Abstract
Femoral cannulation in pediatric patients requiring extracorporeal membrane oxygenation (ECMO) is commonly associated with distal limb ischemia. Authors have previously reported successful lower limb perfusion using various open techniques to cannulate a distal lower extremity artery at the time of initial ECMO cannulation. These procedures include open femoral artery antegrade cannulation and distal posterior tibial artery retrograde cannulation in older children and adults. Such approaches require ample vessel diameters that can accommodate an arteriotomy and catheter insertion, and therefore are of limited utility in smaller children. We hypothesized that following femoral artery cannulation for ECMO, a percutaneous technique of distal limb perfusion might offer unique advantages when treating lower extremity ischemia in small pediatric patients. We report a technique for percutaneous antegrade cannulation in a 4 year old patient shortly following her primary cannulation for venoarterial ECMO via the femoral artery.
Keywords: Extracorporeal membrane oxygenation, Pediatric, Lower Extremity, Ischemia, Cannulation
INTRODUCTION
Pediatric patients who require cannulation for venoarterial extracorporeal membrane oxygenation (ECMO) remain a challenging cohort. The femoral artery is the preferred cannulation site for this population in our institution, but the negligible difference between cannula diameter and the femoral artery lumen places these children at risk for lower extremity ischemia. Many children who survive ECMO may suffer permanent sensory or motor deficits in the affect limb, tissue loss, and even amputation. However, limb-threatening ischemia may be reduced by adopting techniques which augment flow to the hypoperfused distal lower extremity following femoral artery cannulation.
Successful distal limb perfusion in adults has been described using various approaches, including open antegrade cannulation of the femoral artery and open retrograde cannulation of the dorsalis pedis or posterior tibial arteries. [1–3] These techniques are usually performed concurrently with initial ECMO cannulation to prevent possible leg ischemia rather than selectively employed only in cases of clinically apparent ischemia. However, the benefits of preventing tissue hypoxia through universal distal perfusion must be balanced against the added morbidity of a potentially unnecessary second arteriotomy. Furthermore, the techniques described in adults have limited efficacy in children with narrow distal vessels. Clinicians would therefore benefit from a management approach where interval distal perfusion (i.e., hours to days after initial ECMO cannulation) is offered only for “at-risk” limbs, using a technique optimized for small pediatric patients. However, there are no published reports describing either concurrent or interval distal limb cannulation in small children. We describe a percutaneous modification of the adult open distal perfusion technique, performed 48 hours after initial ECMO cannulation, and utilized successfully to restore distal limb perfusion in a 4 year old child.
CASE REPORT
A 4 year old girl who arrested during induction of anesthesia for sinus surgery at an outside hospital presented to our pediatric intensive care unit with an acute exacerbation of previously undiagnosed pulmonary hypertension. Having failed conventional ventilator strategies prior to arrival, she was placed on high frequency oscillatory ventilation with a FiO2 of 1.0 and inhaled nitric oxide at 20 ppm. She required escalating doses of inotropes and vasopressors, including dopamine (15 mcg/kg/min), epinephrine (1.0 mcg/kg/min), milrinone (0.7 mcg/kg/min), and vasopressin (0.0005 U/kg/min). Echocardiogram confirmed super-systemic pulmonary hypertension with a severely dilated, poorly functioning right ventricle, posterior bowing of the atrial septum, dilation of the main pulmonary artery, and moderately decreased left ventricular function. Her acidosis continued to worsen despite maximal support, with an arterial blood gas of 7.12/54/90 and an arterial lactate peaking at 15.5 mmol/L. With progressively worsening ventilation and perfusion, the patient was offered venoarterial (VA) ECMO.
