Abstract
Inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis constitute a major health problem in developed countries. Moreover, IBD predisposes to the development of colorectal cancer. The intracellular NOD-like receptor Nlrp3 is rapidly emerging as a crucial regulator of intestinal homeostasis. This innate immune receptor mediates assembly of the inflammasome complex in the presence of microbial ligands, triggering caspase-1 activation and secretion of IL-1β and IL-18. Recent studies suggest that defective Nlrp3 inflammasome signaling in the gut contributes to IBD through increased permeability across the epithelial barrier and the induction of detrimental immune responses against invading commensals. Here, we review and discuss recent advances of the role of the Nlrp3 inflammasome in colitis and colon tumorigenesis.
Keywords: colitis, Crohn’s disease, NLR, Nlrp3 inflammasome, caspase-1
Nlrp3 in inflammatory bowel disease: for or against?
Crohn’s disease (CD) and ulcerative colitis (UC) represent major remitting and relapsing inflammatory disorders of the gastrointestinal tract and are characterized by chronic inflammation, abdominal pain, rectal bleeding, diarrhea and malnutrition 1. In addition, these inflammatory bowel diseases (IBDs) constitute major risk factors for the development of colorectal cancer 2, thus being responsible for significant health-related costs in the Western world. These ailments differ from each other in location. CD usually starts in the terminal ileum, although it may affect any part of the gastro-intestinal tract. In contrast, inflammation is typically limited to the colon and rectum of UC patients 3. Moreover, pathological lesions associated with UC are restricted to the mucosal layer of the gut lumen, whereas CD patients typically present with transmural inflammation 3. Although the precise etiology of CD and UC remains unclear, aberrant immune responses against commensal microflora are widely thought to underlie IBD 3,4. Multiple receptors of the extracellular interleukin (IL) receptor and Toll-like receptor (TLR) families are expressed on epithelial and immune cells in the gastrointestinal tract and have been implicated in IBD (Figure 1). The biology of these receptors and their roles in IBD are discussed elsewhere 5. More recently, single nucleotide polymorphisms (SNPs) in the gene encoding the NOD-like receptor (NLR) family member Nlrp3 were linked to CD susceptibility 6. Nlrp3 is a cytosolic platform protein that assembles the inflammasome, a protein complex that is responsible for the proteolytic maturation and secretion of the pro-inflammatory cytokines IL-1β and IL-18 7–9. The latter molecules induce inflammation and participate in epithelial repair and healing processes through recruitment and activation of immune cells and by inducing the production of pro-inflammatory cytokines, chemokines and growth factors 10. Consequently, the Nlrp3 inflammasome plays crucial roles in regulating a variety of inflammatory and autoimmune diseases. However, the precise roles of the Nlrp3 inflammasome in IBD are still debated. Early studies suggested IL-1β and IL-18 production to contribute to intestinal inflammation 11–14. However, the concept of inflammasome signaling being detrimental in IBD is being reevaluated based on recent reports suggesting that Nlrp3 inflammasome-induced production of IL-1β and IL-18 confers protection against colitis and colitis-associated tumorigenesis 15–19. Here, we review the current understanding of how the Nlrp3 inflammasome regulates integrity of the intestinal mucosal barrier under homeostatic conditions and discuss its role in shaping the immune response to commensal microbiota during colitis and colitis associated-tumorigenesis.
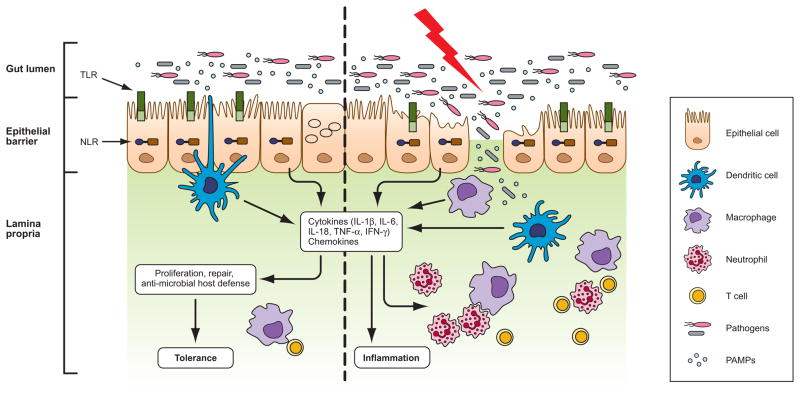
Figure 1. Host-microbe interactions in the gut and development of IBD.
The intestinal epithelial barrier protects underlying mucosal tissues from commensal bacteria present in the gut lumen. In healthy individuals, a state of immune tolerance exists that allows nonpathogenic microbes to live in the gut without any detrimental immune response. Dendritic cells residing in the intraepithelial spaces and lamina propria sample commensal bacteria and induce a regulatory immune response, which provide tolerance to commensal flora. In susceptible hosts, the epithelial barrier is compromised allowing commensal bacteria to invade lamina propria and mucosa. Infiltrated bacteria interact with macrophages, dendritic cells and neutrophils via innate recognition receptors such as Toll-like receptors (TLRs) and NOD-like receptors (NLRs). Activation of innate immune receptors induce the production of proinflammatory cytokines and chemokines which further recruit myeloid derived immune cells to the infected tissue accelerating inflammatory response and leading to the development of inflammatory bowel disease.
