymer and homopolymer. Conjugation with a block copolymer of 2-methyl-2-oxazoline and 2-butyl-2-oxazoline led to the highest cellular uptake as compared to other conjugates. Our data indicates that modification with amphiphilic POx has the potential to modulate and enhance cellular delivery of proteins.
Keywords: Protein engineering, polyoxazolines, biocompatible, endocytosis, MDCK, Caco-2 cells
INTRODUCTION
Development of therapeutic proteins is one of the most thriving areas in today’s pharmaceutical and biotech industry. However, many hurdles need to be overcome before novel protein drugs can reach the marketplace (1, 2). One such problem is the need to increase stability and circulation time of the protein in the body (1). This problem has been successfully addressed by “PEGylation” – a covalent modification of a protein with polyethylene glycol (PEG) (3, 4). Several PEGylated polypeptides have been approved for therapeutic use and few more are under clinical development (4, 5). However, PEGylation cannot address another important limitation of many proteins, which display poor ability to transport across cellular membranes (1, 6). For many years attempts were made to increase membrane interactions of proteins by introducing hydrophobic moieties, which can anchor the proteins onto the cell surface. For example, the modification of proteins with fatty acids was shown to increase binding and internalization of these proteins in cells (7). Furthermore, horseradish peroxidase (HRP) modified by fatty acid also exhibited higher permeability across the blood-brain barrier (BBB) (8). However, in vivo such lipophilic modification also resulted in increased uptake of the protein in peripheral organs (8). Therefore, a fine balance should be found between hydrophilic and lipophilic moieties introduced into a protein to improve its transport across biological barriers while also maintaining favorable pharmacokinetics and stability.
One solution to this problem involves the use of amphiphilic block copolymers for protein modification. For example, conjugation of polypeptides with triblock copolymers poly(ethylene glycol)-b-poly(propylene glycol)-b-poly(ethylene glycol) (PEG-PPG-PEG, also known as Pluronic® or poloxamer) was shown to increase permeability of the polypeptides across BBB while also increasing their stability and circulation time (8–10). In contrast to the fatty acid acylation, the Pluronic® modification of HRP did not appear to increase the peripheral tissue uptake, which along with the increased BBB permeability resulted in the increase of the dose of this protein delivered to the brain (8). Furthermore, Pluronic® conjugates with Cu/Zn superoxide dismutase (SOD1) were shown to internalize in neuronal cells, while SOD1-PEG did not display such ability (11). Therefore, modification of proteins with Pluronic® appears to surpass PEGylation in the ability to deliver proteins into the cells. Importantly, the cellular uptake of Pluronic®-conjugated proteins can be further optimized by changing the lengths of the hydrophilic PEG and hydrophobic PPG blocks (9).
In this study we propose a different approach to polymer conjugation of proteins using amphiphilic block copolymers based on poly(2-oxazoline)s (POx). Recently, POx have attracted considerable attention for drug delivery (12) and other biomedical applications (13). Poly(2-ethyl-2-oxazoline) (PEtOx) and poly(2-methyl-2-oxazoline) (PMeOx) have shown similar properties as PEG, such as stealth (14, 15), protein repellence (16) and ability for rapid renal clearance (17). Trypsin (18), catalase (19), synthetic peptide (20), and various other proteins have been conjugated to PMeOx and PEtOx successfully and these conjugates performed similar to PEGylated proteins. Furthermore, variation in side chain length and architecture gives a tool to impose very subtle changes in the polymer hydrophilic/hydrophobic balance (12). In this work for the first time we report on the conjugation of proteins with amphiphilic POx copolymers and evaluate effects of such modification on the cellular uptake of the protein. With 2-butyl-2-oxazoline (BuOx) as the hydrophobic monomer, two amphiphilic block copolymer P(MeOx-b-BuOx) and P(EtOx-b-BuOx), one random copolymer P(EtOx-co-BuOx) and one homopolymer PMeOx were selected and conjugated with HRP. These conjugates were characterized in different ways and their cellular uptake in MDCK and Caco-2 cells were quantitatively compared.
EXPERIMENTAL SECTION
Materials and Methods
HRP type VI-A, MW 43kDa, anhydrous methanol, dichloromethane, acetone, ethanol, N,N-dimethylformamide (DMF), N,N-diisopropylethylamine (DIPEA), 2,4,6-trinitrobenzenesulfonic acid (TNBS), ultrapure urea, high resolution ampholyte (pH 3.5–10), trichloroacetic acid (TCA), o-phenylenediamine, proteinase K, aprotinin were purchased from Sigma-Aldrich Co. (St-Louis, MO). Dithiobis(succinimidyl propionate) (DSP) and disuccinimidyl propionate (DSS) were from Pierce Biotech Co. (Rockford, IL). Tris-HCl Precast gels (10%) were from Bio-Rad (Hercules, CA). Sephadex LH-20 gel was from GE Healthcare (Waukesha, WI). TSKgel G3000SWXL column (7.8mm ID × 30 cm) was from Tosoh Co. (Japan). Amicon ultra-15 centrifugal filter, MWCO 30K, membrane NMWL was from Millipore Co. (Billerica, MA). Spectro/Por membrane (MWCO 2,000) was from Spectrum Lab Inc. (New Brunswick, NJ). Flexible thin-layer chromatography (TLC) plates were from Whatman Ltd (Mobile, AL). All substances for the preparation of the polymers were purchased from Aldrich (Steinheim, Germany) and Acros (Geel, Belgium) and were used as received unless otherwise stated. 2-Butyl-2-oxazoline was prepared as recently described. Methyl trifluoromethylsulfonate (MeOTf), 2-methyl-2-oxazoline (MeOx), 2-ethyl-2-oxazoline (EtOx), acetonitrile (ACN) and other solvents for polymer preparation were dried by refluxing over CaH2 under dry nitrogen atmosphere and subsequent distillation prior to use. NMR spectra were recorded on a Bruker DRX 500 P, Bruker Avance III 400, Bruker ARX 300 or a Bruker AC 250 at room temperature. The spectra were calibrated using the solvent signals (CDCl3 7.26 ppm, D2O 4.67 ppm). Gel permeation chromatography (GPC) was performed on a Waters system (pump mod. 510, RI-detector mod. 410, precolumn PLgel and two PL Resipore columns (3 µm, 300 × 7,5 mm)) with N,N-dimethylacetamide (DMAc) (57 mmol/L LiBr, 80 °C, 1 mL/min) as eluent and calibrated against PMMA standards. Alternatively a PL120 system using GRAM columns (Polymer Standards Service, Mainz, Germany) with DMAc (57 mmol/L LiBr, 70°C, 1 mL/min) was used.
