Abstract
Objective
To identify the impact of timing of prenatal stress exposure on offspring risk for shortened gestational age (GA), preterm birth (PTB), low birth weight (LBW), and small for gestational age (SGA) using a population-based sample.
Methods
Swedish longitudinal population registries were linked to study all individuals born in Sweden 1973–2004. Prenatal maternal stress exposure was defined as death of the father of the child or first degree relative of the mother. Using linear and logistic regression, timing of stress exposure was examined across pregnancy, by month, and by novel periods created based on month of stress exposure findings.
Results
A total of 2,618,777 live-born, singleton infants without congenital anomalies were included; 32,286 exposed to prenatal maternal stress. Examining associations between stress exposure and outcome by the month revealed that risk increases mid-gestation, particularly following months 5 and 6. Combining months 1–4, 5 and 6, and 7–9 as potential periods of differing vulnerability, it was found that stress during period 2 (months 5 and 6) was associated with the greatest risk for shortened GA (−0.52 days, SE=0.15, p=0.0006), PTB (OR=1.24, 99% CI=1.08–1.42), LBW (OR=1.38, 99% CI=1.19–1.61), and SGA (OR=1.25, 99% CI=1.05–1.49).
Conclusions
Risk for shortened GA, PTB, LBW, and SGA are greater following stress exposure during the 5th and/or 6th month of pregnancy. It may be beneficial to refine future analyses to these months. Possible mechanisms include alterations in the hypothalamic-pituitary-adrenal axis and associated stress-responsive molecular regulators.
Keywords: Stress, pregnancy, timing, preterm birth, low birth weight, small for gestational age
The complexities of prenatal development are only beginning to be understood (1). It is assumed that the fetus is particularly vulnerable to organizing and disorganizing insults because fetal systems are undergoing significant, rapid, and sequential developmental changes (2). Focus has been placed on identifying and understanding potential insults, or prenatal risks, associated with adverse pregnancy outcomes. Unfavorable birth outcomes include preterm birth (<37 weeks of gestation; PTB), low birth weight (<2500g; LBW), and small for gestational age (birth weight ≥ 2 sd below the mean for GA; SGA (3)). These outcomes are associated with increased infant mortality (4–5), lifetime physical and psychological disadvantage (6–10), and staggering medical costs (11–12). Rates of preterm birth and associated adverse pregnancy outcomes are increasing (13). Despite our ability to improve the lives of at-risk infants (14), a mechanistic understanding of the etiology of adverse birth outcomes and a knowledge of the precise window of vulnerability are of paramount importance for prevention and intervention efforts (15).
A growing body of research has examined prenatal maternal stress as a putative risk factor for adverse birth outcomes. Despite mixed findings, sufficient evidence exists to suggest that prenatal maternal stress is moderately associated with adverse birth outcomes and other negative effects across the lifespan (for review see 16). These findings are often situated in the Developmental Origins of Disease hypothesis, also known as fetal programming. This hypothesis asserts that prenatal maternal stress and fetal physiological adaptation influence the health and development of the exposed fetus (17). Although the biological mechanisms explaining such associations have yet to be confirmed, potential pathways through which stress affects development include increased activity of the hypothalamic-pituitary-adrenal (HPA) axis and associated stress-responsive molecular regulators, as well as disruptions in immune and inflammatory systems (18). When a maternal stressor occurs during pregnancy, glucocorticoids, corticotropin-releasing hormone (CRH), and adrenocorticotropic hormone (ACTH) proximally increase in the maternal system (reviewed in 19). During normal pregnancy, glucocorticoids, CRH, and ACTH levels all progressively increase (20), however, the trajectory of CRH increase has been shown to differentiate pregnancies that will be carried to term from those that will end preterm (21). While some degree of glucocorticoid exposure is essential for the development, organization, and maturation of fetal tissue (22), excess and/or untimely exposure may be detrimental to fetal development. Thus, fetal HPA axis development and/or changes in the maternal/fetal system across pregnancy, explained further in the discussion, may play a large part in associations between prenatal insult and adverse birth outcome.
During prenatal development, there may be heightened periods of vulnerability depending on the timetable and rapidity of brain development (23) as well as the successful development of previously or concurrently emerging biological systems, specifically the stress response system. Periods of vulnerability associated with different phenotypic outcomes may vary and the potential mechanisms responsible for associations may depend on timing of insult. For these reasons, timing of insult should be precisely considered when examining associations between prenatal maternal stress and adverse birth outcomes.
Evidence suggesting specific periods of heightened vulnerability to stress during pregnancy is inconsistent. For example, it was recently suggested that first trimester stress carries the greatest risk for reduced gestational length, although the investigation did not adjust for the reduced likelihood of shortened gestational length over advancing pregnancy (24). A different investigation used a smaller sample but controlled for the reduced probability of shortened gestation over time. Results suggested that first trimester exposure to prenatal maternal stress was associated with the greatest risk for shortened gestational length. Additionally, the investigation suggested that the dampening of the maternal stress response system due to pregnancy-induced physiological changes provides protection to stress later in pregnancy (25). Other research, however, has shown that second (26) and early third trimester levels of CRH are predictive of increased risk for preterm birth (27–28). Interestingly, elevated maternal cortisol, a stress-related endocrine signal, early in the second trimester predicts a precocious rise in CRH during the early third trimester (28), thus major stressors affecting the levels of maternal cortisol early in the second trimester may also influence gestational length through a later mechanism.
When predicting birth weight, analyses examining timing of exposure to prenatal maternal severe life events is limited. One study using a similar population and stress exposure indicator suggested that second trimester exposure to prenatal maternal stress is associated with a greater reduction in birth weight as compared with first or third trimester exposure (29). The same investigation also concluded that the largest increased risk for SGA followed second trimester stress exposure, although differences between trimesters were small (29).
