SUMMARY OF RECENT ADVANCES
Oncogenic human papillomaviruses (HPV) are exclusively mucosal pathogens that are non-cytopathic and the basal epithelial cells harboring and maintaining an infection do not produce either capsid antigen or virus. The efficacy of the licensed L1 virus-like particle (VLP) vaccines has encouraged development of several second generation vaccines aimed at expanding the coverage to all oncogenic HPV types and reducing barriers to global implementation. Currently there is no defined immune correlate of protection that can be used to determine if an individual patient is protected and for the evaluation of these second generation vaccines. Surprisingly, passive transfer of neutralizing serum antibody is protective in animal models. Recent studies suggest how neutralizing antibody mediates immunity against mucosal HPV and the possible impact of memory B cells.
INTRODUCTION
Like the hepatitis B vaccine, the development of the two licensed HPV vaccines is a medical triumph in both infectious disease and cancer prevention because the viruses targeted are each responsible for approximately 5% of all cancer cases worldwide. The two licensed HPV vaccines, Cervarix® (GSK) and Gardasil® (Merck), are composed of virus-like particles derived from the major capsid protein L1 of the most prevalent types in cancer, HPV16 and HPV18, and Gardasil also includes L1 VLP of the two benign types HPV6 and HPV11 that cause ~90% of genital warts. While L1 VLP are highly immunogenic alone, both vaccines utilize an adjuvant; Gardasil contains amorphous aluminum hydroxyphosphate sulphate (AAHS), whereas Cervarix utilizes aluminum hydroxide and a toll-like receptor (TLR)-4 agonist, 3-O-desacyl-4’-monophosphoryl lipid A (MPL).
The primary etiologic role of persistent HPV infection in causing ~500,000 cervical cancer cases worldwide each year is firmly established, and thus with the advent of HPV vaccines and continued efforts in cytologic and viral DNA-based screening for precursor lesions (Figure 1) and ablative therapy of high grade squamous intraepithelial neoplasia (SIL), cervical cancer is a preventable disease [1,2]. Further, 40% of penile cancers, 40% of vaginal and vulval cancers, 90% of anal cancers, 3% of mouth cancers and 12% of oro-pharyngeal cancers are also triggered by HPV and potentially preventable through vaccination [3]. HPV is a small, non-enveloped DNA tumor virus and over 120 HPV genotypes have been described [2]. With increasingly sensitive new technologies investigators continue to find new types, but only approximately a dozen sexually-transmitted ‘oncogenic’ HPV types are responsible for more than 95% of cervical cancer cases [1].
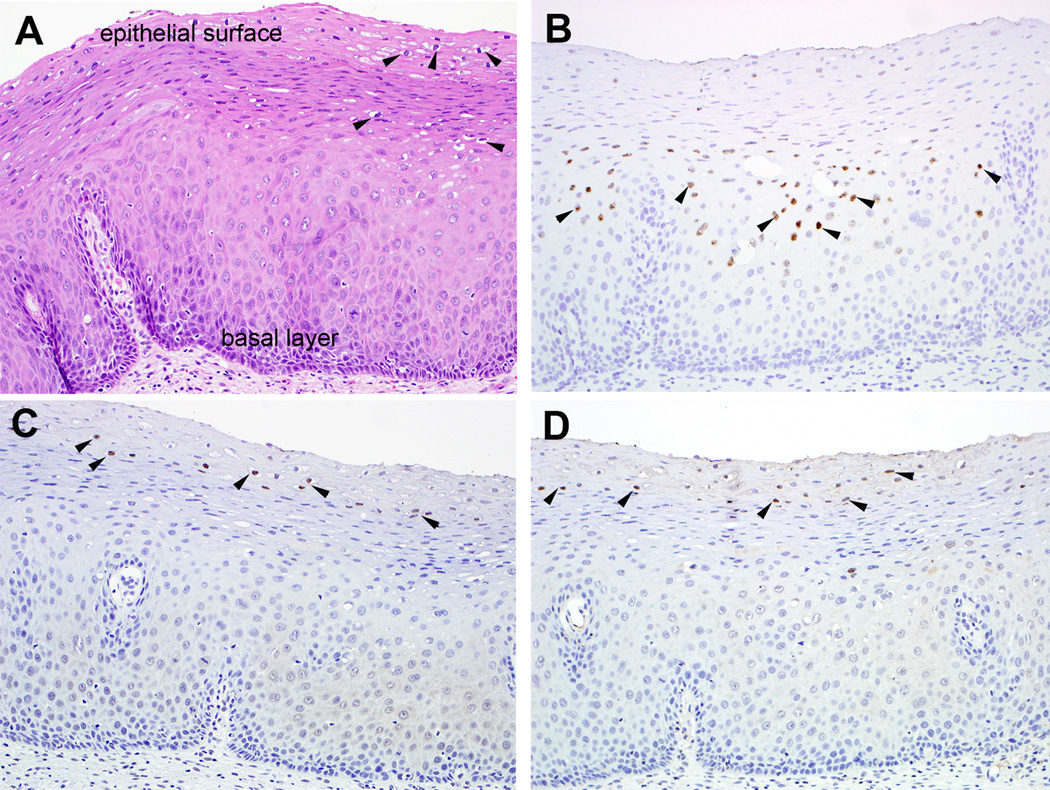
Figure 1. Localization of capsid protein expression and viral genomes in a productive HPV16 lesion (Low grade squamous intraepithelial lesion (LSIL/CIN1)).
