Abstract
Thalamocortical dynamics, the millisecond to second changes in activity of thalamocortical circuits, are central to perception, action and cognition. Generated by local circuitry and sculpted by neuromodulatory systems, these dynamics reflect the expression of vigilance states. In sleep, thalamocortical dynamics are thought to mediate “offline” functions including memory consolidation and synaptic scaling. Here, I discuss thalamocortical sleep dynamics and their modulation by the ascending arousal system and locally-released neurochemicals. I focus on modulation of these dynamics by electrically-silent astrocytes, highlighting the role of purinergic signaling in this glial form of communication. Astrocytes modulate cortical slow oscillations, sleep behavior, and sleep-dependent cognitive function. The discovery that astrocytes can modulate sleep dynamics and sleep-related behaviors suggests a new way of thinking about the brain, in which integrated circuits of neurons and glia control information processing and behavioral output.
Keywords: sleep, thalamocortical circuitry, oscillations, memory, astrocytes, ATP, adenosine
Introduction
Sleep is a behavior that is ancient and pervasive throughout the animal kingdom, yet the cellular and circuit mechanisms underlying sleep regulation are poorly understood. Importantly, the functional need for animals to spend much of their lives in an “offline” state is not clear. The quest for understanding the function(s) and regulation of sleep is one of the most interesting endeavors of modern neuroscience, not only due to the insights it provides for understanding normal brain function but also due to its translational impact given sleep disruptions in most neuropsychiatric disorders, including schizophrenia, bipolar disorder and epilepsy [1]
Phenomenologically, sleep is characterized by a number of criteria that include species-specific postural changes, increased threshold to environmental stimuli and changes in global brain activity [2, 3]. Such criteria have been observed in species ranging from fruit flies to mammals [4], and have been important in establishing sleep as a universal behavior among animals [5] (but see [6]). An additional and perhaps a defining criterion of sleep, as opposed to other expressions of behavioral inactivity, is its “homeostatic regulation”. While sleep is regulated by circadian mechanisms that ensure its appropriate expression in relation to environmental conditions such as the light/dark cycle and food availability [7], sleep drive increases as a function of prior wakefulness independent of circadian control [8]. For example, if sleep expression is hindered at a time when an animal normally sleeps, sleep is compensated in the animal’s active behavioral phase [9]. In humans, deprivation of sleep during the night normally results in enhanced sleep during the following day.
In mammals, global changes in brain activity have classically been described using electroencephalography (EEG); integrated extracellular field potentials reflecting subthreshold membrane potential changes in cortical neurons [10]. Along with the electromyogram (EMG)-- the electrical measurement of muscle activity-- the EEG is considered the gold standard for measurements of vigilance states [11]. The EEG shows changes in frequency and amplitude across the three vigilance states; wakefulness, non-rapid eye movement sleep (NREM) and rapid eye movement sleep (REM). In NREM sleep, the EEG is dominated by slow waves in the 0.5–4Hz range whose amplitude and incidence correlates positively with sleep intensity [12]. Interestingly, slow waves appear to be homeostatically regulated similar to sleep behavior [9]. Brain dynamics during sleep, which include slow waves, are thought to mediate many of the hypothesized function of sleep including synaptic downscaling [13] and memory consolidation [14] (discussed in the following section).
Though much of sleep research in the last century has focused on neuronal mechanisms of sleep regulation, the role of electrically-silent glia in modulation of this behavior has recently been elucidated [15]. Astrocytes, the major subtype of glia in the mammalian neocortex, have classically been thought to play supportive roles. Research over the last two decades has shown that these cells have important neuromodulatory actions [16–18] that can impact circuit function [19] and behavior [15]. In the remainder of this review, I will discuss sleep dynamics in thalamocortical circuits, highlighting the role of neuromodulation in shaping these dynamics. I will subsequently discuss neuromodulatory functions of astrocytes focusing on the role of purinergic gliotransmission in controlling neuronal circuit function, sleep dynamics and sleep behavior.
