Abstract
The total asymmetric synthesis of (+)- and (−)-clusianone and (+)- and (−)-clusianone methyl enol ether is reported. Asymmetric induction is achieved through the use of ACC alkylation, providing the key intermediates with an er of 99:1. The four synthetic compounds were evaluated for their anti-HIV activity. Both (+)- and (−)-clusianone displayed significant anti-HIV activity.
Keywords: ACC ketone alkylation, Asymmetric synthesis, Polycyclic polyprenylated acylphloroglucinols, Viral infection, Anti-HIV activity
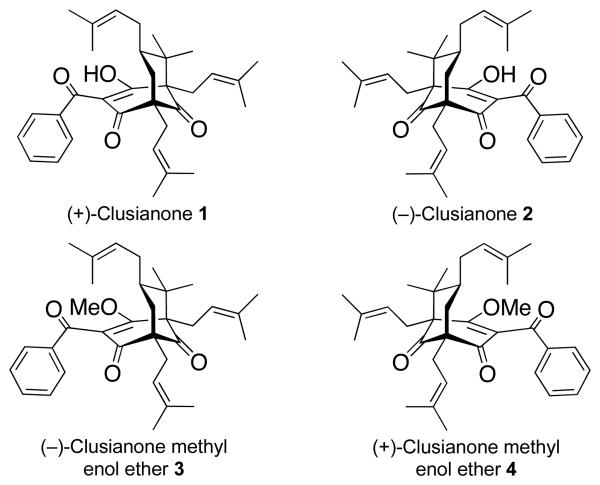
Viral infections are a pervasive form of disease that range from the common cold to much more deadly forms such as AIDS. As with antibiotics,1 current antiviral therapies are becoming less effective due to the development of resistant strains.2 Consequently, there is an urgent need for new anti-viral agents. (+)-Clusianone (1) (Figure 1) is a member of a large class of biologically active natural products termed the polycyclic polyprenylated acylphloroglucinols (PPAPs).3 (+)-Clusianone has been shown to possess antiviral activity against both HIV (EC50 = 0.020 ± 0.003 μM),4 and Epstein-Barr virus (17.4 ± 1.2% of cells were EBV-EA positive in the presence of 32 nmol of 1).5 As such, it is a compelling target for further biological investigation as an antiviral agent. Unfortunately, no asymmetric total synthesis is available, as is necessary for further biological testing and medicinal chemistry efforts.
Figure 1.
The clusianones and their methyl enol ethers.
Not only do PPAPs generally exhibit biological activity, but they also possess intriguing structures.3 Consequently, they have attracted considerable interest from the synthetic community. Despite the impressive advances culminating from the synthetic work carried out to date on the PPAPs, asymmetric total syntheses are rare.6 In the case of clusianone, total syntheses have been reported by Danishefsky,7 Simpkins,8 Porco,9 and Marazano.10 However, only one synthesis of optically active (+)-clusianone has been reported,11 and this was achieved via chiral resolution of a late-stage synthetic intermediate.