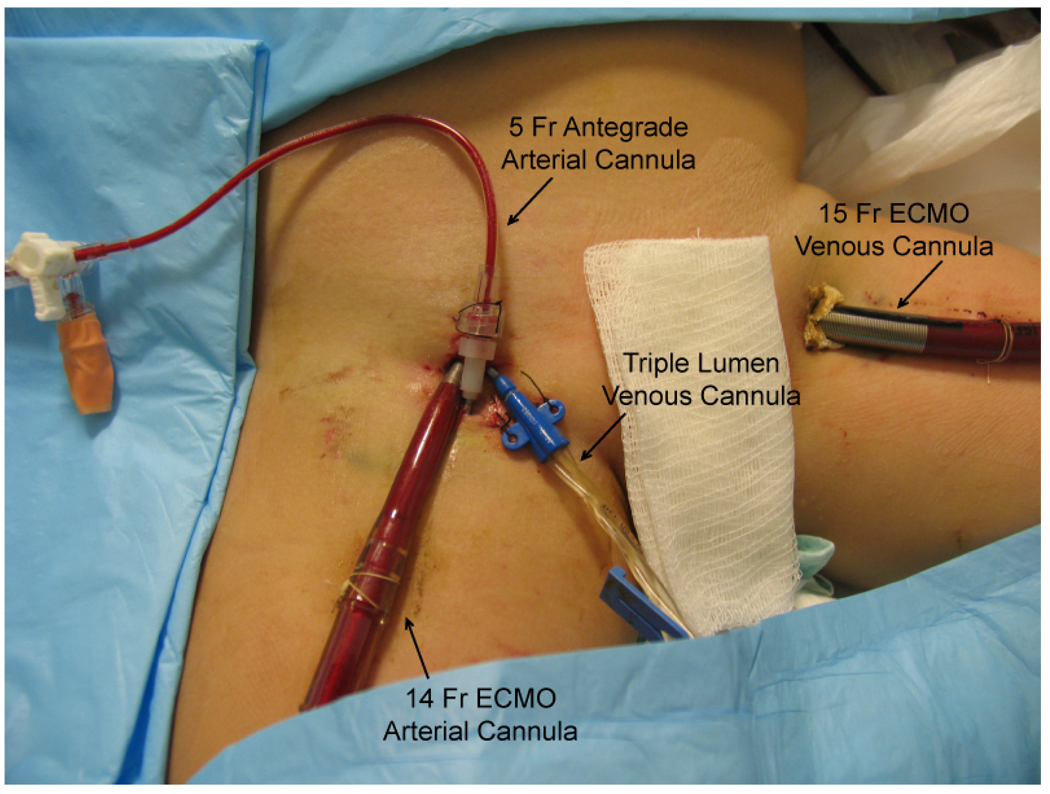
To achieve a cardiac index of 2.4 L/min/m2 in this 17 kg child, a minimum cardiac output of 1.7 L/min was required. Based on in-vitro testing performed on a wet circuit using our circuit configuration and pump, a 14 French, 10 cm Bio-Medicus® arterial cannula (Medtronic, Mineapolis, MN) was chosen because it can sustain a maximum flow of 2.2 L/min while the smaller 12 French cannula can only sustain 1.5 L/min. An existing right femoral arterial line was upsized via serial dilation to the 14 French arterial cannula and a 15 French Bio-Medicus® venous cannula (Medtronic, Mineapolis, MN) was placed percutaneously in the left femoral vein. ECMO flow was initiated using a Jostra Rotaflow™ cetrifugal pump and Quadrox-D™ oxygenator (Maquet, Bridgewater, NJ) at 1.7 L/min. With the exception of milrinone, all vasoactive drugs were weaned completely. In addition, the patient was started on a continuous heparin infusion at the initiation of ECMO support, titrated to maintain activated clotting times between 180 and 220 seconds. The patient’s overall clinical perfusion markedly improved over the first 24hrs, with complete resolution of her acidosis and normalization of her arterial lactate. On ECMO day 2, despite weaning all vasoactive drugs and adequate anticoagulation, her right foot distal to the 14 French arterial cannula showed signs of progressive ischemia, with cyanotic toes and absence of an arterial Doppler signal in the foot. Under ultrasound guidance, the right superficial femoral artery distal to the arterial cannula was identified and accessed with a 21 gauge introducer needle. A 5 French, 10 cm single-lumen Cook® (Bloomington, IN) catheter was advanced distally over a guidewire and connected to the arterial limb of the ECMO circuit (Figure 1). When the stopcock connecting the shunt to the circuit was opened, blood could be seen flowing antegrade into the leg. With re-institution of blood flow, the right foot demonstrated steady clinical improvement on physical examination.
Figure 1.
Percutaneous distal limb perfusion in a small child cannulated for veno-arterial ECMO through the groin.
The patient received aggressive treatment for pulmonary hypertension during her seven day ECMO course, including inhaled nitric oxide (20 ppm), epoprostenol (42 ng/kg/min), and sildenafil (60mg/day). After successfully weaning ECMO support, the arterial bypass catheter and the venous catheter were removed at the bedside and pressure was held until hemostasis was achieved. Following discontinuation of ECMO, sedation was reduced and a limited neurologic exam of the right lower extremity was performed. The patient was able to actively dorsiflex and plantarflex her right foot as well as her toes. She remained hospitalized for a period of 3 months undergoing treatment to manage severe pulmonary hypertension. She was discharged home on a continuous epoprostenol infusion (60ng/kg/min), sildenafil (60mg/day) and 1.5 L/min of oxygen via nasal cannula. A complete neurologic exam prior to discharge revealed no deficits in sensory or motor function in the involved limb.