A. Hematoxylin and eosin-stained section of cervical squamous epithelium; Keratinocytes with markedly enlarged nuclei surrounded by cytoplasmic halo (arrowheads) in the upper epithelial layers characteristic of LSIL, B. In situ hybridization for HPV16 genome; discreet nuclear signals (arrowheads) predominantly in the middle zone of the epithelium where the proliferating cells are just beginning to differentiate, C. Immunohistochemistry of L1 using monoclonal antibody 1H8, D. Immunohistochemistry for L2 using monoclonal antibody RG-1. Positive immunolabeling in the occasional keratinocyte nuclei limited to the upper epithelial layers (arrowheads). The exquisite temporal/spacial regulation of viral genome copy number and late gene expression is apparent in this productive HPV16 lesion. In the basal epithelial cells the viral genome copy number is low (~100 episomes/nucleus), but as the cells divide and move up through the epithelium their differentiation triggers vegetative viral replication (producing a high copy number of ~104 episomes/cell), and in the uppermost regions of the lesion expression of the major L1 and minor L2 capsid proteins.
IMMUNODOMINANT AND COFORMATIONALLY-DEPENDEDNT NEUTRALIZING EPITOPES OF L1 VLP
Since HPV utilizes the host DNA polymerase for replication of its genome at a low error rate, HPV sequences have remained very stable, although it is likely that the different HPV genotypes have emerged over eons based on distinct tissue tropism (e.g. cutaneous versus mucosal epithelia) and to escape immune responses [4]. Indeed, while the sequences of the internal regions of L1 are highly conserved, the surface loops are hypervariable between HPV types and correspond to type-restricted and immune-dominant neutralizing epitopes [5]. This suggests that the preferential accumulation of these changes in the hypervariable loops may reflect both selection to escape protective antibody responses and a lack of structural constraints in these loops. Thus L1 VLP-reactive neutralizing antibodies predominantly recognize only the type against which they were raised, and consequently most genotypes are different serotypes [6]. Another notable feature is that the neutralizing antibodies overwhelmingly recognize conformational/non-linear epitopes, and this may also contribute to the specificity of binding [7]. Thus the licensed vaccines target the two most common HPV types found in cervical cancer, HPV16 and HPV18 (and one also targets the two most prevalent types in benign genital warts, HPV6 and HPV11) and this produces robust protection against persistent infection and intraepithelial lesions caused by these HPV types, but variable efficacy of protection of potentially reduced duration for other related HPV types [8,9]. For example vaccination with HPV16 L1 VLPs can also provide strong protection against HPV31 [10,11]. This is presumably because the sequence of HPV31 L1 is evolutionarily most closely related to HPV16 [12]. However the cross-neutralizing antibody titer is orders of magnitude lower than to the HPV16-specific response (Kemp T et al., Abstract BS2, 26th International Papillomavirus Conference, Montreal, Canada, July 2010). While this suggests that low titers of neutralizing antibody are sufficient for protection, it raises the question of the longevity of the cross-protective response. Similarly, HPV18 L1 VLPs can trigger an HPV45 cross-neutralizing and cross-protective response, but protection against other more divergent types is weak [10,11,13]. This contrasts the T cell responses to HPV L1 VLP which show some type-restriction but in general are very broad, likely reflecting recognition of epitopes within the conserved internal portions of the L1 capsid [14]. As a consequence of the type restricted nature of L1 VLP vaccines, there are ongoing clinical trials of a highly multivalent vaccine, comprising L1 VLPs of the two types found in ~90% of genital warts (HPV6 and HPV11) and the seven most prevalent HPV types detected in ~90% of cervical cancer cases (HPV16, HPV18, HPV31, HPV33, HPV45, HPV52 and HPV58) and efficacy data is expected in 2012.
While the licensed vaccines both utilize an adjuvant, L1 VLPs are remarkably immunogenic and produce robust neutralizing antibody responses even when patients are vaccinated without an adjuvant [15]. Indeed, a single dose of the licensed vaccine provides at least short term protection, although the standard regimen is three immunizations (Kreimer AR et al., Abstract LBA, 26th International Papillomavirus Conference, Montreal, Canada, July 2010). It is likely that several features of L1 VLP contribute to this immunogenicity. Firstly, their highly regular and close-packed display of neutralizing epitopes on the surface of the VLPs provides increased avidity for and cross-linking of reactive B cell receptors lowering the threshold for their activation compared to a monovalent antigen [16]. Secondly, L1 VLP can inherently activate both immature human myeloid and plasmacytoid dendritic cells (but not Langerhans cells) [17–19]. Thirdly, in contrast to typical protein antigens, L1 VLP are rapidly taken up by immune cells facilitating presentation of MHCI and II epitopes, and producing robust cellular immune responses to vaccination [20].
