Abstract
Hookworms are human parasites that have devastating effects on global health, particularly in underdeveloped countries. Ancylostoma ceylanicum infects humans and animals, making it a useful model organism to study disease pathogenesis. A. ceylanicum excretory-secretory protein 2 (AceES-2), a highly immunoreactive molecule secreted by adult worms at the site of intestinal attachment, is partially protective when administered as a mucosal vaccine against hookworm anemia. The crystal structure of AceES-2 determined at 1.75 Å resolution shows that AceES-2 adopts a netrin-like fold similar to those found in tissue inhibitors of matrix metalloproteases (TIMPs), and in complement factors C3 and C5. However, recombinant AceES-2 does not significantly inhibit the ten most abundant human matrix metalloproteases, or complement-mediated cell lysis. The presence of a highly acidic surface on AceES-2 suggests that it may function as a cytokine decoy receptor. Several small nematode proteins that have been annotated as TIMPs or netrin domain-containing proteins display sequence homology in structurally important regions of AceES-2's netrin-like fold. Together, our results suggest that AceES-2 defines a novel family of nematode netrin-like proteins, which may function to modulate the host immune response to hookworm and other parasites.
Keywords: Crystal structure, hookworm parasite, Cytokine decoy receptor, Immunosuppressor, Vaccine candidate
Hookworms are blood feeding intestinal nematodes that rank as top agents of global morbidity1. Nearly one billion people suffer hookworm infection, which is characterized by iron deficiency anemia, malnutrition and suppression of host cellular immune responses.2,3,4 Hookworms and other soil transmitted nematode infections are most prevalent in children in developing countries,5 though infection occurs in all age groups and may exacerbate other common infectious diseases, including tuberculosis, malaria, and human immunodeficiency virus.6,7,8 Although community based deworming programs may have short-term benefit, rapid reinfection rates and declining efficacy of commonly used anthelminthics raise doubts about the long-term value of chemotherapy as an effective means of disease control.9,10 Though there is little evidence that humans develop sterile immunity following natural infection,11,12 vaccination against hookworm could potentially decrease the intensity of infection and augment current hookworm control measures.13
Humans are fully permissive hosts for three hookworm species, Necator americanus, Ancylostoma duodenale and Ancylostoma ceylanicum. Beginning soon after infection, hookworms produce a number of excretory-secretory (ES) proteins that are thought to promote survival within the mammalian host, including inhibitors of thrombosis14,15,16 and complement,17 as well as fatty acid binding proteins18,19 and a small kunitz type inhibitor of host pancreatic enzymes.20 Hookworms also produce proteins that modulate host immune cells, including an inhibitor of neutrophils,21 an orthologue of the mammalian cytokine macrophage migration inhibitory factor (MIF),22 and two putative Tissue Inhibitors of Metalloproteases (TIMPs).23,24 Hookworm ES proteins are believed to function together to facilitate blood feeding, digest tissue and prevent detection or damage by host immune factors at the site of intestinal attachment. Such virulence factors, especially those that function to modulate the host immune response to hookworm infection provide an attractive starting point for vaccine design.25,26
A. ceylanicum excretory-secretory protein 2 (AceES-2) is produced by adult worms soon after infection, and can be detected before the parasites begin to feed on blood from intestinal capillaries.27,28 AceES-2 is an 11.7 kDa protein without significant amino acid sequence homology to other known proteins. Despite its low abundance relative to other ES proteins, AceES-2 induces a strong humoral immune response in infected animals.27,29 While subcutaneous inoculation of hamsters with recombinant AceES-2 is associated with more severe disease AceES-2 administered as an oral vaccine decreases anemia and increases recovery rate following challenge infection.27 Significantly, AceES-2 is the first example of a recombinant protein that confers protection against hookworm following mucosal vaccination.
Here we present the crystal structure of AceES-2. We propose that AceES-2 is representative of a novel family of nematode small netrin-like proteins. Preliminary biochemical studies show that AceES-2 does not have the in vitro activities that are present in the most structurally similar netrin-like proteins, including TIMPs. We suggest that the nematode netrin-like proteins may regulate the host immune response by binding host cytokines. In non-parasitic nematodes, binding of netrin-like proteins to cytokine orthologs may regulate larval development.
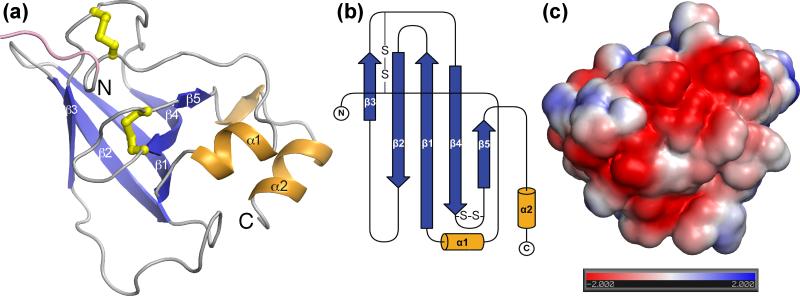
Recombinant AceES-2 (rAceES-2) was expressed in Escherichia coli from a synthetic codon-optimized gene with a 6-histidine tag at either the N- or C-terminus. Both rAceES-2 constructs were purified by nickel-affinity and size-exclusion chromatography, after proteolytic removal of the histidine tag. The crystal structure of rAceES-2 was then determined at 1.75 Å resolution. The protein folds into a five-stranded β-barrel flanked by short N- and C-terminal α-helices that lie parallel to each other on one side of the β-barrel (Fig. 1). The β-barrel is capped by long loops between β-strands. The structure of AceES-2 contains two previously unidentified intramolecular disulfide bonds, Cys3-Cys62 and Cys77-Cys84. One face of AceES-2 has a highly acidic electrostatic potential (Fig. 1c), due to the clustering of the following residues on the surface: E9, D43, D70, D72, D90, D98, E102. The predicted isoelectric point (pI) of AceES-2 is 4.9. Of note, this is only the ninth reported structure of a hookworm (Ancylostoma or Necator) protein.