Proposed functions of sleep
Though sleep may have many functional roles, two “sleep function” hypotheses involving internal computations have recently been introduced and may explain why sensory disengagement is a feature of sleep. One hypothesis is based on the original discovery by Wilson and McNaughton [20], where using multielectrode recordings they found evidence for hippocampal cell “reactivation” during sleep. That is, hippocampal cells that encoded overlapping spatial locations during awake experience, where more likely to fire together during sleep. More recently, sequential “replay” of place cells resembling that seen during awake behavior has been shown [21]. Replay occurs during 200–300Hz field potential hippocampal dynamics called sharp wave ripples (SWR) [22]. Disrupting SWR with electrical stimulation disrupts memory consolidation, providing evidence that hippocampal replay may be essential for memory consolidation [23, 24]. The second hypothesis, proposed by Tononi and Cirelli, is based on the observation that during wakefulness many genes involved in synaptic potentiation are expressed [25]. Corroborated by biochemical and electrophysiological evidence that wakefulness is associated with net potentiation of excitatory synaptic transmission [26, 27] which would increase space and energy demands, they posit that sleep is a state in which synapses are scaled down while preserving their relative strength [13]. Certain thalamocortical sleep dynamics are thought to contribute to synaptic scaling as their low-frequency is conducive to LTD-like synaptic de-potentiation [28]. These two hypotheses are not mutually exclusive, as replay and potentiation of salient memories may occur concurrently with overall net synaptic de-potentiation and scaling and improved mechanistic understanding of these processes will shed light on the requirement of sleep states in brain function.
Thalamocortical dynamics in sleep
The six layered neocortex is a defining feature of the mammalian brain, and is a derivative of the vertebrate dorsal pallium, an evolutionary conserved vertebrate structure [29]. Neocortical evolution has been matched by evolution and expansion of thalamic projections [29, 30]. While thalamocortical projections are classically thought to relay information to the neocortex, recent evidence suggests that thalamocortical neuronal firing impacts neocortical states [31, 32]. Depending on their initial resting membrane potential, thalamocortical (TC) neurons are able to either reliably transmit spike trains or initiate oscillatory burst firing in response to the incoming synaptic drive [33]. This ability is conferred by the repertoire of voltage-gated ion channels that TC neurons express. In particular, T-type Ca2+ channels, which are inactivated at resting membrane potentials of > −65 mV allow for oscillatory burst firing when TC neurons are hyperpolarized below −65 mV [34]. These biophysical properties are shared by neurons of the thalamic reticular nucleus (TRN), which provide the major source of synaptic inhibition to thalamic nuclei [35]. The de-inactivation of T-type Ca2+ channels initiates a low-threshold Ca2+ spike when TC or TRN neurons are synaptically driven. In earlier stages of sleep, with moderate levels of hyperpolarization, synaptic input to TRN and TC neurons results in burst firing and the generation of spindle oscillations (7–14Hz) [36], which are transmitted to the cortex. Interestingly, the incidence of spindles has been shown to positively correlate with the threshold of sensory stimulation required for sleep disruption [37], pointing to a role for spindle-generating circuitry in sensory gating. This is consistent with findings that spindle density is greatly reduced in patients with schizophrenia, a disorder of sensory gating [38]. In addition, spindle oscillations are enhanced after learning [39, 40], and many spindles synchronize with hippocampal SWRs (discussed earlier) [41, 42], pointing to a role for spindles and the underlying thalamic circuitry in sleep-dependent memory consolidation. Understanding precisely how that happens (potentiation of cortical synapses vs. filtering of incoming sensory signals to allow for internal hippocampo-cortical communication), and how it is modulated by subcortical and local factors (see later sections) will be of major importance.
As TC/TRN neurons become more hyperpolarized, their inter-burst interval widens resulting in the appearance of delta oscillations (1–4Hz) [36] , also transmitted to cortex [43]. Evidence that the thalamus is the generator of cortical delta oscillations comes from both slice and in vivo intracellular recordings, showing clock-like delta oscillations in individual thalamocortical neurons [44, 45]. More recently, knockout of potassium channels preferentially expressed by thalamic neurons resulted in marked attenuation of low frequency cortical oscillations during sleep [46, 47]. Importantly, sleep itself was less stable in these mice suggesting that sleep dynamics may drive sleep behavior.