MECHANISM OF PROTECTION AND IMMUNE CORRELATES
An important unanswered question is how vaccination with L1 VLP mediates protection. This question has been addressed in animal models, notably cottontail rabbit papillomavirus (CRPV) challenge of rabbits and canine oral papillomavirus (COPV) challenge of dogs. Specifically, passive transfer of sera from L1 VLP immunized animals protected naïve animals from experimental viral challenge [21,22]. This implies that neutralizing antibodies are sufficient to mediate protection, but does not rule out a contribution of cell-mediated immunity in protection. However, parenteral vaccination with L1 VLP did not impact wart growth in animal models, suggesting minimal impact of L1-specific T cell responses on established lesions. It should also be noted that vaccination with L1 VLP induced type-specific neutralizing antibodies and protection in animals, and that vaccination with denatured L1 failed to induce significant titers of neutralizing antibodies and was not protective.
Clinical studies of potential immune correlates of protection have centered upon L1-specific serum antibody responses, as measured by Competitive Luminex-based Immuno-Assay (CLIA), L1 VLP ELISA, and in vitro neutralization studies [23]. Each of these assays indicates that almost all vaccinated patients generate a robust type-restricted serum antibody response to L1 VLP, corresponding with the high efficacy of type-restricted protection. The serum antibody titers wane to a plateau a few months after the final immunization and appear stable thereafter. In one study of the licensed quadrivalent HPV vaccine, HPV18 L1 VLP specific antibody titers determined by CLIA waned below the threshold of detection in 40% of patients by 48 months although the titers to the three other types in the vaccine remained detectable [24]. Despite the absence of CLIA-detectable HPV18 L1 VLP-specific antibodies, no new HPV18 infections were observed, suggesting that immunity remained [24]. This suggests that either immunity to HPV18 in these patients is cell-mediated, or reactivation of memory B cells or the titer of neutralizing antibody required for protection is very low and its measurement by CLIA lacks adequate sensitivity. Although L1 VLP vaccination induces robust T cell responses in patients, the absence of a profound therapeutic effect of the licensed vaccines upon established HPV infection suggest that it is unlikely that the protection against HPV18 in the absence of a CLIA-detectable antibody response is T cell mediated. Alternatively, it is possible that exposure to the viral inoculums triggers a reactivation of the B cell response and local production of neutralizing antibody in time to prevent an initial infection. However, several factors point to the limited sensitivity of the CLIA for detection of HPV18 L1-specific antibody as an explanation for this observation, and that low, but protective levels of neutralizing antibodies are maintained. Firstly, the threshold of this assay is set using antibody levels in natural infection, and it is likely that these titers are protective. Secondly, CLIA detects responses via competition of patient antibodies with a high avidity neutralizing monoclonal antibody to L1 VLP, and thus primarily detects high avidity responses to a single epitope. It is known that multiple neutralizing epitopes are displayed by L1 VLP, and thus these patients may have protective antibodies to other epitopes, and/or protective antibodies of low avidity that are poorly detected by CLIA.
While the L1 VLP ELISA assay is more sensitive than CLIA, it also detects non-neutralizing and presumably non-protective antibodies and may provide false positives. In vitro neutralization assays measure functional antibodies, but native HPV virions are hard to generate for all types and in sufficient quantities. Furthermore, the readout of infection is early spliced viral mRNA assayed by quantitative RT-PCR, and this is relatively cumbersome. A technology based on infection of 293TT cells with HPV pseudovirions that carry marker genes has greatly simplified the in vitro neutralization assay [25]. Nevertheless, recent passive transfer studies in mice suggest that the in vitro neutralization assay may lack the sensitivity to detect the minimal protective level of antibody, and that the passive transfer approach may provide a better approach, albeit low throughput [26]. This may reflect differing mechanisms of infection and thus neutralization in vivo within the genital mucosa [26] as compared to in vitro with 293TT target cells [27] (Figures 2 and 3 respectively). In vitro neutralization assays also depend upon extensive dilution to measure antibody levels, and it is possible that low avidity antibodies are protective and poorly detected by this in vitro assay [28]. Nevertheless, the range of HPV types cross-neutralized by the sera of L1 VLP-vaccinated patients appears to correspond to the breadth of protection.