Fig. 1.
Overall structure of AceES-2. (a) AceES-2 adopts an NTR-domain fold with a five-stranded β-sheet (blue) capped by long loops (grey). N- and C-terminal α-helices (orange) lie on one face of the protein. Intramolecular disulfide bonds (Cys3-Cys62 and Cys77-Cys84) are shown in yellow, and the nonnative N-terminal portion of recombinant AceES-2 (residues -3 to 0) is shown in pink. The position of Glu1, the N-terminal residue of endogenous AceES-2, is labeled “N”. (b) Schematic of the secondary structure created with TopDraw.57 (c) Solvent accessible surface representation colored by electrostatic potential, calculated with APBS58 assuming physiological ionic strength. The units in the color bar are kBT ec-1, where T = 310 K, kB is the Boltzmann constant, and ec is the charge of an electron. The view is rotated approximately 155° along the y-axis relative to Fig. 1a. Crystals of rAceES-2 grew by hanging drop vapor diffusion at 4°C. rAceES-2 at 24 mg/ml in 10 mM HEPES 7.5, 0.2 M KCl, 0-5% sucrose was mixed with an equal volume of 0.1 M HEPES pH 6.8 and 60% MPD. Lead derivative crystals were obtained by crystallizing rAceES-2 under the same conditions plus 1 mM Pb(NO3)2. Crystals were frozen in liquid nitrogen. Lead derivative crystals belonged to space group C2221 with three molecules per asymmetric unit, while native crystals belonged to space group P43 with seven molecules per asymmetric unit. The structure was determined by single anomalous diffraction (SAD) using a lead derivative crystal. Data were collected at 100 K at the experimentally determined peak anomalous diffraction wavelength of lead (0.9496 Å). Data processing was performed using HKL2000.59 Heavy atom sites were located and experimental phases were calculated using SHELX60 and HKL2MAP.61 Buccaneer62 in the CCP4 suite63 was used for automated model building. In order to take advantage of the higher resolution native data the initial model was placed in the native crystal by molecular replacement with Phaser.64 Six molecules were located in the asymmetric unit of the native crystal, and the model was improved with rounds of model building, refinement and water placement using Coot65 and REFMAC5.66 Initial refinement statistics indicated that the native data were partially twinned. Amplitude-based twin refinement detected 28% merohedral twinning and revealed a seventh molecule in the asymmetric unit in the electron density. Final refinement included optimization of twin and TLS parameters to model anisotropic displacements within the protein,67 yielding Rwork and Rfree of 14.6% and 17.5% respectively. 90% of all residues are in the most favored regions of the Ramachandran plot, and three residues (Ala-31 in subunits B, D and E) are in disallowed regions. See Table S1 for data collection and refinement statistics.
In the absence of significant sequence similarity between AceES-2 and proteins of known function, the Dali server30 was used to search for proteins with structural similarity to AceES-2. The fold of AceES-2 is best classified as a netrin-like (NTR) domain, epitomized by the C-terminal domain of netrins.31 AceES-2 bears significant structural similarity to the inhibitory domain of human tissue inhibitors of metalloproteases (TIMPs), and to the C345C domain of some complement factors (Fig. 2, Table S2).
Fig. 2.
The structure of AceES-2 resembles the NTR domains of TIMPs and the C345C domain of complement C3. (a) Overall structure of AceES-2 (blue). The view is rotated 180° along the y-axis relative to Fig. 1a. (b) The NTR domain of TIMP-1 (green) in complex with MMP-14 (grey, PDB 3MA2 chains C, D). (c) The NTR domain of TIMP-3 (red) in complex with ADAM-17/TACE (grey, PDB 3CKI). (d) The C345C domain of cobra venom factor, a complement C3b analogue, in complex with factor B (PDB 3HRZ). The C345C domain (chain C, residues 1469-1620) is shown in pink; the rest of the structure is shown in grey. Disulfide-bonded cysteines within each NTR domain are shown in yellow. Figures 1a, 1c and 2 were created with Pymol.68
AceES-2 is highly unusual among proteins with NTR domains in that it is a single-domain monomer. The packing of AceES-2 monomers in the crystal does not reveal any potential oligomerization interfaces. This is consistent with the elution profile of AceES-2 in size-exclusion chromatography, which shows that AceES-2 is a monomer in solution (data not shown). The structure of AceES-2 is the first reported of a standalone NTR domain protein. Seven other nematode proteins have been annotated as single-domain NTR proteins (Fig. 3). However, nearly all other NTR domains exist in the context of multidomain proteins including netrins, human TIMPs, complement factors and secreted frizzled-related proteins.
