Abstract
The Mediterranean diet and more specifically certain meats, fruits, vegetables, and olive oil found in certain parts of the Mediterranean region have been associated with a decreased cardiovascular and diabetes risk. More recently, several population based studies have observed with these lifestyle choices have reported an overall reduced risk for several cancers. One study in particular observed an inverse relationship between consumption of Mediterranean herbs such as rosemary, sage, parsley, and oregano with lung cancer. In light of these findings there is a need to explore and identify the anti-cancer properties of these medicincal herbs and to identify the phytochemicals therein. One agent in particular, carnosol, has been evaluated for anti-cancer property in prostate, breast, skin, leukemia, and colon cancer with promising results. These studies have provided evidence that carnosol targets multiple deregulated pathways associated with inflammation and cancer that include nuclear factor kappa B (NFκB), apoptotic related proteins, phosphatidylinositol-3-kinase (PI3 K)/Akt, androgen and estrogen receptors, as well as molecular targets. In addition, carnosol appears to be well tolerated in that it has a selective toxicity towards cancer cells versus non-tumorigenic cells and is well tolerated when administered to animals. This mini-review reports on the pre-clinical studies that have been performed to date with carnosol describing mechanistic, efficacy, and safety/tolerability studies as a cancer chemoprevention and anti-cancer agent.
Keywords: Carnosol, rosemary, sage, antioxidant, cancer
1. Introduction
The identification and characterization of the anti-cancer properties of natural products, especially in the area of cancer chemoprevention, has received significant interest over the years [1–4]. A class of compounds known as diterpenes, is receiving increasing attention for a variety of health promoting properties such as anti-microbial [5], anti-inflammatory [6], neuroprotective [7], anti-oxidant [8], and anti-cancer properties [9]. One agent in particular is carnosol which has been reported to have broad anti-cancer properties in several cell line models including prostate, breast, leukemia as well as others. These studies have begun to identify the pro-apoptotic properties and targeting of multiple deregulated pathways with carnosol, however, a comprehensive evaluation of carnosol as an anti-cancer agent is lacking. This mini-review presents the current knowledge of carnosol as it applies towards several cancers including prostate, breast, skin, leukemia, and colon cancer.
2. Mediterranean Diet and Lifestyle
The Mediterranean diet has received significant attention for its cardiovascular and metabolic health promoting properties [10]. This diet is best understood by the food patterns in Crete, Greece and the southern part of Italy which has high intakes of fresh fruit and vegetables, olive oil, unrefined cereals, and low consumption of meat products. Attention is now being given to how the Mediterranean diet impacts other disease states such as cancer where several population based studies are suggestive of a reduced risk of breast, colon, stomach, and prostate cancers with this type of diet and lifestyle [11–16]. The majority of these studies focus on the consumption of various meats, fruits, vegetables and olive oil.
What is less understood is the impact of Mediterranean herbs such as rosemary, sage, basil, oregano, and others that are often incorporated into various foods and oils. Each of these herbs have a variety of phytochemicals such as diterpenes including carnosol and carnosic acid. For this reason we are focusing on understanding the current knowledge of carnosol as an anticancer agent.
A case-control study in patients diagnosed with lung cancer evaluated the relationship between components of the Mediterranean diet and lung cancer [15]. Eligible participants were living in the Lazio region, which includes the city of Rome, were between the ages of 35 and 90 years of age. Control participants were matched by gender (1:1 for males and 1:2 for females) and age. As a part of the questionnaire participants were evaluated for consumption of herbs including parsley, rosemary, basil, and sage. All of four of these herbs trended towards a decreased overall risk with the most significant overall risk reduction observed with sage [OR = 0.43, 95% CI = 0.29–0.65]. Parsley was also associated with a reduced overall risk [OR = 0.31, 95% CI = 0.11–0.84] while a trend towards a reduced risk was observed with rosemary [OR = 0.66, 95% CI = 0.37–1.15] and basil [OR = 0.63, 95% CI = 0.31–1.30]. These results at a minimum are suggestive that the phytochemicals isolated from these culinary herbs should be investigated for their health promoting properties.
3. Carnosol: Sources, Chemistry, and Synthesis
Mediterranean herbs including rosemary and sage have been used for culinary purposes and for their medicinal properties for millennia. Carnosol was first isolated from sage (Salvia carnosa) in 1942 and the chemical structure was first established in 1964 by Brieskorn et al [17]. Rosemary and sage have been known to contain a variety of polyphenols such as carnosol, carnosic acid, rosmanol, rosmarinic acid as well as others [18]. It has been estimated that approximately 5% of the dry weight of rosemary leaves contains carnosol and carnosic acid, however, this fraction is estimated to account for > 90% of the antioxidant activity [19].
