Abstract
DNA viruses are a significant contributor to human morbidity and mortality. The immune system protects against viral infections through coordinated innate and adaptive immune responses. While the antigen-specific adaptive mechanisms have been extensively studied, the critical contributions of innate immunity to anti-viral defenses have only been revealed in the very recent past. Central to these anti-viral defenses is the recognition of viral pathogens by a diverse set of germ-line encoded receptors that survey nearly all cellular compartments for the presence of pathogens. In this review, we discuss the recent advances in the innate immune sensing of DNA viruses and focus on the recognition mechanisms involved.
Introduction
Infectious diseases as a result of DNA virus infections are a major health concern worldwide. The major pathogenic DNA viruses include cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein-Barr virus, Kaposi's sarcoma-associated herpesvirus, polyoma virus and human papilloma virus. The two major species of herpes viruses such as CMV and HSV are clinically important. Herpes simplex virus is the cause of a wide range of diseases including some serious illnesses such as keratitis and encephalitis. Human cytomegalovirus is the major health risk in the newborn and in the immunocompromised causing congenital abnormalities and systemic diseases, respectively. Moreover, given the ability of DNA viruses to efficiently infect a wide range of cell types, these viruses also have gained clinical importance as potential gene delivery platforms to treat a variety of genetic diseases. The potent immune and inflammatory responses against the viral components however remain the stumbling block to the widespread clinical use of such vectors. Therefore a thorough mechanistic understanding of host anti-viral responses is central to the development not only of anti-viral therapeutics and vaccines but also in order to improve the safety of viral vectors in gene therapies.
Control of viral infections is mediated by coordinate activation of innate and adaptive immune mechanisms involving multiple cell types. CD8 T cells, CD4 T cells, and B cells contribute to anti-viral responses in an antigen-specific manner via IFN-γ production, cytolytic effect, or antibody secretion ensuring long term protection. On the other hand, during the early phase of infection, the antigen-presenting cells, stromal cells and notably NK-cells play a critical role in virus control. Production of the key anti-viral cytokines, the type I Interferons by cells of the innate immune system is the principal mechanism mediating early anti-viral defense. Although type I IFNs are instrumental in coordinating early anti-viral defenses, additional cytokines induced in innate immune cells are also important components of early defenses. These include members of the IL-1 family such as IL-1 and the related cytokine IL-18, as well as IL-12 all of which contribute to early anti-viral immunity. Although the role of these cytokines in immune defenses has been well appreciated for some time, the early events that coordinate the production of these effectors has only been revealed in the last decade or so. What has emerged from this exciting decade or more of intensive research is that innate immune cells express a large repertoire of germ line-encoded pattern recognition receptors (PRRs) that recognize microbial components (Kawai and Akira, 2010). The receptors identified to date include Toll-like receptors (TLRs), Nod-like receptors (NLRs), RIG-I-like receptors (RLRs), and AIM2 like receptors (ALRs).
These germline encoded PRRs bind either directly or indirectly, depending on the class of PRR, microbial ligands and initiate signaling cascades which culminate in the activation of transcription factors such as nuclear factor kappa B (NF-κB), interferon regulatory factors (IRFs), and activating protein-1 (AP-1) involved in the expression of inflammatory and type I IFN genes. The Toll-like receptors (TLRs) were the first set of pattern recognition receptors discovered. A considerable body of work has revealed that these receptors (of which there are 10 or so in humans), survey the presence of microbial products (or endogenous molecules released from damaged or dying cells) on the cell surface or in the endosomal compartment and signal through TIR-domain containing adapter molecules; MyD88 and/or TRIF (reviewed in detail elsewhere) (Kawai and Akira, 2010). In addition, extensive research over the last few years has identified additional PRRs localized in the cytosolic compartment that belong to distinct classes including retinoic acid-inducible gene I (RIG-I)-like helicases and NOD-like receptors and AIM2-like receptors (ALRs). Thus, patrol of the extracellular space as well as intracellular compartments is achieved through a combination of PRRs. In the following sections, we review how DNA viruses are sensed and sampled in various cellular compartments during their life cycle to trigger innate immune defenses.
Cell surface recognition of DNA viruses
The host innate immune system employs PRRs particularly TLRs to detect microbial pathogens at the extracellular milieu. While several of such receptors sense bacteria and fungi, which have a complex outer structure, the detection of viruses at the cell surface is limited to only a very few receptors. Notably, the cell surface localized TLR2 is the predominant plasma membrane-localized sensor involved in anti-viral defenses. TLR2 is expressed by antigen presenting cells and non-immune cells such as mucosal epithelial cells (Iwasaki, 2010). TLR2 along with the co-receptors TLR1 or TLR6 binds cognate ligands, which include bacterial lipoproteins and glycoproteins. The crystallographic data indicate that TLR2 ligands such as, Pam3CSK4, mediate the heterodimerization of the TLR2 and TLR1 receptors through their three acyl chains; two which bind TLR2 and one binds TLR1(Jin et al., 2007) The hererodimerization of TLR1-TLR2 is suggested to promote the dimerization of their intracellular TIR domains. Subsequently, the TLR2-TLR1 complex initiate a signaling cascade via adaptor proteins MAL and MyD88 that leads to the activation of NF-κB and MAP kinases that turn on the genes involved in inflammatory responses (Jin et al., 2007). TLR2 is best studied in the context of anti-bacterial and anti-fungal responses, although in a limited number of cases, TLR2 is also involved in the recognition of viruses where recognition is coupled to the virus entry process. In the case of human cytomegalovirus, a TLR2-TLR1 complex recognize the CMV glycoproteins gB and gH, which mediate virus binding to host integrins and entry into host cell, leading to NF-κB activation (Boehme, Guerrero, and Compton, 2006). Studies in HEK293 cells reconstituted with TLR2 or in TLR2-deficient macrophages have revealed that TLR2 plays a central role in driving inflammatory cytokines following CMV infection (Compton et al., 2003). Consistent with these in vitro findings, impaired TLR2 function is associated with clinical cases of hCMV. In a population of immunocompromised transplant recipients, patients with a point mutation in the cytoplasmic Toll-IL-1 receptor domain of TLR2 that renders it nonfunctional had a higher CMV load indicating that TLR2 recognition is critical to the control of CMV infection (Kijpittayarit et al., 2007). Similarly, TLR2 plays an important role in sensing murine cytomegalovirus (mCMV); the TLR2−/− mice displayed increased susceptibility to mCMV relative to wild type controls as reflected in the increased viral loads in the visceral organs. Mechanistically, TLR2-sensing of mCMV triggers NK-cell activation through IL-18 secretion, which in turn control early infection (Szomolanyi-Tsuda et al., 2006).
TLR2 has also been implicated in sensing other herpes viruses including HSV, Epstein-Barr virus and varicella-zoster virus (Gaudreault et al., 2007; Kurt-Jones et al., 2004; Michaud et al., 2010; Wang et al., 2005). The innate recognition of HSV depends on TLR2 in a cell-type specific manner however. While TLR2 is essential for the recognition of HSV and the production of proinflammatory mediators by macrophages, microglial cells and to a certain extent by myeloid DCs (Aravalli, Hu, and Lokensgard, 2007; Aravalli et al., 2005; Lima et al.), the plasmacytoid DCs sense HSV in a TLR2-independent fashion (Rasmussen et al., 2007; Sato, Linehan, and Iwasaki, 2006). Additionally, Iwasaki and colleagues reported that TLR2 sensing of HSV-1 is virus strain/clone-dependent; TLR2-stimulating activity is restricted to only certain subclones of common laboratory and clinical isolates of HSV-1. Though the molecular mechanism underlying this phenomenon is not known, the inability to trigger TLR2 signaling is suggested to provide a selective advantage to the persistence of the virus in the host (Sato, Linehan, and Iwasaki, 2006). Though the HSV viral component that triggers TLR2 signaling has not been identified, it is possible that TLR2 detects one of the viral envelope glycoproteins as observed in the case of hCMV.