Though spindles and delta oscillations are prominent sleep rhythms, the cortical slow oscillation is the most defining rhythm observed during sleep. The slow oscillation is a <1Hz rhythm that is observed at multiple levels of organization in the brain during sleep [48]. For example, slow oscillations have been described as traveling waves in the human brain [49]. On a cellular level, neurons in a number of different cortical areas fluctuate between depolarized (UP) and hyperpolarized (DOWN) states [50]. The UP state is characterized by barrages of synaptic activity, a plateau depolarization, and action potential firing, while the DOWN state is characterized by cell hyperpolarization and silence [51]. These membrane potential fluctuations are tightly synchronized to the local field potential, in which waves of field potential slow oscillations trigger precise sequential firing of pyramidal neurons [52]. Understanding the detailed circuit mechanism for generating slow oscillations will be important for dissecting other sleep rhythms as slow oscillations organize delta and spindle oscillations [48]. Furthermore, an interest in the role of slow oscillations in mediating sleep function(s) has recently been bolstered by the finding that boosting these rhythms during sleep enhances human memory [53].
Ascending neuromodulators impact thalamocortical dynamics
Though thalamocortical dynamics are generated by synaptic interaction among TRN, TC and neocortical neurons, they are modulated by ascending inputs originating from nuclei in the brainstem, hypothalamus and basal forebrain [54]. Among these is the pedunculopontine and laterodorsal tegmental nuclei (PPT/LDT); brainstem areas containing cholinergic neurons that fire preferentially during wakefulness and REM compared to NREM. Acetylcholine (ACh) released by these neurons can have complex effects on thalamic, hypothalamic and basal forebrain neurons [7]. Monoaminergic neurons in the upper brainstem and caudal hypothalamus, including the noradrenergic locus coeruleus (LC), serotoninergic dorsal raphe (DR), dopaminergic ventral periaqueductal grey matter and histaminergic tuberomammillary neurons, lateral hypothalamic peptidergic neurons (orexin/hypocretin-containing), and basal forebrain neurons (ACh or GABA-containing) [55] project directly to the cortex [56]. Activation of these nuclei promotes behavioral and electrographic arousal [57], and thus these are thought of canonical “arousal centers” [56]. These nuclei receive inhibitory input from the ventrolateral preoptic area (VLPO), a group of GABAergic neurons that fire preferentially during sleep [58]. One synthesis of subcortical sleep/wake neuronal circuitry is proposed by Saper in his flip-flop switch model of sleep regulation [7]. In this model, brainstem and hypothalamic sleep related circuitry is viewed as mutually inhibitory elements in which activity in one of the competing sides shuts down inhibitory inputs from the other side, and therefore disinhibits its own action. This ensures that when arousal centers are “on”, they turn off sleep centers and vice versa, ensuring sharp state-transitions. Though elegant, this model may not be fully compatible with observations of sub-states of arousal, such as tonic alertness, selective attention and sensory-disengagement which exhibit different electrographic signatures and may differentially recruit the canonical sleep wake circuitry.
Local Sleep
Based on studies of unihemispheric sleep in marine mammals, it is widely appreciated that the minimal unit for sleep expression may not encompass the entire brain [59]. However, defining which parts of the brain engage in sleep expression is a challenge. The discovery of regional differences in slow wave expression across the cortex has generated significant excitement, as it supports the notion that sleep may be a use-dependent process, driven by local circuitry[49]. This is further supported by discoveries that local slow wave expression correlates with previous sensory, motor or cognitive demands of the cortex in prior wakefulness [60]. Recently, Krueger and colleagues have suggested that the minimal unit of sleep is the cortical column, and that sleep behavior results from coalescence of many cortical columns engaging in “sleep-like states” [61]. Though our knowledge of regional cortical states is in its infancy, Petersen and colleagues have shown that cortical neurons in the mouse somatosensory cortex can express UP and DOWN states when the mouse is in quiet wakefulness [62], This membrane bistability disappears when the mouse engages in whisking behavior [63]. Work from McCormick’s group has shown that delta oscillations can exist in the visual cortex of ostensibly awake mice [101]. Thus, sleep-like dynamics may be expressed during wakefulness and understanding both their local regulation and link to global sleep behavior would be of great significance, helping to elucidate how and why the brain generates sleep.