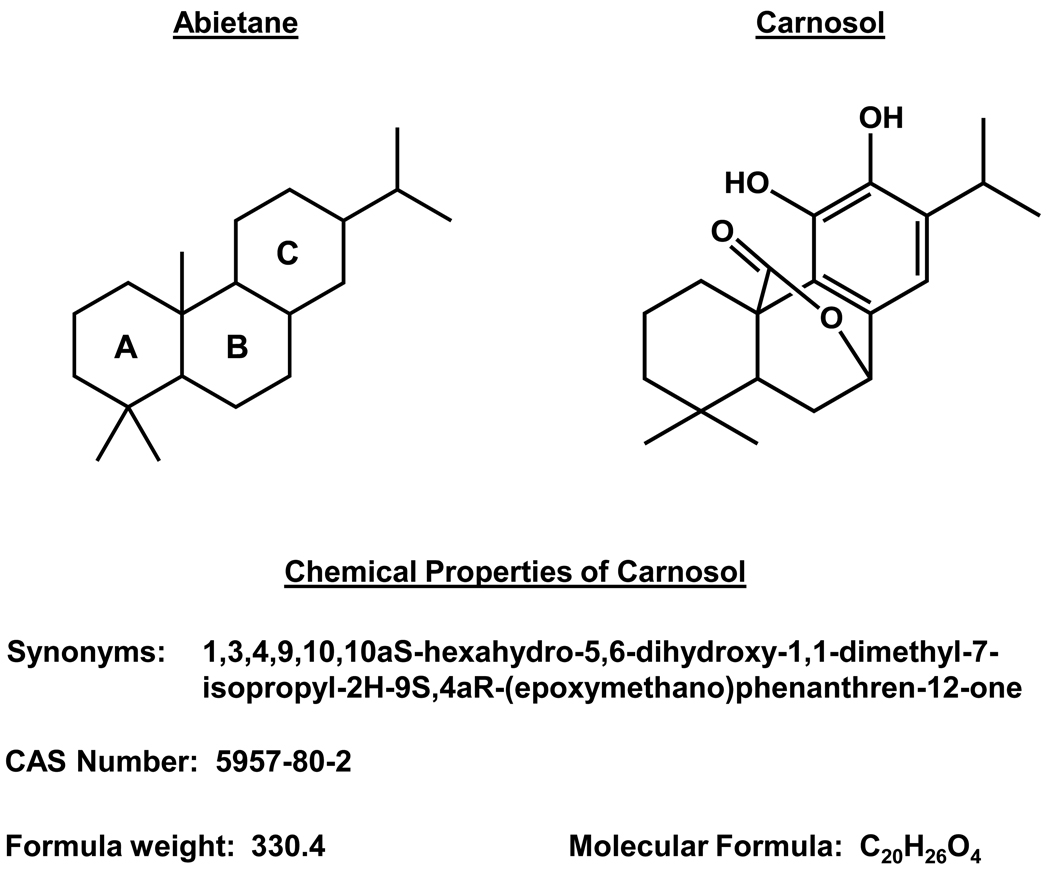
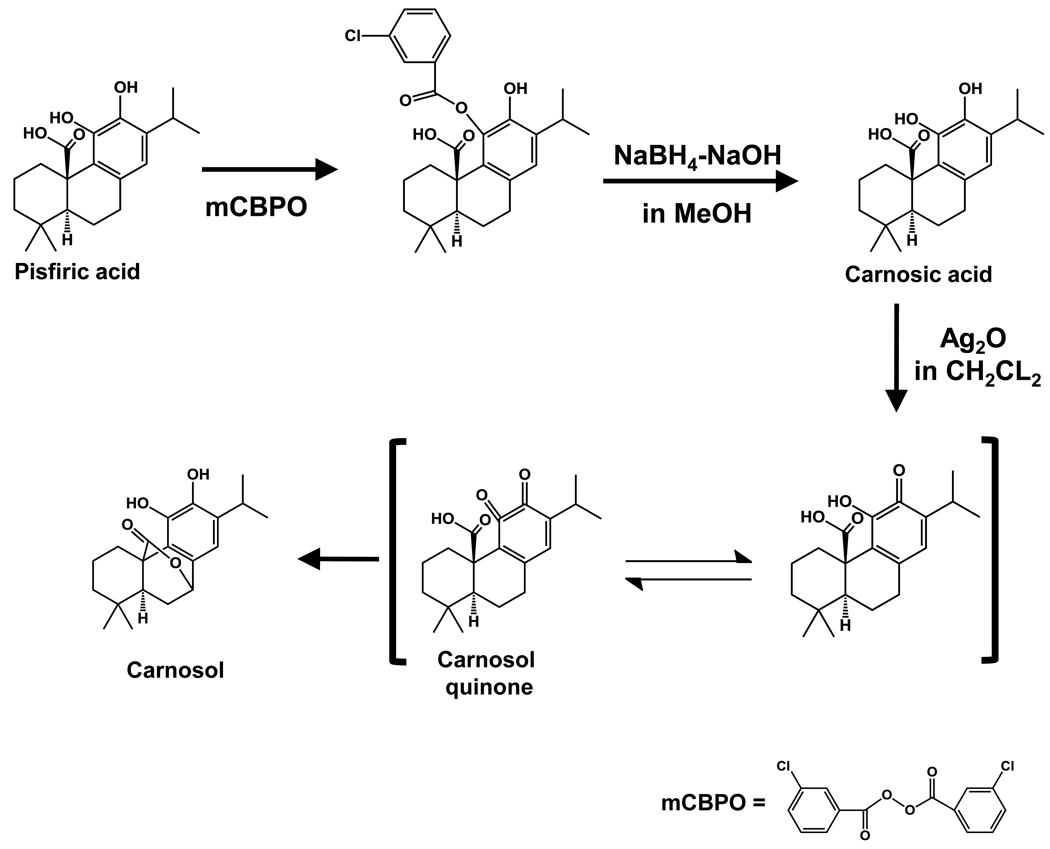
Carnosol is an ortho-diphenolic diterpene with an abietane carbon skeleton (Figure 1) with hydroxyl groups at positions C-11 and C-12 and a lactone moiety across the B ring [20]. Carnosol is the product of oxidative degradation of carnosic acid (Figure 2) [21]. The most popular and expensive way to procure a highly purified form of carnosol is through extraction and purification from natural sources such as rosemary. Recently, a semi-synthetic process has been described using pisiferic acid (Figure 2) extracted from Sawara (Chamaecyparis pisifera) leaves, a cypress tree native to Japan, to synthesize carnosic acid and then to carnosol [22]. Carnosic acid was prepared through oxidation of pisfiric acid using three different methods: a) mCBPO, b) CAMCBP in CH2CL2, or c) IBX in CHCl2-CH3OH. The third semi-synthetic reaction developed had an overall yield of 72% while the first two had considerably lower yields as low as 10%. Carnosol was prepared from carnosic acid in the presence of silver oxide in CH2CL2 with a purified yield of 67%. Alternatively, carnosic acid in the presence of methanol for 1 week at room temperature will oxidize carnosic acid to carnosol [23,24].