The requirement for TLR2 in sensing HSV in vivo depends on the route of infection and the HSV species. In the case of HSV-2, while TLR2 signaling is dispensable for the control of virus replication and dissemination following intraperitoneal infection, the optimal cytokine and chemokine production in the brain in response to a natural vaginal infection was dependent on TLR2 (Sorensen et al., 2008). Genetic studies in humans also revealed a role for TLR2 in HSV-2 infection; two polymorphisms in the TLR2 gene have been found to be associated with increased shedding of virus and higher lesional rates (Bochud et al., 2007). An infection route-dependent role of TLR2 was observed in HSV-1 infection also. Unlike in HSV-2 infection, TLR2 sensing of HSV-1 has been demonstrated to be deleterious to the host following intraperitoneal and ocular infections (Kurt-Jones et al., 2004; Sarangi et al., 2007); severe inflammatory responses characterized by the excessive cytokine and chemokine levels in the brain and the pathological lesions including mononuclear cell infiltration and perivascular cuffing in brain were remarkably reduced in the TLR2 deficiency. In line with these findings, TLR2 deficiency protected both the adult and neonatal mice against HSV-1 induced death (Kurt-Jones et al., 2005; Kurt-Jones et al., 2004). Similarly, in a physiologically relevant corneal infection model, TLR2 deficient mice displayed markedly attenuated proinflammatory cytokine production and stromal keratitic lesions relative to wild type mice (Sarangi et al., 2007). This clearly indicates that TLR2 sensing of HSV-1 in these models triggers exaggerated innate responses that adversely affect the host. In contrast, TLR2 was found to play a minimal role following intranasal infection; though TLR2 detection of HSV-1 contributed to local and/or systemic expression of IFN-γ and IL-1β, it did not play a role in the viral control and host survival. Collectively the data outlined above demonstrate that the invading HSV is sensed by surface TLR2, the contribution of which to innate anti-viral immunity is cell type- and infection route-dependent. TLR2 sensing is also essential for the host inflammatory responses and the virus control in infection with another herpes virus, murine herpes virus 68 (Michaud et al., 2010).
TLR2 is also an integral part of innate detection of additional DNA viruses including pox viruses. TLR2 recognizes vaccinia virus infection triggering NF-κB-dependent production of proinflammatory mediators by DCs (Zhu, Huang, and Yang, 2007). TLR2 activation by vaccinia virus occurs in a virus replication-independent manner and is believed to involve engagement of TLR2 by the envelope or capsid protein(s). Consistent with the in vitro data, a lack of TLR2 signaling hampered both innate and adaptive immune responses, including NK cell and CD8 T cell activation and IFN-γ production, to vaccinia virus in vivo (Zhu et al., 2007). Particularly, NK cell activation appears to be essential for the initial control of vaccinia virus (Zhu et al., 2007). Surprisingly, TLR2 activation in antigen presenting cells and the subsequent IL-1, IL-6 and IL-12 production is not necessary for NK-cell dependent virus control in vivo. In fact, the direct stimulation of TLR2 signaling in NK cells by vaccinia virus is found to be essential for NK cell function and virus control (Martinez, Huang, and Yang). Similarly, TLR2-MyD88 signaling in CD8 T cells rather than in DCs mediates the survival of activated T cells and their development into memory T cells during vaccinia virus infection (Quigley et al., 2009). These lymphocyte–specific functions of TLR2-MyD88 signaling appear to be mediated through the phosphatidylinositol 3-kinase (PI3K)-extracellular signal-regulated kinase (ERK) pathway (Martinez, Huang, and Yang). These findings highlight the fact that TLR2 sensing intrinsic to NK cells and T cells is also a critical event in certain viral infections.
The mechanisms through which TLR2 sensing of viruses contributes to antiviral immunity has further been expanded by additional recent findings (Barbalat et al., 2009; Michaud et al., 2010). Barbalat et al demonstrated that TLR2 activation by DNA viruses such as mCMV and vaccinia virus but not by bacterial TLR2 agonists triggers type I interferons (Barbalat et al., 2009). This was a somewhat surprising finding since TLR2 was thought to be incapable of activating type I IFN gene transcription (Barbalat et al., 2009; Bauernfeind and Hornung, 2009). This phenomenon was found to be unique to a subset of hematopoietic cells; the inflammatory monocytes that are CD11b+Ly6ChiLy6G−, a population distinct from macrophages and dendritic cells. The depletion of this specific cell type in mice revealed that TLR2-dependent type I interferon production is critical for the anti-viral resistance during vaccinia virus infection. These findings raised a key question; how TLR2 differentially responds to virus versus bacterial ligands. It has been speculated that virus recognition uniquely triggers TLR2 translocation from the plasma membrane to endosomes, from where a TLR2-MyD88 complex can activate the TRAF3-IRF3/IRF7 axis leading to the transcription of type I interferon genes, however further studies are required to clarify the molecular basis for this finding.
Adenovirus-based vectors have been developed for gene therapy but the success of these vectors in the clinical setting has been hampered by the rapid and potent inflammatory responses elicited in the host. The liver resident and splenic marginal zone macrophages are the key cell types that trap blood-borne adenovirus and elaborate proinflammatory mediators (Appledorn et al., 2008; Di Paolo et al., 2009). Appledorn et al showed that TLR2 signaling partially contributes to the production of certain chemokines (MCP-1 and RANTES) but not cytokines in a time-dependent manner. Additionally, the humoral responses to adenovirus and adenovirus-encoded transgenes were dependent on TLR2. The limited role of TLR2 indicated that innate recognition of adenovirus involves an additional pathway(s). Recently, Shayakhmetov and colleagues identified cellular β3 integrins as an integral component of innate immune detection of adenovirus (Di Paolo et al., 2009). They demonstrated that the interaction of arginine-glycine-aspartic acid (RGD) motifs of viral homopentameric penton base protein with host β3 integrins during viral entry triggered the production of IL-1α within a few minutes of infection in vivo. IL-1α is synthesized as a preprotein, and is converted to an active cytokine by cytoplasmic proteases such as calpains (Fitzgerald, 2009). β3 integrin signaling appears to trigger the processing of inactive IL-1α rather than its transcription or translation in a MyD88-, TRIF-, and TRAF6-independent fashion (Di Paolo et al., 2009). Surprisingly, adenovirus induced inflammation in vivo is mainly driven by IL-1α signaling; following adenovirus infection, IL-1R-deficient mice and wild type mice treated with anti-IL-1 antibodies displayed reduced inflammatory responses as well as hepatotoxicity (Di Paolo et al., 2009). Furthermore, the sensing of viral RGD motifs by host β3 integrins has also been implicated as a crucial event in mediating chemokine production, leukocyte infiltration as well as corneal inflammation in infections with human adenovirus serotype 37, the causative agent of a highly contagious epidemic keratoconjunctivitis (Chintakuntlawar et al., 2010). Collectively, these findings demonstrated the importance of host integrins as key mediators of inflammation not only to adenoviral vectors but also to pathogenic adenoviruses.
TLR4, the first identified member of the TLR family, is a surface receptor that predominantly recognizes lipid A, the major structural component of bacterial lipopolysaccharide. TLR4 binds lipid A in complex with the accessory protein MD2 which leads to receptor dimerization. Activated receptors then signal via TLR adapter molecules Mal/TIRAP, MyD88, TRAM and TRIF which trigger signaling pathways that culminate in the transcriptional regulation of inflammatory cytokines and type I interferon genes (Kagan et al., 2008). While the role of TLR4 in infections with bacteria has been extensively studied, less was known about the role of TLR4 in anti-viral defenses. TLR4 has been shown to sense the RNA virus, Respiratory Synchitial virus and a recent study also revealed a role for TLR4 in the recognition of DNA viruses (Hutchens et al., 2008a). TLR4 is required for the efficient control and maximal protection against vaccinia virus challenge. Surprisingly, TLR4 deficiency did not impair the production of proinflammatory cytokines in the lungs but rather resulted in exaggerated inflammation in the lungs after vaccinia virus challenge (Hutchens et al., 2008a). Therefore the mechanism of TLR4 mediated protection against vaccinia virus remains not known.