In general, a consensus regarding possible vulnerable prenatal periods has yet to be reached for any measure of birth outcome. Previous research is limited by non-standard prenatal maternal stress measures and small sample sizes (30). Small sample sizes often lead researchers to group prenatal exposure periods into 3-month trimesters contributing to relatively imprecise conclusions regarding sensitive periods of prenatal development. It may be found that the rapidly developing fetal and maternal-fetal systems pass through periods of relative vulnerability within the 3-month trimester period and therefore, by grouping these months together, associations may be attenuated. With a large enough sample size and a precise definition of stress exposure, the statistical power needed for assessing vulnerable periods in greater detail is possible.
The current study uses a large population-based sample of all births in Sweden from 1973 to 2004 to examine sensitive periods during fetal development on the detail of months of exposure. A database was created that includes a measure of prenatal maternal stress exposure, defined as the death of the father of the child or a first degree relative of the mother during pregnancy, as well as the timing of stress exposure according to the day of death during pregnancy (31). Selection of death, a major life event, was used to maximize the likelihood that the exposure resulted in substantial psychological stress. We hypothesized that exposure to a major stressor during pregnancy would be associated with higher risk for adverse birth outcome. Based on previous research (24–25), we hypothesized that the most vulnerable period for shortened GA and PTB would be early in pregnancy. It was also hypothesized that the risk for LBW and SGA would follow mid-gestation stress exposure based on previous cohort research (29) and HPA axis and associated molecular regulator changes around this time of gestation. Due to reduced maternal physiological and psychological reaction to stressors late in pregnancy (25), it was hypothesized that no associations would be found between late pregnancy stress exposure and adverse birth outcome.
Methods
Sample
After Institutional Review Board approval, a large-scale, population-based sample was constructed by linking several Swedish population registries. Information was drawn from: (1) the Multi-Generation Registry, which links extended family members to the target child using unique personal identification numbers, (2) the Swedish Medical Birth Registry, which contains birth outcome and pregnancy information including over 99% of all births in Sweden from 1973 to 2004, and (3) the Cause of Death Registry, which identifies the dates of death for relatives of the mother. Death of the father of the child or first degree relative of the mother (i.e. biological parent, full sibling, or already born biological child) was used as an indicator of stress during pregnancy (31). If the mother experienced two deaths during pregnancy, only timing of the first death was used. Table 1 presents the demographics by stress exposure (32,286 exposed and 2,586,491 unexposed individuals).
Table 1.
Characteristics of all Swedish, live-born, singleton pregnancies without congenital anomalies from 1973 to 2004 by prenatal maternal stress (defined as mother's exposure to death of a first degree relative or the father of the child).
| Characteristic | Stress Exposure |
|
|---|---|---|
| Yes (N=32 286) | No (N=2 586 491) | |
| Male Offspring (n, %) | 16 515 (51.1) | 1 325 675 (51.3) |
| Maternal age (years, n, %) | ||
| ≤24 | 5 475 (17.0) | 661 703 (25.3) |
| 25–29 | 10 261 (31.8) | 960 882 (36.7) |
| 30–34 | 9 942 (30.8) | 678 649 (25.9) |
| ≥35 | 6 608 (20.5) | 285 257 (11.0) |
| Maternal education (years, n, %) | ||
| ≤11a | 16 419 (50.9) | 1 202 309 (46.5) |
| ≥12 | 15 106 (46.8) | 1 334 036 (51.6) |
| Missing | 761 (2.3) | 50 146 (1.9) |
| Maternal country of birth (n, %) | ||
| Nordica | 31 861 (98.7) | 2 543 324 (98.3) |
| Non-Nordic | 419 (1.3) | 42 825 (1.7) |
| Missing | 6 (<0.1) | 342 (<0.1) |
| Parity (n, %) | ||
| Nulliparousa | 11 093 (34.4) | 1 114 403 (43.1) |
| Primiparous | 11 908 (36.9) | 953 326 (36.9) |
| Multiparous | 9 285 (28.8) | 518 762 (20.1) |
| Paternal age (years, n, %) | ||
| ≤24 | 2 934 (9.1) | 341 636 (13.2) |
| 25–29 | 8 330 (25.8) | 841 524 (32.5) |
| 30–34 | 10 404 (32.2) | 817 323 (31.6) |
| ≥35 | 10 618 (32.9) | 586 008 (22.7) |
Reference.
The sample originally contained 2,780,079 target children born between 1973 and 2004 with identified extended family members of the mother. Multiple births (65,753), malformations (45,317), and stillbirths (10,263) were removed from the sample in order to limit the cohort to the most normal prenatal experiences for the current foundational investigation of timing of stress exposure. Birth outcome information was measured at delivery and included GA at birth, PTB, LBW, and SGA. In accordance with Swedish weight-based growth standards (32), SGA was defined as a birth weight of 2 standard deviations below the mean. GA was determined by early second trimester ultrasound or calculated by menstrual dating (33). According to global induction recommendations, a GA cutoff was set at 426/7 weeks (34). Cases with GAs greater than the cutoff or missing were removed from the sample because timing of death was calculated using GA. Similarly, birth weights were considered erroneous if entries were <500g or >5500g and removed from analyses (39,932). Cases with missing parity or other background information were not included in analyses (37). Thus, the final cohort totaled 2,618,777 target children.
Statistical analyses
Linear and logistic regression analyses were used to examine the association between timing of stress exposure and outcome. Logistic regression was employed to predict categorical outcomes following the recommendation that log linear binomial regression is not necessary when predicting rare (<5%) outcomes (35), however, results were verified using this analysis (available upon request).