Figure 1.
The chemical properties of carnosol.
Figure 2.
Semi-synthesis of carnosol from pisiferic acid. Pisiferic acid represents the starting material that has been used for semi-synthetic process of carnosic acid. Carnosic acid is oxidized to form carnosol. This synthesis has been adapted from Tada et al [22].
4. Anti-oxidant Activity of Carnosol
The health promoting properties of rosemary and sage have been attributed to the antioxidant activity of polyphenols present in these extracts [25–28]. Reactive oxygen species and depletion of antioxidant enzymes have been suggested to promote a variety of biological responses including nueurogenerative, inflammatory conditions, cardiovascular disease, and carcinogenesis of various tissues [29]. Rosemary extracts were prepared and shown by the DPPH (2,2-diphenyl-1-pycrilhydrazil hydrate) radical scavenging activity assay to have a radical scavenging activity up to 95.1% [25] with approximately 90% of the antioxidant activity attributed to the diterpenes carnosol and carnosic acid [19]. Carnosol has been shown to inhibit Cu2+ induced LDL oxidation and lipid free radicals in mouse liver microsomes [30] and are good scavengers of peroxyl radicals (CCl302) [19]. The antioxidant response element is believed to be activated through the catechol-hydroxyl groups of carnosol and is converted to a carnosol quinone [8]. This quinone derivative is the main anti-oxidation product of carnosol essentially voiding it of any antioxidant activity [31] and under appropriate conditions the antioxidant activity can be recovered [32].
The glutathione-S-transferase (GST) family of phase II detoxification enzymes catalyze the reaction of glutathione with electrophiles and have been a target of interest for cancer [33]. Carnosol by intraperitoneal administration has been shown to enhance the in vivo activity of GST and quinone reductase in the liver of the female rat [34]. Carnosol (100–400 mg/kg) increased GST activity by 1.6 to 1.9 fold increase.
5. Anti-inflammatory Activity of Carnosol
Deregulated inflammatory signaling including excess nitric oxide (NO) produced by NO synthase (iNOS) occurs during inflammation and the multi-step process of carcinogenesis which has led to the search for agents that decrease inflammatory signaling pathways. Raw 264.7 cells treated with carnosol reduced LPS stimulated NO production with an IC50 of 9.4 uM [35]. This led to an inhibition of the NF-kB, p38 and p44/42 mitogen activated protein kinase (MAPK). In another study, carnosol was shown to activate the peroxisome proliferator-activated receptor gamma. Carnosol has also been shown to reduce the pro-inflammatory leukotrienes in intact polymorphonuclear leukocytes (PMNL), inhibit 5-lipoxygenase, antagonize the intracellular ca2+ mobilization, and inhibit the secretion of leukocyte elastase [6]. In addition, carnosol blocks protein kinase C signaling and inhibits the binding of AP-1 to the COX-2 promoter which should be noted is fundamentally different than the synthetic COX-2 inhibitors (e.g. celecoxib) that function as direct inhibitors of Cox-2 [36].
6. Anti-Cancer properties of Carnosol
6.1 Carnosol and Prostate Cancer
In our laboratory, we are currently investigating the potential role of carnosol for the prevention and treatment of prostate cancer (PCa). Earlier we provided evidence that carnosol promotes G2 cell cycle arrest in PC3 cells decreasing cell viability [37]. The anti-cancer properties of carnosol were associated with a potential to modulate multiple signaling pathways such as the cell cycle related proteins, PI3K/AKT, and apoptotic related proteins [38]. To understand the effects of carnosol in PC3 cells we performed an antibody array that identified 36 proteins downregulated at least 50% and 24 proteins that were up-regulated at least 200% including a target within the 5’-AMP-activated protein kinase (AMPK) protein subunit. AMPK has been shown to regulate the growth and survival of cancer cells. We observed a dose dependent increase in the phosphorylated forms of AMPK-α (Thr172) which has been shown to be upstream of the mammalian target of rapamycin (mTOR). We were also able to show a decrease in the phosphorylation of mTOR (Ser2448) and related downstream targets.