Fig. 3.
AceES-2 defines a family of nematode single domain NTR/TIMP proteins. The signal sequences have been omitted. The AceES-2 secondary structure is shown at the top and the four AceES-2 cysteines involved in disulfide bonds are labeled according to which disulfide bond they belong to. Strictly conserved residues are highlighted in red, substantially conserved residues in yellow. The sequence accession numbers are: AceES-2 Q6R7N7; A. duodenale TIMP ABP88131.1; A. ceylanicum TIMP CB176262; A. caninum TIMP-1 Q963I8; A. caninum TIMP-2 ACB13195.1; H. glycines hypothetical esophageal gland cell secretory protein 12 (SP12) Q9NDF1; C. elegans TIMP Q21265; C. elegans NTR Q21267.
Matrix metalloproteases (MMPs) are secreted zinc dependent enzymes involved in the remodeling of the extracellular matrix.32 Twenty three human MMPs have been identified,32 many of which are secreted by the gut epithelium and immune cells at sites of intestinal inflammation.33 MMPs are regulated at the transcriptional level, and in the cellular matrix by TIMPs. The four human TIMPs each inhibit the soluble MMPs non-selectively via an N-terminal NTR domain.32 Crystal structures of MMPs and related ADAM (a disintegrin and metalloproteinase) metalloproteases bound to TIMPs show that the amine and carbonyl groups of the N-terminal cysteine in the TIMP directly coordinate a zinc ion in the active site.34,35,36,37,38 The overall structure of TIMPs is wedge-like with an N-terminal Cys-X-Cys sequence held by two disulfide bonds that forms a contiguous ridge or edge that slots into the active site of the MMPs thereby blocking access to substrates.
AceES-2 shares significant overall structural similarity with the NTR domain of human TIMP-1 and TIMP-3, with backbone rmsd ≥ 2.1 Å and Z-scores ≤ 9.1 (Fig. 2, Table S2). However, instead of forming a ridge with the N-terminus poised for MMP binding, the N-terminal region of AceES-2 actually inserts into the core of the NTR domain, adding a sixth strand to the central β-barrel (Fig. 1). A disulfide bond near the N-terminus (Cys3-Cys62) also tethers the N-terminal region to the core of the protein. As a result, the overall shape of AceES-2 is globular rather than wedge-like. AceES-2 also lacks a Cys-X-Cys motif.
The loop between strands A and B (AB loop) in TIMPs determines protease-specific binding to certain MMPs and related proteases via aromatic residues at the tip of the loop.38,39 The AB loop of AceES-2 lacks aromatic amino acids and is seven amino acids shorter than the shortest human TIMP AB loop. Together, these structural differences between AceES-2 and the human TIMPs imply that AceES-2 cannot bind or inhibit MMPs or ADAMs by the same mechanism as TIMPs, further evidence that the function of this hookworm secretory protein is distinct from its orthologues (see below).
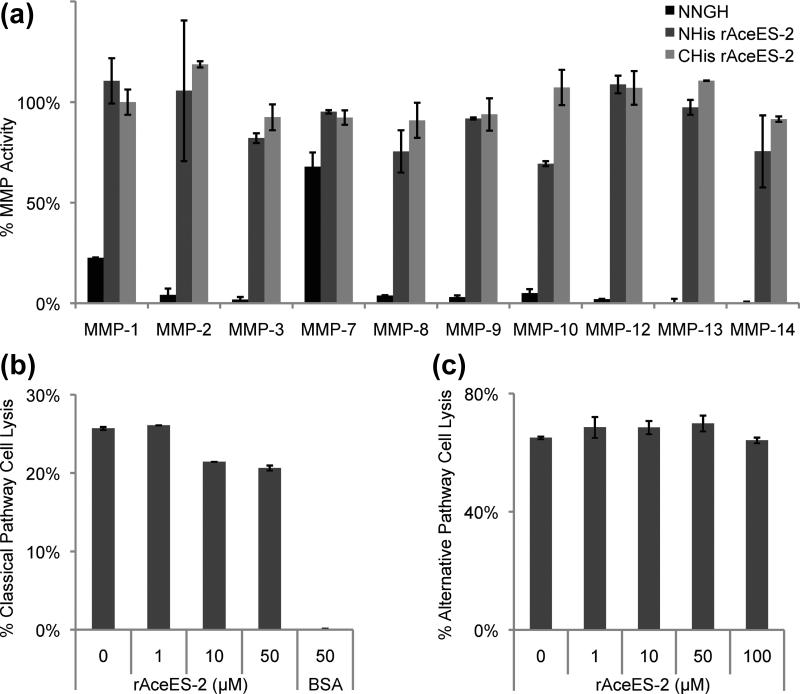
We evaluated the ability of rAceES-2 to inhibit cleavage of a proteolytic substrate by a panel of the ten most abundant human MMPs. At concentrations of up to 20 μM, rAceES-2 did not efficiently inhibit any of the MMPs (Fig. 4a). Although the rAceES-2 used for structure determination had three nonnative N-terminal amino acids left after removal of the purification, a rAceES-2 construct with an intact native N-terminus also failed to inhibit human MMPs. By comparison, the dog hookworm MMP inhibitor A. caninum TIMP-2 (AcTMP-2), which does contain an N-terminal Cys-X-Cys motif, displayed submaximal (56-68% inhibition) inhibition of three of the ten human MMPs tested in an identical assay .24 We cannot rule out the possibility that AceES-2 specifically inhibits another mammalian MMP or protease that has not yet been tested. Alternatively, it is possible that AceES-2 targets an endogenous hookworm metalloprotease, which has been reported for AcTMP-1, another putative TIMP from A. caninum.40,41
Fig. 4.
