Abstract
Research has indicated that people with schizophrenia have deficits in reward representation and goal-directed behavior, which may be related to the maintenance of emotional experiences. Using a laboratory-based study, we investigated whether people with schizophrenia were able to maintain an emotional experience when given explicit instructions to do so. Twenty-eight people with schizophrenia and nineteen people without completed a behavioral task judging their emotional experience of pictures held over a 3 second delay. This emotion maintenance task was compared to a subsequent in-the-moment emotion experience rating of each picture. In addition, all participants completed an analogous brightness experience maintenance and rating task, and patients completed a standardized visual working memory task. Participants with schizophrenia showed normal in-the-moment emotion experience of the emotion pictures; however, they showed decreased performance on emotion maintenance (for both positive and negative emotion) compared to participants without schizophrenia, even after controlling for brightness maintenance. The emotion maintenance deficit was not associated with visual brightness performance nor with performance on the visual working memory task; however, negative emotion maintenance was associated with an interview-based rating of motivation. These findings suggest that some aspects of impaired emotion maintenance in schizophrenia may be related to deficits in motivated behavior.
Keywords: Affective maintenance, motivation, working memory, reward representation
1. Introduction
People with schizophrenia report nearly identical positive and negative experiences to emotion-eliciting material as people without schizophrenia (Kring and Moran, 2008; see Cohen and Minor, 2010 for a meta-analysis and a detailed review), even though they show deficits in the experience of pleasure (i.e., anhedonia) and motivation (e.g., Chapman et al., 1976; Berenbaum et al., 1990; Fenton and McGlashan, 1991; Herbener and Harrow, 2002; Barch, 2005; Gard et al., 2009). Recent research has begun to investigate this seeming paradox by examining the temporal components of emotion experience and how this relates to motivational engagement (e.g., Davidson, 1998; Heerey and Gold, 2007). Further, researchers are beginning to more explicitly define the parameters of motivational problems in schizophrenia (Barch and Dowd, 2010; Medalia and Brekke, 2010)
In an ecological momentary assessment study and a self-report study, we showed that people with schizophrenia may have a deficit in the anticipation of pleasurable experiences, but not in the in-the-moment or consummatory experiences of pleasure (Gard et al., 2007). This distinction clarifies that deficits in the experience of pleasure (anhedonia) may be related to the process of motivation, or ‘looking forward’ to rewarding stimuli, as opposed to a deficit of enjoyment per se (but see Tremeau et al., 2010). Consistent with this notion, Juckel and colleagues (2006) found in an fMRI study that as people with schizophrenia anticipated reward stimuli, they did not recruit brain regions associated with reward anticipation.
In addition to movement towards desired stimuli (appetitive or approach), motivation also requires movement away from aversive stimuli (avoidance), and there is evidence of impairment in this area as well. For example, in a behavioral study of motivational engagement, Heerey and Gold (2007) asked people with and without schizophrenia to view and rate the emotional experience of affective pictures. Participants were then asked to press a button after the images were removed to indicate a preference to see the images again or to avoid them. In spite of their similar self-report ratings, people with schizophrenia made fewer button presses to both positive and negative images than controls, although the overall number of presses did not differ between groups. These findings also suggest that people with schizophrenia may have difficulty translating their emotional experience to appropriate goal-directed behavior – either towards desired stimuli or away from aversive stimuli.
Taken together, the evidence indicates that people with schizophrenia may have difficulty maintaining an emotional experience over time in order to guide behavior. Two recent studies highlight this possibility, also with both positive and negative images. Using the affective startle modulation, we asked people with and without schizophrenia to view emotion-eliciting pictures, wait during a brief delay, and then rate their emotion experiences of the pictures; startle probes were presented either during the picture presentation or during the delay period (Kring et al., in press). Participants with schizophrenia reported similar emotional experiences to the pictures as people without schizophrenia; both groups also showed a common startle response during picture viewing: heightened startle while viewing negative pictures and a diminished startle while viewing positive pictures. However, only people without schizophrenia showed this same pattern seconds after the images were removed. In other words, people with schizophrenia appeared to have difficulty maintaining the emotional response generated by the pictures during the delay period. In a similar design using fMRI, Ursu and colleagues (Ursu et al., in press) showed nearly identical brain activation patterns during the viewing of emotion-eliciting pictures for people with and without schizophrenia (amygdala as well as dorsolateral, orbitofrontal and ventromedial prefrontal cortex), but only those without schizophrenia maintained this activation once the images were removed. Because neither of these studies specifically requested that participants maintain their emotional state, it is unclear whether the lowered performance in the individuals with schizophrenia was due to inattention, distraction, or whether they were unaware that they should be maintaining their emotional state.