Recently, we have shown that carnosol has a unique property where it functions as a dual disruptor of both androgen and estrogen receptor α in PCa [9]. Using a TR-FRET assay we were able to show that carnosol is an antagonist at both the AR and ERα with no agonist properties. To our knowledge this is a unique property as several synthetic agents including fulvestrant, toremifene, and tamoxifen have been investigated as dual disruptors, however, through dose escalation it is not uncommon for these synthetic agents to display agonist properties at the AR [39–42]. Carnosol displayed minimal effects on non-tumorigenic prostate epithelial cells when treated with increasing concentrations and was similar in effect with what was observed with flutamide and tamoxifen. Interestingly, we observed an increase in protein expression of androgen receptor with tamoxifen and flutamide which was reversible with co-treatment with carnosol. In a xenograft study, athymic nude mice were implanted with 22Rv1 cells and treated with carnosol orally five days weekly. At the conclusion of the study mice treated with carnosol had a significant suppression in tumor growth by 36% (P = 0.028) and circulating PSA by 26% (P = 0.0042).
6.2 Carnosol and Breast Cancer
Carnosol and carnosic acid were investigated for their antimicrobial and anti-cancer properties in breast cancer [43]. Both carnosol and carnosic acid were shown to have cytotoxic activity against MCF-7 cells with an IC50 of 82 and 96 µM. We have observed similar results with carnosol decreasing the cell viability of MCF-7 cells [9], AU565, and MDA-MB-231 (unpublished results). Further studies are needed to determine if carnosol preferentially targets ER+ breast cancer cells versus ER− breast cancer cells.
Carnosol was evaluated to determine the ability of carnosol to inhibit the formation of DMBA-DNA adduct formation and DMBA-induced mammary tumorigenesis in the female rat [44]. Intraperitoneal administration of rosemary and carnosol were shown to significantly inhibit mammary adduct formation by 44% and 40%, respectively. At week 20 post DMBA treatment carnosol (100 and 200 mg/kg) administration resulted in a significant inhibition of tumor formation was observed with decreases of 33% (P < 0.001) and 30% (p < 0.005), respectively. The role of carnosol in preventing DMBA-induced mammary tumorigenesis may be partially explained by carnosol inducing detoxification enzymes including GST and quinone reductase which carnosol has been shown to modulate in other studies [34,45]. Another consideration is the microsomal metabolism of endogenous estrogens. CD-1 mice were administered rosemary (2%) in their diet led to an increase in the oxidation and glucuronidation of estradiol and estrone inhibiting their uterotropic action [46].
Using mammary epithelial cells carnosol was shown to block the increased binding of AP-1 to the COX-2 promoter [36]. In addition, carnosol was shown to inhibit the activation of PKC, ERK1/2, p38, and c-jun NH2-terminal kinase mitogen activated protein kinase. Overexpression of c-jun inhibited the suppressive effects of carnosol.
6.3 Carnosol and Skin Cancer
A methanol extract of rosemary was evaluated for its inhibition on tumor initiation and promotion in mouse skin [23]. Several constituents were characterize by HPLC in the rosemary extract and found to contain ursolic acid (16.5–19.2%), carnosol (3.8–4.6%) and carnosic acid (0.1–0.5%). The rosemary extract was found to inhibit the covalent binding of benzo(a)pyrene to skin epidermis DNA. In mice treated with B(a)P and tetradecanoylphorbol-13-acetate (TPA) mice treated with rosemary (1.2 or 3.6 mg) five minutes prior to application of B(a)P led to a reduction in tumors by 54 or 64%, respectively. Topical application of rosemary was also found to decrease TPA induced ornithine decarboxylase (ODC) activity, TPA induced inflammation, arachadonic acid-induced inflammation, TPA induced hyperplasia, and TPA induced tumor promotion. In mice initiated with DMBA followed by TPA for promotion rosemary, (0.4, 1.2, or 3.6 mg) was found to decrease the number of skin tumors by 40, 68 or 99%, respectively. The topical application of carnosol also inhibited TPA induced ear inflammation, ODC activity, and tumor promotion. Carnosol at 1, 3, or 10 µM inhibited the number of skin tumors per mouse by 38, 63, or 78%, respectively.
The migration and invasion of B16/F10 mouse melanoma cells was shown to be inhibited by carnosol [47]. Carnosol treatment resulted in the decrease of MMP-9 mRNA and protein expression with an IC50 value for MMP-9 mRNA at 5 µM suggesting regulation at the transcriptional level. Several upstream regulators of MMP-9 including AKT, p38, and JNK and to a lesser extent Erk1/2 phosphorylation activities were modulated by carnosol. The activity of NF-kB was also inhibited by carnosol (10 µM).
6.4 Carnosol and Leukemia
Carnosol was shown to induce apoptosis by disrupting the mitochondrial membrane potential in three acute leukemia cell lines which included SEM, RS4:11, and MV4:11 [48]. At 18 µM carnosol did not induce cell death of peripheral blood mononuclear cells (PBMCs) isolated from healthy volunteers while the same dose in cell lines resulted in apoptosis. Apoptosis and alterations in mitochondrial membrane potential resulted from carnosol treatment. There is evidence to suggest that carnosol targets the anti-apoptotic members of the Bcl-2 family of proteins. A reduction in Bcl-2 protein expression ranged from 33 to 53% in the three cell lines. Several phytochemicals have been reported to have sensitizing properties when used in combination with chemotherapies in a variety of cancer cell lines. Interestingly, co-treatments of carnosol and AraC, or methotrexate, or vincristine resulted in a delay in chemotherapy induced DNA fragmentation [49]. Further studies are needed to understand how co-treatment of carnosol with chemotherapeutic agents delays DNA fragmentation.