DISCUSSION
Excluding cases of central cannulation, the carotid and femoral arteries are the most commonly used major vessels for venoarterial (VA) ECMO cannulation. In neonates, the right common carotid artery is ligated to obtain the necessary arterial access. Follow up studies of neonatal ECMO survivors have shown that the incidence of major neurological disability following carotid ligation is comparable to that reported in other critically ill neonates not requiring ECMO.[4] However, it remains unclear at what age the brain loses its ability to adapt to carotid ligation. Anecdotally, there is no consensus among pediatric ECMO providers as to what age is most appropriate to avoid using the carotid artery in favor of the femoral artery, the latter representing the preferred vessel for adult VA ECMO cannulation. In the 4 year old child presented here, we decided that the unknown risk of ligating her carotid artery exceeded the risk of utilizing her femoral vessels for ECMO by percutaneously dilating the tracts of catheters already in place. While femoral cannulation successfully spared her carotid circulation, her lower extremity perfusion was critically compromised.
Distal limb ischemia is a well documented complication when the femoral artery is used during cannulation for ECMO support in adults.[2, 3, 5–7] This problem may be magnified in small children whose femoral artery diameter closely approximates the diameter of an appropriate arterial cannula. There are multiple reports describing placement of a small diameter catheter distally to provide antegrade flow to the ischemic limb at the time of initial open ECMO cannulation in adults.[1, 3, 6] Haft, et al. described a technique used in patients >30 kg where the posterior tibial artery is ligated and a retrograde catheter is used to reperfuse the distal extremity.[8] A similar procedure has been reported using the dorsalis pedis artery in an elderly patient with arterial occlusive disease in her femoral vessels.[2] In a child of this age, it is unlikely that the posterior tibial or the dorsalis pedis arteries would be of sufficient size to accommodate a catheter large enough to provide adequate blood flow. Furthermore, this patient had not undergone an open femoral cannulation. An arteriotomy or an open percutaneous approach would have first required exposure of the femoral vessels. The percutaneous procedure spared the patient a groin dissection. The potential complications of percutaneous placement of any small cannula in the femoral artery include infection, sepsis, bleeding, hematoma, ischemia, and psuedoaneurysm. The literature reporting complications of this type of cannulation of the femoral artery involve hemodynamic monitoring catheters and place the overall complication rate at less than 1%.[9] By utilizing ultrasound guidance and a completely percutaneous approach, distal perfusion was restored while minimizing additional trauma to the patient.
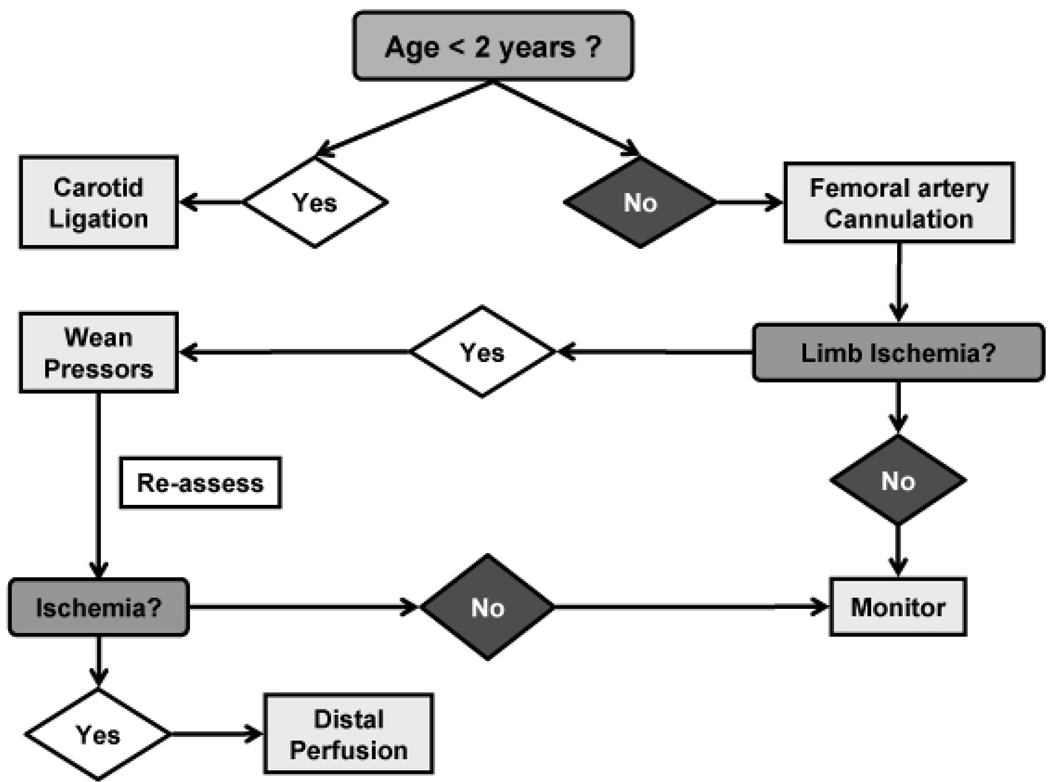
Placing a distal perfusion catheter in all patients during the initial femoral cannulation for VA ECMO may eliminate the multiple local and systemic complications of tissue ischemia and reperfusion. However; in pediatric patients with small vessels a second arteriotomy is not without possible consequences of its own. Interval placement of a second cannula allows time for other factors affecting distal perfusion to resolve. Vasopressors that affect distal perfusion, such as epinephrine, may be weaned off and overall oxygenation should improve after ECMO is initiated. In some patients this is enough to restore adequate blood flow to the distal extremity. Huang, et al. used a flowmeter to measure pressure in the superficial femoral artery distal to the site of cannulation at the time of the initial procedure in adults to select patients for placement of the second catheter.[6] We have introduced an algorithm at our institution using clinical examination to determine which patients require placement of the additional catheter (figure 2).