NLR signaling in IBD
Nlrp3 and the related NLR proteins Nod1 and Nod2 are emerging as crucial regulators of inflammatory responses against commensal microflora in the gut. Notably, the genes encoding Nod1 and Nod2 are mutated in 15–20% of patients suffering from IBD 20–22. Similar to extracellular TLRs and IL receptors, Nod1 and Nod2 activate the transcription factors NF-κB and AP-1 in Paneth cells, epithelial cells and professional antigen-presenting cells 4. The peptidoglycan fragments iE-DAP and muramyl-dipeptide (MDP) from Gram-positive and -negative bacteria activate Nod1 and Nod2, respectively,through binding to the carboxy-terminal leucine-rich repeat (LRR) motifs of these pathogen-recognition receptors (Figure 2). This triggers recruitment of the adaptor proteins RICK and CARD9, resulting in K63-linked ubiquitylation and activation of NF-κB and MAP kinase signaling cascades 4. These pathways culminate in the transcriptional activation of genes encoding cytokines, chemokines and a variety of pro-inflammatory mediators that activate cells of the innate and adaptive immune system. In addition to activating NF-κB, Nod1 and Nod2 were recently shown to regulate autophagy by recruiting the autophagy determinant Atg16L1 to the plasma membrane 23. Interestingly, polymorphisms in the gene encoding Atg16L1 represent another major risk factor for the development of CD 24,25. Moreover, Nod2 mutations that are associated with CD caused defective Atg16L1 recruitment and autophagy induction, suggesting that CD–associated polymorphisms in Nod2 and Atg16L1 might affect autophagy induction via mechanisms distinct from the role of Nod2 in regulating NF-κB activation 23. As described above, a body of recent reports identified Nlrp3 as a third NLR family member that is associated with IBD. Unlike Nod1 and Nod2, however, Nlrp3 activation triggers assembly of a cytosolic caspase-1-activating protein complex referred to as the ‘inflammasome’.
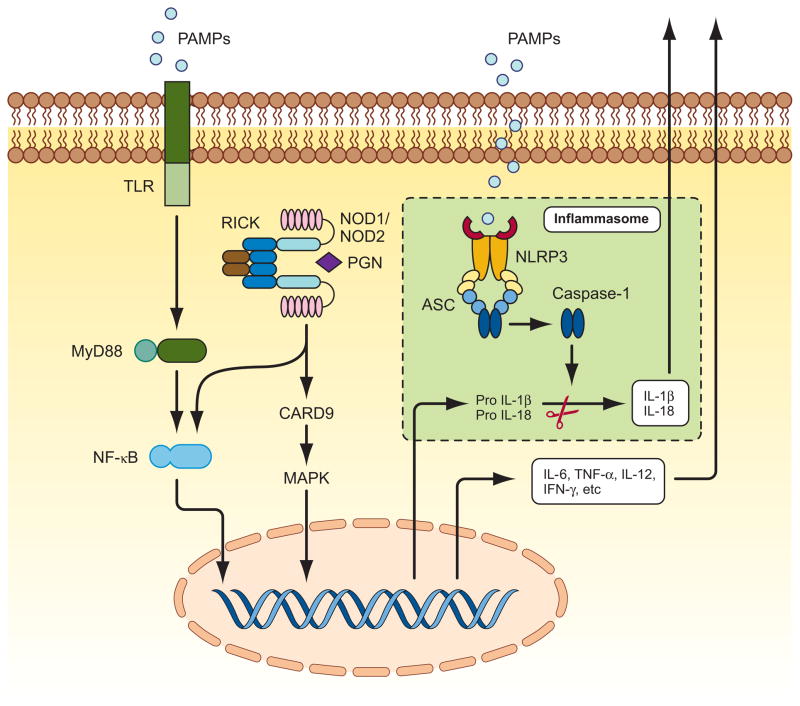
Figure 2. TLRs, NLRs, and inflammasome signaling pathways.
Intestinal epithelial cells and antigen presenting cells (APC) sense the pathogen or pathogen-associated molecular patterns (PAMPS) through pattern recognition receptors TLRs and NLRs. Interaction of PAMPs with cell surface bound TLRs leads to activation of transcription factor NF-κB via effector molecule MyD88. NLR family members NOD1 and NOD2 sense intracellular presence of bacterial cell wall component peptidoglycan (PGN). Direct or indirect ligand recognition by NOD1 and NOD2 induces the recruitment of RICK, which further activates NF-kappa;B and MAP kinases. The NF-κB and MAP kinase pathways are the major signaling pathways that induce the expression of pro-inflammatory cytokines. Another cascade of signaling is mediated by NLR NLRP3, which senses a plethora of microbial and nonmicrobial patterns in the cytosolic compartment and forms a multiprotein complex with ASC and caspase-1called the inflammasome, in which ASC bridges the CARD domain of caspase-1 with pyrin domain of Nlrp3 through homotypic interaction of CARD and pyrin domains. Inflammasome plays a central role in inflammatory process by activating caspase-1, and mediating production of pro-inflammatory cytokines IL-1β and IL-18.
Composition and activation of the Nlrp3 inflammasome
Nlrp3 (also known as Nalp3, Cryopyrin, CIAS1, PYPAF1 and CLR1.1) is an 1016 amino acid protein transcribed from NLRP3 which is located on chromosome 1q44. Mutations in NLRP3 underlie a variety of autosomal-dominant periodic fever syndromes known as familial cold autoinflammatory syndrome (FCAS), Muckle-Wells syndrome (MWS) and chronic infantile neurological cutaneous and articular syndrome (CINCA) 26,27. The protein has a domain architecture characteristic of all NLR members, comprising a centrally located NOD motif, flanked at the carboxy-terminus by an array of 12 leucine-rich repeat (LRR) motifs that are believed to be involved in modulating Nlrp3 activity and sensing microbial ligands and endogenous alarmins 4. At the N-terminus, Nlrp3 contains a pyrin domain that allows homotypic interactions with the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC). This bipartite pyrin/CARD adaptor protein bridges the interaction between Nlrp3 and the cysteine protease caspase-1 28. Together, Nlrp3, ASC and caspase-1 form a large (>700 kDa) multi-protein complex called the “inflammasome” that is sufficient to trigger activation of the caspase-1 under certain in vitro conditions 29 (Figure 2). Once activated, caspase-1 processes the precursor forms of IL-1β and IL-18 to generate the biologically active forms of these pro-inflammatory cytokines 4,30.