6.5 Carnosol and Colon Cancer
Using a model for colon tumorigenesis dietary administration of carnosol was observed to decrease intestinal multiplicity by 46% in the C57BL/6J/Min/+ (Min/+) mouse [50]. Mice were administered a diet with or without carnosol (0.1%). Carnosol was shown to restore E-cadherin and β-catenin to the enterocyte membranes producing a phenotype similar to the APC+/+ wild-type (WT) littermate. Inherited mutations in the APC tumor suppressor gene result in the generation of familial adenomatous polyposis coli with somatic mutations in >80% of sporadic colon cancers [51]. These results suggest that carnosol prevents Apc-associated tumorigenesis in a mouse model, however, further studies are needed.
7. Carnosol Safety and Toxicity
In the Ames Salmonella tester strain TA102 carnosol was found to have significant antioxidant activity with anti-mutagenic activity similar to ascorbic acid [52]. Several studies have suggested that carnosol is protective against environmental toxins in experimental animal models of hepatotoxicity [53–55], bronchial cells [56], and may assist in inducing phase II detoxification enzymes [57]. In the micronucleus test for mutagenesis carnosol was found to be more effective than L-ascorbic acid for gamma-ray radioprotection capacity both before and after radiation exposure [58].
Several animal studies have suggested that daily oral administration of carnosol is well tolerated. In our study, we observed that oral administration of carnosol at 30 mg/kg was well tolerated when administered five days weekly over a 28 day period as evidenced by daily body weight measurements which did not vary between carnosol or vehicle treated mice [9]. In another study, Sprague-Dawley rats were administered an AIN-76A diet with up to 1% carnosol for two weeks with no observable effects on body weight [44]. In a separate experiment with the same group carnosol at 200 mg/kg administered intraperitoneally for five consecutive days was well tolerated as evidenced by measuring liver weight. C57BL/6J/Min/+ (Min/+) Mouse were administered carnosol up to 0.1% in their daily diet was well tolerated over a ten week period [50].
As an alternative approach, future studies should consider evaluating highly characterized rosemary extracts that are standardized to carnosol. This strategy has been employed for studying EGCG (epigallocatechin-3-gallate) in clinical trials through the use of a highly characterized green tea extract standardized to EGCG and other green tea polyphenols [1]. This approach has been recognized by the FDA as seen in their guidance titled, “Guidance for Industry – Botanical Drug Products” and by botanical products receiving investigational new drug (IND) status such as Polyphenon E. The benefit of using a highly characterized extract would be cost to procure carnosol would be cheaper because less purification would be required. A second benefit could be that additional constituents that are found in rosemary would have the opportunity to work synergistically with carnosol. Further studies are needed to evaluate highly characterized rosemary extracts standardized to carnosol. Recently, the European Union has approved a rosemary extract standardized to carnosol for its application towards food preservation and has been adopted into the EU food additive legislation [59]. This extract was well tolerated in short and long term toxicity studies. In a 13 week oral study in male and female rats the NOAEL (no observable adverse event level) of different rosemary extracts was between 180 to 400 mg/kg/bw/day which was equivalent to 20–60 mg/kg /day of carnosol and carnosic acid per day. The adult mean intake of this extract was estimated to be between 500 and 1500 mg of carnosol and carnosic acid per day. Further studies are needed to determine the anti-cancer properties of these rosemary extracts in addition to evaluating isolated constituents from rosemary.
7. Concluding Remarks
The bioavailability and metabolism of carnosol in either animals or humans has not been investigated. The bioavailability of carnosic acid, which shares structural similarities to carnosol are summarized briefly [60]. The Cmax of carnosic acid was 128 µM (42.52 mg/L) when administered intragastrically at 90 mg/kg and the absolute bioavailability of carnosic acid was 65.09%. These results are promising suggesting that diterpenes are well absorbed, however, further studies are needed to determine if carnosol has similar pharmacokinetic parameters to carnosic acid. Pharmacokinetic studies of carnosol will be critical in determining the potential use of carnosol for application as an anti-inflammatory and anti-cancer agent. Several pre-clinical studies have suggested that carnosol selectively targets tumorigenic cell as opposed to non-tumorigenic cells and is safe and tolerable in animals. Further studies are needed to understand molecular interactions of carnosol with deregulated pathways associated with inflammation and cancer. To understand the full potential of carnosol as a chemopreventive or chemotherapeutic agent more mechanistic studies are needed.