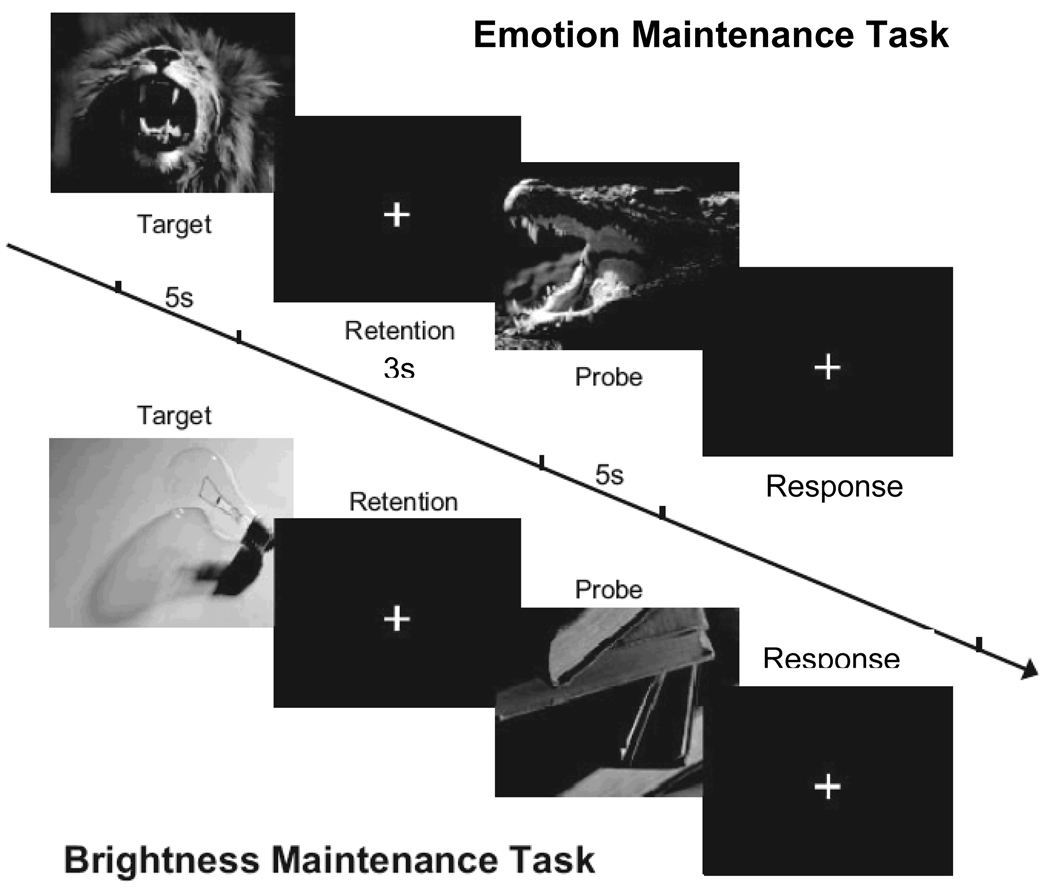
We sought to extend this research by using a behavioral task that measures an individual’s ability to willfully and specifically maintain an emotional experience over a short delay of 3 seconds. This task, designed by Mikels and colleagues (2005; 2008), asks participants to view an emotion-eliciting picture, to hold on to that emotional experience over a short delay, and then to compare that experience to their experience of a subsequent emotion-eliciting picture. Participants later rate their in-the-moment emotion experience to all images (See Figures 1a and 1b). This maintained comparison and the subsequent in-the-moment experience rating allows for an evaluation of emotion maintenance accuracy (i.e., if a participant rates their experience of picture B as more emotionally intense than their experience of picture A after the delay, but during in-the-moment ratings finds their experience of picture A to be more emotionally intense than their experience of picture B, this reflects an inaccuracy of emotional maintenance). Participants also complete an analogous task maintaining and comparing their experience of visual brightness intensity of neutral images, to control for visual working memory abilities. Mikels and colleagues have shown that emotion maintenance and visual brightness maintenance are separable processes. Specifically, in one study, participants did not show impairment in emotion maintenance when concurrently completing verbal and visual working memory tasks during the maintenance period, but did show impairment when given an additional emotion-related task during the emotion maintenance period (Mikels et al., 2008).
Figure 1.
Figure 1a. Emotion maintenance and brightness maintenance task. For the emotion maintenance task a positive or negative target picture was viewed for 5s and the experience of emotional intensity was maintained over a 3s delay. A separate probe picture of the same valence image was viewed for 5s. After the images, participants indicated whether the probe was experienced as more or less emotionally intense than the target. Participants completed an analogous brightness maintenance task which was identical except neutral images were viewed and the experience of brightness intensity was maintained. Figure originally printed in Mikels et al., 2008 Emotion 8(2), 256–266, APA.
Figure 1b. Emotion and brightness rating task. After both maintenance tasks were completed participants rated their in-the-moment experience of emotion to emotion images on emotion intensity, and their in-the-moment brightness experience to the brightness (neutral) images, all using a visual analog scale. Using the computer mouse, participants clicked along the continuum for their experience rating.