Synthesis and Characterization of POx
The polymers were prepared according to our previous accounts (12, 17, 21). Exemplarily for P(EtOx50-b-BuOx20), under dry and inert conditions 10 mg (61 µmol, 1 eq.) of MeOTf and 321 mg (3.24 mmol, 53 eq.) of EtOx were dissolved in 3 mL dry acetonitrile at room temperature. The mixture was subjected to microwave irradiation (150 W maximum, 130 °C) for 5 min. After cooling to room temperature, the monomer for the second block, BuOx (157 mg, 1.23 mmol, 20 eq.) was added and the mixture was irradiated the same way as for the first block. Finally the polymerization was terminated using 150 mg piperazine as a terminating reagent. For precipitation, a solvent mixture of cyclohexane and diethylether (50/50, v/v) was used. The product was obtained as a colorless solid (yield 0.36 g, 77%, molar mass as expected from monomer ([M]0) and initiator concentration ratios ([I]0) Mn(theo.)= 7.8 kg/mol) GPC (DMAc): Mn = 11.5 kg/mol (Mw/Mn = PDI 1.09); 1H-NMR (CDCl3, 298 K): ∂ = 3.45 (br, 276H, (NCH2CH2)); 3.04/2.95 (m, 3H, N-CH3Ini); 2.5–2.2 (m, 144H, CO-CH2-CH3, CO-CH2, CH2Pid); 1.58(br, 37H, -CH2-CH2-CH2-); 1.34 (br, 41H, -CH2-CH3); 1.11 (br, 151H, CO-CH2-CH3); 0.91 ppm (br, 56H, -CH3butyl), Mn = 7.5 kg/mol (EtOx50-b-BuOx19).
Conjugation of HRP with POx
Amine terminated POx were reacted with small molecule linkers, DSS or DSP under two different reaction conditions. Condition A: 110 mg of polymer in 0.5 ml of methanol were mixed with a 10-fold molar excess of DSS (DSP) in 0.5 ml of DMF stored over molecular sieves (4 Å). The mixture was supplemented with 0.1 ml sodium borate buffer (0.1 M, pH 8.0) and incubated for 30 min at 25 °C. Excess of DSS (DSP) was removed by gel filtration on Sephadex LH-20 column (2.5 × 20 cm) in anhydrous methanol and the solvent was removed in vacuo. Condition B: 110 mg of polymer and 10-fold molar excess of DSS (DSP) were dissolved in 1ml of DMF stored over molecular sieves (4 Å). DIPEA (5 µl /10 mg polymer) was added as the organic base. The mixture was incubated for three days at 25 °C. Work-up was performed as under condition A. 1H NMR showed that within experimental error, 100% of polymers were conjugated with DSS or DSP. Activated polymer was subsequently dissolved in 1 ml 20% aqueous ethanol and mixed with 5 mg of HRP in 0.5 ml of 0.1M sodium borate (pH 8.0). The reaction mixture was incubated overnight at 4 °C.
Purification of HRP-POx Conjugates
Purification of HRP conjugates was performed similar to our previous study (8, 9). The HRP conjugates were precipitated in cold acetone to remove excess of non-reacted polymers. Briefly, about 1 ml of the reaction mixture was added dropwise to 30 ml of cold acetone under stirring. HRP conjugates were precipitated and collected by centrifugation at 3,000 rpm for 10 min at 4 °C, washed by cold ethanol (10 ml) and dried in vacuo. The extent of elimination of non-reacted polymers was assayed by TLC on Silica Gel plates in dichloromethane/methanol, 8:2. Under these conditions free polymers migrated (Rf = 0.2), while the conjugate was immobile (The polymer spots were visualized by iodine adsorption). To separate modified and unmodified HRP, the conjugates were further purified on TSKgel G3000SWXL size exclusion column (0.78cm × 30 cm) using a mobile phase consisting of methanol (5%) and pH 6.8, 0.1 M NaH2PO4, 0.2M NaCl buffer (95%). The final conjugates were desalted in Amicon ultra-15 centrifuge tube (MWCO 30 kDa) and lyophilized.
Electrophoresis
Polyacrylamide gel electrophoresis (PAGE) was used to confirm the HRP conjugates which have higher molecular weight than native protein. Briefly, 10 µg of native or modified HRP was mixed with 5 × loading buffer (with DTT) and loaded into the 10% precast Tris-HCl gel. The analysis was performed in Mini-PROTEAN electrophoresis system (Bio-Rad, Hercules, CA) connected with PowerPac Basic Power Supply (Bio-Rad, Hercules, CA). The running condition was 85 V for 45 min and 100 V for 90 min. The gel was stained by Bio-safe coomassie stain (Bio-Rad, Hercules, CA) for 1 hr and destained in water.
Isoelectric Focusing (IEF)
IEF was used to separate the HRP conjugates with different modification degrees based on their shifted isoelectric points. Briefly, denaturing IEF gel (pH 3.5–10) was prepared according to the recipe described elsewhere (22). 20 µg of native or modified HRP was mixed with 2 × loading buffer and loaded into the gel plate. The analysis was performed in Mini-PROTEAN electrophoresis system (Bio-Rad, Hercules, CA) connected with PowerPac High-voltage Power Supply (Bio-Rad, Hercules, CA). The running condition was 100 V for 1 hr, 200 V for 1 hr and 500 V for 30 min. The gel was fixed in 10 % TCA for 10 min and 1% TCA overnight. After fixing, the gel was stained by Bio-safe coomassie stain (Bio-Rad, Hercules, CA) for 1 hr and destained in water.
Degree of Modification by TNBS Assay
TNBS assay was used to determine the degree of protein modification as described earlier (9, 23). Briefly, 10 µl of HRP-POx solutions (protein concentration 0.1 – 0.6 mg/ml) were mixed with 10 µl of TNBS solution (1.7 mM) in 80 µl of sodium borate buffer (0.1 M, pH 9.5) and incubated at 37 °C for 2 hrs. The absorbance was measured at 405 nm using the microplate reader (Spectra Max, MDS, CA). The protein content was measured using MicroBCA kit from Pierce (Rockford, IL). The degree of modification (average number of modified amino groups) was calculated according to the following equation:
| (1) |
where Anative and Amodified were the absorbencies and Cnative and Cmodified were the concentrations of native and modified HRP respectively. The total number of primary amino groups including lysine residues and terminal amine group of HRP is seven (9).
Enzymatic Activity of HRP-POx Conjugates
The use of o-phenylenediamine to determine the HRP enzymatic activity has been described earlier (8, 9). Briefly, 20 µl of 1 to 20 ng/ml HRP-POx were added to 96-well plates and supplemented with 160 µl of citrate buffer (0.1 M, pH 5.0, containing 0.1% Triton X-100 and 1 mg/ml BSA). Freshly prepared o-phenylenediamine (0.5 mg/ml) in the same citrate buffer was mixed with 0.2% H2O2 and 20 µl of the mixture was added to each well immediately. After incubating at 37 °C for 5 min the reaction was stopped by 20 µl of a 0.5% Na2SO3 solution in 2 N H2SO4, and the absorbance was measured at 490 nm using a microplate reader.