Acknowledgements
The original work that was performed with carnosol and prostate cancer was supported by NIH 1KL2RR025012 and K12 RR023268. I would like to thank Professor Hasan Mukhtar for his mentoring and support that he has generously given me over the years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None declared
References
- 1.Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine. 2010;17:3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Saleem M. Lupeol, a novel anti-inflammatory and anti-cancer dietary triterpene. Cancer Lett. 2009;285:109–115. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Syed DN, Khan N, Afaq F, Mukhtar H. Chemoprevention of prostate cancer through dietary agents: progress and promise. Cancer Epidemiol Biomarkers Prev. 2007;16:2193–2203. doi: 10.1158/1055-9965.EPI-06-0942. [DOI] [PubMed] [Google Scholar]
- 5.Weckesser S, Engel K, Simon-Haarhaus B, Wittmer A, Pelz K, Schempp CM. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine. 2007;14:508–516. doi: 10.1016/j.phymed.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Poeckel D, Greiner C, Verhoff M, Rau O, Tausch L, Hornig C, Steinhilber D, Schubert-Zsilavecz M, Werz O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress proinflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem Pharmacol. 2008;76:91–97. doi: 10.1016/j.bcp.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Kim JS, Cho HS, Lee HJ, Kim SY, Kim S, Lee SY, Chun HS. Carnosol, a component of rosemary (Rosmarinus officinalis L.) protects nigral dopaminergic neuronal cells. Neuroreport. 2006;17:1729–1733. doi: 10.1097/01.wnr.0000239951.14954.10. [DOI] [PubMed] [Google Scholar]
- 8.Satoh T, Izumi M, Inukai Y, Tsutsumi Y, Nakayama N, Kosaka K, Shimojo Y, Kitajima C, Itoh K, Yokoi T, Shirasawa T. Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci Lett. 2008;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JJ, Syed DN, Suh Y, Heren CR, Saleem M, Siddiqui IA, Mukhtar H. Disruption of androgen and estrogen receptor activity in prostate cancer by a novel dietary diterpene carnosol: implications for chemoprevention. Cancer Prev Res (Phila) 2010;3:1112–1123. doi: 10.1158/1940-6207.CAPR-10-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piscopo S. The Mediterranean diet as a nutrition education, health promotion and disease prevention tool. Public Health Nutr. 2009;12:1648–1655. doi: 10.1017/S1368980009990504. [DOI] [PubMed] [Google Scholar]
- 11.Brennan SF, Cantwell MM, Cardwell CR, Velentzis LS, Woodside JV. Dietary patterns and breast cancer risk: a systematic review and meta-analysis. Am J Clin Nutr. 2010;91:1294–1302. doi: 10.3945/ajcn.2009.28796. [DOI] [PubMed] [Google Scholar]
- 12.Cottet V, Touvier M, Fournier A, Touillaud MS, Lafay L, Clavel-Chapelon F, Boutron-Ruault MC. Postmenopausal breast cancer risk and dietary patterns in the E3N-EPIC prospective cohort study. Am J Epidemiol. 2009;170:1257–1267. doi: 10.1093/aje/kwp257. [DOI] [PubMed] [Google Scholar]
- 13.Dixon LB, Subar AF, Peters U, Weissfeld JL, Bresalier RS, Risch A, Schatzkin A, Hayes RB. Adherence to the USDA Food Guide, DASH Eating Plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr. 2007;137:2443–2450. doi: 10.1093/jn/137.11.2443. [DOI] [PubMed] [Google Scholar]
- 14.Facchini U, Camnasio M, Cantaboni A, Decarli A, La Vecchia C. Geographical variation of cancer mortality in Italy. Int J Epidemiol. 1985;14:538–548. doi: 10.1093/ije/14.4.538. [DOI] [PubMed] [Google Scholar]
- 15.Fortes C, Forastiere F, Farchi S, Mallone S, Trequattrinni T, Anatra F, Schmid G, Perucci CA. The protective effect of the Mediterranean diet on lung cancer. Nutr Cancer. 2003;46:30–37. doi: 10.1207/S15327914NC4601_04. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez-Chapado M, Olmedilla G, Cabeza M, Donat E, Ruiz A. Prevalence of prostate cancer and prostatic intraepithelial neoplasia in Caucasian Mediterranean males: an autopsy study. Prostate. 2003;54:238–247. doi: 10.1002/pros.10177. [DOI] [PubMed] [Google Scholar]
- 17.Brieskorn CH, Fuchs A, Brendenberg JB, McChesney JD, Wenkert E. The Structure of Carnosol. J Org Chem. 1964;29:2293–2298. [Google Scholar]
- 18.Chang CH, Chyau CC, Hsieh CL, Wu YY, Ker YB, Tsen HY, Peng RY. Relevance of phenolic diterpene constituents to antioxidant activity of supercritical CO(2) extract from the leaves of rosemary. Nat Prod Res. 2008;22:76–90. doi: 10.1080/14786410701591754. [DOI] [PubMed] [Google Scholar]
- 19.Aruoma OI, Halliwell B, Aeschbach R, Loligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 20.Gajhede M, Anthoni U, Nielsen HP, Pederson EJ, Christophersen C. Carnosol. Crystal structure, absolute configuration, and spectroscopic properties of a diterpene. Journal of Chemical Crystallography. 1990;20:165–171. [Google Scholar]