Local neuromodulation of thalamocortical dynamics by adenosine
In addition to circuit modulation via afferent projections, neuromodulatory substances can be released locally within neuronal circuitry via non-synaptic mechanisms. Such substances include peptides such as tumor necrosis factor alpha (TNF-α), insulin and growth hormone (GH) which locally regulate the expression of slow wave activity in the cortex [64], and small molecules such as nitric oxide and adenosine, powerful modulators of sleep homeostasis [65].
On a behavioral level, adenonsine satisfies the criteria for an endogenous sleep factor. Its levels correlate positively with the amount of time spent in wakefulness [65–67], infusing it (or its agonists) into the brain promotes sleep [68, 69], and antagonizing its effects promotes wakefulness [70–72]. Increasing adenosine availability through the infusion of adenosine kinase inhibitors, adenosine deaminase inhibitors, and an adenosine transport blockers also promotes sleep [65, 73]. Interestingly, caffeine, one of the most widely used substances in the world, is thought to exert its wake-promoting effects by adenosinergic mechanisms [74, 75]. Additionally adenosine deaminase polymorphism profoundly impacts sleep intensity and duration in humans [76]. Thus, deconstructing the cellular and circuit mechanism of adenosinergic regulation of sleep is likely to result in significant translational benefits.
On a cellular level, adenosine has been shown to act through several receptor subtypes, namely, A1R, A2AR, A2BR and A3R. In sleep studies, A1Rs and A2aRs have received particular attention due to their expression patterns in the brain, the availability of selective agonists and antagonists and selective molecular manipulation of genes encoding their receptor subtypes[2]. A1R are expressed at high levels throughout the brain, particularly in the cortex, hippocampus, thalamus and cerebellum [77]. A2ARs are expressed most strongly in the striatum [78]. A1R are Gi-coupled, exerting inhibitory effects on neuronal excitability and synaptic transmission, while A2ARs are Gs-coupled, exerting opposite electrophysiological effects. Activation of these two receptors is thought to promote sleep through a number of proposed mechanisms. For example, activation of A1R promotes sleep at the level of ascending arousal system—inhibition of PPT/LDT neuronal activity [79], inhibition of cholinergic basal forebrain neuron [79]. Importantly, A1R activation can have direct sleep-rhythmogenesis enhancing effects on the thalamocortical system. Circuit analysis of thalamocortical neurons in acute brain slices has shown that adenosine enhances burst firing in these neurons in the absence of other modulatory input [80]. A2AR on the other hand may promote sleep by activating sleep-active VLPO neurons [81].
Infusion of adenosine A1R agonists into the brain not only results in enhancement of sleep, but also in enhancement of slow wave activity (SWA; 0.5–4Hz frequency band oscillations) during sleep [69, 82]. SWA activity is a marker of homeostatic sleep pressure, as its power positively correlates with the amount of prior wakefulness [8]. A1R antisense infusion into the basal forebrain of rats resulted in attenuation of the behavioral and electrographic homeostatic sleep response [83].
A1R knockout studies aimed at understanding the role of A1R in sleep regulation were initially controversial. A study by Sternberg et al [84], showed that A1R knockout animals had no sleep phenotype. However, it is important to note that one needs to interpret studies from constitutive knockouts with caution. The constitutive absence of A1R throughout development is likely to result in compensatory changes that may mask important physiological functions that this receptor signaling pathway has. This is especially true given the role of A1R in brain development [85]. In fact, a recent study by Bjorness and Greene has shown an important role for A1R in sleep homeostasis using a conditional, forebrain-specific, A1R knockout animal [86]. In this study, the authors used a novel method for controlled sleep restriction, where sleep time was limited to a total of 4 hours across the course of the total 24 hours. During restricted sleep, the expression of SWA was attenuated by forebrain specific knockout of the A1R.