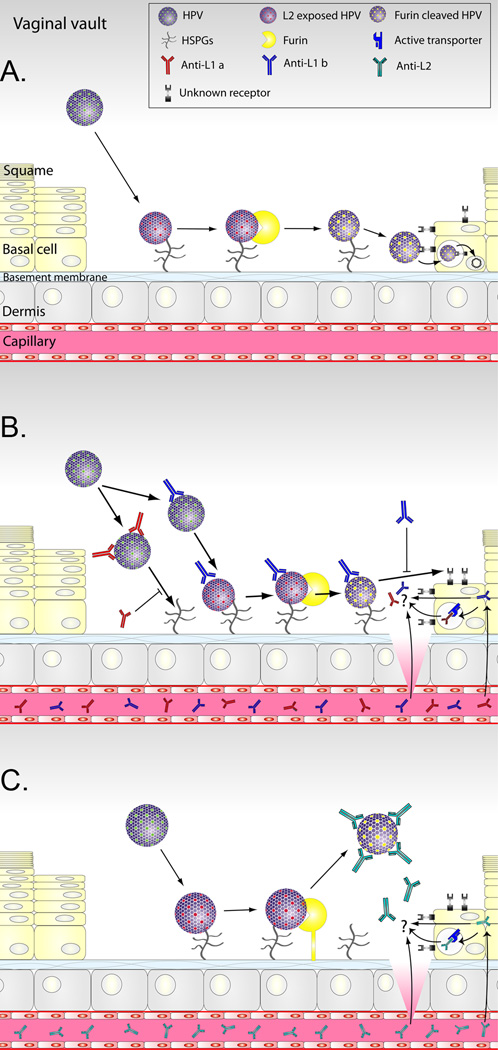
Figure 2. Models for the mechanisms of HPV infection in vivo and antibody-mediated protection.
Day et al. have proposed models of infection and immunity based upon microscopic studies of HPV pseudovirions during vaginal challenge of naïve mice and those immunized with L1 or L2 [26]. A. Infection of vaginal epithelium. Microtrauma to the squamous epithelium of the vagina/and or cervix associated with intercourse provides HPV access to the basement membrane. HPV binds to the basement membrane via heparan sulphate glycosaminoglycans (HSPGs) and this triggers a conformational change in the capsid exposing L2 for N-terminal clipping by secreted furin. The furin-cleaved HPV binds to a viral receptor on the surface of basal epithelial cells during wound healing to initiate infection. B. L1 VLP-specific antibody-mediated protection against vaginal infection. In hosts vaccinated with L1 VLPs, neutralizing IgG passively transudates from the capillaries into the cervical/vaginal fluid and/or is actively exchanged. If the trauma is sufficient, direct exudation from plasma is possible at the site of wounding. High concentrations of L1 VLP-specific antibodies can prevent virions binding to the basement membrane. In the presence of lower antibody levels the binding of HPV to the basement membrane, and the cleavage of L2 by furin still occurs, but the virions are unable to transfer to the viral receptor on the basal epithelial cells and the virion-antibody complexes are released. C. L2-specific antibody-mediated protection against vaginal infection. In the presence of L2-specific antibody, the binding of HPV to the basement membrane, and the exposure of L2 occurs. However, the antibodies bind to epitopes in the N-terminus of L2 after its cleavage by furin and the virions are unable to transfer to the viral receptor on the basal epithelial cells, leading to release of the virion-antibody complexes.
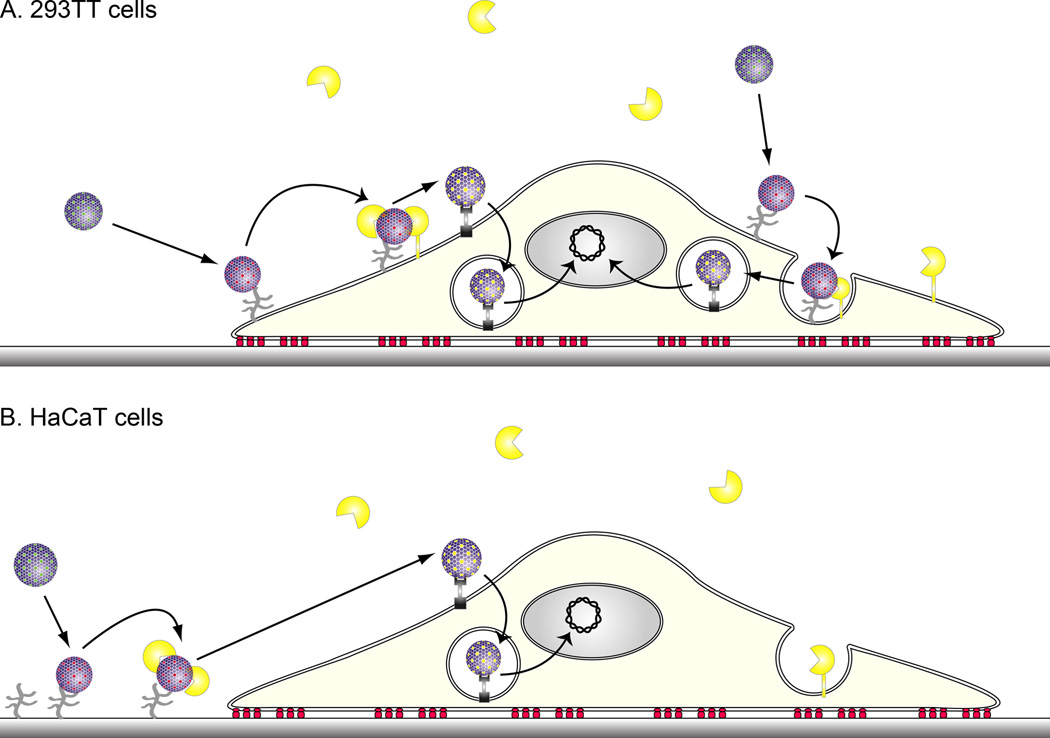
Figure 3. In vitro infection of 293TT and HaCaT cells by HPV.