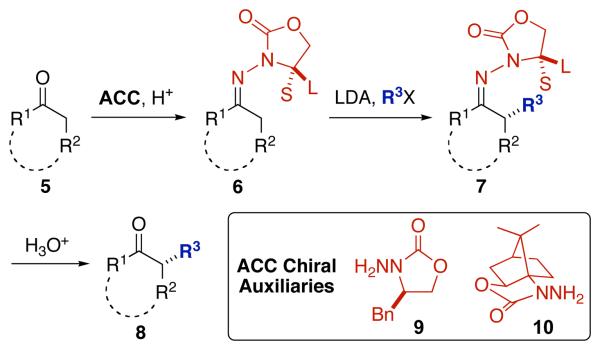
We recently described a new method for the asymmetric α-alkyation of ketones utilizing chiral N-amino cyclic carbamate (ACC) auxiliaries (cf. 9 and 10, Scheme 1).12 ACC auxiliaries react readily with ketones to afford the corresponding hydrazones (5 → 6). These undergo rapid deprotonation to the azaenolates, which can be alkylated on up to a multi-gram scale with excellent stereoselectivity and yield (6 → 7). Moreover, the auxiliaries can be recovered quantitatively and recycled. We realized that this enantio-selective alkylation method could provide the basis for an efficient asymmetric total synthesis of the clusianones. In an earlier communication,13 we described the first asymmetric total synthesis of (+)-clusianone (1) and its methyl enol ether (3). In what follows we describe the extension of this synthetic strategy to the first asymmetric total synthesis of (−)-clusianone (2) and its methyl enol ether (4). Whereas some PPAPs have been isolated in both their (+)- and (−)-forms, clusianone has not, so this constitutes the first report of (−)clusianone. We also report our initial biological investigation of compounds 1–4 with regard to their anti-HIV activity. To our knowledge, this is the first instance in which the two enantiomers of a PPAP have been tested in the same biological assay.
Scheme 1.
Asymmetric ACC α-alkylation of ketones.
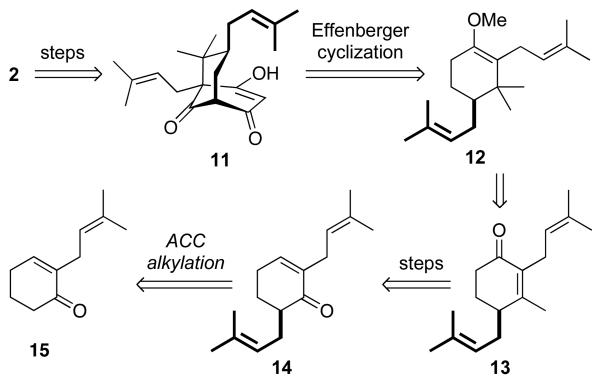
Our general retrosynthetic plan is summarized in Scheme 2 by reference to (−)-clusianone (2), and is analogous to that used previously by us for the synthesis of (+)-Clusianone (1).13 We would engage optically pure enol ether 12 in a diastereoselective Effenberger cyclization14 to generate the advanced bicylic intermediate 11, as done by Simpkins in his synthesis of racemic clusianone.8a This would be elaborated to optically pure 2 using established procedures.8a Conjugate addition and enol ether formation would provide access to 12 from 13. The preparation of 13 would be effected by 1,2-methylation of α,β-unsaturated ketone 14, followed by a Babler-Dauben15 oxidation of the resulting tertiary allylic alcohol. Synthesis of the key optically pure intermediate 14 would be achieved via enantioselective ACC alkylation,12 beginning from known enone 15.
Scheme 2.
Retrosynthetic analysis of 2.
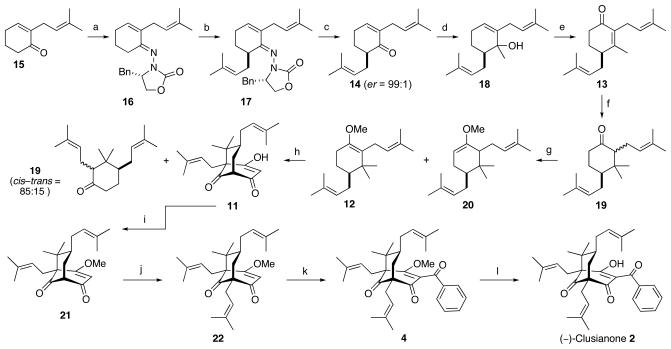
We began our studies by preparing enone 15 using a combination of literature procedures, 10,16,17 which was then subjected to enantioselective ACC alkylation. To begin this process, compound 15 was converted to hydrazone 16 by acid mediated condensation with ACC auxiliary 9.18 Once formed, 16 was subjected to LDA-mediated prenylation, giving 17 in excellent yield, and as a single diastereomer, as judged by 1H and 13C NMR analysis. Auxiliary removal from prenylated hydrazone 17 was achieved hydrolytically by treatment with p-TsOH·H2O in acetone/H2O, producing the key synthetic intermediate 14 in 80% yield. Analysis of synthetic 14 by chiral HPLC revealed an enantiomer ratio of 99:1 indicating that minimal, if any, epimerization occurred during removal of the ACC auxiliary.