- 21.Munne-Bosch S, Schwarz K, Alegre L. Response of abietane diterpenes to stress in Rosmarinus officinalis L.: new insights into the function of diterpenes in plants. Free Radic Res. 1999;31 Suppl:S107–S112. doi: 10.1080/10715769900301391. [DOI] [PubMed] [Google Scholar]
- 22.Tada M, Ohkanda T, Kurabe J. Syntheses of carnosic acid and carnosol, anti-oxidants in Rosemary, from pisiferic acid, the major constituent of Sawara. Chem Pharm Bull (Tokyo) 2010;58:27–29. doi: 10.1248/cpb.58.27. [DOI] [PubMed] [Google Scholar]
- 23.Huang MT, Ho CT, Wang ZY, Ferraro T, Lou YR, Stauber K, Ma W, Georgiadis C, Laskin JD, Conney AH. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54:701–708. [PubMed] [Google Scholar]
- 24.Schwarz K, Ternes W. Antioxidative constituents of Rosmarinus officinalis and Salvia officinalis. II. Isolation of carnosic acid and formation of other phenolic diterpenes. Z Lebensm Unters Forsch. 1992;195:99–103. doi: 10.1007/BF01201766. [DOI] [PubMed] [Google Scholar]
- 25.Yesil-Celiktas O, Nartop P, Gurel A, Bedir E, Vardar-Sukan F. Determination of phenolic content and antioxidant activity of extracts obtained from Rosmarinus officinalis' calli. J Plant Physiol. 2007;164:1536–1542. doi: 10.1016/j.jplph.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J Agric Food Chem. 2007;55:7879–7885. doi: 10.1021/jf0715323. [DOI] [PubMed] [Google Scholar]
- 27.Matsingou TC, Petrakis N, Kapsokefalou M, Salifoglou A. Antioxidant activity of organic extracts from aqueous infusions of sage. J Agric Food Chem. 2003;51:6696–6701. doi: 10.1021/jf034516o. [DOI] [PubMed] [Google Scholar]
- 28.Moreno S, Scheyer T, Romano CS, Vojnov AA. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res. 2006;40:223–231. doi: 10.1080/10715760500473834. [DOI] [PubMed] [Google Scholar]
- 29.Roberts RA, Smith RA, Safe S, Szabo C, Tjalkens RB, Robertson FM. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology. 2010;276:85–94. doi: 10.1016/j.tox.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng HH, Tu PF, Zhou K, Wang H, Wang BH, Lu JF. Antioxidant properties of phenolic diterpenes from Rosmarinus officinalis. Acta Pharmacol Sin. 2001;22:1094–1098. [PubMed] [Google Scholar]
- 31.Masuda T, Kirikihira T, Takeda Y, S Y. Thermal recovery of antioxidant activity from carnosol quinone, the main antioxidation product of carnosol. J Sci Food Agric. 2004;84:1421–1427. [Google Scholar]
- 32.Masuda T, Kirikihira T, Takeda Y. Recovery of antioxidant activity from carnosol quinone: antioxidants obtained from a water-promoted conversion of carnosol quinone. J Agric Food Chem. 2005;53:6831–6834. doi: 10.1021/jf050685s. [DOI] [PubMed] [Google Scholar]
- 33.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 34.Singletary KW. Rosemary extract and carnosol stimulate rat liver glutathione-S-transferase and quinone reductase activities. Cancer Lett. 1996;100:139–144. doi: 10.1016/0304-3835(95)04082-x. [DOI] [PubMed] [Google Scholar]
- 35.Lo AH, Liang YC, Lin-Shiau SY, Ho CT, Lin JK. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappaB in mouse macrophages. Carcinogenesis. 2002;23:983–991. doi: 10.1093/carcin/23.6.983. [DOI] [PubMed] [Google Scholar]
- 36.Subbaramaiah K, Cole PA, Dannenberg AJ. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Res. 2002;62:2522–2530. [PubMed] [Google Scholar]
- 37.Johnson JJ, Syed DN, Heren CR, Suh Y, Adhami VM, Mukhtar H. Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5'-AMP-activated protein kinase (AMPK) pathway. Pharm Res. 2008;25:2125–2134. doi: 10.1007/s11095-008-9552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan N, Afaq F, Mukhtar H. Apoptosis by dietary factors: the suicide solution for delaying cancer growth. Carcinogenesis. 2007;28:233–239. doi: 10.1093/carcin/bgl243. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson JM, Hayes S, Thompson D, Whitney P, Bi K. Compound profiling using a panel of steroid hormone receptor cell-based assays. J Biomol Screen. 2008;13:755–765. doi: 10.1177/1087057108322155. [DOI] [PubMed] [Google Scholar]
- 40.Kawashima H, Tanaka T, Cheng JS, Sugita S, Ezaki K, Kurisu T, Nakatani T. Effect of anti-estrogens on the androgen receptor activity and cell proliferation in prostate cancer cells. Urol Res. 2004;32:406–410. doi: 10.1007/s00240-004-0424-8. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148:2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- 42.Steinsapir J, Mora G, Muldoon TG. Effects of steroidal and non-steroidal antiandrogens on the androgen binding properties of the rat ventral prostate androgen receptor. Biochim Biophys Acta. 1991;1094:103–112. doi: 10.1016/0167-4889(91)90031-r. [DOI] [PubMed] [Google Scholar]