A. In vitro infection of 293TT cells. Early studies of in vitro infection by HPV utilized 293TT or other transformed cell lines as target cells. HPV binds directly to the 293TT cell surface via HSPGs which triggers a conformational change in the capsid such that the amino terminus minor capsid protein L2 becomes exposed. Exposure of L2 renders it accessible to cleavage by furin [50]. Furin-cleavage of L2 is essential to infection and is associated with escape of L2 and the viral genome from the endosome. γ secretase activity is also required for infection, but it is unclear what it cleaves [51]. The viral genome-L2 complex is too large to cross the nuclear envelope, but gains access to the nucleus as during mitosis while its membrane has dissolved [52]. B. In vitro infection of HaCaT cells or primary keratinocytes. In contrast to 293TT cells, HPV binds first to the laminin-5 associated extracellular matrix via HSPGs secreted upon the culture surface prior to infection of the immortalized keratinocyte line HaCaT or primary keratinocytes. Upon attachment to the extracellular matrix, the HPV virions undergo a conformational shift that exposes L2 for cleavage by secreted furin. Only then can the virions bind to a secondary receptor on the keratinocyte surface for subsequent uptake. Antibodies reacting with particular loops on the capsid surface (e.g. H16.V5 and H16.E70 monoclonal antibodies) can prevent virions binding to the extracellular matrix, but these antibody-bound virions can still bind the cell surface, although infection does not occur. In the presence of antibodies reacting with other surface L1 loops (e.g. H16.U4 monoclonal antibody), the binding of HPV to the basement membrane, and the cleavage of L2 by furin still occurs, but the virions are unable to transfer to the viral receptor on the basal epithelial cells [27]. Likewise, L2-specific antibodies (e.g. RG-1 monoclonal antibody) allow the binding of HPV to the basement membrane, and the exposure of L2. The antibody binds to the exposed L2 after its cleavage by furin and the virions are unable to transfer to the viral receptor on the basal epithelial cells [27].
That serum L1 VLP-specific antibody titer better correlates with protection than the robust L1-specific cell mediated response raises several questions. Firstly, why does the L1-specific cellular immune response not clear established HPV infections? This may be explained in part by the unique biology of HPV [29]. HPV does not produce viremia, but rather is confined to epithelial lesions above the basement membrane and systemic T cell responses may not reach these lesions or may be suppressed therein. Most importantly, while all HPV infected cells express early genes E6 and E7, the capsid proteins are only expressed in the upper differentiating and dying layers (Figure 1). Consequently, L1-specific cellular immune responses do not target the basal epithelial cells that harbor the infection, unless some type of bystander response can be triggered [29]. The second surprise is that serum neutralizing IgG titers are the relevant correlate for a purely mucosal infection and begs the question of how these antibodies reach the viral inoculum since systemic vaccination typical fails to induce a local IgA response (Figure 2). L1 VLP-specific IgG is detected in the vaginal fluid of vaccination patients and its level correlates with serum titer, although it varies with the menstrual cycle [30].
This indicates the occurrence of either active transport or passive transudation of the IgG into the vaginal fluid wherein it neutralizes the viral inoculum. However, it does not explain protection at cutaneous sites against HPV6 and HPV11 infections induced by vaccination. A second, not mutually exclusive, possibility is that the microtrauma that is associated with infection during intercourse and facilitating access for HPV to the basal epithelia may trigger a local exudation directly from plasma to the site of infection (Figure 2). It is unclear whether a minimal level of neutralizing antibody must be maintained sufficient to provide sterilizing immunity, or whether the viral inoculum can trigger a rapid anamnestic activation of memory B cells to produce neutralizing antibody locally [31]. The slow course of HPV infection and the ability to neutralize the virus many hours after binding to cell surfaces suggests that the latter is a possibility [32]. Indeed re-vaccination of individuals clearly triggers a robust anamnestic antibody response, but it is unclear if it happens within the window for neutralization [33].