Activation of the Nlrp3 inflammasome in cultured macrophages is achieved with millimolar concentrations of ATP provided the cells are pre-exposed to TLR ligands such as lipopolysaccharide (LPS), to bacterial or viral nucleic acids, or to fungal cell wall components 4,8,9,30–32. ATP triggers opening of the non-selective cation channel of the purinergic P2X7 receptor. The shellfish toxin maitotoxin and the bacterial ionophore nigericin can substitute for ATP in the activation of caspase-1 via Nlrp3 8. Studies in mice with a gene-targeted deletion in Nlrp3 demonstrated that Nlrp3-dependent caspase-1 activation is stimulus-dependent under physiological conditions 8,9,31. The Nlrp3 inflammasome is responsible for caspase-1 activation in macrophages and dendritic cells infected with Staphylococcus aureus 8 and plays a crucial role in the host response against influenza virus 33–35 and the fungal pathogen Candida albicans 36–38. The Nlrp3 inflammasome also drives the inflammatory response in skin keratinocytes exposed to various skin irritants such as ultraviolet B irradiation and chemicals inducing contact hypersensitivity 9,39. Alzheimer’s disease-associated amyloid deposits and medically-relevant crystals such as monosodium urate, calcium pyrophosphate dihydrate, crystalline asbestos and silica all induce Nlrp3-dependent activation of caspase-1 in LPS-primed macrophages 4,30. Also aluminum adjuvant activates the Nlrp3 inflammasome and subsequent release of IL-1β and IL-18 in LPS-pretreated macrophages 40,41. However, the role of the Nlrp3 inflammasome in alum adjuvanticity and antibody production is controversial 42,43.
Inflammasome effector genes are risk alleles for IBD
Decreased secretion of the inflammasome cytokine IL-1β was noted in MDP-stimulated myeloid cells of CD patients 44–46. In addition, polymorphisms in the genes encoding the inflammasome effector IL-18 and the IL-18 receptor accessory protein correlate with increased susceptibility to CD 47,48. These interesting observations raised the possibility that inflammasomes might play a crucial role in IBD. Indeed, a recent study found that SNPs in regulatory elements of Nlrp3 strongly associated with increased susceptibility to CD development in humans 6. These polymorphisms led to decreased Nlrp3 expression and correlated with downregulated IL-1β production from LPS-activated monocytes that were homozygous for the risk alleles 6. These studies suggested diminished expression of IL-1β and IL-18 to be linked to susceptibility to IBD. However, the molecular chain of events linking decreased IL-1β and IL-18 secretion to CD and UC development remained unclear. Understanding the mechanisms by which IL-1β and IL-18 levels modulate gut homeostasis is of particular importance because elevated IL-18 expression in cells of the intestinal mucosa was also observed in affected regions of the diseased gut of CD patients 49,50. Similarly, increased IL-1β expression in the inflamed mucosa of patients with CD or UC has been noted 51. Thus, it appears that homeostasis of the intestinal epithelium is highly sensitive to the expression levels of the inflammasome effectors IL-1β and IL-18, and deregulated expression (culminating in either increased or decreased protein levels) of these cytokines might severely affect the susceptibility of the gastro-intestinal tract to IBD. This might explain why secretion of these inflammatory cytokines is tightly controlled by a two-step process involving NF-κB-dependent transcription of their messengers and the proteolytic maturation of their cytosolic precursors by the inflammasome machinery 28,30 (Figure 2).
The Nlrp3 inflammasome in homeostasis of the intestinal epithelium
The crucial role of the Nlrp3 inflammasome in regulating gut homeostasis was strengthened by recent studies examining the molecular mechanisms by which Nlrp3, ASC and caspase-1 control integrity of the intestinal epithelium and modulate immune responses to microbiota in the gut during experimental colitis. While a complete surrogate model displaying all clinical features of human IBD is not available, various mouse models of experimental colitis that are useful for examining important aspects of human disease have been developed 52. The dextran sodium sulfate (DSS) model is one of the most extensively used to investigate innate immune mechanisms of colitis. Oral administration of this chemical is directly toxic to the colonic epithelium 53 and triggers inflammation by disrupting the compartmentalization of commensal bacteria in the gut 54. The clinical features of the DSS model include loss of body weight, diarrhea, rectal bleeding and mortality. Histopathological analysis typically reveals extensive crypt and epithelial cell damage, significant infiltration of neutrophils and macrophages, tissue edema and ulceration 18.
Notably, Nlrp3−/− mice were found to be more susceptible to DSS-induced colitis 15,17,18. These mice also suffered from increased sensitivity to body weight loss, diarrhea, rectal bleeding and mortality in the acute 2,4,6-trinitrobenzene sulfonate (TNBS)-induced colitis model 17,18. Similar to Nlrp3−/− mice, deficiency in the inflammasome proteins ASC and caspase-1 caused greater colitis-associated lethality and more severe histopathological changes during both the acute and chronic phases of DSS-induced colitis 15,16,18. Thus, activation of the Nlrp3 inflammasome following cytotoxic assaults on the intestinal epithelium might trigger repair responses characterized by increased division of stem cells at the base of crypts to replace damaged enterocytes 55. Concurrently, decreased proliferation of epithelial cells lining the gastro-intestinal tract was noted in Nlrp3−/− and caspase-1−/− mice during acute colitis 18. Defective IL-1β and IL-18 production downstream of the Nlrp3 inflammasome activation hampered these repair mechanisms and led to increased permeability of the gut epithelium as evidenced by the retrieval of significantly higher FITC-dextran levels in serum of DSS-fed Nlrp3−/− and caspase-1−/− mice18,56. Increased permeability of the epithelial layer in the gut of Nlrp3−/− and caspase-1−/− mice was also apparent from the systemic dispersion of gut microbiota in animals of these genotypes 16,18.