Using this experimental task, our hypotheses are as follows: 1) participants with schizophrenia would show a deficit in brightness maintenance relative those without schizophrenia (due to visual working memory deficits typically seen in schizophrenia (e.g., Tek et al., 2002)); 2) people with schizophrenia will show a deficit in both positive and negative emotion maintenance relative to people without schizophrenia, even when deficits in brightness maintenance are controlled for; 3) for people with schizophrenia positive and negative emotion maintenance ability will be related to clinician ratings of motivation (i.e., PANSS avolition item and QLS motivation item).
2. Method
2.1. Participants
Twenty-eight clinically stable outpatients with schizophrenia (N = 18) or schizoaffective disorder (N = 10) and 19 healthy control subjects participated in the study. All schizophrenia participants were recruited from a larger ongoing study of cognitive training in schizophrenia, and were run in the present study either prior to cognitive training (N = 5) or were recruited after participating in the control condition (N = 23). The average time since the completion of the control task condition for these participants was 1.53 years. People with schizophrenia were recruited from psychiatric outpatient services and community mental health clinics in the San Francisco Bay area. Diagnoses were confirmed using the DSM-IV-Clinician Version (SCID: First et al., 1997). People with schizophrenia were also given the Positive and Negative Syndrome Scale-Extended interview (PANSS: Kay and Sevy, 1990; Poole et al., 2000) and the Quality of Life Scale (QLS: Heinrichs et al., 1984); behavioral measures of motivation used for this study were the PANSS “avolition” rating (defined as “disturbance in the willful initiation, sustenance and control of one’s thoughts, behavior, movements and speech”) and the QLS “motivation” item (defined as “the extent to which the person is able to initiate or sustain goal-directed activity due to adequate drive”). Exclusion criteria included a history of head trauma/loss of consciousness, substance abuse in the last six months, neurological disorders, and non-fluency in English, significant changes in medication or dosage in the previous 30 days, or hospitalization in the previous three months. Healthy comparison subjects were recruited through community postings and bulletin boards in the San Francisco Bay area. There were no differences in any demographic measures between groups (see Table 1).
Table 1.
Demographic characteristics for people with and without schizophrenia.
| Schizophrenia Patients (N = 28) |
Healthy Controls (N = 19) |
|
|---|---|---|
| Male – N | 18 | 13 |
| Age (SD) | 44.21 (11.53) | 45.16 (10.79) |
| Education – years (SD) | 13.50 (1.50) | 14.18 (1.48) |
| Mother education – years (SD) | 13.50 (3.07) | 13.72 (2.76) |
| Father education – years (SD) | 14.13 (3.09) | 15.45 (2.70) |
| Employment – N | ||
| Full time employed – N | 0 | 2 |
| Part time employed – N | 8 | 7 |
| Unemployed – N | 17 | 8 |
| Student – N | 3 | 2 |
| Ethnicity - N | ||
| Caucasian | 15 | 13 |
| African-American | 4 | 2 |
| Asian-American | 4 | 2 |
| Latino | 3 | 0 |
| Other | 2 | 2 |
| Diagnosis – N | ||
| Schizophrenia | 18 | - |
| Schizoaffective | 10 | - |
| Chlorpromazine Eq. (SD) | 803.91 (957.99) | - |
| PANSS1-Total, mean rating (SD) | 2.30 (0.56) | - |
| PANSS1-Avolition, mean rating (SD) | 2.48 (1.34) | - |
| QLS2 - Motivation, mean rating (SD) | 3.39 (1.57) | - |
Positive and Negative Syndrome Scale
Quality of Life Scale
2.2. Procedures
After having all experimental procedures explained to them, participants provided written informed consent for the emotion maintenance and brightness tasks. Nominal payment was given for participation in this study. All participants completed the emotion and brightness maintenance comparison tasks as described in Mikels (2005); each maintenance task was counterbalanced by participant so that half of the participants started with the emotion maintenance task and half started with brightness maintenance task. The in-the-moment rating tasks were completed after both maintenance tasks were finished and these were also counterbalanced. Forty emotionally evocative images pairs (20 positive pairs, 20 negative pairs) and 40 neutral image pairs (80 neutral images) were chosen from the International Affective Picture System (IAPS: Lang et al., 2005). Emotion-eliciting pictures depicted a wide range of content (e.g., animal and human attack, pollution, food, action scenes) and neutral images depicted household items, common daily objects, and people. All image pairs were similar in valence and arousal ratings based on normed ratings (Lang et al., 2005)1. The means (and standard deviations) of valence and arousal for all pictures used in the study (from the published norms) are as follows: valence: positive M = 7.14 (0.66); negative M = 3.45 (0.97); neutral = M = 5.31 (0.69); arousal: positive M = 5.20 (1.04); negative M = 5.40 (0.89); neutral = M = 3.45 (0.88). Images were presented on a Compaq laptop 12-inch monitor approximately 30-inches from the participant. Stimuli presentation was administered through E-Prime Version 2.0. All images were presented in a random order for each participant (within the emotion or brightness conditions). The neuropsychological battery and diagnostic interview were completed on a separate day.