CANDIDATES FOR SECOND GENERATION HPV VACCINES
The identification of a correlate of protection is important to identify whether an individual immunization is successful, to validate batches or sources of L1 VLP vaccine produced by different manufacturers and/or in different systems (e.g. bacteria or plants [34,35]) and also in the development of second generation preventive HPV vaccines. One such potential second generation vaccine comprises L1 capsomers (the pentameric subunit of the VLP) that can be readily produced in bacteria [36]. The L1 capsomer-based vaccine offers potential advantages of reduced cost, stability at ambient temperature that could facilitate introduction into low resource settings where HPV vaccines are most needed. Although L1 capsomers induce lower neutralizing antibody titers than VLP, protection is robust even without adjuvant and the use of an adjuvant can potentially close this gap [36,37]. Another approach of HPV vaccination is the use of naked DNA vectors, which are simple to manufacture, heat stable and delivered by gene gun, tattoo or electroporation to express codon-modified L1 in vivo [38]. The use of needles for immunization also provides a barrier to widespread use, and there are efforts to use live recombinant vectors, such as an L1 recombinant version of the orally administered typhoid vaccine S. typhi [39]. The VLP vaccine could also be combined with other vaccines, either by mixing together, or by introducing the L1 gene, for example into measles vaccine, or the tuberculosis vaccine BCG etc, to deliver L1 [40,41].
In selecting a correlate of protection, it should be both simple to use, readily standardized into pre-defined international units and ideally not specific to L1. The latter point is relevant because of several efforts to second generation HPV vaccines based upon the minor capsid antigen L2 [42]. Vaccination with L2 protects animals from experimental viral challenge, but this immunity lacks the type restriction associated with L1 VLP vaccines [43,44]. The protection is mediated by broadly neutralizing antibodies that recognize conserved linear epitopes in the N-terminus of L2 [43,45]. Residues within the protective region of L2 play a critical role in viral infection and thus their sequence is conserved even in diverse HPV types [46,47] (Figure 3). The L2 cross-neutralizing epitope appears to be displayed on the capsid surface only during infection, and thus vaccination with virions or L1/L2 VLPs induces limited, if any, L2-specific antibody [27,44]. Unfortunately L2 does not form a particulate structure alone and therefore is significantly less immunogenic than L1 VLP, suggesting the need for an adjuvant for vaccination or for multimeric display of L2 in an immune-dominant epitope of a recombinant VLP [48,49].
CONCLUSIONS
The licensed HPV vaccines will dramatically reduce the incidence of cervical cancer in the years to come. However, the global impact upon HPV-associated cancer rates will depend greatly upon the extent of implementation of vaccination. Second generation HPV vaccines are being developed to overcome barriers to global implementation and expand the breath of protection. The development of robust immune correlate of protection with international units and a more detailed understanding of the immunologic mechanisms underlying the efficacy of the licensed HPV vaccines are important to further the development of such second generation HPV vaccines. It is also important to note that HPV vaccines targeting the viral capsid antigens alone do not appear to impact the course of preexisting infections, and that continued efforts to develop therapeutic HPV vaccines (or even combination preventive and therapeutic HPV vaccines) should remain a priority given the current high prevalence of HPV-associated disease.
ACKNOWLEDGEMENTS
Funding was provided by the National Institutes of Health (National Cancer Institute, R01CA118790, R01 CA133749 and SPORE in Cervical Cancer, P50 CA098252 to RBSR. We apologize to those whose work was not cited due to constraints in the number of references that could be included, and for those whose important work was not highlighted because it is more than 2 years past.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: RBSR has served as a paid consultant of Merck & Co. RBSR is an inventor on L2 patents licensed to Shantha Biotechnics, PaxVax, Inc., Sanofi Pasteur and GlaxoSmithKline. The terms of these arrangements are being managed by Johns Hopkins University in accordance with its conflict of interest policies.