Circular Dichroism (CD) Spectra
Modified or unmodified HRP was dissolved in phosphate-buffered saline (PBS) (pH 7.4) at the concentration of 0.5 mg/ml determined by MicroBCA assay. Far-UV (200 to 260 nm) and near-UV-vis (250 to 450 nm) CD spectra were recorded using an Aviv Circular Dichroism Model 202SF spectrometer (Lakewood, NJ) with a cuvette having 1 cm path length. Spectra were obtained from 450 to 200 nm in 1 nm decrements and the reported spectra correspond to the average of three wavelength scans. All the CD spectra of protein were obtained by subtracting the spectra of blank solvent. The mean residue molar ellipticity [θ] was calculated based on the following equation:
| (2) |
where θ is the observed ellipticity (deg), M is the mean residue molecular weight (g/mol), Cis the protein concentration (g/ml) and l is the optical path length (cm) (24).
Cellular Uptake
MDCK cells (from ATCC, CCL-34) were seeded in 96-well plates at a density of 20,000 cells/well in Dulbecco's Modified Eagle Medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 1% penicillin/streptomycin and 10% fetal bovine serum (Invitrogen, Carlsbad, CA). The cells were cultured at 37°C with 95% humidity and 5% CO2, and grown for two days until 80 ~ 90% confluence. Caco-2 cells (from ATCC, HTB-37) were seeded in collagen-coated 96-well plates at a density of 5000 cells/well and grown to 80% confluence (5–6 days) in the same medium and culture condition as MDCK. The cells were washed twice in assay buffer containing 122 mM NaCl, 25 mM NaHCO3, 10 mM glucose, 3 mM KCl, 1.2 mM MgSO4, 0.4 mM K2HPO4, 1.4 mM CaCl2 and 10 mM HEPES. Cells were exposed to unmodified or modified HRP in assay buffer for various time intervals (10 to 120 min) at 37 °C, then washed with cold PBS 5 times and lysed in 1% Triton X-100. No cellular toxicity was observed during the treatment. Aliquots of cell lysates (20 µl) were taken for HRP activity determination as described above. Separate calibration curves were used for unmodified and modified HRP. The amounts of cell associated HRP were normalized for the cell protein as determined by MicroBCA assay.
The method to determine the internalized and membrane bound fractions of HRP was described before (7). Briefly, Caco-2 cells were exposed to unmodified or modified HRP in assay buffer for 30 min. at 37°C, then washed with cold PBS 5 times, and incubated 60 min. with or without proteinase K (0.1 mg/ml) in assay buffer at 4°C. The medium was replaced by assay buffer containing aprotinin (10 U/ml) for 10 min, then the cells were washed by cold PBS 3 times and lysed in 1% Triton X-100. The HRP activity and concentration were determined as described above. The internalized protein was determined in proteinase K treated samples. Membrane-bound fraction was determined as the difference between the total uptake measured in the samples without proteinase K treatment and the internalized protein.
Statistical Analysis
Statistical analysis was done using one-way ANOVA (LSD multiple comparisons). A minimum p value of 0.05 was regarded as the significance level for all tests.
RESULTS AND DISCUSSION
Synthesis and Purification of HRP-POx conjugates
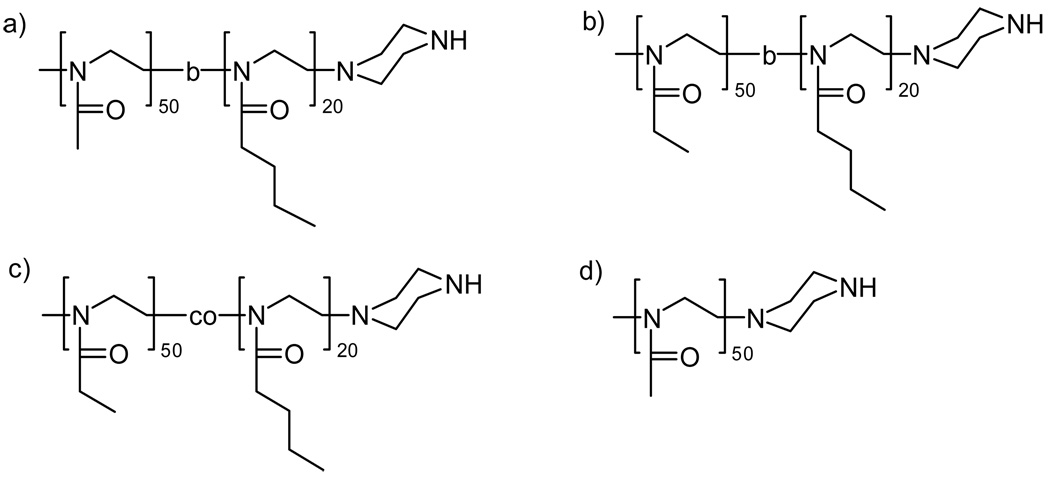
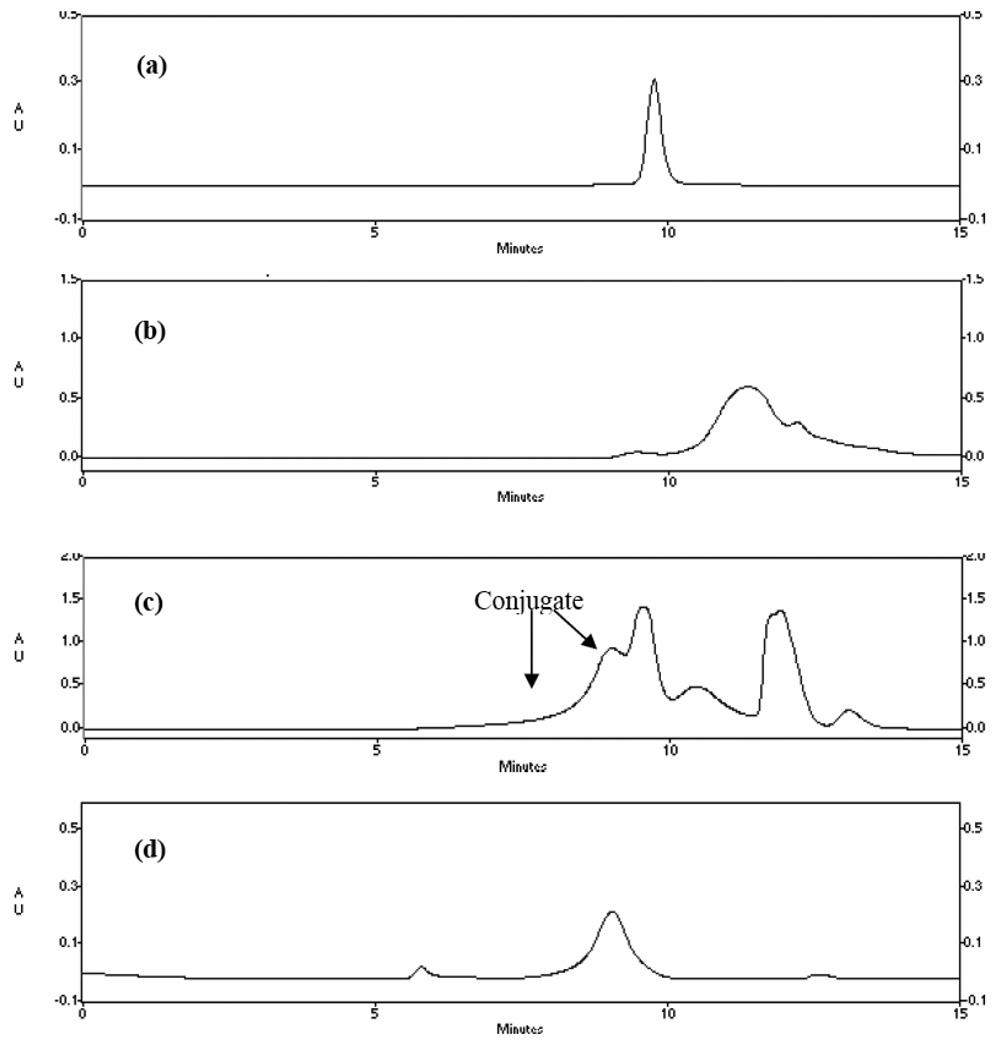
The synthetic routes for HRP-POx are presented in Scheme 1. Four different POx were used for the conjugation: 1) a block copolymer of MeOx and BuOx, P(MeOx-b-BuOx); 2) a block copolymer of EtOx and BuOx, P(EtOx-b-BuOx); 3) a random copolymer, P(EtOx-co-BuOx); and 4) a homopolymer, PMeOx. Structures and analytical data of all polymers are summarized in Figure 1 and Table 1. The numbers of the hydrophilic repeating units (MeOx or EtOx) were kept the same in all four polymers. The block and random copolymers also had the same number of the hydrophobic units, BuOx. All polymers were well defined with PDI below 1.2. In order to use these polymers for conjugation with HRP we used piperazine as a terminating reagent. First, this approach is fast, proceeds without side reactions and under mild conditions resulting in amine terminated POx derivatives (26). Second, such derivatives can be