Innate sensing of DNA viruses in the endosomal compartment
Following their interaction with the cell membrane, viruses enter the host cell primarily via (Mercer, Schelhaas, and Helenius, 2010). As the internalized virus traffics in the endosomal compartment, it undergoes disassembly exposing the hitherto-concealed viral products, particularly the genome to endosomally localized PRRs such as TLR3, 7, 8 and 9. Innate-surveillance in the endosomal compartment relies on the sensing of viral nucleic acids and is mediated through TLR3, TLR7, TLR8 and TLR9. While TLR3 recognizes double stranded RNA, TLR7 and TLR8 have been shown to detect ssRNA mainly from viral infections and in some rare cases in response to bacterial infections (Kawai and Akira, 2010). TLR9 recognizes hypo/unmethylated CpG-rich DNA that is frequently present in the genomes of microbes. In contrast in mammalian cells this CpG motif is methylated. New evidence indicates that TLR9 can also recognize natural DNA with a phospho-diester backbone in a manner dependent on the 2-deoxyribose sugar rather than the CpG motif (Haas et al., 2008). Thus nucleic acid sensing by all the endosomal TLRs appears to be mostly sequence-independent. Upon binding their ligands, TLR7 and 9 recruit MyD88 to initiate the signaling that lead to the activation of IRF7 and the expression of proinflammatory genes including type I interferons. The MyD88-IRF7 signaling pathway is the major IFN-α/β-inducing pathway in pDCs because the nucleic acid ligands are retained in the endosomes for a longer duration in this cell type leading to the sustained activation of IRF7. In contrast, TLR3 signals via TRIF to activate the IRF3 transcription factor. All of the endosomal TLR pathways converge on either IRF3 or IRF7. These IRFs are strong inducers of type I interferons (Kawai and Akira, 2010). Once activated, the phospho-IRF3 and -IRF7 translocate into the nucleus as homodimers or heterodimers and form a transcription complex termed the enhanceosome together with NF-κB and ATF2-c-jun. The assembly of the enhanceosome leads to a strong induction of type I IFN-β (Honda and Taniguchi, 2006).
In DNA viral infections, TLR9 is the primary sensing mechanism in the endosome. A number of DNA viruses have been shown to trigger TLR9. The innate control of mCMV infection is dependent at least in part on TLR9 signaling; TLR9−/− mice had significantly increased viral titers following systemic infection with mCMV (Krug et al., 2004a; Tabeta et al., 2004). TLR9-dependent detection of mCMV results in the production of IL-12, MIP-1 and IFN-α/β and thereby activation and proliferation of NK-cells, the crucial cell type responsible for the early control of mCMV. Consistent with these attenuated immune responses, TLR9 deficiency impaired the survival of mCMV-infected mice (Tabeta et al., 2004). As the innate recognition of mCMV is virus replication-independent (Krug et al., 2004a), TLR9 is suggested to sense the DNA of the incoming mCMV virions following endocytosis/uncoating in the endosome. Though the CMV genome is rich in unmethylated CpG motifs, it is still not clear whether TLR9 is activated by the CpG motifs or the 2-deoxyribose sugar backbone of the viral DNA. Though TLR9 deficiency significantly hampered the immune response to mCMV in the above-mentioned studies, those responses particularly, type I IFN production and virus control were not completely abrogated as observed in the absence of MyD88 (Hokeness-Antonelli et al., 2007). In other words, MyD88−/− mice are more susceptible than TLR9−/− mice to mCMV infection suggesting an additional MyD88-dependent pathway sensing CMV infection. Indeed, the TLR7 pathway which is also capable of inducing IFN-α/β in pDCs also contributes to the detection of mCMV in vivo (Delale et al., 2005). Though TLR7 deficiency alone did not significantly impair pDC production of IFN-α/β, IL-12, and TNF-α in vivo, the deficiency of both TLR7 and TLR9 abolished the host responses to mCMV (Delale et al., 2005). Moreover, the TLR7−/−TLR9−/− double KO mice were more susceptible than the respective single KO mice to mCMV infection (Delale et al., 2005). Together, these findings demonstrated that TLR7 and TLR9 cooperate in the innate sensing of mCMV. The mechanism underlying TLR7 detection of mCMV is not yet known. One possibility is that the autophagic machinery may directly participate in the infected cells to deliver the viral RNA to endosomes as indicated in certain RNA viral infections (Lee et al., 2007). Alternatively, during phagocytosis of infected cells by pDCs the viral RNAs CMV may become accessible to TLR7 in the endosomal compartment. Further studies are required to define the role of the autophagy machinery during infection with DNA viruses however.
The role of TLR9 in the detection of the α herpes viruses is cell type-specific and limited to certain responses. While TLR9 mediates the early but not late production of interferon-α/β by pDCs in response to HSV-1 and HSV-2 infection in vivo, the production of other proinflammatory cytokines including TNF-α, IL-6, and KC by pDCs occurs in a TLR9-independent manner (Rasmussen et al., 2007). Similarly, HSV-driven innate responses including interferon production by conventional DCs, macrophages, and MEFs does not appear to require TLR9 signaling. A parallel trend has also been observed in humans. HSV-driven IFN-α/β secretion by peripheral blood mononuclear cells (PBMCs) cells from patients with IRAK-4 deficiency, which abolishes interferon production in response to TLR7, TLR8, TLR9 but not TLR3 and TLR4 ligands, was normal (Yang et al., 2005). In addition, TLR9 is dispensable for the control of HSV replication in vitro in several cell types, particularly, pDCs (Rasmussen et al., 2007). The role of TLR9 in the control of virus in vivo depends on both the route and dose of infection. The HSV-1 load at the local sites after footpad injection was comparable between TLR9−/− and wild type mice (Krug et al., 2004b). In the corneal infection model, while TLR9 did not contribute to virus control in the cornea and trigeminal nerves after infection with a higher dose of HSV-1, following a lower dose infection the optimal virus clearance in the cornea was dependent on TLR9 (Wuest et al., 2006). While the contribution of TLR9 to virus clearance is minimal, TLR9 plays a modest role in the pathogenesis of HSV-1-induced disease (Sarangi et al., 2007; Wuest et al., 2006). After corneal scarification with HSV-1, TLR9−/− mice displayed a partial reduction in the production of cytokines and chemokines such as CXCL-9 and CXCL-10 and the infiltration of cornea with neutrophils. Accordingly, the neovascularization of the cornea and the development of keratitic lesions were modestly attenuated in the absence of TLR9 signaling.
TLR9 sensing of HSV-2 plays a protective role in a murine model of genital herpes (Lund et al., 2006). Following intravaginal infection with HSV-2, TLR9−/− mice and pDC-depleted mice displayed reduced IFN-α secretion, increased leukocyte infiltration, severe vaginal inflammation, and increased local viral loads compared to wild type mice. Consistent with this, the deficiency of TLR9 or depletion of pDCs significantly compromised the survival of infected mice. The mechanism of TLR9 sensing of HSV is similar to that observed with β-herpes viruses. TLR9 recognition required virus entry by endocytosis and endosomal acidification indicating that the DNA, most likely the CpG motifs, of the invading virus is the ligand for TLR9 (Lund et al., 2003; Rasmussen et al., 2007). Overall, TLR9 sensing plays a relatively minimal role in eliciting anti-viral responses in HSV-1 infection when compared to CMV infection. Perhaps, the stimulation of TLR9 by HSV DNA may be weak in the physiological conditions possibly due to the limited accessibility of viral DNA to TLR9. Alternatively, TLR9 mediated responses to HSV-1 are redundant with additional pathways. For instance, the strong activation of TLR2 signaling by HSV-1 may compensate for the lack of TLR9 signaling resulting in normal anti-viral responses in TLR9-deficient cells/mice.