In addition to an increased transmural permeability, colonic crypts of Nlrp3−/− mice were shown to exert reduced antimicrobial activity, which correlated with altered expression of colonic defensins 17. Defensins are a family of short peptides with bactericidal activity. Their secretion by cells of the colonic crypt represents a crucial mechanism for guarding homeostasis of the intestinal epithelium as illustrated by the observation that diminished expression of these antimicrobial compounds also occurs in CD patients 57,58. However, whether the Nlrp3 inflammasome modulates defensin expression directly or downstream of IL-1β and/or IL-18 secretion remains to be determined. Regardless, these observations indicate a key role for the Nlrp3 inflammasome in protection against colitis. Notable in this regard is that mice lacking caspase-1 were consistently found more susceptible to colitis than Nlrp3-deficient mice, suggesting that additional NLRs may regulate caspase-1 activation. Indeed, a recent study showing that activation of the Nlrc4 inflammasome in the gut also confers protection against colitis-associated colon tumorigenesis 59.
Nlrp3 activation in cells of the intestinal tract
Because Nlrp3 is expressed in both immune and epithelial cells 60, bone marrow chimera mice were used to determine the cellular compartments contributing to Nlrp3 inflammasome-mediated protection against colitis. Nlrp3 signaling in non-hematopoietic cells was concluded to be crucial because expression of Nlrp3 18 and caspase-1 16 in these cells prevented the aggravated disease symptoms seen in DSS-administered Nlrp3−/− and caspase-1−/− mice. This effect is likely to originate from intestinal epithelial cells because activation of inflammatory and repair signaling pathways in these cells was previously demonstrated to play a crucial role in protection against colitis 54,61. However, a crucial role for Nlrp3 inflammasome signaling in epithelial cells does not imply that its activation in myeloid cells doesn’t contribute to protection against colitis. In this regard, reconstituting Nlrp3-deficient mice with wild type bone marrow was recently shown to confer resistance against colon tumorigenesis in the chronic azoxymethane (AOM)/DSS model15. This suggests that Nlrp3 inflammasome activity in radiosensitive cells of the lamina propria may also contribute to protection against chronic colitis. It is thus likely that, depending on spatiotemporal parameters, inflammasome activation in cells of the epithelial layer and the lamina propria may variably contribute to homeostasis of the gut epithelium and protection against colitis. In support of this model, colitis-associated tumorigenesis was recently demonstrated to be more severe in caspase-1−/− mice relative to animals lacking caspase-1 in either the epithelial or myeloid compartments, respectively 59.
The inflammasome cytokines IL-1β and IL-18 protect against colitis
Previous work demonstrating a crucial role for the inflammasome effectors IL-1β and IL-18 in repair and restitution of the ulcerated epithelium is in agreement with the ‘epithelial guard’ hypothesis for the Nlrp3 inflammasome discussed above 62. Once secreted, inflammasome-matured IL-1β and IL-18 might exert their functions through ligation of their respective receptors expressed on intestinal epithelial cells and local immune cells in the gut. Such role for IL-18 is supported by data showing that Il18−/− and Il18r1−/− mice are hyper-susceptible to DSS-induced colitis, which is associated with higher mortality rates and more severe histopathological changes in these mice 63. Similarly, Il1r−/− mice also show increased intestinal damage and histopathology during DSS-induced colitis 64. Finally, DSS-induced colitis is more severe in mice lacking the adaptor protein MyD88 54,65,66, which is required for the production of IL-1β and IL-18 as well as for signaling downstream of their respective receptors. These observations suggest that defective production of IL-1β and/or IL-18 may underlie the DSS-susceptibility phenotypes of Nlrp3−/− and caspase-1−/− mice. Rescue experiments involving exogenous administration of recombinant IL-18 to DSS-fed caspase-1−/− mice provided direct evidence for the crucial role of IL-18 in mediating the effects of the Nlrp3 inflammasome during colitis 16,18.
Noteworthy, the results from the gene-deleted mouse models described above are sometimes in conflict with reports using biochemical approaches for neutralization of caspase-1 and IL-18. For instance, studies using the chemical caspase-1 inhibitor pralnacasan suggested a detrimental role for this protease in DSS-induced colitis 14,67,68. In contrast, four recent studies reported caspase-1−/− mice to be hypersusceptible to DSS-induced colitis 15,18,56,69 . Similarly, experiments in Il18−/− and Il18r1−/− mice suggested a beneficial role for IL-18 production during DSS-induced colitis 63, whereas IL-18 neutralization with recombinant IL-18 binding protein 11 and IL-18 antibodies suggested a detrimental role for IL-18 12. In addition to differences in experimental design, characteristics inherent to biochemical neutralization and gene-deleted mouse models may have contributed to the different outcomes. Chemical and biochemical inhibitors are ideal for therapeutic intervention in patients, but they are unlikely to achieve a complete and enduring neutralization of the desired target throughout the body. Moreover, they may suffer from off-target effects that could influence disease outcome. On the other hand, gene-targeted deletion in mice is a surer approach for removing the protein under study and therefore generally considered a more elegant approach to study gene function. However, disease outcome in knockout mice might also be influenced by a number of parameters including the protocol used to induce gut inflammation, the genetic background of the mice and the composition of their gut microbiota. This may explain why one recent study proposed Nlrp3−/− mice to be protected from DSS-induced colitis70, while four other studies showed a hypersusceptible response in these mice 15–18.