directly conjugated to the protein. This is an advantage compared to OH-terminated POx, produced upon conventional termination in aqueous solution, which would require multi-step activation prior to the attachment to a protein. The secondary amine of the terminal piperazine group of the polymers was coupled to the primary amine of HRP using small bi-functional linkers, DSS or DSP. DSS is a non-degradable linker, while DSP contains disulfide bonds, which are cleaved in the reducing environment within the cell (27). The conjugation was a two-step procedure. First, the polymers were reacted with the linkers to generate N-hydroxysuccinimide terminated POx derivatives. Second, these activated polymers were reacted with HRP in 20% aqueous ethanol. For this reaction we selected pH 8.0 based on our previous experience with HRP-Pluronic synthesis that employed similar conjugation chemistries (9). The HRP-POx conjugates were purified by cold acetone precipitation and then by size exclusion chromatography (Figure 2). Based on the chromatography profiles the yields of the conjugates varied from about 20 to 30% for block copolymers to about 40% for homopolymer.
Fig. 1.
Structures of POx used in the present study: (a) P(MeOx50-b-BuOx20); (b) P(EtOx50-b-BuOx20); (c) P(EtOx50-co-BuOx20); (d) PMeOx50.
Table 1.
Structure and analytical data of polymers used in this study.
| Polymer | Mn(theo.), kg/mola | Mn, kg/mol (PDI) b | Yieldc% | CMC, % w/wd |
|---|---|---|---|---|
| PMeOx50 | 4.3 | 5.5 (1.17) | 84 | n/a |
| P(EtOx50-b-BuOx20) | 7.8 | 11.5 (1.09) | 77 | 0.001 |
| 6.9 | 11.0 (1.15) | 76 | 0.001 | |
| P(EtOx50-co-BuOx20) | 7.0 | 9.3 (1.19) | 85 | 0.04 |
| P(MeOx50-b-BuOx20) | 7.0 | 11.9 (1.07) | 73 | 0.002 |
obtained from [M]0/[I]0
as obtained by GPC
recovered yield
critical micelle concentration (CMC) in aqueous solution values at 37°C as determined using pyrene probe (25).
Fig. 2.
Representative chromatographic profiles of HRP-POx conjugates purification using TSKgel G3000SWXL column (0.78cm × 30 cm): (a) HRP; (b) P(MeOx-b-BuOx); (c) reaction mixture of HRP-P(MeOx-b-BuOx) before purification; (d) HRP-P( MeOx-b-BuOx), after purification. UV absorbance was detected at 220 nm. The mobile phase was methanol (5%) and pH 6.8, 0.1 M NaH2PO4, 0.2M NaCl buffer (95%).
Characterization of HRP-POx Conjugates
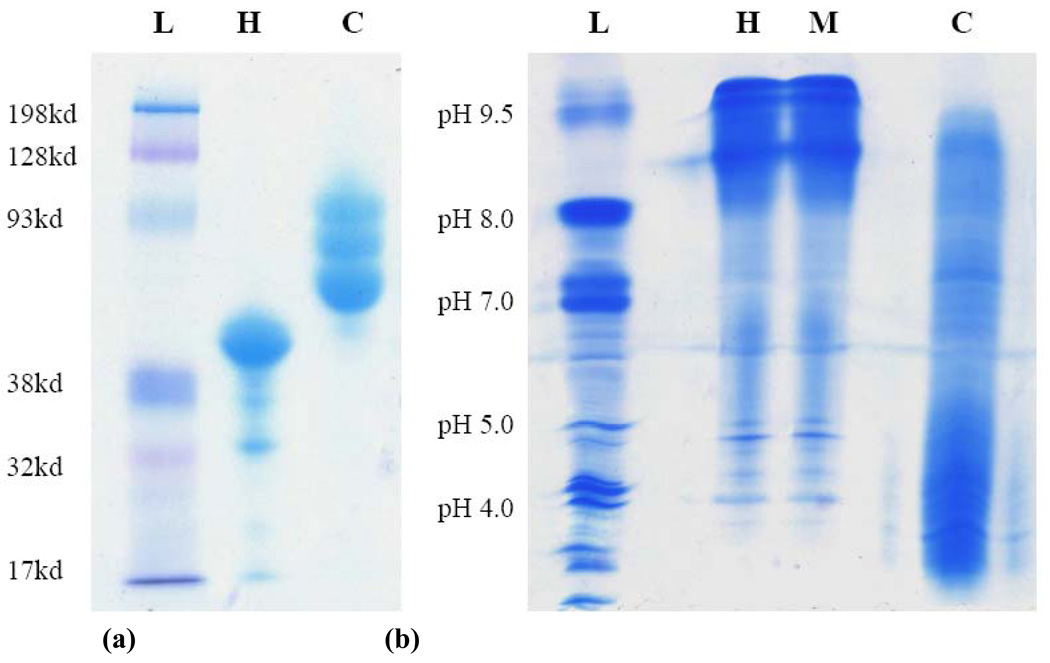
Analytical data for the HRP-POx conjugates are summarized in Table 2. The typical mean modification degree of these conjugates ranged from ca. 0.7 (for P(MeOx-b-BuOx) with DSP linker and P(EtOx-co-BuOx)) to ca. 1.6 (for P(MeOx-b-BuOx) with DSS linker) polymer chains per protein as determined by titration of the protein free amino groups by TNBS. In most cases the residual enzymatic activity of HRP after conjugation was relatively high (70% – 90%). The formation of the HRP-POx conjugates was further confirmed by PAGE, which revealed increases in the molecular masses of the conjugates compared to unmodified HRP (44 kDa) (Figure 3a). Furthermore, the high resolution IEF (28) allowed use to separate HRP-POx conjugates with different modification degrees, which had different isoelectric points (Figure 3b). Specifically, after conjugation and chromatographic separation of the conjugates the unmodified HRP virtually disappeared and was replaced by several new bands corresponding to the conjugates with lower isoelectric points (Figure 3b, lanes C). This result was consistent with the TNBS assay suggesting POx was attached to HRP through amino groups. However, in contrast to TNBS, which provided average degrees of modification, PAGE and IEF clearly revealed that the conjugates contained a mixture of HRP derivatives with different numbers of polymer chains attached (9). There are only six lysine residues in HRP of which three (Lys174, Lys232, and Lys241) are exposed at the surface of the protein globule and are believed to be accessible for chemical modification (29). Such a small number of amino groups explains significant shift in the isoelectric point upon modification. Notably, simple mixing of protein and polymer did not change the isoelectric characteristics of HRP.