With optically active ketone 14 in hand, we initiated the methylation/carbonyl transposition sequence. Treatment of 14 with the Grignard reagent MeMgBr efficiently produced tertiary allylic alcohol 18. This underwent Babler-Dauben oxidation upon exposure to PCC producing enone 13, marking a convergence point with Simpkins' synthesis of racemic clusianone.8b α,β-Unsaturated ketone 13 was, therefore, subjected to conjugate addition using a methyl cuprate to produce 19. The methyl enol ethers 12 and 20 were generated from 19 upon treatement with t-BuOK and Me2SO4, setting the stage for the Effenberger cyclization. Careful acid/base extraction was required following this transformation to separate the desired product (11) from 19, and the latter was able to be recycled to produce more of 12 and 20. Methylation of crude cyclized product 11 gave 21 in 18% yield over the two steps (35% based on recovered 19). Next, 21 was subjected to bridgehead prenylation, which gave 22 in excellent yield. Subsequent addition of the benzoyl group gave rise to (+)-clusianone methyl enol ether (4). Finally, simple hydrolysis was used to afford (−)-clusianone (2).
(+)-Clusianone (1) and the corresponding (−)-clusianone methyl enol ether (3) were prepared from ketone 15, but using the (R)-benzyl-2-oxazolidinone-derived ACC auxiliary.13
To determine the enantiomeric purity of our synthetic material, the above synthetic sequence was carried out in a racemic fashion to the stage of the penultimate compound.13 Thus, enone 15 underwent LDA-mediated prenylation, producing (±)-14. This compound was then subjected to the transformations developed above to give a racemic mixture of 3 and 4. Conditions were established that gave baseline resolution of 3 and 4 by chiral HPLC. Subsequent analysis of synthetic 3 produced via asymmetric ACC alkylation (Scheme 3) revealed an er = 99:1. Similar analysis of synthetic 4 gave an equally high er.13
Scheme 3.
Synthesis of (−)-Clusianone (2). a) 9, p-TsOH·H2O, CH2Cl2, reflux, 95%; b) LDA, THF, −78 °C, then prenyl bromide, to 0 °C, 90%; c) p-TsOH·H2O, acetone, H2O, 80%; d) MeMgBr, Et2O, −78 °C to rt, 88%; e) PCC, 3 Å MS, CH2Cl2, 67%; f) MeMgBr, CuBr·SMe2, TMSCl, HMPA, then 10% HCl, 88%; g) t-BuOK, DMSO, then Me2SO4, 63%; h) malonyl dichloride, Et2O, −20 °C, then KOH, BnEt3NCl, H2O, 35%; i) (MeO)3CH, p-TsOH·H2O, MeOH, 50 °C, 60%; j) LDA, THF, −78 °C, then prenyl bromide, 90%; k) LTMP, THF, −78 °C, then BzCl, 65%; l) LiOH, dioxane, H2O, 90 °C, 79%.