Timing of stress exposure was first examined across the entire pregnancy period. Stress exposure windows were then limited to months. Inclusion in the sample was contingent on still being in utero during the window of stress exposure examined. For example, while investigating risk for LBW following stress exposure during prenatal month 7, all children born before or during prenatal month 6 were excluded from analyses. Partial adjustment included adjusting for year of birth and target child's sex. Fully adjusted models were adjusted for year of birth, target child's sex, maternal age, maternal education, mother's country of origin [i.e. Nordic (Sweden, Denmark, Finland, Iceland, or Norway) or non-Nordic], parity, paternal age, and month of last menstrual period (outlined in Table 1). Because no associations were found between month of last menstrual period (to account for possible seasonal influences) and birth outcomes, this variable was subsequently dropped from all models. For analyses predicting PTB, only exposures prior to week 37 were considered.
Results
A total of 32,286 mothers experienced stress during pregnancy in the form of death of a parent, sibling, already born child, or father of the child during pregnancy. Table 2 presents the number of prenatal stress exposures per month of pregnancy as well as the average length of gestation and number of PTB, LBW, and SGA for stress exposed and unexposed pregnancies.
Table 2.
Number of cases of Swedish, live-born, singleton pregnancies without congenital anomalies born 1973 to 2004 that were exposed to prenatal maternal stress (defined as mother's exposure to death of a first degree relative or the father of the child) and gestational age, preterm birth, low birth weight, and small for gestational age by prenatal stress exposure status.
| Days |
Cases(%) |
||||
|---|---|---|---|---|---|
| Gestational Age | Preterm Birth | Low Birth Weight | Small for Gestational Age | ||
| Unexposed | |||||
|
| |||||
| Cases | |||||
| 2 586 491 | 279.19 | 123 800 (4.79) | 88 595 (3.43) | 71 384 (2.77) | |
| Exposed | |||||
|
| |||||
| Month | Cases | ||||
| 1 | 2 981 | 278.82 | 150 (5.03) | 126 (4.23) | 98 (3.30) |
| 2 | 3 148 | 278.99 | 157 (4.99) | 120 (3.81) | 103 (3.28) |
| 3 | 3 293 | 279.18 | 146 (4.43) | 106 (3.22) | 107 (3.26) |
| 4 | 3 388 | 278.45 | 184 (5.43) | 135 (3.98) | 99 (2.93) |
| 5 | 3 255 | 278.39 | 191 (5.87) | 154 (4.73) | 124 (3.82) |
| 6 | 3 254 | 278.89 | 190 (5.84) | 156 (4.79) | 106 (3.27) |
| 7 | 3 286 | 278.71 | 174 (5.30) | 126 (3.83) | 96 (2.93) |
| 8 | 3 327 | 279.69 | 164 (4.93) | 117 (3.52) | 102 (3.07) |
| 9 | 3 220 | 79 (2.45) | 91 (2.83) | ||
Risk for adverse birth outcome was first examined across the entire prenatal period. Experiencing stress at any time point during pregnancy was associated with greater risk for all outcomes although the magnitude of associations were small. Exposure to prenatal maternal stress was associated with an unexpected increase in gestational length of 0.20 (SE=0.07, p=0.004) days. Exposure to prenatal maternal stress tended to be associated with elevated risk of PTB (Odds ratio (OR) =1.05; 99% confidence intervals (CI), 0.98–1.12) and LBW (OR=1.04; 99% CI, 0.96–1.12), albeit non-significant because of relatively large confidence intervals. The association between stress exposure and risk for outcome was strongest for SGA (OR=1.12; 99% CI, 1.03–1.22).
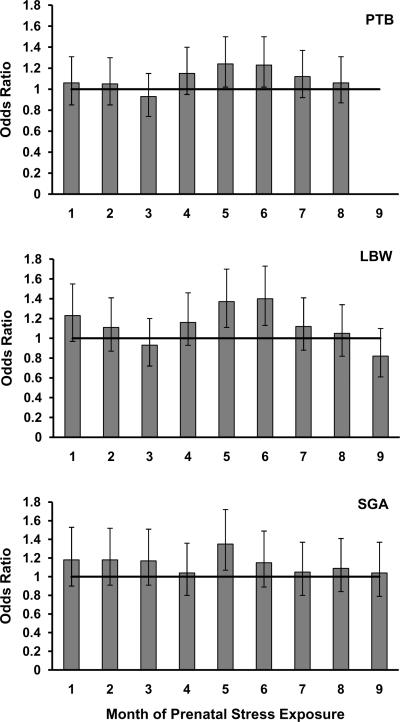
Timing of stress exposure was next limited to month exposure periods in order to examine possible sensitive periods with greater precision. Inclusion in analyses was contingent on still being in utero during the month being examined. Across all outcomes, the middle months of pregnancy appeared to be at heightened sensitivity to the detrimental effects of prenatal maternal stress exposure. As shown in Table 3, fully adjusted linear regression analyses revealed that .72 (SE=0.21, p=0.0007) and .77 (SE=0.22, p=0.0003) gestational days were lost on average following stress exposure during prenatal months 4 and 5 respectively. For categorical outcomes, logistic regression was employed. Logits, the logarithm of the odds of an event occurrence, represent an increase or decrease in risk across pregnancy using the date of conception as reference and are listed in Table 3. For ease of interpretation, logits have been converted [e(b*day)] to OR. When predicting PTB, months 5 (OR=1.24, 99% CI=1.02–1.50) and 6 (OR=1.23, 99% CI=1.02–1.50) were significant and robust to covariates. Similarly, when predicting LBW, months 5 (OR=1.37, 99% CI=1.11–1.70) and 6 (OR=1.40, 99% CI=1.13–1.73) were also significant and robust to covariates. Associations between stress exposure and LBW were verified by predicting continuous birth weight. For continuously measured birth weight, the same pattern emerged and risk for the greatest decrease in birth weight followed after months 4 (b=−27.16, SE=9.25, p=0.003), 5 (b= −35.36, SE=9.43, p=0.0002) and 6 (b= −27.67, SE=9.44, p=0.003) stress exposure (full results available upon request). When predicting SGA, month 5 (OR=1.35, 99% CI=1.07–1.72) (b=0.30, SE=0.09, p=0.001) also emerged as significant. Figure 1 presents the associations between month of exposure and risk for categorically measured adverse birth outcomes. As can be observed in Figure 1, months 5 and 6 appear to be heightened periods of vulnerability to stress due to the slightly larger associations with adverse birth outcomes after stress exposure.