Table 2.
Characteristics of HRP-POx conjugates
| Conjugate | Linker | Experimental condition a |
Modification degree |
Residual activity, % unmodified |
|---|---|---|---|---|
| HRP-P(MeOx-b-BuOx)* | DSS | B | 1.62 | 87.0 |
| HRP-SSP(MeOx-b-BuOx) | DSP | A | 0.67 | N/D |
| HRP-P(EtOx-b-BuOx)* | DSS | B | 1.04 | 76.8 |
| HRP-SSP(EtOx-b-BuOx) | DSP | A | 1.16 | 70.1 |
| HRP-P(EtOx-co-BuOx)* | DSS | B | 0.76 | 77.9 |
| HRP-PMeOx* | DSS | B | N/D | 87.5 |
Condition A: Reaction was conducted in 500 µl methanol, 500 µl DMF, and 100 µl sodium borate buffer (pH 8.0), at room temperature for 30 min; Condition B: 1ml DMF and 5 µl (per 10 mg polymer) DIPEA, at room temperature for 3 days.
Those conjugates were used for CD analysis and cellular uptake studies.
Fig. 3.
Representative (a) PAGE and (b) IEF analysis of HRP and HRP-POx: L - Ladder; H - HRP; M - mixture of HRP and P(MeOx-b-BuOx) (1:10); C - HRP-P(MeOx-b-BuOx) conjugate. The HRP-POx conjugates were purified by size exclusion chromatography as shown in Figure 2.
Conformation Stability
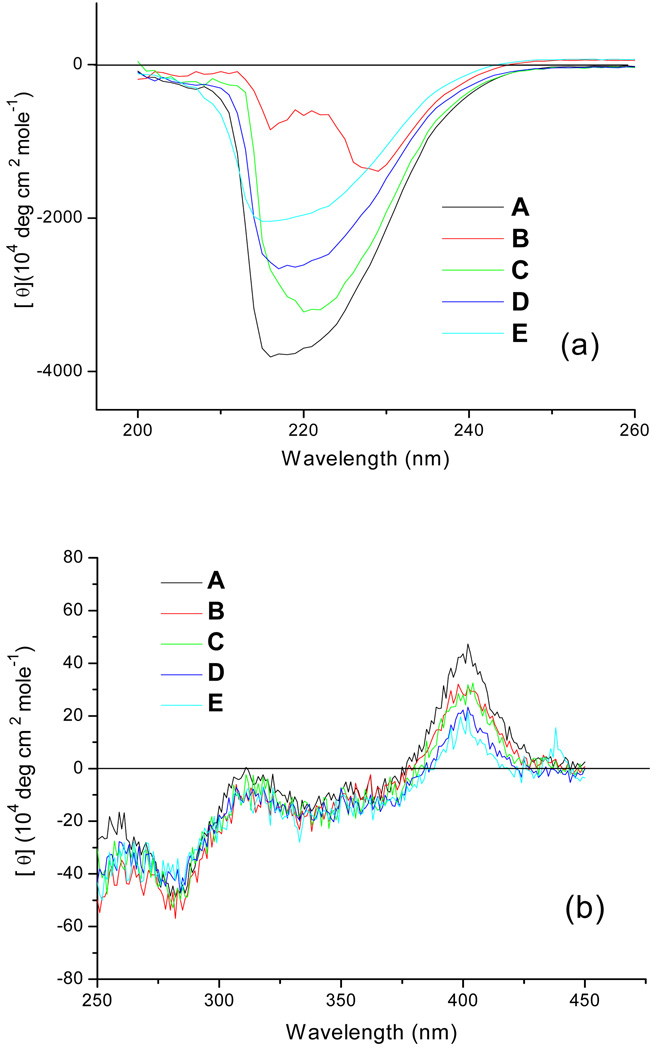
Possible changes of secondary and tertiary structure of a protein during chemical modification may lead to its aggregation and deactivation (29). Therefore, we examined the HRP structure after modification by CD spectroscopy. Both Far-UV (190 to 260 nm) and Near-UV-vis (250 to 450 nm) CD spectra of HRP and HRP-POx were recorded and analyzed (Figure 4). The Far-UV CD spectra of the conjugates revealed considerable decreases in the CD signals in the major bands at 215 nm and 225 nm and a shift of 225 nm band to 230 nm compared to the unmodified protein (Figure 4a). These CD changes were most pronounced for HRP-P(MeOx-b-BuOx) and are indicative of changes in the secondary structure of the HRP apo-protein (30–31). At the same time there was little if any changes of CD signal in the Near-UV-vis spectra of the conjugates (Figure 4b) suggesting that the tertiary structure of HRP apo-protein and the microenvironment of the prosthetic heme remained unaffected by the conjugation (30–31), even though the secondary structure of apo-protein was partially disrupted. The changes of the secondary structure may be ascribed to possible interactions between the block copolymer chain and α-helices on the surface of the apo-protein. This interaction may be responsible for the partial loss of the enzyme activity after the modification. Yet the enzyme was still catalytically active as its tertiary structure was more robust probably due to the presence of stabilizing structural elements (two Ca2+ ions and four disulfide bridges) in HRP molecules (32).
Fig. 4.
CD spectra of HRP and HRP-POx: (a) Far-UV (200 to 260 nm) CD; (b) Near-UV-vis (250 to 450 nm) CD. A – HRP; B - HRP-P(MeOx-b-BuOx); C - HRP-P(EOx-b-BuOx); D - HRP-P(EtOx-co-BuOx); E - HRP-PMeOx. All samples were dissolved in PBS (PH 7.4) at the concentration of 0.5mg/ml (determined by MicroBCA assay). The modification degrees of HRP-POx are the same as those in cellular uptake studies.