MMP activity and complement-mediated cell lysis inhibition assays with rAceES-2. (a) A colorimetric kit was used to test the ability of rAceES-2 to inhibit cleavage of a thiopeptide substrate by human MMPs 1, 2, 3, 7, 8, 9, 10, 12, 13 and 14 (Enzo Life Sciences). The cleavage product reacts with 5,5’-dithiobis(2-nitrobenzoic acid) to yield 2-nitro-5-thiobenzoic acid, which can be detected by absorbance at 412 nm. Final MMP concentrations in the reactions were 17-126 nM according the manufacturer's protocol. AceES-2 with either N- or C-terminal histidine tags (NHis and CHis, respectively) do not significantly inhibit any of MMPs tested. Shown is one representative experiment using 2.0 μM rAceES-2, which corresponds to a rAceES-2:MMP molar ratio of between 15:1 and 115:1, depending on the concentration of MMP used. Similar results where obtained using up to 20 μM rAceES-2. The general peptide inhibitor NNGH was used as an inhibitory control. (b) The ability of rAceES-2 to complement pathways was tested using standard erythrocyte lysis assays (see Supplementary Data for detailed methods). rAceES-2 does not significantly inhibit complement-mediated lysis of sheep erythrocytes in the classical pathway relative to a buffer control. Bovine serum albumen (BSA) at 50 μM inhibits this pathway non-specifically. (c) rAceES-2 does not significantly inhibit complement-mediated lysis of rabbit erythrocytes in the alternative pathway.
The complement system relies on a proteolytic cascade to enhance the adaptive immune response to hookworms and other pathogens.42 Many pathogen proteins inhibit complement by mimicking complement factors and thus sequestering and inactivating other complement factors.43 For example, a calreticulin-like protein from the hookworm N. americanus inhibits complement factor C1q.17
AceES-2 resembles the C345C domains of complement C3 and C5, and of cobra venom factor (CVF), a complement C3b analogue, with backbone rmsd ≥ 2.4 Å and Z-score ≤ 8.6 (Fig. 2; Table S2). Recent structural studies show that the key interaction between the CVF-C345C and factor B is the direct coordination by the C-terminal carboxylate group of the C345C domain of an essential magnesium ion within factor B.44,45 However, the C-terminal region of AceES-2 adopts a different structure than that of CVF-C345C (Fig. 2). The C-terminal helix of AceES-2 is one and a half turns shorter, and AceES-2 lacks the disulfide bond that stabilizes the structure of the CVF-C345C C-terminal region.
If AceES-2 were able to mimic the C345C domain of a complement component, AceES-2 might compete with the C345C domain of C3b for binding to Factor B. This could in turn prevent the recruitment of Factor B to C3b, thereby inhibiting the complement pathway. We tested rAceES-2 for its ability to inhibit complement mediated cell lysis. In these assays, rAceES-2 did not inhibit the classical or alternative complement pathways (Fig. 4).
Several small proteins from the nematode species A. duodenale, A. caninum, A. ceylanicum, Heterodera glycines and Caenorhabditis elegans have been annotated as TIMPs or NTR proteins.24,41,46,47 Since AceES-2 resembles the NTR domain of human TIMPs, we sought to align the sequences of AceES-2 with those of annotated nematode TIMPs and NTR proteins. We found that the regions of the AceES-2 sequence that are important for the secondary and tertiary structures of the NTR domain could be aligned with significant homology to the nematode small NTR/TIMP sequences (Fig. 3).
In contrast to the human TIMPs, the nematode NTR/TIMPs appear to be single-domain proteins. Moreover, like AceES-2, the hookworm AcTMP-1 and C. elegans NTR proteins have shorter AB loops than any of the human TIMPs. In addition, the Cys3-Cys62 disulfide bond (AceES-2 numbering) is conserved in all of the nematode proteins. We therefore propose that AceES-2 and the related nematode sequences, many of which are currently annotated as TIMPs, define a novel family of nematode small NTR proteins. Of note, this level of sequence homology would not have been detectable without structural information, providing a striking example of the value of knowing the three-dimensional structure of a protein.
Among the nematode NTR family members, the most closely related to AceES-2 is a TIMP sequence from the hookworm A. duodenale (Fig. 3). This sequence is uniquely similar to AceES-2 in that it also lacks an N-terminal Cys-X-Cys motif and has cysteines at the same four positions as AceES-2, suggesting that is has the same disulfide bonding pattern and possibly a very similar structure to AceES-2. The absence of the Cys-X-Cys motif in AceES-2 and A. duodenale TIMP suggests that these two hookworm proteins may not function as protease inhibitors in vivo.