Astrocytes release ATP which contributes to extracellular adenosine
The discovery that astrocytes release chemical transmitters has revolutionized the study of the brain, as it added a layer of complexity to neuronal network function in which electrically silent glia can have neuromodulatory functions [16, 18, 87–89]. Gliotransmission, the process of transmitter release by astrocytes is mechanistically diverse and may occur through multiple molecular pathways including synaptic-like vesicles [90], large peptide-containing vesicles [91], lysosomes [92] anion channels [93], and connexin/pannexin hemichannels [94]. Additionally, astrocytes can release a number of transmitters including ATP, glutamate and D-serine [95]. [see contribution by Lalo et al in this issue]
The use of molecular genetics to understand the role of vesicular gliotransmission in neurophysiology resulted in the discovery that astrocytes release ATP through a SNARE-dependent mechanism [96]. Astrocytic ATP is rapidly (~200msec) degraded to adenosine which preferentially acts on A1R [96, 97]. These discoveries were made possible by the generation of transgenic animals in which the cytoplasmic domain of synaptobrevin II was specifically and conditionally expressed in astrocytes[96]. Given the role of synaptobrevin II in the formation of the SNARE-complex that is necessary for vesicular docking and fusion [98, 99], the overexpression of its cytoplasmic domain is thought to inhibit vesicular release by a dominant negative mechanism (dnSNARE).
Electrophysiological experiments carried out on acute hippocampal slices derived from dnSNARE animals revealed that the molecular attenuation of vesicular gliotransmission resulted in enhancement of basal synaptic transmission, attenuation of paired-pulse facilitation and attenuation of theta-burst induced long term potentiation (LTP) [96]. These electrophysiological effects were mimicked in wild type slices by A1R antagonists [96], and the dnSNARE electrophysiological phenotype was rescued by CCPA an A1R agonist [96]. Phenotypic rescue was also possible with extracellular perfusion of ATP, an effect that disappeared when extracellular 5’-ectonucleotidase inhibitor (ARL67156) was additionally perfused or when the A1R antagonist (CPT) was perfused. Finally, optical imaging of extracellular ATP revealed a reduction in slices derived from dnSNARE animals compared to wild type littermates. The combination of the aforementioned experiments demonstrated that astrocytes, through a SNARE-dependent mechanism, release ATP that is rapidly degraded to adenosine and that astrocytic adenosine has powerful modulatory effects on hippocampal synaptic transmission and plasticity.
More recently, astrocytes of the brainstem chemoreceptor area have been shown to respond to local increase in pH by an increase in their intracellular Ca2+ [100]. In response to this increase in Ca2+, astrocytes release ATP which causes activation of nearby chemoreceptor neurons. Driving astrocytes optogenetically, by activating the light-activated ion channel, channelrhodopsin 2 (ChR2) resulted in astrocytic release of ATP and an enhancement of the respiratory rhythm in vivo [100]. In contrast, driving astrocytes optogenetically in the subthalamic nucleus resulted in neuronal suppression likely due to ectonucleotidase-mediated degradation of ATP to adenosine, which activated A1R on these neurons. Thus, it may be plausible to hypothesize that the relative activity of ectonucleotidases in brain regions determines the sign of purinergic gliotransmission.
Astrocytic modulation of sleep and cognition
The discovery that astrocytes regulate extracellular adenosine in multiple forebrain regions led to the hypothesis that astrocytes may regulate sleep homeostasis. Reversible attenuation of gliotransmission in mice allowed for the discovery that astrocytes, indeed, regulate sleep homeostasis by adenosinergic mechanisms [15]. EEG recordings revealed an attenuated SWA phenotype under baseline conditions when gliotransmission was genetically inhibited. This reduction in spectral power of the EEG was state-specific, as SWA activity was only attenuated in NREM, but not wakefulness or REM in dnSNARE mice. This decrease in SWA was reversible when transgene expression was suppressed in dnSNARE mice as they exhibited a similar phenotype to their wildtype littermate when gliotransmission was restored. Importantly, this SWA activity phenotype manifested at the cellular and circuit level, where intracellular recordings of cortical neurons in vivo revealed a decrease in UP state probability of cortical neurons when gliotransmission was attenuated. Local field potential (LFP) recordings demonstrated the local network correlate of this phenomenon, demonstrating that gliotransmission can impact the genesis of sleep rhythms at the local circuit level by impacting synaptic transmission at multiple loci [19].