Table 3.
Risk of adverse birth outcomes for all Swedish, live-born, singleton pregnancies without congenital anomalies from 1973 to 2004 by month of prenatal maternal stress exposure (defined as mother's exposure to death of a first degree relative or the father of the child).
| b (SE) |
Logits (SE) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Gestational Age |
Preterm Birth |
Low Birth Weight |
Small for Gestational Age |
|||||
| Month Exposed | Partially adjusteda | Adjustedb | Partially adjusteda | Adjustedb | Partially adjusteda | Adjustedb | Partially adjusteda | Adjustedb |
| 1 | −0.44 (0.23) | −0.32 (0.23) | 0.06 (0.08) | 0.05 (0.08) | 0.21 (0.09) | 0.20 (0.09) | 0.15 (0.10) | 0.16 (0.10) |
| 2 | −0.28 (0.22) | −0.17 (0.22) | 0.05 (0.08) | 0.05 (0.08) | 0.10 (0.09) | 0.10 (0.09) | 0.14 (0.10) | 0.16 (0.10) |
| 3 | −0.07 (0.22) | −0.02 (0.21) | −0.08 (0.08) | −0.08 (0.08) | −0.07 (0.09) | −0.07 (0.10) | 0.14 (0.10) | 0.16 (0.10) |
| 4 | − 0.82 (0.21) | − 0.72 (0.21) | 0.14 (0.08) | 0.14 (0.08) | 0.15 (0.09) | 0.15 (0.09) | 0.02 (0.10) | 0.04 (0.10) |
| 5 | − 0.88 (0.22) | − 0.77 (0.22) | 0.22 (0.07) | 0.21 (0.07) | 0.33 (0.08) | 0.32 (0.08) | 0.30 (0.09) | 0.30 (0.09) |
| 6 | −0.38 (0.22) | −0.28 (0.22) | 0.21 (0.07) | 0.21 (0.08) | 0.35 (0.08) | 0.33 (0.08) | 0.14 (0.10) | 0.14 (0.10) |
| 7 | − 0.58 (0.21) | −0.48 (0.21) | 0.11 (0.08) | 0.12 (0.08) | 0.11 (0.09) | 0.11 (0.09) | 0.02 (0.10) | 0.04 (0.10) |
| 8 | 0.27 (0.20) | 0.37 (0.20) | 0.06 (0.08) | 0.06 (0.08) | 0.06 (0.09) | 0.05 (0.09) | 0.07 (0.10) | 0.08 (0.10) |
| 9 | −0.06 (0.09) | −0.05 (0.09) | −0.19 (0.11) | −0.20 (0.11) | 0.02 (0.11) | 0.04 (0.11) | ||
Numbers in bold are significant at p<.01.
Adjusted for fetal sex and year of birth.
Adjusted for fetal sex, year of birth, mother's age, education, country of origin, parity, and father's age.
Figure 1.
Odds ratios predicting preterm birth (PTB), low birth weight (LBW), and small for gestational age (SGA) outcomes after exposure to prenatal maternal stress by month of pregnancy with reference line.
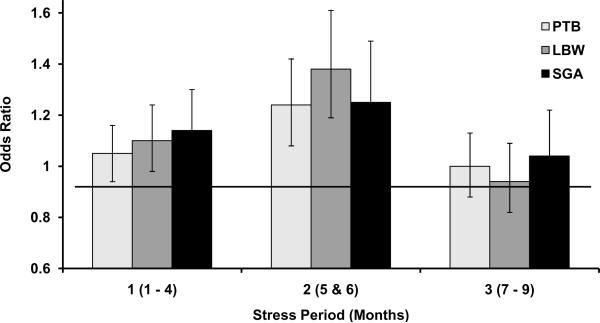
To test for differences between months 5 and 6 stress exposure to exposure during other months, new groups (periods) were created based on our findings from months of stress exposure. Period 1 consisted of months 1–4, period 2 consisted of months 5 and 6, and period 3 consisted of months 7–9. Predicted rates of adverse birth outcomes from these stress periods are presented in Table 4. Period 1 stress exposure was associated with a decrease of 0.30 (SE=0.11, p=0.006) days of gestation, period 2 with a significant decrease of 0.52 (SE=0.15, p=0.0006) days, and period 3 stress exposure did not significantly affect the length of gestation. Also shown in Table 4, associations between period exposure to prenatal maternal stress and other adverse birth outcomes (i.e. PTB, LBW, and SGA) were generally highest following period 2 (months 5 and 6). Logits were converted to OR and are shown in Figure 2. When predicting PTB, risk following period 2 stress exposure was the highest (OR=1.24, 99% CI=1.08–1.42). When predicting LBW, risk following period 2 was also highest (OR=1.38, 99% CI=1.19–1.61). There was no significant difference in the first and second period exposure when predicting SGA, although OR were larger following period 2 exposure (ORPeriod1=1.14, 99% CI=1.00–1.30; ORPeriod2=1.25, 99% CI=1.05–1.49). It can be observed that limiting the second exposure group to months 5 and 6 of pregnancy reveals relatively strong associations between stress during this period and adverse birth outcome as compared with stress exposure during the first or last period. Differences can be particularly noted when predicting PTB and LBW. In the case of SGA, grouping month 5 with month 6 may actually dampen the differential effects of stress exposure found for month 5.