TIMPs also perform functions that are independent of metalloprotease inhibition. For example, human TIMP-1 and TIMP-2 exhibit growth factor-like activity and can inhibit angiogenesis.48 In the case of hookworm proteins, injection of the recombinant AcTMP-1 into mice modulates the function of dendritic cells by inducing the expression of IL-10 and TGF-β and decreasing the expression of MHC molecules.23,24 These combined effects lead to the development of anti-inflammatory responses from CD4+ and CD8+ T cells and the suppression of proliferation responses in vitro. Evidence for an immunomodulatory role for AceES-2 includes data from studies showing that the route of vaccination with recombinant protein modifies the immune response in a subsequent challenge infection.27 Infected hamsters previously vaccinated subcutaneously with rAceES-2 in the adjuvant aluminum hydroxide (alum) exhibited more severe anemia and growth delay compared to adjuvant immunized controls despite comparable intestinal worm burdens. By contrast, a single oral immunization with rAceES-2 conferred partial protection and accelerated recovery from hookworm-associated anemia, again despite no difference in worm burden. These data suggest that the cellular immune response to AceES-2 in animals that have been previously exposed through vaccination can either be accelerated or blunted based on the route of priming. By contrast, subcutaneous vaccination of dogs with recombinant AcTMP-1 was not protective against challenge infection, nor was there any difference in hematological parameters between vaccinated and control animals.49 These data further suggest that there are functional differences between AceES-2 and AcTMP-1, both in terms of their roles in disease pathogenesis, as well as vaccine potential.
The low pI of AceES-227 and its acidic electrostatic surface potential on one face suggest that it may bind to a cytokine. Many cytokines have highly basic surfaces that bind glycosaminoglycans on the cell surface, and cytokine receptors typically have complementary acidic surfaces. Some pathogens control host inflammation by secreting cytokine decoy receptors, which often bear no structural resemblance to the host receptor.50,51,52 The potential role of AceES-2 and the other hookworm NTR proteins as cytokine decoy receptors is consistent with the observed effects of rAceES-2 and AcTMP-1 in prior studies.24,27 Further studies are required to determine the mechanism through which AceES-2 exerts its immunomodulatory effect, and whether it does so via a mechanism similar to the related hookworm protein AcTMP-1.
Nematodes, both free-living and parasitic, rely on cytokine orthologs for biological functions that are not related to parasitism. In C. elegans, for example, orthologs of the cytokines TGF-β and four distinct migration inhibitory factor (MIF) genes regulate larval development under low nutrient conditions.53,54 Moreover, C. elegans contains orthologs of proteins that regulate cytokines, including a TRAF (TNF receptor-associated factor),55 and ced-3, a protein similar to mammalian IL-1β-converting enzyme proposed to act as a cysteine protease in the initiation of programmed cell death during embryogenesis.56 Similarly, the nematode small NTR proteins may participate in the regulation of larval development or programmed cell death by sequestering nematode cytokine orthologs.
In conclusion, we have shown that AceES-2 defines a novel family of nematode small NTR proteins, which includes a number of sequences annotated as TIMPs based on primary amino acid sequence similarity. Despite its structural similarity to the NTR domains present in the TIMP and complement protein families, respectively, data from biochemical studies suggest that rAceES-2 inhibits neither human MMPs nor complement. By providing a structural framework for understanding the potential function of AceES-2, we hope to further define its mechanism of action and fully exploit the vaccine potential of this novel family of nematode netrin-like (NTR) proteins.
Supplementary Material
Acknowledgements
This work was supported by a Burroughs Wellcome Investigator Award to YM; a National Science Foundation Graduate Research fellowship to KK; MC is supported by NIH grant AI 058980 and a Clinical Research Grant from the March of Dimes Birth Defects Foundation. We thank Raj Kanagalaghatta and other staff at the NE-CAT beamlines of the Advanced Photon Source (APS). We thank Annie Héroux and other staff at the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory. Use of APS and NSLS is supported by the Offices of Biological and of Basic Energy Sciences of the US Department of Energy.
Abbreviations Used
- rAceES-2
recombinant Ancylostoma ceylanicum excretory-secretory protein 2
- MMP
matrix metalloprotease
- TIMP
tissue inhibitor of matrix metalloprotease
- CVF
cobra venom factor
- AcTMP-2
A. caninum TIMP-2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession number
AceES-2 coordinates and experimental amplitudes have been deposited in the Protein Data Bank with accession code PDB 3NSW.
Supplementary Data
Supplementary data associated with this article can be found, in the online version, at doi:XXX.