To test whether this electrographic phenotype had a behavioral consequence, the homeostatic sleep response was investigated while gliotransmission was manipulated. It was found that dnSNARE animals exhibited an attenuated behavioral and electrographic homeostatic sleep response to six hours of sleep deprivation at the beginning of their rest cycle compared to their wildtype littermates; both the enhancement in SWA and increased sleep time following sleep deprivation were attenuated. Interestingly, both these phenoptyes were reversed upon transgene suppression in dnSNARE animals. Thus, reversible inhibition of gliotransmission reversibly attenuated the homeostatic sleep response, a fundamental aspect of sleep behavior. This phenotype was mimicked by intracerebroventricular infusion of A1R antagonist (CPT) via microosmotic minipumps into wildtype animals. Additionally, dnSNARE animals showed an attenuated behavioral response to acute injections of CPT but not to the A2aR-selective antagonist (ZM 241385), concluding that the dnSNARE phenotype can be explained by purinergic mechanisms. Furthermore, while accumulation of sleep pressure prevented memory consolidation in wild type animals, dnSNARE mice showed resistance to the effects of sleep deprivation on memory. A detailed investigation of this phenomenon is likely to provide insight into mechanisms of cognitive impairment secondary to sleep loss. Additionally, region-specific manipulation of astrocytic function using genetic, pharamocologic and optic approaches will provide mechanistic insight into how precisely astrocytes regulate SWA and sleep behavior, as many outstanding questions regarding whether the effects seen in dnSNARE animals can be attributed to local cortical modulation or more global modulation at the level of basal forebrain and/or brainstem.
Conclusions
Though sleep signals behavioral inactivity, the brain remains quite active during this state. In particular, the thalamocortical system generates a number of dynamics including, spindles, delta and slow oscillations which may be key to mediating hypothesized functions of sleep, such as synaptic scaling and memory consolidation. Much progress has been made on understanding the genesis and modulation of these dynamics. In addition to afferent input from the canonical ascending arousal system, the thalamocortical circuitry can be modulated by locally released neurochemicals. One such chemical is adenosine, a substance whose extracellular levels are controlled by electrically-silent astrocytes. These cells release ATP through a vesicular pathway, which is degraded to adenosine and acts on neuronal A1Rs to regulate synaptic transmission. Genetic inhibition of astrocytic vesicular transmitter release has revealed that astrocytes modulate sleep rhythmogenesis, sleep behavior, and sleep-related cognitive functioning by purinergic mechanisms. This highlights the integrated nature of brain circuits in which slow-signaling glia can modulate fast neuronal processes by releasing neuromodulators (Summary Figure). Investigating neuron-glial interaction in sleep will likely further our understanding of its function and regulation, and may provide therapeutic targets of sleep disruption in neuropsychiatric illnesses.