From a broad perspective, a protective role for Nlrp3 during colitis is more aligned with other findings. Firstly, polymorphisms leading to decreased Nlrp3 expression are associated with increased risk for developing CD 6. Secondly, mice lacking other inflammasome components (Nlrc4, ASC and caspase-1) 15–19,59, the inflammasome substrates IL-1β and IL-18 63 or their cognate receptors63,64 were all found to be more susceptible to DSS-induced colitis. Finally, deletion of the adaptor protein MyD88, which is required for both the production of the caspase-1 substrates IL-1β and IL-18 as well as for signaling downstream of their respective receptors, renders mice hyper-susceptible to DSS-induced colitis 54,65,66. Thus, both genetic evidence from IBD patients as well as experimental colitis studies using mice lacking signaling molecules in inflammasome pathways indicate a protective role for the Nlrp3 inflammasome in IBD. As described later, this model is further supported by the observation that inflammasome signaling is required for protection against colitis-associated tumorigenesis.
The Nlrp3 inflammasome in colitis-associated tumorigenesis
Inflammation is generally considered a beneficial host response to injury and infection. However, chronic intestinal inflammation is increasingly recognized as a risk factor for the development of colorectal cancer and IBD patients are at increased risk of developing colorectal cancer 71. Recent studies implicate defective NLR activation in priming the intestinal mucosa for increased cell proliferation and tumorigenesis. For instance, defective Nod1 signaling aggravates permeability of the intestinal epithelium during colitis and promotes development of colitis-associated tumors 72. Moreover, polymorphisms in Nod2 are linked with increased susceptibility to gastrointestinal tumorigenesis 73. Although genetic linkage studies for Nlrp3 are currently lacking, its association with increased susceptibility to CD development 6 prompted analysis of its role in colitis-associated tumorigenesis 15,19. As a result of elevated inflammatory responses and destruction of the epithelial barrier, Nlrp3−/− mice suffered from increased dysplasia and tumor formation when subjected to the widely used azoxymethane (AOM)/DSS tumorigenesis model 15,19. Similar observations were noted for mice with targeted deletions in the inflammasome components ASC and caspase-1 15,19, confirming a crucial role for the Nlrp3 inflammasome in protection against colitis-associated tumorigenesis. Despite the crucial role intestinal epithelial cells play in protection against colitis-associated inflammation 16,18, bone marrow chimera studies demonstrated a major role for the hematopoietic compartment in protecting against colitis-associated tumorigenesis 15. As already discussed, this suggests that the intestinal epithelium and local immune cells are important for protection against colitis and colitis-associated tumorigenesis at different stages of disease progression.
What is the mechanism by which the Nlrp3 inflammasome regulates colitis-associated tumorigenesis? Several lines of evidence point to IL-18 as the main effector of this process. Firstly, IL-18 exerts anti-tumor effects in a number of experimental tumor models of sarcoma and melanoma 74–77. It inhibits tumor growth and angiogenesis 78–80 and promotes repair and restitution of ulcerated epithelium 16,19,62. Secondly, IL-18 production is significantly reduced in colons of DSS-fed 16,18 and AOM/DSS-treated Nlrp3−/−and caspase-1−/−mice 15,19. Thirdly, administration of recombinant IL-18 markedly reduces disease progression in AOM/DSS-treated caspase-1−/− mice 19. Finally, colons of AOM/DSS-treated Il-18−/− and Il18r1−/− mice recapitulate the increased tumor burdens seen in mice lacking Nlrp3 or caspase-1 19,81. Notably, unlike in the caspase-1-deficient mice, AOM/DSS-treated Il1r1−/− mice contain similar numbers of intestinal polyps as their wild-type littermates 81. These observations suggest a crucial role for IL-18 production downstream of the Nlrp3 inflammasome in protection against colitis-associated neoplasia. They also suggest that while IL-18 promotes enterocyte proliferation to repair chemically-induced injury of colonic epithelium, it inhibits hyperplasia during chronic stages of colitis. This apparent discrepancy might be explained by differential roles of IL-18 during the acute and chronic stages of colitis (Figure 3). During acute phase of disease, IL-18 might contribute to restoring epithelial barrier integrity by inducing controlled proliferation of stem cells at the crypt base and turnover of damaged epithelial cells. This prevents systemic dispersion of commensal microflora and the induction of exaggerated inflammatory responses. However, during remission and chronic stages of colitis, IL-18 may inhibit epithelial cell proliferation in neoplastic regions of the colon epithelium. This might be achieved, at least in part, through the induction of IFN-γ production because the production of IFN-γ is markedly reduced in colons of AOM/DSS-treated Nlrp3−/− and caspase-1−/− mice 19. IFN-γ mediates its effect through the IFN-γReceptor (IFN-γR)-mediated phosphorylation and nuclear translocation of the transcription factor STAT1 (Figure 3). Consistent with deregulated IFN-γ signaling, phosphorylated STAT1 levels were markedly reduced in colons of AOM/DSS-treated caspase-1−/− mice, but restored upon stimulation with either IFN-γ or IL-18. In agreement with a biphasic role for Nlrp3-mediated IL-18 production in colitis-associated tumorigenesis, a recent report described a biphasic role for IFN-γ during DSS-induced colitis with promotion of intestinal epithelial cell proliferation at early stages and induction of anti-proliferative responses at later stages 82.