References
- 1.Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- 2.Geiger SM, Massara CL, Bethony J, Soboslay PT, Correa-Oliveira R. Cellular responses and cytokine production in post-treatment hookworm patients from an endemic area in Brazil. Clinical and Experimental Immunology. 2004;136:334–340. doi: 10.1111/j.1365-2249.2004.02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loukas A, Constant SL, Bethony JM. Immunobiology of hookworm infection. Fems Immunology and Medical Microbiology. 2005;43:115–124. doi: 10.1016/j.femsim.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: Cellular and molecular mechanisms. Nature Reviews Immunology. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 5.Awasthi S, Bundy DA, Savioli L. Helminthic infections. Bmj. 2003;327:431–433. doi: 10.1136/bmj.327.7412.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roussilhon C, Brasseur P, Agnamey P, Perignon JL, Druilhe P. Understanding human-Plasmodium falciparum immune interactions uncovers the immunological role of worms. PLoS One. 2010;5:e9309. doi: 10.1371/journal.pone.0009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bundy D, Sher A, Michael E. Good worms or bad worms: do worm infections affect the epidemiological patterns of other diseases? Parasitol Today. 2000;16:273–274. doi: 10.1016/s0169-4758(00)01689-6. [DOI] [PubMed] [Google Scholar]
- 8.Onyemelukwe GC, Musa BO. T-lymphocyte subsets in patients with hookworm infection in Zaria, Nigeria. Afr J Med Med Sci. 2001;30:255–259. [PubMed] [Google Scholar]
- 9.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 10.Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–1210. doi: 10.1016/j.ijpara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Behnke JM. Do hookworms elicit protective immunity in man? Parasitol Today. 1987;3:200–206. doi: 10.1016/0169-4758(87)90060-3. [DOI] [PubMed] [Google Scholar]
- 12.Loukas A, Prociv P. Immune responses in hookworm infections. Clin Microbiol Rev. 2001;14:689–703. doi: 10.1128/CMR.14.4.689-703.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotez PJ, Zhan B, Bethony JM, Loukas A, Williamson A, Goud GN, Hawdon JM, Dobardzic A, Dobardzic R, Ghosh K, Bottazzi ME, Mendez S, Zook B, Wang Y, Liu S, Essiet-Gibson I, Chung-Debose S, Xiao SH, Knox D, Meagher M, Inan M, Correa-Oliveira R, Vilk P, Shepherd HR, Brandt W, Russell PK. Progress in the development of a recombinant vaccine for human hookworm disease: The Human Hookworm Vaccine Initiative. International Journal for Parasitology. 2003;33:1245–1258. doi: 10.1016/s0020-7519(03)00158-9. [DOI] [PubMed] [Google Scholar]
- 14.Cappello M, Vlasuk GP, Bergum PW, Huang S, Hotez PJ. Ancylostoma caninum anticoagulant peptide: a hookworm-derived inhibitor of human coagulation factor Xa. Proc Natl Acad Sci U S A. 1995;92:6152–6156. doi: 10.1073/pnas.92.13.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Valle A, Jones BF, Harrison LM, Chadderdon RC, Cappello M. Isolation and molecular cloning of a secreted hookworm platelet inhibitor from adult Ancylostoma caninum. Mol Biochem Parasitol. 2003;129:167–177. doi: 10.1016/s0166-6851(03)00121-x. [DOI] [PubMed] [Google Scholar]
- 16.Stassens P, Bergum PW, Gansemans Y, Jespers L, Laroche Y, Huang S, Maki S, Messens J, Lauwereys M, Cappello M, Hotez PJ, Lasters I, Vlasuk GP. Anticoagulant repertoire of the hookworm Ancylostoma caninum. Proc Natl Acad Sci U S A. 1996;93:2149–2154. doi: 10.1073/pnas.93.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper G, Brown A, Eberl M, Vallar L, Kieffer N, Berry C, Girdwood K, Eggleton P, Quinnell R, Pritchard DI. A calreticulin-like molecule from the human hookworm Necator americanus interacts with C1q and the cytoplasmic signalling domains of some integrins. Parasite Immunology. 2001;23:141–152. doi: 10.1046/j.1365-3024.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 18.Basavaraju S, Zhan B, Kennedy MW, Liu Y, Hawdon J, Hotez PJ. Ac-FAR-1, a 20 kDa fatty acid- and retinol-binding protein secreted by adult Ancylostoma caninum hookworms: gene transcription pattern, ligand binding properties and structural characterisation. Mol Biochem Parasitol. 2003;126:63–71. doi: 10.1016/s0166-6851(02)00253-0. [DOI] [PubMed] [Google Scholar]
- 19.Fairfax KC, Vermeire JJ, Harrison LM, Bungiro RD, Grant W, Husain SZ, Cappello M. Characterisation of a fatty acid and retinol binding protein orthologue from the hookworm Ancylostoma ceylanicum. Int J Parasitol. 2009;39:1561–1571. doi: 10.1016/j.ijpara.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milstone AM, Harrison LM, Bungiro RD, Kuzmic P, Cappello M. A broad spectrum Kunitz type serine protease inhibitor secreted by the hookworm Ancylostoma ceylanicum. Journal of Biological Chemistry. 2000;275:29391–29399. doi: 10.1074/jbc.M002715200. [DOI] [PubMed] [Google Scholar]