BIBLIOGRAPHY
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Munoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature Rev Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24 Suppl 3:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 4.Yamada T, Manos MM, Peto J, Greer CE, Munoz N, Bosch FX, Wheeler CM. Human papillomavirus type 16 sequence variation in cervical cancers: a worldwide perspective. J Virol. 1997;71:2463–2472. doi: 10.1128/jvi.71.3.2463-2472.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–567. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- 6.Roden RB, Greenstone HL, Kirnbauer R, Booy FP, Jessie J, Lowy DR, Schiller JT. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J Virol. 1996;70:5875–5883. doi: 10.1128/jvi.70.9.5875-5883.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89:12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomized double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 9.Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, Jenkins D, Schuind A, Costa Clemens SA, Dubin G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 10.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 11. Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Tay EH, Garcia P, et al. The Impact of Quadrivalent Human Papillomavirus (HPV; Types 6, 11, 16, and 18) L1 Virus-Like Particle Vaccine on Infection and Disease Due to Oncogenic Nonvaccine HPV Types in Generally HPVNaive Women Aged 16–26 Years. J Infect Dis. 2009;199:926–935. doi: 10.1086/597307.Reveals the spectrum of protection among oncogenic HPV types provided by the licensed quadrivalent HPV vaccine
- 12. Bernard HU, Burk RD, Chen Z, van Doorslaer K, Hausen H, de Villiers EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. 401:70–79. doi: 10.1016/j.virol.2010.02.002.The updated phylogeny of papillomaviruses
- 13.Roden RB, Hubbert NL, Kirnbauer R, Christensen ND, Lowy DR, Schiller JT. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J Virol. 1996;70:3298–3301. doi: 10.1128/jvi.70.5.3298-3301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto LA, Viscidi R, Harro CD, Kemp TJ, Garcia-Pineres AJ, Trivett M, Demuth F, Lowy DR, Schiller JT, Berzofsky JA, et al. Cellular immune responses to HPV-18, -31, and -53 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. Virology. 2006;353:451–462. doi: 10.1016/j.virol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, Mast TC, Robinson R, Murphy BR, Karron RA, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93:284–292. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 16.Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science. 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 17.Lenz P, Thompson CD, Day PM, Bacot SM, Lowy DR, Schiller JT. Interaction of papillomavirus virus-like particles with human myeloid antigen-presenting cells. Clin Immunol. 2003;106:231–237. doi: 10.1016/s1521-6616(02)00039-6. [DOI] [PubMed] [Google Scholar]
- 18.Lenz P, Lowy DR, Schiller JT. Papillomavirus virus-like particles induce cytokines characteristic of innate immune responses in plasmacytoid dendritic cells. Eur J Immunol. 2005;35:1548–1556. doi: 10.1002/eji.200425547. [DOI] [PubMed] [Google Scholar]
- 19.Fausch SC, Da Silva DM, Rudolf MP, Kast WM. Human papillomavirus virus-like particles do not activate Langerhans cells: a possible immune escape mechanism used by human papillomaviruses. J Immunol. 2002;169:3242–3249. doi: 10.4049/jimmunol.169.6.3242. [DOI] [PubMed] [Google Scholar]
- 20.Rudolf MP, Fausch SC, Da Silva DM, Kast WM. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitopespecific human T cell responses in vitro. J Immunol. 2001;166:5917–5924. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- 21.Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, Schiller JT, Lowy DR. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–3963. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzich JA, Ghim SJ, Palmer-Hill FJ, White WI, Tamura JK, Bell JA, Newsome JA, Jenson AB, Schlegel R. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci U S A. 1995;92:11553–11557. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frazer IH. Measuring serum antibody to human papillomavirus following infection or vaccination. Gynecol Oncol. 2010;118:S8–S11. doi: 10.1016/j.ygyno.2010.04.003. Describes the assays for immune responses of patients to HPV vaccines
- 24. Joura EA, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, et al. HPV antibody levels and clinical efficacy following administration of a prophylactic quadrivalent HPV vaccine. Vaccine. 2008;26:6844–6851. doi: 10.1016/j.vaccine.2008.09.073.Describes continued protection despite the absence of CLIA-detectable HPV18 L1 VLP-specific antibodies in patients four years after vaccination
- 25.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Kruger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–216. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 26. Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 8:260–270. doi: 10.1016/j.chom.2010.08.003. Microscopic analysis of the mechanism of protection of mice against vaginal challenge with HPV after vaccination with L1 VLP or multimeric L2 protein
- 27. Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by L2 cross-neutralizing and L1 type-specific antibodies. J Virol. 2008;82:4638–4646. doi: 10.1128/JVI.00143-08.Analysis of the mechanisms of in vitro neutralization by L1 VLP and L2-specific antibodies