Table 4.
Adjusteda risk of adverse birth outcomes for all Swedish, live-born, singleton pregnancies without congenital anomalies from 1973 to 2004 by novel and empirically supported period of stress exposure (defined as mother's exposure to death of a first degree relative or the father of the child).
| b (SE) |
Logits (SE) |
||||
|---|---|---|---|---|---|
| Period Exposed |
Prenatal Months |
Gestational Age |
Preterm Birth |
Low Birth Weight |
Small for Gestational Age |
| 1 | 1 – 4 | − 0.30 (0.11) | 0.05 (0.04) | 0.10 (0.05) | 0.13 (0.05) |
| 2 | 5 & 6 | − 0.52 (0.15) | 0.21 (0.05) | 0.32 (0.06) | 0.23 (0.07) |
| 3 | 7 – 9 | 0.07 (0.12) | −0.001 (0.05) | −0.06 (0.06) | 0.04 (0.06) |
Numbers in bold are significant at p<.01.
Adjusted for fetal sex, year of birth, mother's age, education, country of origin, parity, and father's age.
Figure 2.
Odds ratios predicting preterm birth (PTB), low birth weight (LBW), and small for gestational age (SGA) outcomes after exposure to prenatal maternal stress by empirically created periods during pregnancy with reference line. Period 1 includes months 1 through 4, period 2 includes months 5 and 6, and period 3 includes months 7–9.
Discussion
Results suggest that exposure to prenatal maternal stress during mid-gestation, indicated by the death of the father of the child or first degree relative of the mother, is associated with an increased risk for adverse birth outcomes. Vulnerability depends on the timing of exposure to stress. Months 4 and 5 showed heightened vulnerability to stress when predicting shortened GA. Months 5 and 6 emerged as periods of heightened vulnerability when predicting PTB and LBW. Gestational month 5 exposure showed increased risk for SGA. When grouped together and tested against month groups 1–4 and 7–9, months 5 and 6 were more strongly associated with adverse birth outcomes particularly when predicting PTB and LBW. Overall, findings suggest that the risk for adverse birth outcomes after prenatal maternal stress exposure is highest following exposure during gestational months 5 and 6. Future investigations using more precise vulnerability windows of months 5 and 6, rather than the whole trimester spanning months 4 to 6, may aide in identifying the relatively small, yet meaningful associations between stress exposure and later adverse developmental outcomes. Identification of increased vulnerability in months 5 and 6 may also be helpful for targeted intervention and prevention efforts.
This is the largest population-based study to date that specifically examined the impact of death of a first degree relative or father of the child during pregnancy on adverse birth outcomes. In addition, the present investigation examined the timing of the psychological stress-associated exposures per month, thereby providing more detail than the typical categorization per trimester. Indexing prenatal maternal stress with the well-defined and objective exact date of death allowed for the testing of single-month exposure periods and avoided potential maternal self-reporting biases that may have affected previous investigations. Despite these advantages, there are also limitations to indexing stress in this way. For example, hospital admissions for family members may have occurred several months before the date of death, thereby increasing the level of stress in the mother before the death of the family member occurred (29, 36–37). Future research would be aided by including cause of death and hospital admission dates (29) to address stress onset and duration. Additionally, creating a composite score of stress during pregnancy that includes other potential stressors (e.g. job loss, divorce, residential move, natural disaster) may provide a clearer, more thorough picture of prenatal conditions for the mother and fetus (38–39) as well as lead to stronger associations with adverse birth outcomes and greater comparability to other studies.
Although the current positive associations between stress exposure and shortened GA and PTB support previous research,vulnerable periods identified in the current investigation do not agree with previous findings. Previous research has suggested that first trimester (24–25) exposure is associated with the greatest decrease in gestational length and risk for PTB whereas the current findings suggest that mid-gestation stress exposure, coincident with second trimester, may be the most vulnerable period during pregnancy. These discrepancies may be due to the use of different types of stressors (24–25) and, most importantly, smaller sample sizes in previous literature (24–25). Although not examined in the current investigation, previous research has also suggested that preconception exposure to severe life events may have a detrimental impact on the length of the future gestational period (40).
Our results do support previous research showing that stress due to the death of any relative during the prenatal period is associated with increased risk of LBW and SGA (29) and that increased risk for these outcomes occurs after mid-gestation stress exposure. It should also be noted, however, that preconception severe life event exposure has been associated with increased risk for SGA in preterm infants (41), but preconception exposure was not examined in the current study. Associations between stress exposure and LBW were verified by examining associations between stress exposure and continuously measured birth weight. Associations between stress and SGA were tested with a more conservative categorization of SGA than previous research: birth weight for GA 2 standard deviations below the mean as opposed to birth weight for GA less than the 10th percentile (29). Regardless, the associations found between months 5 and 6 stress exposure and SGA are larger than previous associations found following trimester 2 stress exposure. Our larger overall sample size, more conservative definition of SGA, and more precise treatment of timing of stress exposure may have been responsible for the increased strength in association.
In conjunction with the Developmental Origins of Disease hypothesis (17) there are several non-exclusive biobehavioral hypotheses of the mechanisms through which maternal stress during mid-gestation could affect fetal development and birth outcome (42). These hypotheses posit 1) a direct effect of stress-responsive molecular regulators of the HPA axis on fetal development and parturition (18, 43), 2) alterations in immunological functioning, inflammation, and infection risk (18, 44), and 3) indirect effects of alterations in maternal behavior (39). Depending on the timing of stress, different mechanisms may act to produce similar outcomes (equifinality) or similar mechanisms may produce different results (multifinality) in part because of the dramatically changing fetal (2) and maternal stress response system (25, 45).