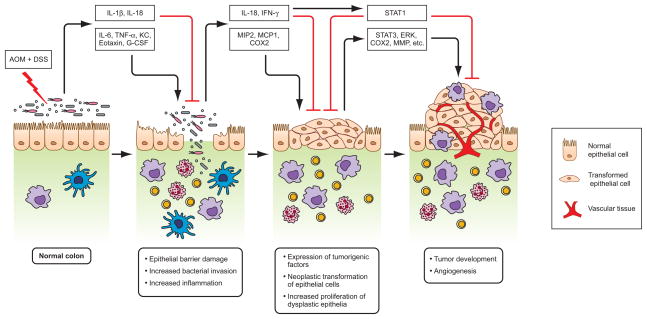
Figure 3. Model for Nlrp3 inflammasome-mediated protection against AOM plus DSS-induced colitis and colorectal tumorigenesis in mice.
A single treatment of DNA methylating agent azoxymethane (AOM) followed by repeated exposure of dextran sodium sulphate (DSS) induces chronic colitis and colorectal tumor formation in mice. Due to injury of the epithelium caused by chemicals, gut microflora invade the deeper tissue and activate immune cells to produce cytokines and chemokines. An uncontrolled production of cytokines such as IL-6 and TNF-α, and chemokines like KC, Eotaxin, G-CSF and MCP induce inflammatory response by recruiting immune cells such as macrophages, neutrophils and T cells, and helps neoplastic transformation of intestinal epithelial cells. Immune cells particularly macrophages have profound role in shaping microenvironment of tumor development. Macrophages produce tumorigenic factors such as COX2 and MIP2, activates MMPs, and promotes angiogenesis. The Nlrp3 inflammasome, which mediates IL-1β and IL-18 production, plays a protective role against deregulated inflammatory response and tumor induction. The beneficial function of Nlrp3 inflammasome is mainly exerted by IL-18, which induces production of IFN-γ and its downstream antitumor signaling through activation of transcription factor STAT1. IL-18-mediated downstream signals protect epithelial barrier damage, and maintain cellular proliferation, and regulate inflammatory responses. In the absence of functional inflammasome, a balance of antitumor signaling shifts towards pro-tumor signaling, such as STAT3, and to promote developing adenomatous polyps.
Concluding remarks
Our understanding of the biological mechanisms underlying IBD has markedly improved in recent years with the discovery of the major roles of the NLR proteins Nod1 and Nod2 in CD and UC 20–23. Further progress in understanding the mechanisms underlying IBD was achieved with the discovery that SNPs in the gene encoding Nlrp3 linked with increased susceptibility to CD 6. As discussed above, the previously held model of inflammasome signaling being detrimental during colitis 67,83–86 is being revisited in light of an important number of recent observations that suggest a protective role for this pathway during colitis and colitis-associated tumorigenesis 87. IL-18 production especially is emerging as a crucial effector mechanism by which inflammasomes confer protection against colitis-associated inflammation 16,18 and neoplasia 19,81. IL-18 may possibly exert these effects by modulating permeability of the intestinal epithelium 16,18, the production of antimicrobial peptides 17 and the activation levels of the tumor suppressors IFN-γ and STAT1 19, respectively. Undoubtedly, further insight into the complex roles NLRs and inflammasomes play in regulating IBD severity will be gained by detailed characterization of the cell types involved and the signaling mechanisms operating downstream of IL-18. Further progress may open up new strategies to preventing and treating IBD-associated inflammation and colorectal tumor development in the context of chronic inflammation.
Box 1. The Nlrp3 inflammasome in maintenance of the colonic barrier.
The mucosal immune system faces the challenging task of existing in peaceful coexistence with the commensal flora, while defending the host against pathogens. Initiation of inflammatory process is therefore thought to be due to a breach in the epithelial barrier. Deterioration of the mucus layer of the colon is prominent in patients with IBD, and mice having defective epithelial barrier function have been shown to be susceptible to develop colitis. Therefore, maintaining epithelial barrier integrity is crucial factor for protection against IBD development. Recent studies demonstrated that Nlrp3-inflammasome deficient mice are susceptible to colitis, and this is because of, at least in part, increased epithelial barrier damage 18. Compromised epithelial barrier in Nlrp3-inflammasome defective mice was evident by increased translocation of commensal bacteria into colon tissue and their dissemination in other organs like MLN, spleen and liver 16,18. Permeability assay using FITC-dextran further confirmed that there is increased colonic epithelial damage in Nlrp3-deficient mice during acute colitis induced by DSS 18.
Epithelial damage induces a localized repair response characterized by increased division of stem cell at the base of crypts to replace damaged enterocytes 55. Proliferation assay using BrdU and Ki67 staining revealed that the inflammasome-deficient mice have decreased proliferation of epithelial cells in colon during acute DSS colitis 16,18. These results led to postulate that Nlrp3 inflammasome plays crucial role in maintaining epithelial barrier by promoting proliferation of epithelial stem cells 18. Further investigation on the molecular basis of Nlrp3-mediated protection of epithelial barrier suggests that of the two cytokines downstream of the Nlrp3 inflammasome, IL-18 is crucial for epithelial protection 15,16,18. It was shown that during DSS induced colitis IL-1β is barely increased, while IL-18 is induced in several folds 15,16,18. Previous studies also documented that IL-18 is expressed in epithelial cells of colon of CD patients 49,62. Moreover, IL-18 has been shown to play role in proliferation of epithelial cells and repair response of damaged epithelia 62,63. Therefore, IL-18 production by the Nlrp3 inflammasome in colonic epithelia was identified as a crucial mediator of repair of the mucosal barrier and protection against DSS-induced colitis 16,18. Our understanding on exactly how IL-18 promotes epithelial proliferation and repair is currently lacking. However, our work also demonstrated that IFN-γ and its downstream STAT-1 signaling pathway are abrogated in Nlrp3-inflammasome-deficient mice in a manner dependent on IL-18, which was initially described as IFN-γ-inducing factor 19. IFN-γ is an important cytokines that regulates proliferation and repair process and involved in protection against colitis 82. It is also possible that IL-18 or IFN-γ downstream of inflammasome regulates several other cytokines and factors involved in proliferation, survival and repair process.