TLR9 also appears to contribute to the innate response to gammaherpesviruses where TLR9-dependent sensing is important in viral pathogenesis and organ-specific immunity during both lytic infection and latency (Guggemoos et al., 2008). TLR9 signaling is essential for the optimal proinflammatory responses characterized by the production of IL-12, IFN-α, and IL-6 by Flt3L-derived DCs upon stimulation with murine gammaherpesvirus 68. In a systemic infection model, mice lacking TLR9 were susceptible to gammaherpesvirus 68 and displayed higher latent viral loads while no difference was observed with intranasal challenge (Guggemoos et al., 2008)
The role of TLR9 in immunity to poxviruses has also been revealed and appears to be virus species-specific. The maturation and interferon production by pDCs in response to ectromelia virus (ECTV) was mediated by TLR9 (Samuelsson et al., 2008). TLR9 is also an essential component of innate sensing of ECTV in vivo as the TLR9−/− mice are highly susceptible to ECTV infection (Samuelsson et al., 2008). In contrast, the proinflammatory responses induced by live modified vaccinia virus Ankara (MVA) were TLR9-independent (Samuelsson et al., 2008; Waibler et al., 2007). Though both ECTV and MVA belong to the same genus Orthopoxvirus, the mechanism responsible for the differential requirement for TLR9 signaling is not known. Likewise, TLR9 plays little or no role in infections with vaccinia-related viruses (Zhao et al., 2009). In fact a recent study showed that vaccinia virus-induced activation of pDCs is mediated by TLR8 and that this TLR8-dependent immune responses is critical in innate control of VV infection in vivo (Martinez, Huang, and Yang, 2010). TLR8 is phylogenetically related toTLR7 and while human TLR8 recognizes ssRNA, the mouse version was originally believed to be nonfunctional (Kawai and Akira, 2010). However, the recent findings indicate that TLR8 plays a role in the regulation of innate immunity (Demaria et al., 2010). The molecular mechanism by which vaccinia virus activates TLR8 however remains unknown.
TLR9 also contributes to the innate immune response to adenovirus in a cell-specific manner (Cerullo et al., 2007; Zhu, Huang, and Yang, 2007). Dendritic cell recognition of helper dependent-adenovirus does not involve TLR9 (Cerullo et al., 2007). However, macrophages, the major cell type driving inflammation following adenoviral delivery, sense helper dependent-adenovirus through TLR9 (Cerullo et al., 2007). A similar phenomenon has also been observed for the recombinant E1- and E3-deleted adenovirus (Zhu, Huang, and Yang, 2007). The type I IFN production by pDCs in response to the recombinant adenovirus depends on TLR9-MyD88 signaling, whereas that by conventional DCs and macrophages is TLR9-independent. Importantly, TLR9 deficiency attenuated acute proinflammatory responses including IFN-α production elicited by adenoviral vectors in vivo. Similarly, in a mouse adenovirus keratitis model, though TLR9 contributed to IL-6 production and sustained monocytic infiltration of the cornea, chemokine secretion and keratitis development were TLR9-independent (Chintakuntlawar et al., 2010). Furthermore, empty adenoviral particles devoid of genomic DNA are poor inducers of innate responses indicating that the DNA is a major immunostimulatory molecule of adenovirus ((Cerullo et al., 2007); (Iacobelli-Martinez and Nemerow, 2007)). All of the inflammatory responses elicited by adenovirus in vitro and in vivo are not solely attributed to TLR9 signaling indicating the existence of an additional sensor(s) of adenoviral DNA in the cell (Appledorn et al., 2008); (Cerullo et al., 2007; Zhu, Huang, and Yang, 2007).
TLR3 is also expressed in the endosomes where it detects dsRNA. Unlike TLR2 and TLR9 which signal via MyD88, TLR3 activates a MyD88-independent pathway that requires TRIF (Kawai and Akira, 2010). The formation of dsRNA structures has been observed during the replication of many DNA viruses such as vaccinia virus, adenovirus, and herpes viruses. The dsRNA may reach the endosome either via autophagy or by the phagocytosis of infected cells by the bystander cells. Though dsRNA structures are commonly noticed in several classes of DNA viruses, TLR3 sensing has been shown to play a role mainly in immunity to herpes viruses. A dominant negative mutant allele of TLR3 characterized by P554S aminoacid substitution has been found to be associated with herpes simplex virus 1 (HSV-1) encephalitis in children. This P554S mutation in the extracellular leucine-rich repeat domain, which is essential for dsRNA binding and receptor multimerization, renders TLR3 nonfunctional. The heterozygosity for this P544S TLR3 mutation in humans has been suggested to impair antiviral responses and virus control in the central nervous system (Zhang et al., 2007). The innate immune response to mCMV is partly mediated by TLR3; TLR3 is essential for maximal interferon and cytokine production following mCMV infection in vivo as well as for virus clearance at later but not at early time points (Delale et al., 2005; Tabeta et al., 2004). Epstein-Barr virus, a gammmaherpes virus, encodes small noncoding RNAs that form dsRNA–like structures. These dsRNAs released by the EBV-infected cells are present in the serum from patients with active EBV infections where they activate TLR3 (Iwakiri et al., 2009). Furthermore, TLR3 is essential for sensing Kaposi's sarcoma-associated herpesvirus (KSHV) in human THP-1 monocytes (West and Damania, 2008). Collectively, the endosomal recognition of infections with several classes of herpes viruses such as α, β, and γ is mediated by TLR3. The recent findings indicate a deleterious role for TLR3 in sensing pox viruses. TLR3 deficient mice displayed enhanced virus control and were protected from vaccinia virus induced lethality (Hutchens et al., 2008b). However, it appears that TLR3 is not activated by majority of other classes of DNA viruses or is largely redundant with other innate pathways.
Cytosolic sensing of DNA viruses
Most of the viruses access the cytosolic compartment of host cells via multiple mechanisms during their life cycle. In most cases, following endocytosis the internalized virus gains access to the cytosol by fusion and uncoating of the virus in the cytosol or by lysing of the endosome as in the case of adenovirus (Greber, Singh, and Helenius, 1994). Certain viruses including HSV directly fuse their envelope with the plasma membrane leading to the delivery of the capsid into the cytoplasm (Marsh and Helenius, 2006). During the process of replication, virions and/or their components accumulate in the cytosol. This is particularly relevant in the case of pox viruses as they replicate in the cytosol exposing potentially a whole array of viral ligands to the host cytosolic surveillance system. Therefore the detection of viral products in the cytosol is integral to innate anti-viral defense. One of the most commonly observed virus-associated molecular patterns in the cytosol is viral nucleic acids, the detection of which forms the basis of the cytosolic sensing of DNA viruses. Accumulating evidence over the last decade has demonstrated that unlike at the cell surface and in the endosomes, where virus sensing is restricted to a few TLRs, an expanding repertoire of PRRs contribute to innate viral sensing in the cytosol (Hornung and Latz, 2010b). The recognition of viral DNA by these mechanisms leads to two distinct types of responses characterized by the production of type I interferons/inflammatory cytokines and caspase 1-dependent secretion of IL-1β.
Interferon-inducing cytosolic sensors
Type I interferon production by the host is the frontline anti-viral defense strategy and it is one of main outcomes of the cytosolic sensing of DNA. The identification of the upstream receptors and signaling components that mediate the cytosolic interferon response has been the subject of intense investigation over the past few years. Multiple receptors namely, DAI (also known as ZBP1 or DLM-1), RNA polymerase III, LRRFIP1, DDX36/DHX9 and IFI16 have all been implicated (discussed below). A common signaling pathway exists downstream of these receptors to turn on interferon production at the transcriptional level; the cytosolic DNA recognition pathways converge on STING (stimulator of interferon genes), a transmembrane protein expressed on the endoplasmic reticulum membranes and the outer mitochondrial membrane (Ishikawa, Ma, and Barber, 2009). STING relocalizes with TANK-binding kinase 1 (TBK1), an IKK-related kinase that phosphorylates and activates IRF3 and IRF7 (Hornung and Latz, 2010b; Ishikawa, Ma, and Barber, 2009). The STING-TBK1 axis is central to the cytosolic DNA- and RNA- driven interferon responses and host resistance against DNA viral infections (Ishikawa, Ma, and Barber, 2009).
DAI
DNA-dependent activator of IRFs (DAI) was the first molecule to be identified as a DNA sensor in the cytosol (Takaoka et al., 2007). Initial knockdown approaches showed that DAI mediates TBK1-IRF3 dependent type I interferon production in responses to synthetic DNA and HSV-1 infection. Human CMV-driven interferon production by human fibroblasts was also shown to dependent on DAI (DeFilippis et al., 2010). However, the DAI-deficient mice and various cell types derived from them including macrophages and mouse embryonic fibroblasts displayed normal responses to synthetic DNA and DNA viruses (Ishii et al., 2008; Wang et al., 2008). These later studies indicated that DAI plays a redundant and/or cell-type specific role in the sensing of cytosolic DNA.