- 28.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 29.Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005;32 Suppl 1:S7–S15. doi: 10.1016/j.jcv.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De Grandi P. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J Natl Cancer Inst. 2003;95:1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 31. Dauner JG, Pan Y, Hildesheim A, Harro C, Pinto LA. Characterization of the HPV-specific memory B cell and systemic antibody responses in women receiving an unadjuvanted HPV16 L1 VLP vaccine. Vaccine. 28:5407–5413. doi: 10.1016/j.vaccine.2010.06.018. Analysis of memory B cells, antibody avidity and neutralizing antibodies in women of an HPV vaccine trial
- 32.Christensen ND, Cladel NM, Reed CA. Postattachment neutralization of papillomaviruses by monoclonal and polyclonal antibodies. Virology. 1995;207:136–142. doi: 10.1006/viro.1995.1059. [DOI] [PubMed] [Google Scholar]
- 33.Olsson SE, Villa LL, Costa RL, Petta CA, Andrade RP, Malm C, Iversen OE, Hoye J, Steinwall M, Riis-Johannessen G, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007;25:4931–4939. doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 34.Chen XS, Casini G, Harrison SC, Garcea RL. Papillomavirus capsid protein expression in Escherichia coli: purification and assembly of HPV11 and HPV16 L1. J Mol Biol. 2001;307:173–182. doi: 10.1006/jmbi.2000.4464. [DOI] [PubMed] [Google Scholar]
- 35.Maclean J, Koekemoer M, Olivier AJ, Stewart D, Hitzeroth II, Rademacher T, Fischer R, Williamson AL, Rybicki EP. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J Gen Virol. 2007;88:1460–1469. doi: 10.1099/vir.0.82718-0. [DOI] [PubMed] [Google Scholar]
- 36.Yuan H, Estes PA, Chen Y, Newsome J, Olcese VA, Garcea RL, Schlegel R. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J Virol. 2001;75:7848–7853. doi: 10.1128/JVI.75.17.7848-7853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jagu S, Kwak K, Garcea RL, Roden RB. Vaccination with multimeric L2 fusion protein and L1 VLP or capsomeres to broaden protection against HPV infection. Vaccine. 28:4478–4486. doi: 10.1016/j.vaccine.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pokorna D, Rubio I, Muller M. DNA-vaccination via tattooing induces stronger humoral and cellular immune responses than intramuscular delivery supported by molecular adjuvants. Genet Vaccines Ther. 2008;6:4. doi: 10.1186/1479-0556-6-4. Describes naked DNA vectors expressing L1 as potential second generation HPV vaccines
- 39.Nardelli-Haefliger D, Benyacoub J, Lemoine R, Hopkins-Donaldson S, Potts A, Hartman F, Kraehenbuhl JP, De Grandi P. Nasal vaccination with attenuated Salmonella typhimurium strains expressing the Hepatitis B nucleocapsid: dose response analysis. Vaccine. 2001;19:2854–2861. doi: 10.1016/s0264-410x(01)00009-3. [DOI] [PubMed] [Google Scholar]
- 40. Cantarella G, Liniger M, Zuniga A, Schiller JT, Billeter M, Naim HY, Glueck R. Recombinant measles virus-HPV vaccine candidates for prevention of cervical carcinoma. Vaccine. 2009;27:3385–3390. doi: 10.1016/j.vaccine.2009.01.061. Shows the possibility of combining measles and HPV vaccination
- 41.Govan VA, Christensen ND, Berkower C, Jacobs WR, Jr, Williamson AL. Immunisation with recombinant BCG expressing the cottontail rabbit papillomavirus (CRPV) L1 gene provides protection from CRPV challenge. Vaccine. 2006;24:2087–2093. doi: 10.1016/j.vaccine.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 42.Karanam B, Jagu S, Huh WK, Roden RB. Developing vaccines against minor capsid antigen L2 to prevent papillomavirus infection. Immunol Cell Biol. 2009;87:287–299. doi: 10.1038/icb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaukroger JM, Chandrachud LM, O'Neil BW, Grindlay GJ, Knowles G, Campo MS. Vaccination of cattle with bovine papillomavirus type 4 L2 elicits the production of virus-neutralizing antibodies. J Gen Virol. 1996;77:1577–1583. doi: 10.1099/0022-1317-77-7-1577. [DOI] [PubMed] [Google Scholar]
- 44.Roden RB, Yutzy WHt, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270:254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- 45.Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RB. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of Human Papillomavirus L2. J Virol. 2007;81:13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gambhira R, Jagu S, Karanam B, Day PM, Roden R. Role of L2 cysteines in papillomavirus infection and neutralization. Virol J. 2009;6:176. doi: 10.1186/1743-422X-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jagu S, Karanam B, Gambhira R, Chivukula SV, Chaganti RJ, Lowy DR, Schiller JT, Roden RB. Concatenated multitype L2 fusion proteins as candidate prophylactic pan-human papillomavirus vaccines. J Natl Cancer Inst. 2009;101:782–792. doi: 10.1093/jnci/djp106. Describes the potential of multimers of L2 to induce very broad cross-type neutralizing antibodies and protection
- 49. Schellenbacher C, Roden R, Kirnbauer R. Chimeric L1-L2 virus-like particles as potential broad-spectrum human papillomavirus vaccines. J Virol. 2009;83:10085–10095. doi: 10.1128/JVI.01088-09.Shows the possibility of mutlimeric L2 display in the context of the L1 VLP
- 50.Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc Natl Acad Sci U S A. 2006;103:1522–1527. doi: 10.1073/pnas.0508815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Karanam B, Peng S, Li T, Buck C, Day PM, Roden RB. Papillomavirus infection requires gamma secretase. J Virol. 84:10661–10670. doi: 10.1128/JVI.01081-10.Describes a surprizing role for γ secretase in HPV infection
- 52. Pyeon D, Pearce SM, Lank SM, Ahlquist P, Lambert PF. Establishment of human papillomavirus infection requires cell cycle progression. PLoS Pathog. 2009;5:e1000318. doi: 10.1371/journal.ppat.1000318.Describes a requirement for active cell division for HPV infection