Cellular Uptake of HRP-POx
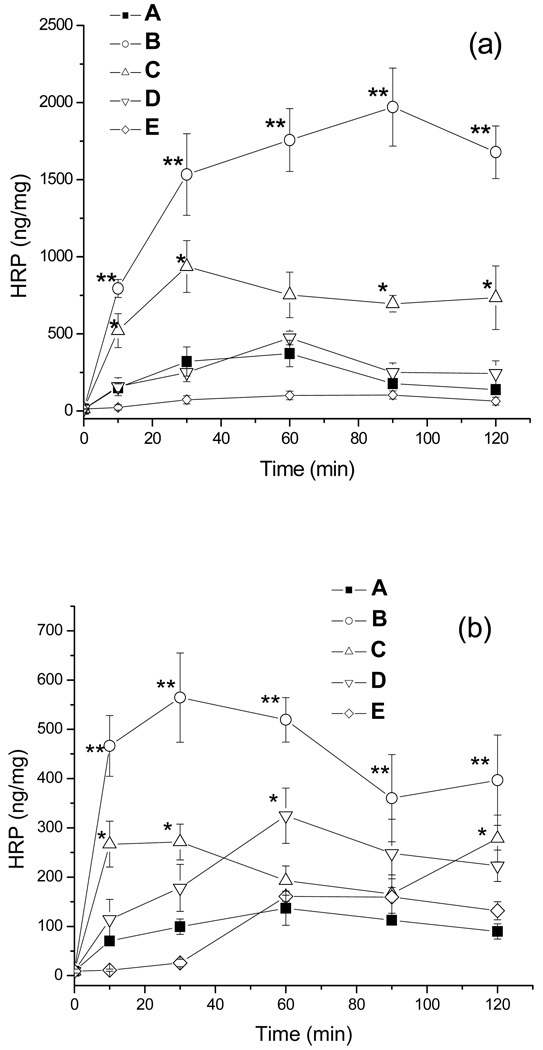
The cellular uptake of the HRP-POx was examined using MDCK and Caco-2 cells as models. The cells were exposed to the unmodified protein or the conjugates (50 µg/ml) for different time intervals (up to 120 min) in serum-free condition and the total amounts of HRP bound with cell membranes or internalized into cells were measured as previously described (7). In both cell models, HRP-P(MeOx-b-BuOx) exhibited significantly enhanced cellular uptake (3–6 fold) compared to unmodified protein (p<0.01) and other conjugates (p<0.05). An increase of uptake was also observed for HRP-P(EtOx-b-BuOx) conjugate (2–3 fold, p<0.05) but it was not statistically significant at 60 min for MDCK cells and 60 min and 80 min for Caco-2 cells. The conjugate of the HRP with a random copolymer, HRP-P(EtOx-co-BuOx), did not show any increase of uptake in MDCK cells but had somewhat increased uptake in Caco-2 cells (p<0.05 at 60 min). A conjugate with the hydrophilic homopolymer, HRP-PMeOx exhibited similar cellular uptake as unmodified HRP in Caco-2 cells, and even lower uptake in MDCK cells (p<0.1, marginally significant). A simple mix of HRP and polymers at 1:10 (molar ratio) did not show any effect on the uptake (data not shown).
Furthermore, we carried out a detailed analysis of the internalized and membrane bound fractions of HRP-P(EtOx-b-BuOx) and HRP-PMeOx (Table 3). In this experiment the internalized fraction was determined after the proteinase K treatment, and the membrane bound fraction was computed as the difference of the total uptake and internalized fractions. After 30 min exposure of the Caco-2 cells approximately 77% of the unmodified HRP were internalized in the cells and 23% were bound on the cell surface. This is consistent with non-specific fluid-phased endocytosis of HRP. In the case of HRP-P(EtOx-b-BuOx) both the internalized and membrane fractions were considerably increased and accounted for approximately 62% and 38% of the total uptake, respectively. Based on this it appears that modification of HRP with the block copolymer may enhance the adsorption component of endocytosis, presumably due to the increased interaction of the conjugate with the cell membrane. In contrast, the internalized fraction of HRP-PMeOx was decreased compared to both unmodified HRP and HRP-P(EtOx-b-BuOx). Interestingly, for HRP-PMeOx there was a considerable increase in the membrane bound fraction compared to the unmodified HRP. This membrane bound fraction of HRP-PMeOx was almost as high as the membrane-bound HRP-P(EtOx-b-BuOx) and amounted to 69% of the total uptake (while only 31% was internalized).
Table 3.
Uptake of HRP and HRP-POx in Caco-2 cells.
| Samples | Cell-associated HRP, ng/mg total protein (% fraction) a | ||
|---|---|---|---|
| Total uptake | Internalized | Membrane-bound | |
| HRP | 87.8 ± 11.2 (100) | 67.8 ± 5.6(77) | 10.0 (23) |
| HRP-P(EtOx-b-BuOx) | 165.0 ± 9.1(100) | 102.6 ± 6.2(62) | 62.4 (38) |
| HRP-PMeOx | 75.6 ± 5.7(100) | 23.2 ± 4.5(31) | 52.4 (69) |
Fifty µg/ml HRP or HRP-POx conjugates were incubated with Caco-2 cells for 30 min. at 37°C. Internalized protein was determined after proteinase K treatment. Membrane-bound fraction was determined as the difference between the total uptake and internalized protein.