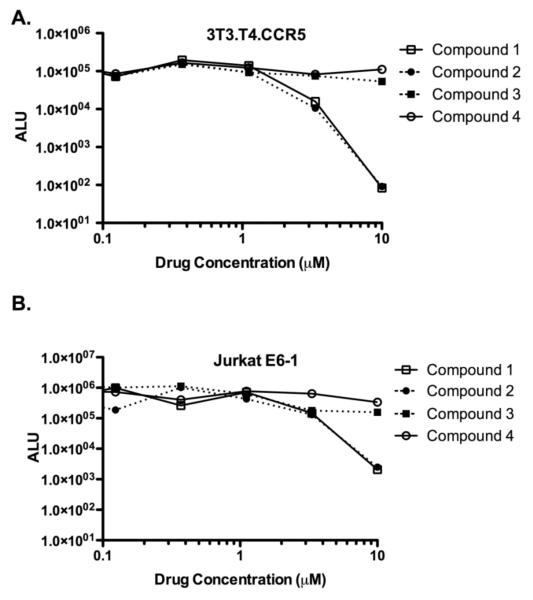
Having completed the asymmetric total synthesis of compounds 1–4, we undertook an investigation into their anti-HIV activity. To do so, we created HIV-1 Env-psuedoviruses and tested the ability of compounds 1–4 to block their infectivity in a mammalian cell culture system. Pseudoviruses were made according to the methods of Montefiori et al.19 Briefly, 293T-cells were co-transfected with an env-deficient HIV-1 genome that contains a firefly luciferase gene inserted into the HIV nef gene (pNLCH5.1)20 and a second plasmid containing an envelope gene (pNL4.3 [CXCR4-tropic] or pJRFL [CCR5-tropic], both obtained from the AIDS Reagent Repository). Target cells for these HIV-1 Env-pseudoviruses were 3T3.T4.CCR5 and Jurkat E6-1 cells, both of which were obtained from the NIAID AIDS Reference Program. 3T3 cells were incubated with a 1:4 dilution of the pJRFL HIV-1 Envpseudoviruses and Jurakat E6-1 cells were incubated with a 1:4 dilution of the pNL4.3 HIV-1 Env-pseudoviruses. When the Env-psuedoviruses successfully infect the target cells, the viral genome becomes integrated into the cellular genome and luciferase is produced. Luciferase activity was measured with the Promega GloMax system according to the manufacturers instructions. To test the ability of compounds 1–4 to inhibit viral infectivity, the compounds were serially diluted 3-fold from 10 μM to 4.6 nM, all at a final concentration of 1% DMSO. All infections were carried out in duplicate; the 3T3 results are representative of three experiments and the Jurkat results are from one experiment. IC50 values were calculated with Graphpad Prism.
Interestingly, despite being enantiomers of one another, (+)- and (−)-clusianone (1 and 2) exhibited very similar IC50 values of 1.53 and 1.13 μM, respectfully, in the 3T3 system.21 However, compounds 3 and 4 showed no inhibition (Figure 2A). Similar results were obtained in the Jurkat cells, although the IC50 of compounds 1 and 2 were lower than the IC50 observed in the 3T3 cells (Figure 2B). The IC50 values of the clusianone compounds are higher than the reported IC50 values for AZT (~120 nM)22 and nevirapine (~40 nM).23 As mentioned above, some PPAPs have been isolated in both their (+)- and (−)- forms. These include the enantiomeric pairs hyperibone G24 and propolone D,25 hyperibone A24 and garcinielliptone I,26 guttiferone E27–30 and carcinol,28 and isoxanthochymol27,28 and isogarcinol.31 However, this appears to be the first instance in which an enantiomeric pair of PPAPs has been evaluated in the same biological assay.
Figure 2.
Compounds 1 and 2 block HIV-1 infection. HIV-1 Env-pseudoviruses were incubated with 3×104 3T3.T4.CCR5 (Figure 2A) or Jurkat E6-1 (Figure 2B) cells at varying concentrations of compounds 1–4. Two days post-infection luciferase activity was measured. Cells with no virus had luciferase values less than 100 arbitrary light units (ALU). Cells with no drug and 1% DMSO had ALU values similar to the values obtained at the 0.005 μM concentration of the drug.