LRRFIP1
LRRFIP1 has been recently shown to recognize both cytosolic RNA and DNA (AT-rich B-form dsDNA as well as GC-rich Z-form dsDNA) and subsequently recruit β-catenin through an unknown mechanism to enhance IFNB production. The association of β-catenin with LRRFIP1 leads to its phosphorylation at Ser552 and translocation into the nucleus. The activation of β-catenin leads to the recruitment of p300-histone acteyltransferase to the Ifnb1 promoter through IRF3, which ultimately enhances the expression of IFN-β gene. Though LRRFIP1 is essential for maximal interferon production in response to a cytosolic bacterium, Listeria monocytogenes or a RNA virus, vesicular stomatitis virus, the role of LRRFIP1 in sensing infections with DNA viruses remains unknown (Rathinam, Sharma, and Fitzgerald, 2010; Yang et al., 2010).
IFI16
IFI16 (interferon-inducible protein 16) and its closest murine homolog p204 belong to the interferon inducible PYHIN protein family (pyrin and HIN200 domain-containing proteins; also known as p200 or HIN200 proteins). Bowie and colleagues recently demonstrated that IFI16 is a sensor of cytosolic DNA. IFI16 recognizes DNA through DNA-binding HIN domains and subsequently interacts with STING to activate a TBK1-IRF3 axis resulting in type I interferon gene expression (Unterholzner et al., 2010). RNAi-mediated knockdown of IFI16 indicated that IFI16 is essential for innate responses driven by HSV-1 infection or a synthetic DNA motif that is common in the vaccinia virus genome. The generation of mice deficient in p204 will allow further analysis of the relative role of IFI16 in sensing DNA and DNA viruses.
DHX36 and DHX9
Aspartate-glutamate-any amino acid-aspartate/histidine (DExD/H)-box helicase 36 (DHX36) and DExD/H-box helicase 9 (DHX9) have recently been shown to sense CpG-A and CpG-B DNA, respectively, in the cytosol of human pDCs (Kim et al., 2010). In contrast to other cytosolic sensors described so far that signal through the STING-TBK1 pathway, DHX36 and DHX9 activate IFN via MyD88. Upon binding CpG DNA, DHX9 and DHX36 recruit MyD88 via interactions between the HA2 and DUF domains of DHX and the TIR domain of MyD88 leading to the activation of IRF7 and NF-κB. RNAi-mediated knockdown of DHX36 and DHX9 expression blunted the proinflammatory responses of human pDCs to HSV infection indicating the involvement of these proteins in the innate recognition of DNA viruses.
RNA polymerase III
A novel RNA polymerase III-dependent mechanism of cytosolic DNA has also been revealed (Ablasser et al., 2009; Chiu, MacMillan, and Chen, 2009). RNA polymerase III present in the cytoplasm recognizes AT-rich DNA and transcribes it into immunostimulatory RNA transcripts characterized by the presence of uncapped 5′ triphosphate group. These RNA species then stimulate RIG-I which signals via IPS1/MAVS to turn on IFN and cytokine genes. RNA polymerase III has been shown to play a role in sensing certain DNA viral infections. Adenovirus-induced IFN-β expression in murine cells is dependent on RNA polymerase III (Chiu, MacMillan, and Chen, 2009). Furthermore, Epstein-Barr virus induces type I interferon expression via EBV-encoded small RNA 1 and 2, (EBER1 and EBER2) and this response is mainly mediated by RNA polymerase III (Ablasser et al., 2009). However, the role of RNA polymerase III in sensing the GC-rich HSV-1 DNA is not clear as the published findings on the requirement of RNA polymerase III for HSV-1-induced type I interferon expression are not consistent, warranting further studies (Chiu, MacMillan, and Chen, 2009; Melchjorsen et al., 2010; Unterholzner et al., 2010).
MDA5/MAVS
Recent studies also indicate an RNA polymerase III-independent mechanism of sensing of immunostimulatory viral RNAs in the cytosol. In vaccinia virus infected cells, MDA (melanoma differentiation-associated protein 5), the cytosolic sensor for long stretches of dsRNA has been shown to detect higher-order structures of RNA comprising both single-stranded RNA and dsRNA and subsequently mediate type I IFN responses (Pichlmair et al., 2009). Similarly, the MDA-MAVS pathway elicits interferon responses to HSV-1 infection in human primary macrophage in a RNA polymerase III-independent manner adding to the complexity of the innate immune detection of HSV (Melchjorsen et al.). Overall these studies together with those on RNA polymerase III indicate that at least two mechanisms operate in the cytosol of DNA virus infected cells to generate immunostimulatory RNA and that each of these mechanisms stimulates distinct cytosolic RNA sensors with a certain level of redundancy.
These studies therefore reveal the complexity of the mechanisms by which the innate immune system senses the presence of DNA in the cytosol. It is likely that over the next few years, we will gain a comprehensive understanding of how these complex mechanisms coordinate anti-viral defenses.
Inflammasome-dependent recognition of DNA viruses
The immune response to DNA in the cytosol is not limited to the activation of type I interferon responses, but also stimulates the production of an important group of cytokines belonging to IL-1 family of pro-inflammatory cytokines (Hornung and Latz, 2010b). IL-1β and IL-18 play a central role in host defense against a variety of bacterial and fungal pathogens (Martinon, Mayor, and Tschopp, 2009). Growing evidence supports the importance of these cytokines in anti-viral defenses (Kanneganti, 2010; Rathinam and Fitzgerald, 2010). IL-1β stimulates innate and adaptive mechanisms of antimicrobial resistance through its action on neutrophils, macrophages, CD4 and CD8 T cells. Similarly, IL-18 is critical for IFN-γ production by NK cells and T cells (Rathinam and Fitzgerald, 2010). In contrast to the transcriptional regulation of other proinflammatory cytokines, IL-1β and IL-18 are regulated post-translationally. IL-1β and IL-18 are synthesized as inactive proteins following the stimulation of pattern recognition pathways, particularly TLRs (Dinarello, 2010). The conversion of proforms of IL-1β and IL-18 into their respective bioactive forms is mediated by a large multiprotein complex in the cytosol referred to as inflammasomes (Martinon, Burns, and Tschopp, 2002; Martinon, Mayor, and Tschopp, 2009). In response to a distinct set of stimuli of microbial as well as endogenous origins, an upstream receptor that belongs to the NOD-like receptor family in most cases forms a complex with procaspase 1, a cysteine protease via an adaptor protein ASC. The assembly of this complex leads to the cleavage of inactive procaspase 1 to caspase 1, which then catalyzes the proteolytic processing of proIL-1β and proIL-18 into IL-1β and IL-18 (Bryant and Fitzgerald, 2009). Of all the inflammasome complexes identified so far, NLRP3 and AIM2 inflammasomes play a role in sensing DNA viruses (Rathinam and Fitzgerald, 2010).
The NLRP3 inflammasome
A diverse range of stimuli of microbial and endogenous origins such as bacterial pore-forming toxins, uric acid and ATP, triggers NLRP3 to associate with ASC and procaspase 1 and form an active inflammasome complex (Bryant and Fitzgerald, 2009). The heterogenous nature of the stimuli that activate NLRP3 indicate that NLRP3 does not recognize these ligands directly but rather senses a common downstream cellular event that is triggered in cells in response to these various stimuli, which include the generation of reactive oxygen species, potassium efflux. Several mechanisms have been proposed and have been reviewed extensively, elsewhere (Hornung and Latz, 2010a; Latz, 2010; Martinon, 2010). The role of NLRP3 inflammasome in viral infections is best characterized in influenza A infection. NLRP3 is required for the optimal inflammasome-dependent responses and host resistance following infection with a high dose of influenza A virus (Allen et al., 2009; Ichinohe et al., 2009; Thomas et al., 2009). NLRP3 has been shown to sense infections with DNA viruses such as adenovirus and modified vaccinia virus Ankara strain. NLRP3 and ASC are essential for the adenovirus-driven processing of IL-1β by mouse macrophages (Muruve et al., 2008). Moreover, the inflammatory responses elicited by adenovirus in the liver and spleen of infected mice was shown to be dependent on NLRP3. Similarly, NLRP3 mediates the production of active IL-1β in response to modified vaccinia virus Ankara strain (Delaloye et al., 2009). The precise molecular mechanism by which DNA viruses activate NLRP3 inflammasomes is not yet clear. Furthermore, whether NLRP3 plays an important role in the host protective immune responses to DNA viruses in vivo remains largely unknown.