It was previously reported that structure of copolymers has a marked influence on the cellular uptake of the protein-copolymer conjugates. For example, previous study of HRP conjugates with Pluronic® block copolymers suggested that the lengths of hydrophilic and hydrophobic blocks of the copolymer as well as the number of the copolymer chains attached to the protein have major effects on the cellular uptake of such conjugates (9). Long hydrophobic blocks can increase the binding of protein with cell membrane but may also cause aggregation of protein conjugates. A shorter hydrophilic block is more desirable in terms of cellular uptake but its length should be carefully chosen in order to avoid aggregation. Hence, tight control over the hydrophilic/lipophilic balance is a necessity and POx are ideally suited in this respect (12). In the case of HRP-POx conjugates presented in this work we observed the highest uptake for HRP-P(MeOx-b-BuOx) followed by HRP-P(EtOx-b-BuOx). Both block copolymers used for conjugation have the same hydrophobic PBuOx block, and nearly the same lengths of the hydrophilic PMeOx and PEtOx blocks. However, the PMeOx is more hydrophilic than PEtOx. The difference in the uptake of these conjugates was most likely due to the higher modification degree of HRP-P(MeOx-b-BuOx) (1.62) compared to HRP-P(EtOx-b-BuOx) (1.04). The relatively low levels of uptake of HRP-P(EtOx-co-BuOx) compared to HRP-P(EtOx-b-BuOx) in MDCK cells may suggest that the random copolymer without ordered block structure cannot elicit appropriate interactions of the conjugates with the cellular membrane to increase cellular uptake. At the same time, in the case of Caco-2 cells, the uptake of the latter two conjugates (HRP-P(EtOx-b-BuOx) and HRP-P(EtOx-co-BuOx)) was still comparable. We recently reported on the cellular uptake of different copolymers based on N-(2-hydroxypropyl)-methacrylamide (33). In that case, we found markedly higher uptake for the random copolymers than for the block copolymers. However, these systems were of a different polymer, without proteins, and the observed internalization behavior appeared to be related to the micellization – block copolymers capable of aggregation into stable micelles were less efficiently internalized than random copolymers, which yielded less stable or ordered aggregates (33). Finally, hydrophilic and flexible polymers, such as PEG, PMeOx and PEtOx are well known to be able to enhance water solubility of covalently attached molecules such as drugs and proteins and reduce their interaction with other proteins, surfaces and interfaces. Therefore, the reduced cellular uptake of HRP-PMeOx compared to HRP-P(MeOx-b-BuOx) and HRP-P(EtOx-b-BuOx) could be expected. However, an unexpected result was that binding of this conjugate with the cell membranes was increased while internalization was decreased compared to HRP. Therefore, efficient internalization of protein-polymer conjugate is not a simple function of the membrane binding and “unproductive” binding can occur, which is not accompanied with an increased internalization. In this regard a clear relationship between the internalization efficiency, membrane binding and copolymer structure was reported for different free Pluronic copolymers (34). In this study the best internalization was observed for the copolymers with intermediate hydrophobicity. The copolymers with very long hydrophilic blocks were not entering the cells, and the copolymers with very long hydrophobic blocks remained strongly bound with the membranes and also unable to internalize.
In summary, we successfully synthesized HRP-POx conjugates with different polymer structures and linkers, using a well-established conjugation procedure. These conjugates bear from about 0.7 to 1.7 polymer moieties per protein and retain high enzymatic activity. Conformation analysis reveals that polymer modification changed the secondary structure, but not the tertiary structure and heme environment of HRP important for the catalytic activity. HRP-P(MeOx-b-BuOx) and HRP-P(EtOx-b-BuOx) showed significantly enhanced cellular uptake in MDCK and Caco-2 cells, which was not found in HRP-P(EtOx-co-BuOx) and HRP-PMeOx conjugates. The inability of these two polymer conjugates to increase cellular uptake is probably due to the lack of structurally ordered hydrophobic block, which can assist the hydrophilic protein to transport into a cell. Combined, these data show that modification by amphiphilic POx block copolymers is a promising strategy to enhance cellular delivery and transport of protein drugs.
Supplementary Material
Figure 5.
Cellular uptake of HRP and HRP-POx in (a) MDCK cells and (b) Caco-2 cells: A– HRP;B-HRP-P(MeOx-b-BuOx); C-HRP-P(EOx-b-BuOx); D-HRP-P (EtOx-co-BuOx); E - HRP-PMeOx. The cells were exposed to the unmodified protein or the conjugates (50 µg/ml) for different time intervals in serum-free condition. Data presented as means ± SEM (n = 6).Statistical analysis was done using one-way ANOVA (LSD multiple comparisons): * p < 0.05, ** p < 0.01.
Acknowledgements
This study was supported by the United States National Institute of Health (NIH) RO1 grant NS051334, the United States Department of Defense (DoD) USAMRMC 06108004 and by the Nanomaterials Core Facility of the Nebraska Center of Nanomedicine supported by NIH COBRE grant RR021937 (all awarded to A.V.K.). R.L. is also thankful to the Deutsche Akademischen Austauschdienst (DAAD) for a postdoctoral fellowship and to the King Abdullah University of Science and Technology (KAUST) Award No. KUK-F1-029-32 for partial salary support. Likewise X.Y. has been in part supported by the American Heart Association (AHA) Midwest Predoctoral Fellowship 0910040G. We also gratefully acknowledge Professor Luis Marky (College of Pharmacy, UNMC) for kind assistance in CD experiments.
References
- 1.Brown LR. Commercial Challenges of Protein Drug Delivery. Expert Opin. Drug Deliv. 2005;2:29–42. doi: 10.1517/17425247.2.1.29. [DOI] [PubMed] [Google Scholar]
- 2.Malik DK, Baboota S, Ahuja A, Hasan S, Ali J. Recent Advances in Protein and Peptide Drug Delivery Systems. Curr. Drug Deliv. 2007;4:141–151. doi: 10.2174/156720107780362339. [DOI] [PubMed] [Google Scholar]
- 3.Bailon P, Won CY. PEG-Modified Biopharmaceuticals. Expert Opin. Drug Deliv. 2009;6:1–16. doi: 10.1517/17425240802650568. [DOI] [PubMed] [Google Scholar]
- 4.Katre NV. The Conjugation of Proteins with Polyethylene Glycol and Other Polymers. Adv. Drug. Deliv. Rev. 1993;10:91–114. [Google Scholar]
- 5.Duncan R. The Dawning Era of Polymer Therapeutics. Nat. Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 6.Begley DJ. Delivery of Therapeutic Agents to the Central Nervous System: The Problems and the Possibilities. Pharmacol. Ther. 2004;104:29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Slepnev VI, Phalente L, Labrousse H, Melik-Nubarov NS, Mayau V, Goud B, Buttin G, Kabanov AV. Fatty Acid Acylated Peroxidase as a Model for the Study of Interactions of Hydrophobically-Modified Proteins with Mammalian Cells. Bioconjug. Chem. 1995;6:608–615. doi: 10.1021/bc00035a016. [DOI] [PubMed] [Google Scholar]