Acknowledgments
We acknowledge researchers who have contributed to this field, whose work was not cited or was cited through review articles because of space limitations. This work is supported by National Institute of Health Grants AR056296 and AI088177, a NIAMS Centers of Excellence for Influenza Research and Surveillance (CEIRS) grant and the American Lebanese Syrian Associated Charities (ALSAC) to TD-K. M.L. is supported by the European Union Framework Program 7 Marie-Curie grant 256432 and by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
Abbreviations
- CD
Crohn’s disease
- IBD
Inflammatory bowel disease
- IL
interleukin
- LPS
lipopolysaccharide
- MDP
muramyl-dipeptide
- NLR
NOD-like receptor
- DSS
dextran sodium sulfate
- TLR
Toll-like receptor
- TNBS
2,4,6-trinitrobenzene sulfonate
- UC
ulcerative colitis
Footnotes
Competing interest statement
The author declares no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8 (6):458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 2.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115 (1):182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 3.Goyette P, et al. Molecular pathogenesis of inflammatory bowel disease: genotypes, phenotypes and personalized medicine. Ann Med. 2007;39 (3):177–199. doi: 10.1080/07853890701197615. [DOI] [PubMed] [Google Scholar]
- 4.Kanneganti TD, et al. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27 (4):549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Kaser A, et al. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villani AC, et al. Common variants in the NLRP3 region contribute to Crohn's disease susceptibility. Nat Genet. 2009;41 (1):71–76. doi: 10.1038/ng285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanneganti TD, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440 (7081):233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 8.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440 (7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 9.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24 (3):317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 11.Sivakumar PV, et al. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50 (6):812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegmund B, et al. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281 (4):R1264–1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 13.Siegmund B. Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation. Biochem Pharmacol. 2002;64 (1):1–8. doi: 10.1016/s0006-2952(02)01064-x. [DOI] [PubMed] [Google Scholar]
- 14.Bauer C, et al. The ICE inhibitor pralnacasan prevents DSS-induced colitis in C57BL/6 mice and suppresses IP-10 mRNA but not TNF-alpha mRNA expression. Dig Dis Sci. 2007;52 (7):1642–1652. doi: 10.1007/s10620-007-9802-8. [DOI] [PubMed] [Google Scholar]
- 15.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207 (5):1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 32(3):367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Hirota SA, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. 2010 doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaki MH, et al. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32 (3):379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaki MH, et al. IL-18 Production Downstream of the Nlrp3 Inflammasome Confers Protection against Colorectal Tumor Formation. J Immunol. 2010 doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411 (6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 21.Rioux JD, Abbas AK. Paths to understanding the genetic basis of autoimmune disease. Nature. 2005;435 (7042):584–589. doi: 10.1038/nature03723. [DOI] [PubMed] [Google Scholar]
- 22.McGovern DP, et al. Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum Mol Genet. 2005;14 (10):1245–1250. doi: 10.1093/hmg/ddi135. [DOI] [PubMed] [Google Scholar]
- 23.Travassos LH, et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 11(1):55–62. doi: 10.1038/ni.1823. [DOI] [PubMed] [Google Scholar]
- 24.Rioux JD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39 (5):596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hampe J, et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39 (2):207–211. doi: 10.1038/ng1954. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman HM, et al. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29 (3):301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmann J, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71 (1):198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamkanfi M, Kanneganti TD. Nlrp3: an immune sensor of cellular stress and infection. Int J Biochem Cell Biol. 42(6):792–795. doi: 10.1016/j.biocel.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinon F, et al. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14 (21):1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 30.Lamkanfi M, Dixit VM. The inflammasomes. PLoS Pathog. 2009;5 (12):e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanneganti TD, et al. Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J Biol Chem. 2006;281(48):36560–36568. doi: 10.1074/jbc.M607594200. [DOI] [PubMed] [Google Scholar]
- 32.Muruve DA, et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452 (7183):103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 33.Thomas PG, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30 (4):566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ichinohe T, et al. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206 (1):79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen IC, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30 (4):556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gross O, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459 (7245):433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 37.Hise AG, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5 (5):487–497. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joly S, et al. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183 (6):3578–3581. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17 (13):1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 40.Eisenbarth SC, et al. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453 (7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, et al. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181 (1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38 (8):2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKee AS, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183 (7):4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Heel DA, et al. Muramyl dipeptide and toll-like receptor sensitivity in NOD2-associated Crohn's disease. Lancet. 2005;365 (9473):1794–1796. doi: 10.1016/S0140-6736(05)66582-8. [DOI] [PubMed] [Google Scholar]
- 45.Li J, et al. Regulation of IL-8 and IL-1beta expression in Crohn's disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13 (16):1715–1725. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 46.van Beelen AJ, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27 (4):660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Tamura K, et al. IL18 polymorphism is associated with an increased risk of Crohn's disease. J Gastroenterol. 2002;37(Suppl 14):111–116. doi: 10.1007/BF03326428. [DOI] [PubMed] [Google Scholar]