- 21.Moyle M, Foster DL, Mcgrath DE, Brown SM, Laroche Y, Demeutter J, Stanssens P, Bogowitz CA, Fried VA, Ely JA, Soule HR, Vlasuk GP. A Hookworm Glycoprotein That Inhibits Neutrophil Function Is a Ligand of the Integrin Cd11b Cd18. Journal of Biological Chemistry. 1994;269:10008–10015. [PubMed] [Google Scholar]
- 22.Cho YS, Jones BF, Vermeire JJ, Leng L, DiFedele L, Harrison LM, Xiong HB, Kwong YKA, Chen Y, Bucala R, Lolis E, Cappello M. Structural and functional characterization of a secreted hookworm macrophage migration inhibitory factor (MIF) that interacts with the human MIF receptor CD74. Journal of Biological Chemistry. 2007;282:23447–23456. doi: 10.1074/jbc.M702950200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuellar C, Wu W, Mendez S. The hookworm tissue inhibitor of metalloproteases (Ac-TMP-1) modifies dendritic cell function and induces generation of CD4 and CD8 suppressor T cells. PLoS Negl Trop Dis. 2009;3:e439. doi: 10.1371/journal.pntd.0000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhan B, Gupta R, Wong SP, Bier S, Jiang D, Goud G, Hotez P. Molecular cloning and characterization of Ac-TMP-2, a tissue inhibitor of metalloproteinase secreted by adult Ancylostoma caninum. Mol Biochem Parasitol. 2008;162:142–148. doi: 10.1016/j.molbiopara.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Bungiro R, Cappello M. Hookworm infection: new developments and prospects for control. Current Opinion in Infectious Diseases. 2004;17:421–426. doi: 10.1097/00001432-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Loukas A, Bethony J, Brooker S, Hotez P. Hookworm vaccines: past, present, and future. Lancet Infect Dis. 2006;6:733–741. doi: 10.1016/S1473-3099(06)70630-2. [DOI] [PubMed] [Google Scholar]
- 27.Bungiro RD, Jr., Solis CV, Harrison LM, Cappello M. Purification and molecular cloning of and immunization with Ancylostoma ceylanicum excretory-secretory protein 2, an immunoreactive protein produced by adult hookworms. Infect Immun. 2004;72:2203–2213. doi: 10.1128/IAI.72.4.2203-2213.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bungiro RD, Jr., Cappello M. Detection of excretory/secretory coproantigens in experimental hookworm infection. Am J Trop Med Hyg. 2005;73:915–920. [PubMed] [Google Scholar]
- 29.Bungiro RD, Jr., Sun T, Harrison LM, Shoemaker CB, Cappello M. Mucosal antibody responses in experimental hookworm infection. Parasite Immunol. 2008;30:293–303. doi: 10.1111/j.1365-3024.2008.01023.x. [DOI] [PubMed] [Google Scholar]
- 30.Holm L, Kaariainen S, Rosenstrom P, Schenkel A. Searching protein structure databases with DaliLite v.3. Bioinformatics. 2008;24:2780–2781. doi: 10.1093/bioinformatics/btn507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 32.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Medina C, Radomski MW. Role of matrix metalloproteinases in intestinal inflammation. J Pharmacol Exp Ther. 2006;318:933–938. doi: 10.1124/jpet.106.103465. [DOI] [PubMed] [Google Scholar]
- 34.Gomis-Ruth FX, Maskos K, Betz M, Bergner A, Huber R, Suzuki K, Yoshida N, Nagase H, Brew K, Bourenkov GP, Bartunik H, Bode W. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature. 1997;389:77–81. doi: 10.1038/37995. [DOI] [PubMed] [Google Scholar]
- 35.Grossman M, Tworowski D, Dym O, Lee MH, Levy Y, Murphy G, Sagi I. The Intrinsic Protein Flexibility of Endogenous Protease Inhibitor TIMP-1 Controls Its Binding Interface and Affects Its Function. Biochemistry. 2010;49:6184–6192. doi: 10.1021/bi902141x. [DOI] [PubMed] [Google Scholar]
- 36.Maskos K, Lang R, Tschesche H, Bode W. Flexibility and variability of TIMP binding: X-ray structure of the complex between collagenase-3/MMP-13 and TIMP-2. J Mol Biol. 2007;366:1222–1231. doi: 10.1016/j.jmb.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 37.Arumugam S, Van Doren SR. Global orientation of bound MMP-3 and N-TIMP-1 in solution via residual dipolar couplings. Biochemistry. 2003;42:7950–7958. doi: 10.1021/bi034545s. [DOI] [PubMed] [Google Scholar]
- 38.Wisniewska M, Goettig P, Maskos K, Belouski E, Winters D, Hecht R, Black R, Bode W. Structural determinants of the ADAM inhibition by TIMP-3: crystal structure of the TACE-N-TIMP-3 complex. J Mol Biol. 2008;381:1307–1319. doi: 10.1016/j.jmb.2008.06.088. [DOI] [PubMed] [Google Scholar]
- 39.Williamson RA, Hutton M, Vogt G, Rapti M, Knauper V, Carr MD, Murphy G. Tyrosine 36 plays a critical role in the interaction of the AB loop of tissue inhibitor of metalloproteinases-2 with matrix metalloproteinase-14. J Biol Chem. 2001;276:32966–32970. doi: 10.1074/jbc.M101843200. [DOI] [PubMed] [Google Scholar]
- 40.Feng J, Zhan B, Liu Y, Liu S, Williamson A, Goud G, Loukas A, Hotez P. Molecular cloning and characterization of Ac-MTP-2, an astacin-like metalloprotease released by adult Ancylostoma caninum. Mol Biochem Parasitol. 2007;152:132–138. doi: 10.1016/j.molbiopara.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhan B, Badamchian M, Meihua B, Ashcom J, Feng J, Hawdon J, Shuhua X, Hotez PJ. Molecular cloning and purification of Ac-TMP, a developmentally regulated putative tissue inhibitor of metalloprotease released in relative abundance by adult Ancylostoma hookworms. Am J Trop Med Hyg. 2002;66:238–244. doi: 10.4269/ajtmh.2002.66.238. [DOI] [PubMed] [Google Scholar]