- 8.Batrakova EV, Vinogradov SV, Robinson SM, Niehoff ML, Banks WA, Kabanov AV. Polypeptide Point Modifications with Fatty Acid and Amphiphilic Block Copolymers for Enhanced Brain Delivery. Bioconjug. Chem. 2005;16:793–802. doi: 10.1021/bc049730c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi X, Batrakova EV, Banks WA, Vinogradov SV, Kabanov AV. Protein Conjugation with Amphiphilic Block Copolymers for Enhanced Cellular Delivery. Bioconjug. Chem. 2008;19:1071–1077. doi: 10.1021/bc700443k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price TO, Farr SA, Yi X, Vinogradov SV, Batrakova EV, Banks WA, Kabanov AV. Transport across the Blood-Brain Barrier of Pluronic Leptin. J. Pharmacol. Exp. Ther. 2010;333:253–263. doi: 10.1124/jpet.109.158147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi X, Zimmerman MC, Yang R, Tong J, Vinogradov SV, Kabanov AV. Pluronic-Modified Superoxide Dismutase 1 (SOD 1) Attenuates Angiotensin II-Induced Increase in Intracellular Superoxide in Neurons. Free Radic. Biol. Med. 2010 doi: 10.1016/j.freeradbiomed.2010.04.039. (Accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luxenhofer R, Schulz A, Roques C, Li S, Bronich TK, Batrakova EV, Jordan R, Kabanov AV. Doubly Amphiphilic Poly(2-oxazoline)s as High Capacity Delivery Systems for Hydrophobic Drugs. Biomaterials. 2010 doi: 10.1016/j.biomaterials.2010.02.057. (In Press), doi:10.1016/j.biomaterials.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogenboom R. Poly(2-oxazoline)s: A Polymer Class with Numerous Potential Applications. Angew. Chem. Int. Ed. 2009;48:7978–7994. doi: 10.1002/anie.200901607. [DOI] [PubMed] [Google Scholar]
- 14.Zalipsky S, Hansen CB, Oaks JM, Allen TM. Evaluation of Blood Clearance Rates and Biodistribution of Poly(2-oxazoline)-Grafted Liposomes. J. Pharm. Sci. 1996;85:133–137. doi: 10.1021/js9504043. [DOI] [PubMed] [Google Scholar]
- 15.Woodle MC, Engbers CM, Zalipsky S. New Amphipatic Polymer-Lipid Conjugates Forming Long-Circulating Reticuloendothelial System-Evading Liposomes. Bioconjug. Chem. 1994;5:494–496. doi: 10.1021/bc00030a001. [DOI] [PubMed] [Google Scholar]
- 16.Konradi R, Pidhatika B, Mühlebach A, Textor M. Poly-2-methyl-2-oxazoline: A Peptide-Like Polymer for Protein-Repellent Surfaces. Langmuir. 2008;24:613–616. doi: 10.1021/la702917z. [DOI] [PubMed] [Google Scholar]
- 17.Gaertner FC, Luxenhofer R, Blechert B, Jordan R, Essler M. Synthesis, Biodistribution and Excretion of Radiolabeled Poly(2-alkyl-2-oxazoline)s. J. Controlled Release. 2007;119:291–300. doi: 10.1016/j.jconrel.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Mero A, Pasut G, Via LD, Fijten MWM, Schubert US, Hoogenboom R, Veronese FM. Synthesis and Characterization of Poly(2-ethyl 2-oxazoline)-Conjugates with Proteins and Drugs: Suitable Alternatives to PEG-Conjugates? J. Controlled Release. 2008;125:87–95. doi: 10.1016/j.jconrel.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto M, Naka K, Shiozaki M, Chujo M, Saegusa T. Preparation and Enzymatic Activity of Poly[(N-acylimino)ethylene]-Modified Catalase. Macromolecules. 1990;23:3201–3205. [Google Scholar]
- 20.Velander WH, Madurawe RD, Subramanian A, Kumar G, Sinai-Zingde G, Riffle JS, Orthner CL. Polyoxazoline-Peptide Adducts That Retain Antibody Avidity. Biotech. Bioeng. 1992;39:1024–1030. doi: 10.1002/bit.260391006. [DOI] [PubMed] [Google Scholar]
- 21.Ivanova R, Komenda T, Bonné TB, Lüdtke K, Mortensen K, Pranzas PK, Jordan R, Papadakis CM. Micellar Structures of Hydrophilic/Lipophilic and Hydrophilic/Fluorophilic Poly(2-oxazolines) Diblock Copolymers in Water. Macromol. Chem. Phys. 2008;209:2248–2258. [Google Scholar]
- 22.Giulian GG, Moss RL, Greaser M. Analytical Isoelectric Focusing Using a High-Voltage Vertical Slab Polyacrylamide Gel System. Anal. Biochem. 1984;142:421–436. doi: 10.1016/0003-2697(84)90486-x. [DOI] [PubMed] [Google Scholar]
- 23.Habeeb AFSA. Determination of Free Amino Groups in Proteins by Trinitrobenzenesulfonic Acid. Anal. Biochem. 1965;14:328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- 24.Goycoolea FM, Lollo G, Remunñán-López C, Quaglia F, Alonso MJ. Chitosan-Alginate Blended Nanoparticles as Carriers for the Transmucosal Delivery of Macromolecules. Biomacromolecules. 2009;10:1736–1743. doi: 10.1021/bm9001377. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm M, Zhao CL, Wang Y, Xu R, Winnik MA, Mura JC, Riess G, Croucher MD. Poly (styrene-ethylene oxide) Block Copolymer Micelle Formation in Water: A Fluorescence Probe Study. Marcomolecules. 1991;24:1033–1040. [Google Scholar]
- 26.Nuyken O, Maier G, Groß A, Fischer H. Systematic Investigations on the Reactivity of Oxazolinium Salts. Macromol. Chem. Phys. 1996;197:83–95. [Google Scholar]
- 27.Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK. Pharmacokinetics and Biodistribution of Genetically-Engineered Antibodies. Q J. Nucl. Med. 1998;42:225–241. [PubMed] [Google Scholar]
- 28.Snider J, Neville C, Yuan LC, Bullock J. Characterization of the Heterogeneity of Polyethylene Glycol-Modified Superoxide Dismutase by Chromatographic and Electrophoretic Techniques. J. Chromatography. 1992;599:141–155. doi: 10.1016/0021-9673(92)85467-8. [DOI] [PubMed] [Google Scholar]
- 29.Hassani L, Ranjbar B, Khajeh K, Naderi-Manesh H, Naderi-Manesh M, Sadeghi M. Horseradish Peroxidase Thermostabilization: The Combinatorial Effects of the Surface Modification and the Polyols. Enzyme Microbial. Technol. 2006;38:118–125. [Google Scholar]
- 30.Akita M, Tsutsumi D, Kobayashi M, Kise H. Structural Change and Catalytic Activity of Horseradish Peroxidase in Oxidative Polymerization in Phenol. Biosci. Biotechnol. Biochem. 2001;65:1581–1588. doi: 10.1271/bbb.65.1581. [DOI] [PubMed] [Google Scholar]
- 31.Strickland EH. Circular Dichroism of Horseradish Peroxidase and Its Enzyme-Substrate Compounds. Biochem. Biophys. Acta. 1968;151:70–75. doi: 10.1016/0005-2744(68)90162-9. [DOI] [PubMed] [Google Scholar]
- 32.Tsaprailis G, Chan DWE, English AM. Conformational States in Denaturants of Cytochrom c and Horseradish Peroxidases Examined by Fluorescence and Circular Dichroism. Biochemistry. 1998;37:2004–2016. doi: 10.1021/bi971032a. [DOI] [PubMed] [Google Scholar]
- 33.Barz M, Luxenhofer R, Jordan R, Kabanov AV. The Uptake of N-(2-hydroxypropyl)-methacrylamide Based Homo, Random and Block Copolymers by Human Multi-Drug Resistant Breast Adenocarcinoma Cells. Biomaterials. 2009;30:5682–5690. doi: 10.1016/j.biomaterials.2009.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal Structure Requirements for Pluronic Block Copolymers in Modifying P-Glycoprotein Drug Efflux Transporter Activity in Bovine Brain Microvessel Endothelial Cells. J. Pharmacol. Exp. Ther. 2003;304:845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.