2.3. Measures
2.3.1. Emotion intensity maintenance
Each trial began with a positive or negative picture (target) presented for 5 seconds, followed by a fixation maintenance period of 3 seconds. Participants were asked to view the target and hold in mind the ‘emotional intensity’ experience of the picture throughout the maintenance period. A second picture of the same valence (probe) was presented for 5 seconds. Again, participants were asked to experience the emotional intensity of the probe picture. Following the probe picture, a response window was available in which participants were asked to choose whether the experience of the second picture was higher or lower in emotional intensity than the experience of first picture, and to respond by pressing the corresponding labeled keyboard key (H for higher, L for lower). The order of the images was presented randomly such that each image could either serve as the target or the probe (i.e., no participant saw the same images as targets or probes). There was no limit on the amount of time for a response to occur. Immediately following the response, the next trial began (See Figure 1a).
2.3.2. Brightness intensity maintenance
All participants completed an analogous task with neutral pictures. In this task, participants viewed a neutral target picture for 5 seconds and held in mind the experience of the brightness intensity of the picture during a 3 second delay, followed by a neutral probe picture, presented for 5 seconds. Similar to the emotion intensity maintenance task participants chose whether the second picture was experienced as more or less intense in brightness than the first picture.
2.3.3. Emotion intensity rating
In a separate task, each emotion-eliciting picture used in the emotion intensity maintenance task was presented in random order to participants. The task was to simply indicate the emotional intensity experienced for each picture. Under each picture there was a continuous visual analog scale that ranged from not intense to extremely intense. Using the computer mouse participants indicated on the continuum how intense their experience was (See Figure 1b). The computer assigned this mouse click a numerical value based on where the participant clicked on the continuum. For each trial, participants had as much time as needed to respond.
2.3.4. Brightness intensity rating
Participants completed an analogous rating task with the neutral pictures viewed in the brightness maintenance task, rating the experience of brightness intensity of each picture on a visual analog continuum, ranging from not intense to extremely intense, using the computer mouse.
2.3.5. Neuropsychological measure of visual working memory
The WMS-R Visual Memory Span was used to compare subjects’ performance on brightness maintenance to a standard test of visual working memory.
2.4. Data Analytic Plan
A maintenance percentage accuracy score was computed for both positive and negative emotion tasks and for the brightness task for all participants. This was calculated for each participant by comparing the response in the maintenance task (H or L) to the response in the rating task (from the continuous rating). An ‘inaccurate maintenance’ on a particular trial occurred if the in-the-moment experience ratings differed from the maintenance comparisons. For example, if the experience of image B was rated higher than the experience of image A, but the continuous in-the-moment experience of image A was higher than B, this was considered an inaccurate maintenance. Note that the computer gave a numerical value to the continuous rating. In the event that a participant rated their in-the-moment experience of the two images identically (i.e., clicked on the exact spot on the continuous rating scale), these trials were not included in the analysis. Percentage accuracy for the emotion task was completed separately for positive images and negative images. We also investigated participants’ comparisons to normed experience ratings (e.g., Mikels et al., 2008); and since these findings were nearly identical, we have limited our presentation to accuracy levels only (and not concordance rates) for the sake of brevity.
In order to verify that participants with schizophrenia had the same in-the-moment emotional and brightness experiences to the stimuli as did the comparison subjects, we performed an ANOVA with participant group as the between-subjects factor for ratings of the in-the-moment experience of emotional intensity and of brightness intensity. In order to test the hypothesis that the participants with schizophrenia would show a deficit in emotion maintenance independent of their performance on brightness maintenance, we first performed an ANOVA on brightness maintenance accuracy. Once the expected deficit on brightness maintenance was found (given the known deficits in visual working memory (Tek et al., 2002)), we performed an ANCOVA on positive emotion maintenance accuracy and on negative emotion maintenance accuracy, with group as the between-subjects factor controlling for accuracy scores on the brightness maintenance task. In order to verify that brightness maintenance assessed (non-emotional) visual working memory, we performed a Pearson’s bivariate correlation (one-tailed) between brightness maintenance and WMS-R Spatial Memory in the schizophrenia participant group. Finally, to determine whether the deficit in emotion maintenance showed an association with behavioral measures of motivation in the schizophrenia participants, we performed a Pearson’s bivariate correlation (one-tailed due to a priori hypotheses) between positive and negative emotion maintenance and PANSS “avolition,” and between positive and negative emotion maintenance and QLS “motivation,” controlling for brightness maintenance. As emotion maintenance could potentially differ by gender we included this factor in all analyses. However, no gender differences were found and so this analysis was excluded here for the sake of brevity. Effect sizes are reported as eta squared.