In conclusion, (+)- and (−)-clusianone and (+)- and (−)-clusianone methyl enol ether have been synthesized in a highly enantioselective (er = 99:1) fashion using asymmetric ACC alkylation. Biological analysis of these compounds was conducted to determine their potential anti-HIV activity. While the degree of antiviral activity depended on cell type, both (+)- and (−)-clusianone (1 and 2, respectively) displayed significant anti-HIV activity. Despite their enantiomer relationship, compounds 1 and 2 exhibited essentially the same inhibitory activity. The mechanism of this activity is currently unknown.
Supplementary Material
Scheme 4.
Preparation of a racemic mixture of 3 and 4.
Acknowledgements
M.R.G. is grateful for support from the Pharmacological Sciences Training Program – Duke University. This work was supported by Duke University, NCBC (2008-IDG-1010), the National Institute of Health (1R01AI084110-01 and 5U19AI067854 to J.J.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Experimental procedures and analytical data for all new compounds is available.
References and notes
- 1.(a) Walsh C. Nature. 2006;406:775. doi: 10.1038/35021219. [DOI] [PubMed] [Google Scholar]; (b) Fisher JF, Meroueh SO, Mobashery S. Chem. Rev. 2005;105:395. doi: 10.1021/cr030102i. [DOI] [PubMed] [Google Scholar]
- 2.Strasfeld L, Chou S. Infect. Dis. Clin. N. Am. 2010;24:809. doi: 10.1016/j.idc.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Ciochina R, Grossman RB. Chem. Rev. 2006;106:3963. doi: 10.1021/cr0500582. [DOI] [PubMed] [Google Scholar]
- 4.Piccinelli AL, Cuesta-Rubio O, Chica MB, Mahmood N, Pagano B, Pavone M, Barone V, Rastrelli L. Tetrahedron. 2005;61:8206. [Google Scholar]
- 5.Ito C, Itoigawa M, Miyamoto Y, Onoda S, Rao KS, Mukainaka T, Tokuda H, Nishino H, Furukawa H. J. Nat. Prod. 2003;66:206. doi: 10.1021/np020372g. [DOI] [PubMed] [Google Scholar]
- 6.(a) Shimizu Y, Shi SL, Usuda H, Kanai M, Shibasaki M. Angew. Chem. Int. Ed. 2010;49:1103. doi: 10.1002/anie.200906678. [DOI] [PubMed] [Google Scholar]; (b) Rodeschini V, Simpkins NS, Wilson C. J. Org. Chem. 2007;72:4265. doi: 10.1021/jo070225t. [DOI] [PubMed] [Google Scholar]
- 7.Tsukano C, Siegel DR, Danishefsky SJ. Angew. Chem. Int. Ed. 2007;46:8840. doi: 10.1002/anie.200703886. [DOI] [PubMed] [Google Scholar]
- 8.(a) Ahmad NM, Rodeschini V, Simpkins NS, Ward SE, Blake AJ. J. Org. Chem. 2007;72:4803. doi: 10.1021/jo070388h. [DOI] [PubMed] [Google Scholar]; (b) Rodeschini V, Ahmad NM, Simpkins NS. Org. Lett. 2006;8:5283. doi: 10.1021/ol0620592. [DOI] [PubMed] [Google Scholar]
- 9.Qi J, Porco JA. J. Am. Chem. Soc. 2007;129:12682. doi: 10.1021/ja0762339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuhant P, David M, Pouplin T, Delpech B, Marazano C. Org. Lett. 2007;9:287. doi: 10.1021/ol062736s. [DOI] [PubMed] [Google Scholar]
- 11.Rodeschini V, Simpkins NS, Wilson C. J. Org. Chem. 2007;72:4265. doi: 10.1021/jo070225t. [DOI] [PubMed] [Google Scholar]
- 12.(a) Lim D, Coltart DM. Angew. Chem. Int. Ed. 2008;47:5207. doi: 10.1002/anie.200800848. [DOI] [PubMed] [Google Scholar]; (b) Krenske EH, Houk KN, Lim D, Wengryniuk SE, Coltart DM. J. Org. Chem. 2010;75:8578. doi: 10.1021/jo1019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garnsey MR, Lim D, Yost JM, Coltart DM. Org. Lett. 2010;12:5234. doi: 10.1021/ol1022728. [DOI] [PubMed] [Google Scholar]
- 14.Schonwalder KH, Kollat P, Stezowski JJ, Effenberger F. Chem. Ber. Recl. 1984;117:3280. [Google Scholar]
- 15.(a) Dauben WG, Michno DM. J. Org. Chem. 1977;42:682. [Google Scholar]; (b) Babler JH, Coghlan MJ. Synth. Commun. 1976;6:469. [Google Scholar]
- 16.Clark RD, Heathcock CH. J. Org. Chem. 1976;41:636. doi: 10.1021/jo00870a023. [DOI] [PubMed] [Google Scholar]
- 17.See the Supporting Information for details.