- 43.Hussein AA, Meyer JJ, Jimeno ML, Rodriguez B. Bioactive diterpenes from Orthosiphon labiatus and Salvia africana-lutea. J Nat Prod. 2007;70:293–295. doi: 10.1021/np0680376. [DOI] [PubMed] [Google Scholar]
- 44.Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104:43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- 45.Singletary KW, Rokusek JT. Tissue-specific enhancement of xenobiotic detoxification enzymes in mice by dietary rosemary extract. Plant Foods Hum Nutr. 1997;50:47–53. doi: 10.1007/BF02436042. [DOI] [PubMed] [Google Scholar]
- 46.Zhu BT, Loder DP, Cai MX, Ho CT, Huang MT, Conney AH. Dietary administration of an extract from rosemary leaves enhances the liver microsomal metabolism of endogenous estrogens and decreases their uterotropic action in CD-1 mice. Carcinogenesis. 1998;19:1821–1827. doi: 10.1093/carcin/19.10.1821. [DOI] [PubMed] [Google Scholar]
- 47.Huang SC, Ho CT, Lin-Shiau SY, Lin JK. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-kappa B and c-Jun. Biochem Pharmacol. 2005;69:221–232. doi: 10.1016/j.bcp.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Dorrie J, Sapala K, Zunino SJ. Carnosol-induced apoptosis and downregulation of Bcl-2 in B-lineage leukemia cells. Cancer Lett. 2001;170:33–39. doi: 10.1016/s0304-3835(01)00549-3. [DOI] [PubMed] [Google Scholar]
- 49.Zunino SJ, Storms DH. Carnosol delays chemotherapy-induced DNA fragmentation and morphological changes associated with apoptosis in leukemic cells. Nutr Cancer. 2009;61:94–102. doi: 10.1080/01635580802357360. [DOI] [PubMed] [Google Scholar]
- 50.Moran AE, Carothers AM, Weyant MJ, Redston M, Bertagnolli MM. Carnosol inhibits betacatenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Res. 2005;65:1097–1104. [PubMed] [Google Scholar]
- 51.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 52.Minnunni M, Wolleb U, Mueller O, Pfeifer A, Aeschbacher HU. Natural antioxidants as inhibitors of oxygen species induced mutagenicity. Mutat Res. 1992;269:193–200. doi: 10.1016/0027-5107(92)90200-l. [DOI] [PubMed] [Google Scholar]
- 53.Fahim FA, Esmat AY, Fadel HM, Hassan KF. Allied studies on the effect of Rosmarinus officinalis L. on experimental hepatotoxicity and mutagenesis. Int J Food Sci Nutr. 1999;50:413–427. doi: 10.1080/096374899100987. [DOI] [PubMed] [Google Scholar]
- 54.Sotelo-Felix JI, Martinez-Fong D, Muriel De la Torre P. Protective effect of carnosol on CCl(4)-induced acute liver damage in rats. Eur J Gastroenterol Hepatol. 2002;14:1001–1006. doi: 10.1097/00042737-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Sotelo-Felix JI, Martinez-Fong D, Muriel P, Santillan RL, Castillo D, Yahuaca P. Evaluation of the effectiveness of Rosmarinus officinalis (Lamiaceae) in the alleviation of carbon tetrachloride-induced acute hepatotoxicity in the rat. J Ethnopharmacol. 2002;81:145–154. doi: 10.1016/s0378-8741(02)00090-9. [DOI] [PubMed] [Google Scholar]
- 56.Offord EA, Mace K, Ruffieux C, Malnoe A, Pfeifer AM. Rosemary components inhibit benzo[a]pyrene-induced genotoxicity in human bronchial cells. Carcinogenesis. 1995;16:2057–2062. doi: 10.1093/carcin/16.9.2057. [DOI] [PubMed] [Google Scholar]
- 57.Fiander H, Schneider H. Dietary ortho phenols that induce glutathione S-transferase and increase the resistance of cells to hydrogen peroxide are potential cancer chemopreventives that act by two mechanisms: the alleviation of oxidative stress and the detoxification of mutagenic xenobiotics. Cancer Lett. 2000;156:117–124. doi: 10.1016/s0304-3835(00)00368-2. [DOI] [PubMed] [Google Scholar]
- 58.Del Bano MJ, Castillo J, Benavente-Garcia O, Lorente J, Martin-Gil R, Acevedo C, Alcaraz M. Radioprotective-antimutagenic effects of rosemary phenolics against chromosomal damage induced in human lymphocytes by gamma-rays. J Agric Food Chem. 2006;54:2064–2068. doi: 10.1021/jf0581574. [DOI] [PubMed] [Google Scholar]
- 59.Aguilar F, Autrup H, Barlow S, Castle L, Crebelli R, Dekrant W, Engel K, Gontard N, Gott D, Grilli S, Gurtler R, Larsen J, Leclerq C, Leblanc J, Malcata FX, Mennes W, Milana MR, Pratt I, Rietjens I, Tobback P, Toldra F. Use of Rosemary Extracts as a food addtive - Scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. The EFSA Journal. 2008;8:1–29. [Google Scholar]
- 60.Yan H, Wang L, Li X, Yu C, Zhang K, Jiang Y, Wu L, Lu W, Tu P. High-performance liquid chromatography method for determination of carnosic acid in rat plasma and its application to pharmacokinetic study. Biomed Chromatogr. 2009;23:776–781. doi: 10.1002/bmc.1184. [DOI] [PubMed] [Google Scholar]