3. Results
3.1. In-the-moment emotion and brightness experience ratings
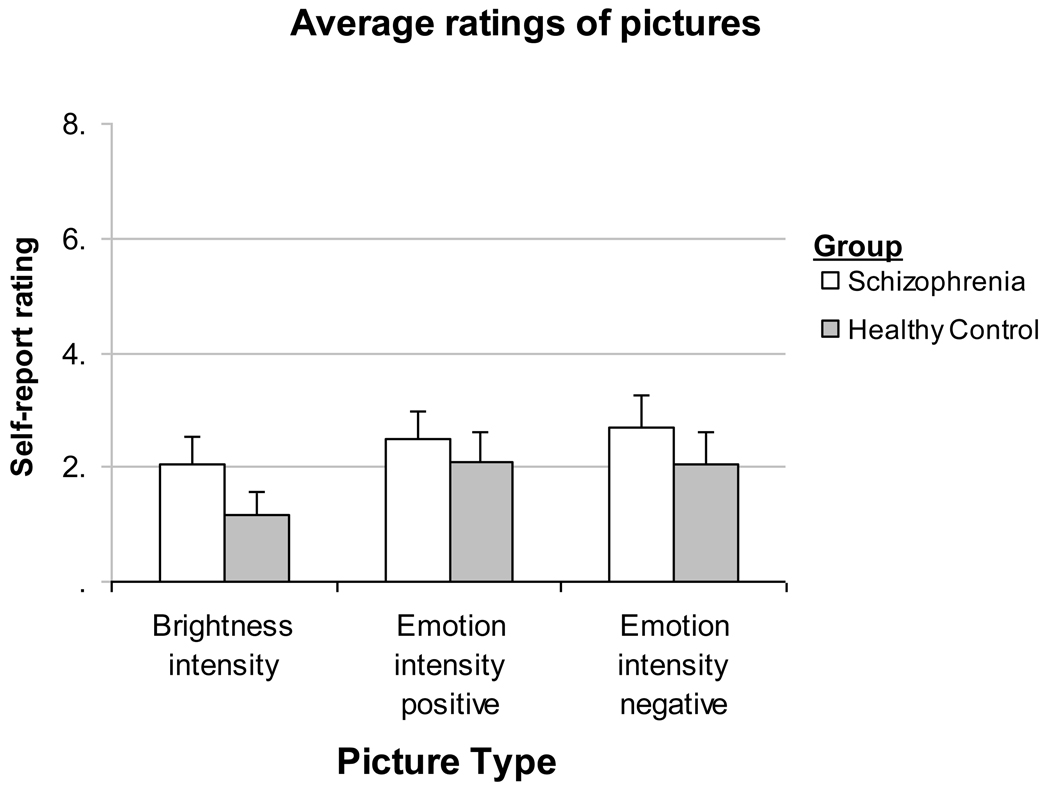
As expected, participants with schizophrenia did not differ from those without schizophrenia in their in-the-moment experience ratings of emotional intensity for positive images F(1, 46) = 0.56, P = 0.46) or negative images F(1, 46) = 2.07, P = 0.16). Participants with schizophrenia rated their experience of the neutral images as more bright, than those without schizophrenia F(1, 46) = 5.67, P < 0.05, η2 = .11) (See Figure 2).
Figure 2.
People with (n=28) and without (n=19) schizophrenia report similar in-the-moment experiences of emotional intensity of positive pictures and negative pictures. People with schizophrenia rated the brightness experience of the images in the brightness condition as more bright (p<.05) compared to people without schizophrenia (means and standard errors presented).
3.2. Emotion maintenance accuracy
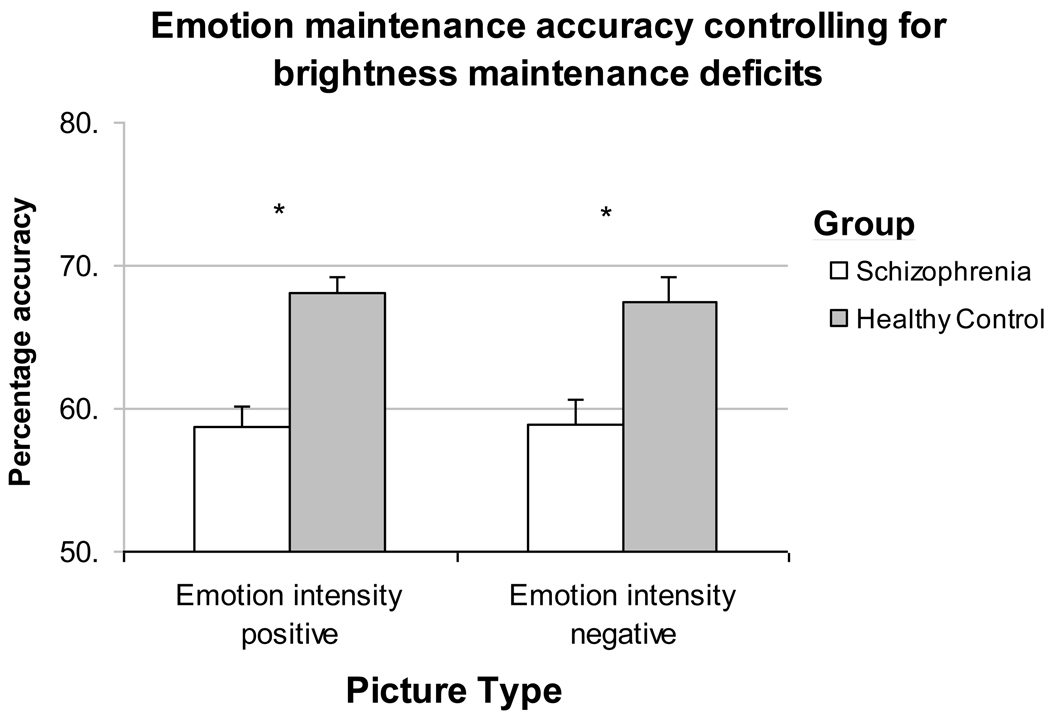
People with schizophrenia were significantly more impaired in their ability to maintain the experience of emotional intensity of the target image over a delay for positive images F(1, 46) = 8.22, P < 0.01, η2 = 0.15), and for negative images F(1, 46) = 7.19, P = 0.01, η2 = 0.14). They also showed a significant deficit in brightness maintenance accuracy (i.e., visual working memory) relative to people without schizophrenia F(1, 46) = 15.45, P < 0.001, η2 = 0.26). Neither positive nor negative emotion maintenance accuracy was associated with brightness maintenance accuracy for people with (r = 0.04, P = 0.42; r = 0.13, P = 0.26) or without schizophrenia (r = 0.16, P = 0.26; r = −0.28, P = 0.12). For people with schizophrenia, brightness maintenance was significantly related to performance on WMS-R Visual Memory (r = 0.56, P < 0.01), but positive and negative emotion maintenance was not (r = −0.07, P = 0.73; r = 0.26, P = 0.28). After controlling for accuracy in brightness maintenance, participants with schizophrenia continued to show significant impairment in the emotional maintenance, positive images F(1, 46) = 4.82, P < 0.05, η2 = 0.10), and negative images F(1, 46) = 5.33, P < 0.05, η2 = 0.11) (See Figure 3).
Figure 3.
People with schizophrenia (n=28) continue to show significant deficits in emotion maintenance for both positive and negative images relative to people without schizophrenia (n=19) when controlling for deficits in brightness maintenance (i.e., visual working memory). Mean percentages are presented with standard error bars.
NOTE: *p < 0.05
3.3. Relationship of Emotion Maintenance Accuracy to Behavioral Measures of Motivation