Due to the findings concerning mid gestation in the current study, a possible mechanism of the associations is a change in the maternal/fetal HPA axis and associated molecular regulators. Prenatal maternal stress may exert an effect on fetal development through placental function modification via maternal glucocorticoid stimulation of CRH production. This will in turn activate the fetal HPA axis. Changes in CRH levels during mid-gestation may be particularly important for the outcomes studied (21), but particularly important for shortened GA and PTB. CRH is the primary molecular regulator of the HPA axis and is critical for the onset and timing of spontaneous labor and delivery. The slope of the increase in CRH levels across pregnancy differentiates pregnancies that will end preterm, term, or post-term (21). Similar to the months of importance identified in the current study, CRH levels as early as prenatal months 4 and 5 differentiate preterm from term pregnancies (21, 26–28). Elevated maternal cortisol, a stress-related endocrine signal, early in the second trimester predicts a precocious rise in CRH during the early third trimester (28), and may also be a contributing factor. Also, CRH trajectories may differentiate between types of PTB: spontaneous preterm labor, preterm premature rupture of membranes, or induced early delivery on obstetric indication (43). Separating PTB into categories by obstetric precursor could explain our findings further and begin to address HPA axis-related mechanisms. Thus, CRH is part of an especially important candidate mechanism behind the association between stress and gestational length and PTB. Additionally, based on similarities in vulnerability related to stress timing found here, CRH may also play a role in other adverse birth outcomes.
Placental 11B-hydroxysteroid dehydrogenase type 2 (11β-HSD2) inactivates glucocorticoids until late in pregnancy thereby providing a barrier that decreases the risk of untimely action at glucocorticoid-responsive tissues during earlier fetal development. Therefore, 11β-HSD2 may also be a candidate mechanism for the current associations. Premature reduction in the expression or activity of 11β-HSD2 may lead the fetus to be at increased vulnerability to the effects of prenatal maternal stress (46). Research has shown that acute prenatal stress can influence 11β-HSD2 activity in rats (47) and early exponential increases in CRH concentrations have been shown to be associated with a comparable decrease in 11β-HSD2 later in pregnancy (21). Previous human and non-human animal research has shown that reduced placental 11β-HSD2 correlates with lower fetal weight (46), and intrauterine growth restriction (48). Further, the response of 11β-HSD2 may be mediated by genetic vulnerability (49).
Additionally, as pregnancy advances, mothers become less physiologically (45) and emotionally (25) sensitive to the effects of stress (19) which may contribute to decreased associations between later prenatal maternal stress and adverse birth outcomes. Other possible mechanisms including alterations in immunological functioning, inflammation, and infection (18, 44) as well as alterations in maternal behavior (39). An elaboration of the definition of prenatal maternal stress exposure to include maternal immunological (e.g. hospital visits) and behavioral changes (e.g. smoking during pregnancy) will explore these possibilities and help to more specifically test possible mediators of the associations found.
Although novel, this study is not without limitations. First, our results may not generalize optimally to other populations. Prenatal care is advanced in Sweden with about 95% of the pregnant population participating in antenatal care by the 15th week of gestation (50). This suggests, however, that identified associations between prenatal stress and poor developmental outcome may be stronger in countries with less advanced prenatal care. Second, abortion legislation is liberal in Sweden and neither abortions nor miscarriages were addressed in the current study. If stress exposure were great enough, the pregnancy may have been terminated with or without intention (51). In other words, this sample may be slightly biased towards resilient fetuses (i.e. pregnancies that do not end in miscarriage) or more determined mothers (i.e. mothers that, in the face of highly stressful circumstances, do not decide to abort the fetus). Finally, it should be noted that this investigation does not prove that stress causes poor birth outcomes. In fact, it may be that a common underlying risk factor contributes to both the stressor and outcome. Investigations into genetic and epigenetic factors will begin to address this issue (52–54) and our large sample size provides the opportunity to examine possible familial confounding (55). Additionally, with further elaboration of the maternal stress conditions, environmental or behavioral factors that may contribute to both the risk and outcome (e.g. hospitalization of the mother) can also be explored.
With the knowledge that months 5 and 6 may be most vulnerable to the disorganizing effects of prenatal maternal stress associated with the death of a first-degree relative or father of the child, future research using major life events to index prenatal maternal stress may consider examining these months specifically. After further investigation, replication, and an elucidation of the contributing mechanisms, intervention and prevention efforts may benefit from targeting these months of pregnancy. Differing rates of PTB, LBW and SGA among countries (56) suggests that improvements in care and prenatal health can be made and will improve fetal development and ultimately save lives.
Acknowledgments
This work was graciously supported by an Indiana Clinical and Translational Sciences Institute Career Development Award to QAC (5TL1RR025759-03, PI A. Shekhar). Support was also granted by Indiana University Faculty Research Support Program, NARSAD, and the Swedish Research Council to BMD. The Swedish Research Council also supported NL. The authors would also like to thank Dr. Curt Sandman for his insightful editorial comments.