- 48.Zhernakova A, et al. Genetic analysis of innate immunity in Crohn's disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am J Hum Genet. 2008;82 (5):1202–1210. doi: 10.1016/j.ajhg.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pizarro TT, et al. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162 (11):6829–6835. [PubMed] [Google Scholar]
- 50.Monteleone G, et al. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999;163 (1):143–147. [PubMed] [Google Scholar]
- 51.Papadakis KA, Targan SR. Role of cytokines in the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2000;51:289–298. doi: 10.1146/annurev.med.51.1.289. [DOI] [PubMed] [Google Scholar]
- 52.Pizarro TT, et al. Mouse models for the study of Crohn's disease. Trends Mol Med. 2003;9 (5):218–222. doi: 10.1016/s1471-4914(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 53.Kitajima S, et al. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48 (3):137–143. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 54.Rakoff-Nahoum S, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118 (2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307 (5717):1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 56.Dupaul-Chicoine J, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32 (3):367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Simms LA, et al. Reduced alpha-defensin expression is associated with inflammation and not NOD2 mutation status in ileal Crohn's disease. Gut. 2008;57 (7):903–910. doi: 10.1136/gut.2007.142588. [DOI] [PubMed] [Google Scholar]
- 58.Wehkamp J, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53 (11):1658–1664. doi: 10.1136/gut.2003.032805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kummer JA, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55 (5):443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- 61.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446 (7135):557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 62.Reuter BK, Pizarro TT. Commentary: the role of the IL-18 system and other members of the IL-1R/TLR superfamily in innate mucosal immunity and the pathogenesis of inflammatory bowel disease: friend or foe? Eur J Immunol. 2004;34 (9):2347–2355. doi: 10.1002/eji.200425351. [DOI] [PubMed] [Google Scholar]
- 63.Takagi H, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38 (8):837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- 64.Lebeis SL, et al. Interleukin-1 receptor signaling protects mice from lethal intestinal damage caused by the attaching and effacing pathogen Citrobacter rodentium. Infect Immun. 2009;77 (2):604–614. doi: 10.1128/IAI.00907-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Araki A, et al. MyD88-deficient mice develop severe intestinal inflammation in dextran sodium sulfate colitis. J Gastroenterol. 2005;40 (1):16–23. doi: 10.1007/s00535-004-1492-9. [DOI] [PubMed] [Google Scholar]
- 66.Fukata M, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288 (5):G1055–1065. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 67.Siegmund B, et al. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci U S A. 2001;98 (23):13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loher F, et al. The interleukin-1 beta-converting enzyme inhibitor pralnacasan reduces dextran sulfate sodium-induced murine colitis and T helper 1 T-cell activation. J Pharmacol Exp Ther. 2004;308 (2):583–590. doi: 10.1124/jpet.103.057059. [DOI] [PubMed] [Google Scholar]
- 69.Hirota SA, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis. doi: 10.1002/ibd.21478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bauer C, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59 (9):1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- 71.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287 (1):G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 72.Chen GY, et al. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 2008;68 (24):10060–10067. doi: 10.1158/0008-5472.CAN-08-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts RL, et al. Caspase recruitment domain-containing protein 15 mutations in patients with colorectal cancer. Cancer Res. 2006;66 (5):2532–2535. doi: 10.1158/0008-5472.CAN-05-4165. [DOI] [PubMed] [Google Scholar]
- 74.Micallef MJ, et al. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57 (20):4557–4563. [PubMed] [Google Scholar]
- 75.Micallef MJ, et al. In vivo antitumor effects of murine interferon-gamma-inducing factor/interleukin-18 in mice bearing syngeneic Meth A sarcoma malignant ascites. Cancer Immunol Immunother. 1997;43 (6):361–367. doi: 10.1007/s002620050345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Osaki T, et al. Potent antitumor effects mediated by local expression of the mature form of the interferon-gamma inducing factor, interleukin-18 (IL-18) Gene Ther. 1999;6 (5):808–815. doi: 10.1038/sj.gt.3300908. [DOI] [PubMed] [Google Scholar]
- 77.Osaki T, et al. IFN-gamma-inducing factor/IL-18 administration mediates IFN-gamma- and IL-12-independent antitumor effects. J Immunol. 1998;160 (4):1742–1749. [PubMed] [Google Scholar]
- 78.Cao E, et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26 (3):311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Coughlin CM, et al. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101 (6):1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hegardt P, et al. Nitric oxide synthase inhibitor and IL-18 enhance the anti-tumor immune response of rats carrying an intrahepatic colon carcinoma. Cancer Immunol Immunother. 2001;50 (9):491–501. doi: 10.1007/s002620100230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salcedo R, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J Exp Med. 2010;207 (8):1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nava P, et al. Interferon-gamma regulates intestinal epithelial homeostasis through converging beta-catenin signaling pathways. Immunity. 2010;32 (3):392–402. doi: 10.1016/j.immuni.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kanai T, et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology. 2001;121 (4):875–888. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- 84.Nakamura S, et al. IFN-gamma-dependent and -independent mechanisms in adverse effects caused by concomitant administration of IL-18 and IL-12. J Immunol. 2000;164 (6):3330–3336. doi: 10.4049/jimmunol.164.6.3330. [DOI] [PubMed] [Google Scholar]
- 85.Ten Hove T, et al. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001;121 (6):1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- 86.Wirtz S, et al. Treatment of T cell-dependent experimental colitis in SCID mice by local administration of an adenovirus expressing IL-18 antisense mRNA. J Immunol. 2002;168 (1):411–420. doi: 10.4049/jimmunol.168.1.411. [DOI] [PubMed] [Google Scholar]
- 87.Siegmund B. Interleukin-18 in intestinal inflammation: friend and foe? Immunity. 32(3):300–302. doi: 10.1016/j.immuni.2010.03.010. [DOI] [PubMed] [Google Scholar]