Positive emotion, negative emotion and brightness maintenance accuracy were all unrelated to the PANSS symptom rating of avolition (r = 0.21, P = 0.15 and r = 0.09, P = 0.34, respectively). For the QLS measure of motivation, brightness maintenance was significantly related (r = 0.39, P < 0.05) as was negative emotion maintenance (r = 0.35, P < 0.05), while positive emotion maintenance was unrelated (r = −0.20, P = 0.17). After controlling for brightness maintenance, the association between negative emotion maintenance and QLS motivation remained significant (r = 0.32, P = 0.05). Finally, the QLS motivation item was related to WMS-R Visual Memory (r = 0.61, P < 0.01). (See Table 2).
Table 2.
Bivariate correlations for positive and negative emotion and brightness accuracy with visual working memory z-score, PANSS Avolition rating, and QLS motivation rating for people with schizophrenia (n=28).
| Brightness Maintenance |
Negative Emotion Maintenance |
Positive Emotion Maintenance |
Visual Working Memory |
PANSS Avolition |
QLS Motivation |
|
|---|---|---|---|---|---|---|
| Brightness Maintenance | - | 0.13 | 0.04 | 0.56** | −0.22 | 0.39* |
| Negative Emotion Maintenance | - | 0.21 | 0.33 | 0.09 | 0.35* | |
| Positive Emotion Maintenance | - | - | −0.11 | 0.21 | −0.20 |
p < 0.01
p < 0.05
PANSS: Positive and Negative Syndrome Scale
QLS: Quality of Life Scale
4. Discussion
The present study indicates that, when given explicit instructions to maintain an emotional experience, people with schizophrenia have a deficit in this ability relative to healthy control subjects, yet show no difference in the experience of emotion when presented with emotion-eliciting stimuli. Further, this deficit appears to be independent of problems with visual working memory. This is in line with recent studies of emotion maintenance, using the affective startle modulation method and fMRI (Kring et al., in press; Ursu et al., in press). In addition, we found that impaired performance in the maintenance of negative emotion experience was associated with the QLS rating of motivation, suggesting that this deficit may be related to motivated behavior. Certainly, goal-directed behavior requires one to ‘hold in mind’, or represent, the desired or to-be-avoided stimulus outside the presence of that stimulus (Burbridge and Barch, 2007; Gold et al., 2008). It is possible that deficits in the ability to engage in motivated behavior seen in people with schizophrenia are due in part to difficulty maintaining the representation of an affective or emotional experience. Indeed, much of daily life involves having an emotional experience (positive or negative) and then using this experience to guide movement towards desired goals and away from undesirable outcomes (Carver and Scheier, 1998). The fact that negative emotion maintenance was associated with clinician ratings of motivation highlights the important point that motivation is not solely movement towards rewards, but also the avoidance of problematic outcomes, something that negative emotion maintenance may help accomplish.