Acronyms used in text
- ACTH
adrenocorticotropic hormone
- CRH
corticotrophin-releasing hormone
- GA
gestational age
- HPA
hypothalamic pituitary adrenal
- LBW
low birth weight
- PAPP-A
pregnancy-associated plasma protein-A
- PTB
preterm birth
- SGA
small for gestational age
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levitt P. Structural and functional maturation of the developing primate brain. Journal of Pediatrics. 2003;143:S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 2.Nathanielsz PW. Life in the womb: The origin of health and disease. Promethean Press; New York, NY: 1999. [Google Scholar]
- 3.Resnik R. Intrauterine growth restriction. Obstetrics and Gynecology. 2002;99:490–96. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- 4.Pulver LS, Guest-Warnick G, Stoddard GJ, Byington CL, Young P. Weight for gestational age affects the mortality of late preterm infants. Pediatrics. 2009;123:1072–77. doi: 10.1542/peds.2008-3288. [DOI] [PubMed] [Google Scholar]
- 5.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2005 period: Linked birth/infant death data set. National vital statistics reports. 2008;57:1–32. [PubMed] [Google Scholar]
- 6.Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. Journal of the American Medical Association. 2002;288:728–37. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 7.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. The New England Journal of Medicine. 2008;359:262–73. doi: 10.1056/NEJMoa0706475. [DOI] [PubMed] [Google Scholar]
- 8.Costello EJ, Worthman C, Erkanli A, Angold A. Prediction from low birth weight to female adolescent depression: A test of competing hypotheses. Archives of General Psychiatry. 2007;64:338–44. doi: 10.1001/archpsyc.64.3.338. [DOI] [PubMed] [Google Scholar]
- 9.Bohnert KM, Breslau N. Stability of psychiatric outcomes of low birth weight. Archives of General Psychiatry. 2008;65:1080–86. doi: 10.1001/archpsyc.65.9.1080. [DOI] [PubMed] [Google Scholar]
- 10.Strang-Karlsson S, Raikkonen K, Pesonen A, Kajantie E, Paavonen J, Lahti J. Very low birth weight and behavioral symptoms of Attention Deficit Hyperactivity Disorder in young adulthood: The Helsinki study of very low birth weight adults. American Journal of Psychiatry. 2008;165:1345–53. doi: 10.1176/appi.ajp.2008.08010085. [DOI] [PubMed] [Google Scholar]
- 11.Zupancic JAF. A systematic review of costs associated with prematurity. In: Behrman R, Butler AS, editors. Preterm birth: Causes, consequences, and prevention. The National Academies Press; Washington, D. C: 2006. [PubMed] [Google Scholar]
- 12.Gilbert WM, Nesbitt TS, Danielson B. The cost of prematurity: Quantification by gestational age and birth weight. Obstetrics and Gynecology. 2003;102:488–92. doi: 10.1016/s0029-7844(03)00617-3. [DOI] [PubMed] [Google Scholar]
- 13.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S. Births: Final data for 2005. National vital statistics reports. 2007;56 [PubMed] [Google Scholar]
- 14.EXPRESS Group One-year survival of extremely preterm infants after active perinatal care in Sweden. Journal of the American Medical Association. 2009;301:2225–33. doi: 10.1001/jama.2009.771. [DOI] [PubMed] [Google Scholar]
- 15.Behrman R, Butler AS, editors. Preterm birth: Causes, consequences, and prevention. The National Academies Press; Washington DC: 2007. p. 790. [PubMed] [Google Scholar]
- 16.Beydoun H, Saftlas AF. Physical and mental health outcomes of prenatal maternal stress in human and animal studies: A review of recent evidence. Pediatric and Perinatal Epidemiology. 2008;22:438–66. doi: 10.1111/j.1365-3016.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 17.Barker DJP. Mothers, babies and health in later life. 2nd ed. Churchill Livingstone; Edinburgh: 1998. [Google Scholar]
- 18.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, Hobel CJ, Chicz-DeMet A, Dunkel-Schetter C, Garite TJ, Glynn L. Stress, infection and preterm birth: A biobehavioural perspective. Paediatric & Perinatal Epidemiology. 2001;15:17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 19.de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy--a review. Neuroscience & Biobehavioral Reviews. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Liu JH, Rebar RW. Endocrinology of pregnancy. Saunders; Philadelphia: 1999. [Google Scholar]
- 21.McLean M, Bistis A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nature Medicine. 1995;1:460–63. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 22.Meaney MJ, Diorio J, Fancis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 23.Dobbing J, Sands J. Quantitative growth and development of human brain. Archives of Disease in Childhood. 1973;48:757. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lederman SA, Rauh V, Weiss L, Stein JL, Hoepner LA, Becker M, Perera FP. The effects of the World Trade Center event on birth outcomes among term deliveries at three lower Manhattan hospitals. Environmental Health Perspectives. 2004;112:1772–78. doi: 10.1289/ehp.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynn L, Wadhwa PD, Schetter CD, Chicz-DeMet A, Sandman CA. When stress happens matters: Effects of earthquake timing on stress responsivity in pregnancy. American Journal of Obstetrics and Gynecology. 2001;184:637–42. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 26.Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstetrics and Gynecology. 2001;97:657–63. doi: 10.1016/s0029-7844(00)01209-6. [DOI] [PubMed] [Google Scholar]
- 27.Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. American Journal of Obstetrics & Gynecology. 1998;179:1079–85. doi: 10.1016/s0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 28.Sandman CA, Glynn L, Dunkel-Schetter C, Wadhwa PD, Garite TJ, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Khashan AS, McNamee R, Abel KM, Pedersen MG, Webb RT, Kenny LC, Mortensen PB, Baker PN. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosomatic Medicine. 2008;70:688–94. doi: 10.1097/PSY.0b013e318177940d. [DOI] [PubMed] [Google Scholar]
- 30.Dunkel-Schetter C, Glynn L. Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary research. In: Contrada R, Baum A, editors. Handbook of Stress. in press. [Google Scholar]