Figure 2.
Proposed decision algorithm for distal limb ischemia following femoral artery cannulation in the pediatric ECMO population.
Distal limb ischemia in a patient femorally cannulated for VA ECMO is a problem we encounter at our institution once or twice a year. Other institutions that perform femoral artery cannulations in the pediatric ECMO population likely have a similar incidence, but we identified no published cases specific to pediatric patients. Additionally, the Extracorporeal Life Support Organization (ELSO) International Registry does not track complications specifically related to lower extremity ischemia such as loss of neurological function, tissue loss, or amputation. Therefore, the true incidence of lower limb ischemia following femoral cannulation in children remains unknown. Given the potential long term sequela of limb ischemia, it would be beneficial to include this information in future data collection.
The percutaneous modification we describe may be performed with relative ease at the bedside to provide distal blood flow in small children with signs of persistent distal ischemia while allowing an appropriate interval to spare small arteries the trauma of a second arteriotomy when unnecessary. The algorithm we propose may provide clinicians with a strategy to better manage pediatric ECMO patients who require femoral artery cannulation.
REFERENCES
- 1.Hendrickson SC, Glower DD. A method for perfusion of the leg during cardiopulmonary bypass via femoral cannulation. Ann Thorac Surg. 1998;65:1807–1808. doi: 10.1016/s0003-4975(98)00302-6. [DOI] [PubMed] [Google Scholar]
- 2.Kimura N, Kawahito K, Ito S, et al. Perfusion through the dorsalis pedis artery for acute limb ischemia secondary to an occlusive arterial cannula during percutaneous cardiopulmonary support. J Artif Organs. 2005;8:206–209. doi: 10.1007/s10047-005-0300-5. [DOI] [PubMed] [Google Scholar]
- 3.Madershahian N, Nagib R, Wippermann J, et al. A simple technique of distal limb perfusion during prolonged femoro-femoral cannulation. J Card Surg. 2006;21:168–169. doi: 10.1111/j.1540-8191.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 4.Glass P, Wagner AE, Papero PH, et al. Neurodevelopmental status at age five years of neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1995;127:447–457. doi: 10.1016/s0022-3476(95)70082-x. [DOI] [PubMed] [Google Scholar]
- 5.Greason KL, Hemp JR, Maxwell JM, et al. Prevention of distal limb ischemia during cardiopulmonary support via femoral cannulation.[see comment] Ann Thorac Surg. 1995;60:209–210. [PubMed] [Google Scholar]
- 6.Huang S-C, Yu H-Y, Ko W-J, et al. Pressure criterion for placement of distal perfusion catheter to prevent limb ischemia during adult extracorporeal life support. J Thorac Cardiovasc Surg. 2004;128:776–777. doi: 10.1016/j.jtcvs.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 7.Read R, St Cyr J, Tornabene S, et al. Improved cannulation method for extracorporeal membrane oxygenation. Ann Thorac Surg. 1990;50:670–671. doi: 10.1016/0003-4975(90)90219-v. [DOI] [PubMed] [Google Scholar]
- 8.Haft JW, Bartlett RH. Lower Extremity Perfusion During VA ECMO via Femoral Cannulation. The 23rd Annual CNMC Sypmosium: ECMO & the Advanced Therapies for Respiratory Failure; Keystone, CO. 2007. [Google Scholar]
- 9.Scheer B, Perel A, Pfeiffer UJ. Clinical review: complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Crit Care. 2002;6:199–204. doi: 10.1186/cc1489. [DOI] [PMC free article] [PubMed] [Google Scholar]