Neither emotion nor brightness maintenance was associated with the PANSS avolition rating, in contrast to their association with QLS motivation. Unlike this latter measure, the PANSS avolition item does not focus on the presence of goal-directed behavior per se, but rather on “control of ones thoughts, behavior, movements and speech” (Poole et al., 2000), Thus, it may be better described as a measure of willful control of cognitions and actions, rather than a measure of motivational state per se, possibly accounting for its lack of association with emotion and brightness maintenance.
Our findings also suggest that real-world motivated behavior, as assessed via QLS motivation, is associated with intact visual working memory. Specifically, we found that both brightness maintenance and WMS-R Visual Memory were significantly related to the QLS rating of motivation. This provides further evidence that motivational problems are linked to some basic aspects of neurocognitive functioning, particularly working memory (e.g., Nakagami et al., 2008; Gard et al., 2009). Future studies will need to tease apart the relative contributions of emotion maintenance, reward representation, and working memory impairment to problems with motivated behavior.
Interestingly, the emotion and brightness maintenance tasks were unrelated for both people with and without schizophrenia, and although the brightness maintenance task was related to a standard measure of visual working memory, the emotion maintenance task (for both positive and negative emotion) was not. Similar to our findings in schizophrenia in the present study, Mikels and colleagues (2008) showed that visual working memory and emotion maintenance (i.e., “affective working memory”) are separable processes in healthy individuals. This separation of working memory from emotion maintenance appears to conflict with findings that have linked working memory and emotion impairments in schizophrenia. For example, Burbridge and Barch (2007) found that working memory moderated the relationship between the symptom of anhedonia and self-reported experience of enjoyment of pleasurable stimuli. Likewise, working memory has been shown to be related to problems delaying future rewards (Heerey et al., 2007) and in using potential outcomes as a guide on a reward decision making task (Heerey et al., 2008). The key difference in the current study is that participants were asked to actively and willfully maintain the representation of emotional intensity – i.e., the emotional experience – rather than to project themselves into the future and imagine the appropriate reward. One possibility is that working memory is helpful for keeping a goal in mind, while emotion maintenance is important for tagging the emotional valence of a goal. This process is similar to what Barch and Dowd (2010) and others have referred to as “working memory for value”.
Although the primary manipulation of the study involves maintenance of emotional and brightness intensities, we cannot rule out the possibility that the deficits observed here might be due to difficulty in comparing emotional experiences, a fundamental aspect of the task. However, the fact that emotion maintenance differences remained significant after controlling for brightness maintenance implies that whatever comparison differences exist between the groups were not accounted for in any comparison problems that existed in the brightness task. One additional limitation of the present study is that our only measures of motivated behavior were based on clinician ratings (PANSS and QLS). Certainly it is important that motivation be carefully defined from a variety of angles (self-report, observation, behavior) and this definition should map on to clear aspects of known impairment in schizophrenia (Barch, 2005).
Future studies must address whether this laboratory-based emotion maintenance deficit in schizophrenia is related to ecologically meaningful problems with motivation, as measured by self and observer report, and other behavioral assessments and daily life examples. If this proves to be the case, then emotion maintenance may be a useful area to target in order to remediate motivational deficits. For example, it may be possible to design computerized exercises that train patients to maintain affective responses over increasingly longer periods of time, similar to exercises that are designed to improve verbal working memory in schizophrenia (Fisher et al., 2009; 2010). Recent work in our laboratory has shown that the emotion maintenance measure used in the present study is reliable over a one week period (Broome et al., under review), and therefore may be useful in measuring change in response to therapeutic interventions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
IAPS picture numbers used in this study were: POSITIVE: 1340, 1440, 1500, 1650, 1810, 1811, 1850, 1942, 2040, 2070, 2260, 2311, 2345, 2550, 4598, 4599, 5201, 5594, 5621, 5626, 5629, 5830, 5831, 5890, 5910, 5950, 5982, 5991, 7195, 8030, 8034, 8116, 8117, 8180, 8185, 8250, 8460, 8490, 8497, 8501; NEGATIVE: 1019, 1113, 2050, 2120, 2141, 2271, 2490, 2682, 2692, 3022, 3210, 3230, 3280, 3500, 5940, 5971, 6010, 6200, 6210, 6211, 6260, 6300, 6370, 6410, 6840, 6930, 8230, 8231, 9110, 9160, 9230, 9280, 9290, 9331, 9373, 9404, 9440, 9480, 9561, 9622; NEUTRAL: 1121, 1313, 1560, 1670, 2020, 2130, 2190, 2220, 2383, 2385, 2410, 2480, 2485, 2487, 2514, 2516, 2570, 2575, 2580, 2600, 2620, 2681, 2702, 2840, 2850, 2890, 4571, 4610, 5000, 5120, 5130, 5220, 5410, 5455, 5500, 5510, 5520, 5530, 5532, 5533, 5534, 5731, 5740, 5779, 5800, 5920, 6150, 7002, 7004, 7709, 7030, 7034, 7035, 7060, 7080, 7090, 7095, 7140, 7150, 7160, 7170, 7175, 7190, 7224, 7234, 7283, 7320, 7351, 7402, 7490, 7224, 7234, 7283, 7320, 7351, 7402, 7490, 7560, 7620, 7705, 7710, 7820, 7830, 7920, 8311, 9070, 9700.