- 31.Arbuckle NW, de Vries B. The long-term effects of later life spousal and parental bereavement on personal functioning. The Gerontologist. 1995;35:637–47. doi: 10.1093/geront/35.5.637. [DOI] [PubMed] [Google Scholar]
- 32.Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatrica. 1996;85:843–48. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- 33.National Board on Health and Welfare . Cf Epidemiology. Stockholm, Sweden: 2003. The Swedish Medical Birth Register - A summary of content and quality. Report 112-3. [Google Scholar]
- 34.Cuervo LG. Induction of labour for improving birth outcomes for women at or beyond term: RHL commentary. The WHO Reproductive Health Library; 2006. [Google Scholar]
- 35.Robbins AS, Chao SY, Fonseca VP. What's the relative risk? A method to directly estimate risk ratios in cohort studies of common outcomes. Annals of Epidemiology. 2002;12:452–54. doi: 10.1016/s1047-2797(01)00278-2. [DOI] [PubMed] [Google Scholar]
- 36.Hansen D, Lou HC, Olsen J. Serious life events and congenital malformations: A national study with complete follow-up. Lancet. 2000;356:875–80. doi: 10.1016/S0140-6736(00)02676-3. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Vestergaard M, Obel C, Precht DH, Christensen J, Lu M, Olsen J. Prenatal stress and cerebral palsy: A nationwide cohort study in Denmark. Psychosomatic Medicine. 2009;71:615–18. doi: 10.1097/PSY.0b013e3181a56ca1. [DOI] [PubMed] [Google Scholar]
- 38.Lobel M, Dunkel-Schetter C, Scrimshaw SC. Prenatal maternal stress and prematurity: A prospective study of socioeconomically disadvantaged women. Health Psychology. 1992;11:32–40. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- 39.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychology. 2008;27:604–15. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 40.Khashan AS, McNamee R, Abel KM, Mortensen PB, Kenny LC, Pedersen MG, Webb RT, Baker PN. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Human Reproduction. 2009;24:429–37. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- 41.Precht DH, Andersen PK, Olsen J. Severe life events and imparied fetal growth: a nation-wide study with complete follow-up. Acta Obstet Gynecol Scand. 2007;86:266–75. doi: 10.1080/00016340601088406. [DOI] [PubMed] [Google Scholar]
- 42.Paarlberg KM, Vigerhoets AJJM, Passchier J, Dekker GA, Geijn HPV. Psychosocial factors and pregnancy outcomeL A review with emphasis on methodological issues. Journal of Psychosomatic Research. 1995;39:563–95. doi: 10.1016/0022-3999(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 43.McGrath S, McLean M, Smith D, Bisits A, Giles WB, Smith R. Maternal plasma corticotropin-releasing hormone trajectories vary depending on the cause of preterm delivery. American Journal of Obstetric Gynecology. 2002;186:257–60. doi: 10.1067/mob.2002.119635. [DOI] [PubMed] [Google Scholar]
- 44.Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Maternal & Child Health Journal. 2001;5:127–34. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- 45.Schulte HM, Weisner D, Allolio B. The corticotropin releasing hormone test in late pregnancy: Lack of adrenocorticotropin and cortisol response. Clinical Endocrinology. 1990;33:99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 46.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Frontiers in Behavioral Neuroscience. 2009;3:1–9. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welberg LAM, Thrivikraman KV, Plotsky PM. Chronic maternal stress inhibits the capacity to up-regulate placental 11B-hydroxysteroid dehydrogenase type 2 activity. Journal of Endocrinology. 2005;186:R7–R12. doi: 10.1677/joe.1.06374. [DOI] [PubMed] [Google Scholar]
- 48.Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, Afnan M, Stewart PM. 11B-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Human Reproduction. 1998;13:799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- 49.Lucassen PJ, Bosch OJ, Jousma E, Kromer SA, Andrew R, Seckl JR, Neumann ID. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but notlow, anxiety: Possible key role of placental 11B-hydroxysteroid dehydrogenase type 2. European journal of Neuroscience. 2009;29:97–103. doi: 10.1111/j.1460-9568.2008.06543.x. [DOI] [PubMed] [Google Scholar]
- 50.Lindmark G, Cnattinguis S. The scientific basis of antenatal care. Report from a state-of-the-art conference. Acta Obstetricia & Gynecologica Scandinavica. 1991;70:105–09. doi: 10.3109/00016349109006190. [DOI] [PubMed] [Google Scholar]
- 51.Arck PC, Rucke M, Rose M, Szekeres-Bartho J, Douglas AJ, Pritsch M, Blois SM, Pincus MK, Barenstrauch N, Dudenhausen JW, Nakamura K, Sheps S, Klapp BF. Early risk factors for miscarriage: A prospective cohort study in pregnant women. Reproductive BioMedicine Online. 2008;17:101–13. doi: 10.1016/s1472-6483(10)60300-8. [DOI] [PubMed] [Google Scholar]
- 52.Plunkett J, Muglia LJ. Genetic contributions to preterm birth: Implications from epidemiological and genetic association studies. Annals of Medicine. 2008;40:167–95. doi: 10.1080/07853890701806181. [DOI] [PubMed] [Google Scholar]
- 53.Chaudhari BP, Plunkett J, Ratajczak CK, Shen TT, DeFranco EA, Muglia LJ. The genetics of birth timing: Insights into a fundamental component of human development. Clinical Genetics. 2008;74:493–501. doi: 10.1111/j.1399-0004.2008.01124.x. [DOI] [PubMed] [Google Scholar]
- 54.Mesquita AR, Wegerich Y, Patchev AV, Oliveira M, Leao P, Sousa N, Almeida OFX. Glucocorticoids and neuro- and behavioral development. Seminars in Fetal and Neotatal Medicine. 2009;14:130–35. doi: 10.1016/j.siny.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Drake AJ, Walker BR. The intergenerational effects of fetal programming: Nongenomic mechanisms for the inheritance of low birth weight and cardiovascular risk. Journal of Endocrinology. 2004;180:1–16. doi: 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- 56.Gissler M, Rahkonen O, Mortensen L, Arntzen A, Cnattinguis S, Nybo Anderson AM, Hemminki E. Sex differences in child and adolescent mortality in the Nordic countries. Scandinavian Journal of Public Health. 2009;37:340–46. doi: 10.1177/1403494809103905. [DOI] [PubMed] [Google Scholar]