- 18.Auxiliary 9 is prepared in one step from commercially available (S)-benzyl-2-oxazolidinone. See the Supporting Information for details.
- 19.http://www.hiv.lanl.gov/content/nab-reference-strains/html/Protocol-Preparation-and-Titration-of-HIV-1-Env-Pseuodoviruses-July-2008.pdf
- 20.Foulkes JE, Prabu-Jeyabalan M, Cooper D, Henderson GJ, Harris J, Swanstrom R, Schiffer CA. J. Virol. 2006;80:6906. doi: 10.1128/JVI.01900-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(+)-Clusianone obtained from natural sources was tested in C8166 human T lymphoblastoid cells infected with HIV-1MN and was found to have EC50 = 0.020 ± 0.003 μM. See reference 4.
- 22.Grob PM, Wu JC, Cohen KA, Ingraham RH, Shih C-K, Hargrave KD, Mctague TL, Merluzzi VJ. AIDS Res. Hum. Retroviruses. 1992;8:145. doi: 10.1089/aid.1992.8.145. [DOI] [PubMed] [Google Scholar]
- 23.Erice A, Balfour HH, Jr, Myers DE, Leske VL, Sannerud KJ, Kuebelbeck V, Irvin JD, Uckun FM. Antimicrob. Agents Chemother. 1993;37:835. doi: 10.1128/aac.37.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuhisa M, Shikishima Y, Takaishi Y, Honda G, Ito M, Takeda Y, Shibata H, Higuti T, Kodzhimatov OK, Ashurmetov O. J. Nat. Prod. 2002;65:290. doi: 10.1021/np010310a. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez IM, Fernandez MC, Cuesta-Rubio O, Piccinelli AL, Rastrelli L. J. Nat. Prod. 2005;68:931. doi: 10.1021/np0495884. [DOI] [PubMed] [Google Scholar]
- 26.Weng JR, Lin CN, Tsao LT, Wang AP. Chem. Eur. J. 2003;9:5520. doi: 10.1002/chem.200305209. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson KR, Blunt JW, Munro MHG, Fuller RW, Mckee TC, Cardellina JH, Mcmahon JB, Cragg GM, Boyd MR. Tetrahedron. 1992;48:10093. [Google Scholar]
- 28.Baggett S, Protiva P, Mazzola EP, Yang H, Ressler ET, Basile MJ, Weinstein IB, Kennelly EJ. J. Nat. Prod. 2005;68:354. doi: 10.1021/np0497595. [DOI] [PubMed] [Google Scholar]
- 29.Sahu A, Das B, Chatterjee A. Phytochemistry. 1989;28:1233. [Google Scholar]
- 30.Cuesta Rubio O, Cuellar Cuellar A, Rojas N, Velez Castro H, Rastreli L, Aquino R. J. Nat. Prod. 1999;62:1013. doi: 10.1021/np980339n. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurthy N, Lewis YS, Ravindranath B. Tetrahedron Lett. 1981;22:793. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.