- 42.Carroll MC. The complement system in regulation of adaptive immunity. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 43.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–142. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torreira E, Tortajada A, Montes T, Rodriguez de Cordoba S, Llorca O. 3D structure of the C3bB complex provides insights into the activation and regulation of the complement alternative pathway convertase. Proc Natl Acad Sci U S A. 2009;106:882–887. doi: 10.1073/pnas.0810860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janssen BJ, Gomes L, Koning RI, Svergun DI, Koster AJ, Fritzinger DC, Vogel CW, Gros P. Insights into complement convertase formation based on the structure of the factor B-cobra venom factor complex. EMBO J. 2009;28:2469–2478. doi: 10.1038/emboj.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitreva M, McCarter JP, Arasu P, Hawdon J, Martin J, Dante M, Wylie T, Xu J, Stajich JE, Kapulkin W, Clifton SW, Waterston RH, Wilson RK. Investigating hookworm genomes by comparative analysis of two Ancylostoma species. BMC Genomics. 2005;6:58. doi: 10.1186/1471-2164-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banyai L, Patthy L. The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 1999;8:1636–1642. doi: 10.1110/ps.8.8.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stetler-Stevenson WG, Seo DW. TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005;11:97–103. doi: 10.1016/j.molmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Hotez PJ, Ashcom J, Bin Z, Bethony J, Williamson A, Hawdon JM, Jianjun F, Dobardzic A, Rizo I, Bolden J, Jin Q, Yan W, Dobardzic R, Chung-Debose S, Crowell M, Datu B, Delaney A, Dragonovski D, Jiang Y, Yueyuan L, Ghosh K, Loukas A, Brandt W, Russell PK, Zook BC. Effect of vaccinations with recombinant fusion proteins on Ancylostoma caninum habitat selection in the canine intestine. J Parasitol. 2002;88:684–690. doi: 10.1645/0022-3395(2002)088[0684:EOVWRF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 50.Alexander JM, Nelson CA, van Berkel V, Lau EK, Studts JM, Brett TJ, Speck SH, Handel TM, Virgin HW, Fremont DH. Structural basis of chemokine sequestration by a herpesvirus decoy receptor. Cell. 2002;111:343–356. doi: 10.1016/s0092-8674(02)01007-3. [DOI] [PubMed] [Google Scholar]
- 51.Carfi A, Smith CA, Smolak PJ, McGrew J, Wiley DC. Structure of a soluble secreted chemokine inhibitor vCCI (p35) from cowpox virus. Proc Natl Acad Sci U S A. 1999;96:12379–12383. doi: 10.1073/pnas.96.22.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elde NC, Malik HS. The evolutionary conundrum of pathogen mimicry. Nat Rev Microbiol. 2009;7:787–797. doi: 10.1038/nrmicro2222. [DOI] [PubMed] [Google Scholar]
- 53.Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 54.Marson AL, Tarr DE, Scott AL. Macrophage migration inhibitory factor (mif) transcription is significantly elevated in Caenorhabditis elegans dauer larvae. Gene. 2001;278:53–62. doi: 10.1016/s0378-1119(01)00706-5. [DOI] [PubMed] [Google Scholar]
- 55.Wajant H, Muhlenbeck F, Scheurich P. Identification of a TRAF (TNF receptor-associated factor) gene in Caenorhabditis elegans. J Mol Evol. 1998;47:656–662. doi: 10.1007/pl00006423. [DOI] [PubMed] [Google Scholar]
- 56.Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 57.Bond CS. TopDraw: a sketchpad for protein structure topology cartoons. Bioinformatics. 2003;19:311–312. doi: 10.1093/bioinformatics/19.2.311. [DOI] [PubMed] [Google Scholar]
- 58.Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci U S A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otwinowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 60.Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 61.Pape T, Schneider TR. HKL2MAP: a graphical user interface for macromolecular phasing with SHELX programs. Journal of Applied Crystallography. 2004;37:843–844. [Google Scholar]
- 62.Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallographica Section D-Biological Crystallography. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- 63.CCP4 Collaborative computational project number 4, The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 64.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J. Appl. Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 66.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 67.Winn MD, Isupov MN, Murshudov GN. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr D Biol Crystallogr. 2001;57:122–133. doi: 10.1107/s0907444900014736. [DOI] [PubMed] [Google Scholar]
- 68.DeLano WL. The PyMOL Molecular Graphics System, Version 1.3. Schrödinger, LLC; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.