AIM2 inflammasome
DNA in the cytosolic compartment is a potent trigger of mature IL-1β and IL-18 production (Muruve et al., 2008) and is mediated not by NLRP3, but rather by AIM2 (Absent in melanoma), an interferon inducible protein that belongs to the same family as IFI16 (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Roberts et al., 2009). AIM2 recognizes cytosolic dsDNA of self and nonself origin including viral DNA via its HIN domain in a sequence-independent manner. Contrary to other cytosolic sensors of DNA, the recognition of DNA by AIM2 triggers the assembly of an inflammasome complex. Upon DNA binding, AIM2 likely undergoes oligomerization and associates with ASC via homotypic pyrin-pyrin domain interactions, which in turn recruits procaspase 1. Published data has shown that the AIM2 inflammasome is an integral component of innate sensing of DNA viruses (Rathinam et al., 2010). AIM2 is essential for the activation of caspase 1 and proteolytic processing of IL-1β and IL-18 in antigen presenting cells in response to infection with mCMV and vaccinia virus. Furthermore, AIM2-ASC dependent IL-18 secretion and NK-cell activation is critical in the early control mCMV infection in vivo. Interestingly, AIM2 does not sense all herpes viruses; for example, it has been shown that HSV-1-driven processing of IL-1β by macrophages does not require AIM2. This is somewhat surprising, since HSV-1 DNA (not in association with viral capsids) has been shown to accumulate in the cytosol of infected cells and it activates other DNA sensors such as IFI16 in the cytosol (Unterholzner et al., 2010). It is of critical importance to determine the molecular basis for differential recognition of herpes viruses by AIM2.
Conclusions and future perspectives
Intense research over the last decade has undoubtedly advanced our understanding of the early critical events in the generation of anti-viral immunity. The Innate immune system employs germ-line encoded receptors to sense viruses and/or their components in various cellular compartments. While the recognition of viruses at the cell surface and in the endosomes is almost exclusively by TLRs, the cytosolic sensing relies on a diverse set of receptors. While the recent findings provide deeper insights into innate sensing of viruses, they raise many key questions. First of all, DNA viruses such as herpes viruses replicate in the nucleus. Since every cellular compartment that viruses have access to have been heavily guarded by innate receptors, it raises the possibility that the nucleus should also be under innate immune surveillance. How nuclear surveillance would occur and enable the host to discriminate between pathogenic and host DNA is an area worthy of investigation. This could become particularly relevant for the Aim2-like receptor, IFI16, which is present in the nucleus and to a much lesser degree in the cytosol. The chromatinization of incoming viral DNA and mounting DNA damage responses to the viral DNA fragments by the cellular machinery as in the case of HSV-1 infection are some of the known surveillance mechanisms in the nucleus (Knipe and Cliffe, 2008; Lilley et al., 2010). It is important to understand whether additional mechanism(s) exist in the nucleus that trigger innate and inflammatory responses upon sensing viral products.
Compared to the cell surface and endosomal compartment, the cytosolic sensing of viruses is complex and there appears to be considerable redundancy, with several sensors contributing to the overall anti-viral responses. Though such phenomenon would minimize the possibility of viral evasion of one particular pathway, the biological significance of this redundancy is not yet clear. Additionally, the detection of DNA is the basis of cytosolic sensing and it is important to determine whether these receptors recognize the naked viral DNA or DNA-viral protein complexes. With the existence of several receptors it is also possible that distinct aspects of DNA sequence and or structure is recognized by distinct receptors. The inflammasome-dependent sensing of DNA viruses has been limited to NLRP3 and AIM2 only whereas additional inflammasomes play a role in sensing other classes of pathogenic microbes such as bacteria. Therefore it is of interest to determine whether DNA viruses also activate any novel inflammasome pathways. Importantly, the clinical significance of the innate sensing pathways in human cases of viral diseases should be thoroughly assessed by comprehensive whole genome studies. This will be a major step towards translating the basic research findings to protect humans from infectious diseases.
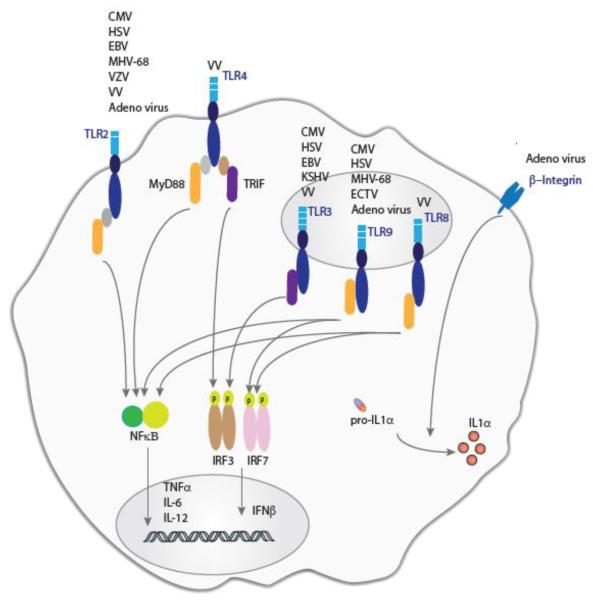
Figure 1.
Cell surface and endosomal recognition of DNA viruses. A majority of DNA viruses such as CMV, HSV, vaccinia virus and adenovirus are recognized at the cell surface by TLR2. Vaccinia virus is also detected by TLR4. The stimulation of TLR2 and TLR4 leads to the activation of NF-κ B and IRF-3 dependent immune responses. In contrast the recognition of adenovirus by β3-integrins at the cell surface leads to post-translational processing of proIL-1α to IL-1α. The recognition of DNA viruses in the endosomes is exclusively mediated by TLRs such as TLR3, 7, 8, and 9 which sense nucleic acids. The activation the endosomal TLRs results in a strong activation of IRF3/7-dependent type I interferon responses as well as NF-κB-dependent cytokine expression.
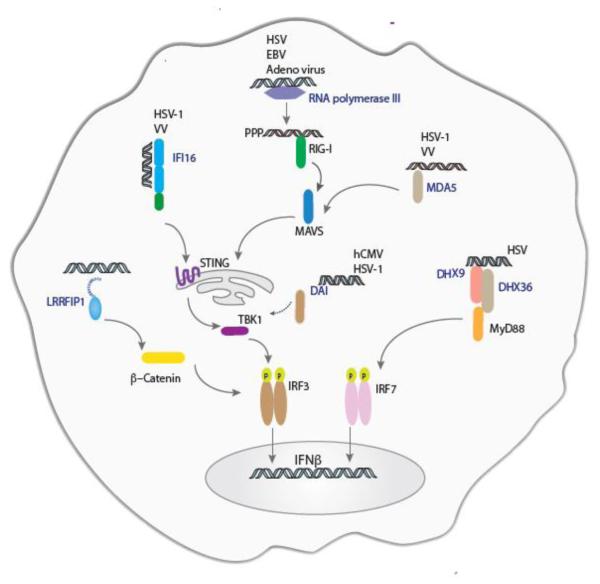
Figure 2.
Detection of DNA viruses by interferon-inducing cytosolic sensors. Cytosolic sensing DNA viruses is a complex phenomenon involving a plethora of receptors including IFI16, RNA polymerase III and MDA5. These receptors sense viral nucleic acids and activate type I interferon responses through a common signaling platform consisting of STING and TBK1.
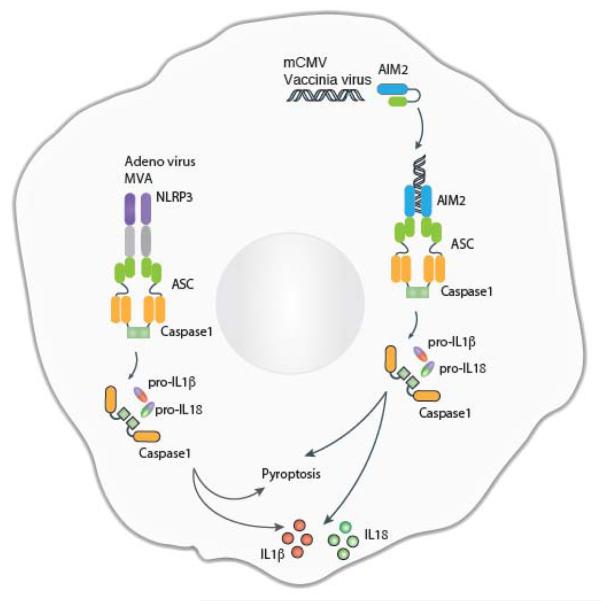
Figure 3.