References
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophrenia Bulletin. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophrenia Bulletin. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF, Gottesman II. Hedonic capacity in schizophrenics and their twins. Psychological Medicine. 1990;20:367–374. doi: 10.1017/s0033291700017682. [DOI] [PubMed] [Google Scholar]
- Broome R, Gard DE, Mikels JA. Reliability of a delayed response affective working memory task. under review. [Google Scholar]
- Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. Journal of Abnormal Psychology. 2007;116:30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. On the Self-regulation of Behavior. New York: Cambridge University Press; 1998. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for Physical and Social Anhedonia. Journal of Abnormal Psychology. 1976;85 doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Minor KS. Emotional Experience in Patients With Schizophrenia Revisited: Meta-analysis of Laboratory Studies. Schizophrenia Bulletin. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- Fenton WS, McGlashan TH. Natural history of schizophrenia subtypes: II. Positive and negative symptoms and long-term course. Archives of General Psychiatry. 1991;48:978–986. doi: 10.1001/archpsyc.1991.01810350018003. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticitybased auditory training to improve verbal memory in schizophrenia. American Journal of Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophrenia Bulletin. 2010;36:869–879. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Fisher M, Garrett C, Genevsky A, Vinogradov S. Motivation and its relationship to neurocognition, social cognition, and functional outcome in schizophrenia. Schizophrenia Research. 2009;115:74–81. doi: 10.1016/j.schres.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Germans Gard M, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophrenia Bulletin. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biological Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RR, Gold JM. Delay discounting in schizophrenia. Cognitive Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: An instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Herbener ES, Harrow M. The course of anhedonia during 10 years of schizophrenic illness. Journal of Abnormal Psychology. 2002;111:237–248. [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, Wrase J, Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kay SR, Sevy S. Pyramidical Model of Schizophrenia. Schizophrenia Bulletin. 1990;16:537–545. doi: 10.1093/schbul/16.3.537. [DOI] [PubMed] [Google Scholar]
- Kring AM, Germans Gard M, Gard DE. Emotion Deficits in Schizophrenia: Timing Matters. Journal of Abnormal Psychology. doi: 10.1037/a0021402. in press. [DOI] [PubMed] [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bulletin. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainsville, FL: University of Florida; 2005. International affective picture system (IAPS). Affective ratings of pictures and instruction manual. [Google Scholar]
- Medalia A, Brekke J. In search of a theoretical structure for understanding motivation in schizophrenia. Schizophrenia Bulletin. 2010;36:912–918. doi: 10.1093/schbul/sbq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels JA, Larkin GR, Reuter-Lorenz PA, Cartensen LL. Divergent trajectories in the aging mind: Changes in working memory for affective versus visual information with age. Psychology and Aging. 2005;20:542–553. doi: 10.1037/0882-7974.20.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels JA, Reuter-Lorenz PA, Beyer JA, Fredrickson BL. Emotion and working memory: Evidence for domain-specific processes for affective maintenance. Emotion. 2008;8:256–266. doi: 10.1037/1528-3542.8.2.256. [DOI] [PubMed] [Google Scholar]
- Nakagami E, Xie B, Hoe M, Brekke JS. Intrinsic motivation, neurocognition and psychosocial functioning in schizophrenia: Testing mediator and moderator effects. Schizophrenia Research. 2008;105:95–104. doi: 10.1016/j.schres.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Poole JH, Tobias FC, Vinogradov S. The functional relevance of affect recognition errors in schizophrenia. Journal of the International Neuropsychological Society. 2000;6:649–658. doi: 10.1017/s135561770066602x. [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Archives of General Psychiatry. 2002;59:146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Tremeau F, Antonius D, Cacioppo JT, Ziwich R, Butler P, Malaspina D, Javitt DC. Anticipated, on-line and remembered positive experience in schizophrenia. Schizophrenia Research. 2010;122:199–205. doi: 10.1016/j.schres.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Ursu S, Kring AM, Germans Gard M, Minzenberg M, Yoon J, Ragland D, Solomon M, Carter CS. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2010.09081215. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]