Inflammasome-dependent recognition of DNA viruses. NLRP3 and AIM2 sense DNA viral infections and form caspase 1-containing inflammasome complexes through the adapter protein ASC. The activation of caspase 1 results in the proteolytic processing of proIL-1β and proIL-18 to IL-1β and IL-18, respectively. The DNA viruses sensed by the inflammasome pathways include adenovirus, modified Ankara vaccinia virus (MAV), mCMV, and vaccinia virus (VV).
Acknowledgements
V.A.R is supported by a postdoctoral fellowship from the New England Regional Center of Excellence for Biodefense and Emerging Infectious Diseases (NERCE; NIH/NIAID AI057159). K.A.F is supported by NIH grants AI083713 and AI067497.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ablasser A, Bauernfeind F, Hartmann G, Latz E, Fitzgerald K, Hornung V. RIG-I-dependent sensing of poly (dA: dT) through the induction of an RNA polymerase IIIñtranscribed RNA intermediate. Nature Immunology. 2009;10(10):1065–1072. doi: 10.1038/ni.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30(4):556–65. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, Amalfitano A. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181(3):2134–44. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- Aravalli RN, Hu S, Lokensgard JR. Toll-like receptor 2 signaling is a mediator of apoptosis in herpes simplex virus-infected microglia. J Neuroinflammation. 2007;4:11. doi: 10.1186/1742-2094-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol. 2005;175(7):4189–93. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10(11):1200–1207. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind F, Hornung V. TLR2 joins the interferon gang. Nat Immunol. 2009;10(11):1139–1141. doi: 10.1038/ni1109-1139. [DOI] [PubMed] [Google Scholar]
- Bochud PY, Magaret AS, Koelle DM, Aderem A, Wald A. Polymorphisms in TLR2 are associated with increased viral shedding and lesional rate in patients with genital herpes simplex virus Type 2 infection. J Infect Dis. 2007;196(4):505–9. doi: 10.1086/519693. [DOI] [PubMed] [Google Scholar]
- Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177(10):7094–102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- Bryant C, Fitzgerald KA. Molecular mechanisms involved in inflammasome activation. Trends in Cell Biology. 2009;19(9):455–464. doi: 10.1016/j.tcb.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, Superti-Furga G. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10(3):266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, Bertin TK, Rodgers JR, Lee B. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol Ther. 2007;15(2):378–85. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- Chintakuntlawar AV, Zhou X, Rajaiya J, Chodosh J. Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis. PLoS Pathog. 2010;6(4):e1000841. doi: 10.1371/journal.ppat.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y, MacMillan J, Chen Z. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell. 2009;138(3):576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. Human Cytomegalovirus Activates Inflammatory Cytokine Responses via CD14 and Toll-Like Receptor 2. J. Virol. 2003;77(8):4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFilippis V, Alvarado D, Sali T, Rothenburg S, Fruh K. Human cytomegalovirus induces the interferon response via the DNA sensor ZBP1. Journal of virology. 2010;84(1):585. doi: 10.1128/JVI.01748-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delale T, Paquin A, Asselin-Paturel C, Dalod M, Brizard G, Bates EE, Kastner P, Chan S, Akira S, Vicari A, Biron CA, Trinchieri G, Briere F. MyD88-dependent and -independent murine cytomegalovirus sensing for IFN-alpha release and initiation of immune responses in vivo. J Immunol. 2005;175(10):6723–32. doi: 10.4049/jimmunol.175.10.6723. [DOI] [PubMed] [Google Scholar]
- Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, Pantaleo G, Esteban M, Calandra T. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5(6):e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Demaria O, Pagni PP, Traub S, de Gassart A, Branzk N, Murphy AJ, Valenzuela DM, Yancopoulos GD, Flavell RA, Alexopoulou L. TLR8 deficiency leads to autoimmunity in mice. J Clin Invest. 2010;120(10):3651–62. doi: 10.1172/JCI42081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, Papayannopoulou T, Shayakhmetov DM. Virus Binding to a Plasma Membrane Receptor Triggers Interleukin-1[alpha]-Mediated Proinflammatory Macrophage Response In Vivo. Immunity. 2009;31(1):110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. IL-1: Discoveries, controversies and future directions. European Journal of Immunology. 2010;40(3):599–606. doi: 10.1002/eji.201040319. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu J, Datta P, Wu J, Alnemri E. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458(7237):509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald K. Integr-ating IL-1 [alpha] in Antiviral Host Defenses. Immunity. 2009;31(1):7–9. doi: 10.1016/j.immuni.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Gaudreault E, Fiola S, Olivier M, Gosselin J. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J Virol. 2007;81(15):8016–24. doi: 10.1128/JVI.00403-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber UF, Singh I, Helenius A. Mechanisms of virus uncoating. Trends in microbiology. 1994;2(2):52–56. doi: 10.1016/0966-842x(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Guggemoos S, Hangel D, Hamm S, Heit A, Bauer S, Adler H. TLR9 contributes to antiviral immunity during gammaherpesvirus infection. J Immunol. 2008;180(1):438–43. doi: 10.4049/jimmunol.180.1.438. [DOI] [PubMed] [Google Scholar]
- Haas T, Metzger J, Schmitz F, Heit A, Müller T, Latz E, Wagner H. The DNA Sugar Backbone 22 Deoxyribose Determines Toll-like Receptor 9 Activation. Immunity. 2008;28(3):315–323. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Hokeness-Antonelli KL, Crane MJ, Dragoi AM, Chu W-M, Salazar-Mather TP. IFN-{alpha}beta-Mediated Inflammatory Responses and Antiviral Defense in Liver Is TLR9-Independent but MyD88-Dependent during Murine Cytomegalovirus Infection. J Immunol. 2007;179(9):6176–6183. doi: 10.4049/jimmunol.179.9.6176. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458(7237):514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010a;40(3):620–3. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Latz E. Intracellular DNA recognition. Nat Rev Immunol. 2010b;10(2):123–130. doi: 10.1038/nri2690. [DOI] [PubMed] [Google Scholar]
- Hutchens M, Luker K, Sonstein J, N'Òez G, Curtis J, Luker G. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 2008a;4:e1000153. doi: 10.1371/journal.ppat.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens M, Luker K, Sottile P, Sonstein J, Lukacs N, Nunez G, Curtis J, Luker G. TLR3 increases disease morbidity and mortality from vaccinia infection. The Journal of Immunology. 2008b;180(1):483. doi: 10.4049/jimmunol.180.1.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobelli-Martinez M, Nemerow GR. Preferential activation of Toll-like receptor nine by CD46-utilizing adenoviruses. J Virol. 2007;81(3):1305–12. doi: 10.1128/JVI.01926-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 2009;206(1):79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kawagoe T, Koyama S, Matsui K, Kumar H, Kawai T, Uematsu S, Takeuchi O, Takeshita F, Coban C. TANK-binding kinase-1 delineates innate and adaptive immune responses to DNA vaccines. Nature. 2008;451(7179):725–729. doi: 10.1038/nature06537. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakiri D, Zhou L, Samanta M, Matsumoto M, Ebihara T, Seya T, Imai S, Fujieda M, Kawa K, Takada K. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J Exp Med. 2009;206(10):2091–9. doi: 10.1084/jem.20081761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10(10):699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, Paik S-G, Lee H, Lee J-O. Crystal Structure of the TLR1-TLR2 Heterodimer Induced by Binding of a Tri-Acylated Lipopeptide. Cell. 2007;130(6):1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Kagan J, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon. Nature Immunology. 2008;9(4):361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanneganti T. Central roles of NLRs and inflammasomes in viral infection. Nature Reviews Immunology. 2010;10(10):688–698. doi: 10.1038/nri2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kijpittayarit S, Eid AJ, Brown RA, Paya CV, Razonable RR. Relationship between Toll-Like Receptor 2 Polymorphism and Cytomegalovirus Disease after Liver Transplantation. Clinical Infectious Diseases. 2007;44(10):1315–1320. doi: 10.1086/514339. [DOI] [PubMed] [Google Scholar]
- Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V, Bover L, Plumas J, Chaperot L, Qin J. Aspartate-glutamate-alaninehistidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proceedings of the National Academy of Sciences. 2010;107(34):15181. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Micro. 2008;6(3):211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004a;21(1):107–19. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood. 2004b;103(4):1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. The role of toll-like receptors in herpes simplex infection in neonates. J Infect Dis. 2005;191(5):746–8. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A. 2004;101(5):1315–20. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E. The inflammasomes: mechanisms of activation and function. Curr Opin Immunol. 2010;22(1):28–33. doi: 10.1016/j.coi.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lund J, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315(5817):1398. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 2010;29(5):943–955. doi: 10.1038/emboj.2009.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima GK, Zolini GP, Mansur DS, Freire Lima BH, Wischhoff U, Astigarraga RG, Dias MF, Silva MD, Bela SR, do Valle Antonelli LR, Arantes RM, Gazzinelli RT, Bafica A, Kroon EG, Campos MA. Toll-Like Receptor (TLR) 2 and TLR9 Expressed in Trigeminal Ganglia are Critical to Viral Control During Herpes Simplex Virus 1 Infection. Am J Pathol. doi: 10.2353/ajpath.2010.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198(3):513–20. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177(11):7510–4. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus Entry: Open Sesame. Cell. 2006;124(4):729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Huang X, Yang Y. Direct TLR2 Signaling Is Critical for NK Cell Activation and Function in Response to Vaccinia Viral Infection. PLoS Pathog. 6(3):e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Huang X, Yang Y. Toll-like receptor 8-mediated activation of murine plasmacytoid dendritic cells by vaccinia viral DNA. Proc Natl Acad Sci U S A. 2010;107(14):6442–7. doi: 10.1073/pnas.0913291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F. Signaling by ROS drives inflammasome activation. Eur J Immunol. 2010;40(3):616–9. doi: 10.1002/eji.200940168. [DOI] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. r. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Molecular Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annual Review of Immunology. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- Melchjorsen J, Rintahaka J, Soby S, Horan KA, Poltajainen A, Ostergaard L, Paludan SR, Matikainen S. Early Innate Recognition of Herpes Simplex Virus in Human Primary Macrophages Is Mediated via the MDA5/MAVS-Dependent and MDA5/MAVS/RNA Polymerase III-Independent Pathways. J. Virol. 84(21):11350–11358. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchjorsen J, Rintahaka J, Soby S, Horan KA, Poltajainen A, Ostergaard L, Paludan SR, Matikainen S. Early Innate Recognition of Herpes Simplex Virus in Human Primary Macrophages Is Mediated via the MDA5/MAVS-Dependent and MDA5/MAVS/RNA Polymerase III-Independent Pathways. J Virol. 2010;84(21):11350–8. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annual review of biochemistry. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- Michaud F, Coulombe F, Gaudreault, Kriz J, Gosselin J, Jeyaseelan S. Involvement of TLR2 in Recognition of Acute Gammaherpesvirus-68 Infection. PLoS One. 2010;5(10):468–1372. doi: 10.1371/journal.pone.0013742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve D, PÈtrilli V, Zaiss A, White L, Clark S, Ross P, Parks R, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452(7183):103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Pichlmair A, Schulz O, Tan C-P, Rehwinkel J, Kato H, Takeuchi O, Akira S, Way M, Schiavo G, Reis e Sousa C. Activation of MDA5 Requires Higher-Order RNA Structures Generated during Virus Infection. J. Virol. 2009;83(20):10761–10769. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M, Martinez J, Huang X, Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113(10):2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SB, Sorensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J Virol. 2007;81(24):13315–24. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam V, Fitzgerald K. Inflammasomes and Anti-Viral Immunity. Journal of clinical immunology. 2010:1–6. doi: 10.1007/s10875-010-9431-4. [DOI] [PubMed] [Google Scholar]
- Rathinam V, Sharma S, Fitzgerald K. Catenin'on to nucleic acid sensing. Nature Immunology. 2010;11(6):466–468. doi: 10.1038/ni0610-466. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, Hornung V, Vogel SN, Szomolanyi-Tsuda E, Fitzgerald KA. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11(5):395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts T, Idris A, Dunn J, Kelly G, Burnton C, Hodgson S, Hardy L, Garceau V, Sweet M, Ross I. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science. 2009;323(5917):1057. doi: 10.1126/science.1169841. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Hausmann J, Lauterbach H, Schmidt M, Akira S, Wagner H, Chaplin P, Suter M, O'Keeffe M, Hochrein H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest. 2008;118(5):1776–84. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi PP, Kim B, Kurt-Jones E, Rouse BT. Innate recognition network driving herpes simplex virus-induced corneal immunopathology: role of the toll pathway in early inflammatory events in stromal keratitis. J Virol. 2007;81(20):11128–38. doi: 10.1128/JVI.01008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc Natl Acad Sci U S A. 2006;103(46):17343–8. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol. 2008;181(12):8604–12. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. Role for TLR2 in NK Cell-Mediated Control of Murine Cytomegalovirus In Vivo. J. Virol. 2006;80(9):4286–4291. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, Beutler B. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101(10):3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A, Wang Z, Choi M, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- Thomas PG, Dash P, Aldridge JR, Jr., Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, Doherty PC, Kanneganti TD. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30(4):566–75. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterholzner L, Keating S, Baran M, Horan K, Jensen S, Sharma S, Sirois C, Jin T, Latz E, Xiao T. IFI16 is an innate immune sensor for intracellular DNA. Nature Immunology. 2010 doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waibler Z, Anzaghe M, Ludwig H, Akira S, Weiss S, Sutter G, Kalinke U. Modified vaccinia virus Ankara induces Toll-like receptor-independent type I interferon responses. Journal of virology. 2007;81(22):12102. doi: 10.1128/JVI.01190-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JP, Kurt-Jones EA, Shin OS, Manchak MD, Levin MJ, Finberg RW. Varicella-Zoster Virus Activates Inflammatory Cytokines in Human Monocytes and Macrophages via Toll-Like Receptor 2. J. Virol. 2005;79(20):12658–12666. doi: 10.1128/JVI.79.20.12658-12666.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Choi M, Ban T, Yanai H, Negishi H, Lu Y, Tamura T, Takaoka A, Nishikura K, Taniguchi T. Regulation of innate immune responses by DAI (DLM-1/ZBP1) and other DNA-sensing molecules. Proceedings of the National Academy of Sciences. 2008;105(14):5477. doi: 10.1073/pnas.0801295105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Damania B. Upregulation of the TLR3 pathway by Kaposi's sarcoma-associated herpesvirus during primary infection. Journal of virology. 2008;82(11):5440. doi: 10.1128/JVI.02590-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuest T, Austin BA, Uematsu S, Thapa M, Akira S, Carr DJ. Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J Neuroimmunol. 2006;179(1-2):46–52. doi: 10.1016/j.jneuroim.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Puel A, Zhang S, Eidenschenk C, Ku C, Casrouge A, Picard C, von Bernuth H, Senechal B, Plancoulaine S. Human TLR-7-,-8-, and-9-Mediated Induction of IFN-[alpha]/[beta] and-[lambda] Is IRAK-4 Dependent and Redundant for Protective Immunity to Viruses. Immunity. 2005;23(5):465–478. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, An H, Liu X, Wen M, Zheng Y, Rui Y, Cao X. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a [beta]-catenin-dependent pathway. Nature Immunology. 2010;11(6):487–494. doi: 10.1038/ni.1876. [DOI] [PubMed] [Google Scholar]
- Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317(5844):1522–7. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- Zhao Y, De Trez C, Flynn R, Ware CF, Croft M, Salek-Ardakani S. The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol. 2009;182(10):6278–86. doi: 10.4049/jimmunol.0803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81(7):3170–80. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-{beta} Blood. 2007;109(2):619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]