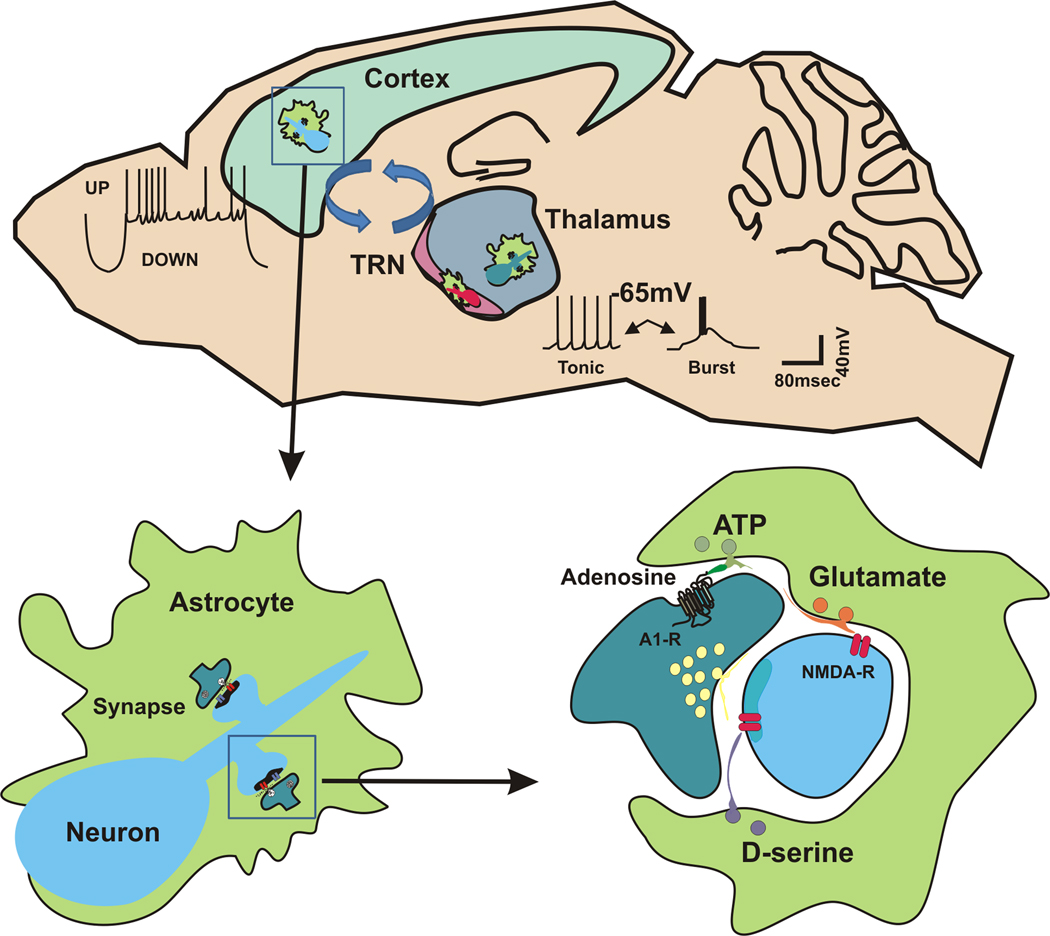
Figure. Thalamocortical sleep dynamics emerge from astrocytic-neuronal networks.
Top: Sagittal section of mouse brain highlighting cortex, thalamus and thalamic reticular nucleus (TRN) as an interactive system (arrows). Cellular-level oscillations are shown next to the relevant circuits which give rise to sleep dynamics. Bottom Left: three dimensional relationship between astrocytes and neurons; astrocytes enwrap neuronal cell bodies and processes. Bottom right: the tripartite synapse; astrocytes release a number of neuromodulatory substances, namely glutamate, D-serine and ATP. Glutamate and D-serine act on neuronal NMDA receptors while ATP is degraded to adenosine and act on adenosine A1-receptors, a modulator of sleep rhythms and sleep behaviors.
Acknowledgement
I would like to thank Christa Van Dort and Jennifer Gatchel for helpful comments on earlier versions of this manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- 2.Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol. 2009;7:238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 5.Greenspan RJ, Tononi G, Cirelli C, Shaw PJ. Sleep and the fruit fly. Trends Neurosci. 2001;24:142–145. doi: 10.1016/s0166-2236(00)01719-7. [DOI] [PubMed] [Google Scholar]
- 6.Siegel JM. Sleep viewed as a state of adaptive inactivity. Nat Rev Neurosci. 2009;10:747–753. doi: 10.1038/nrn2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 8.Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- 9.Tononi G. Slow wave homeostasis and synaptic plasticity. J Clin Sleep Med. 2009;5:S16–S19. [PMC free article] [PubMed] [Google Scholar]
- 10.Steriade M. Brain activation, then (1949) and now: coherent fast rhythms in corticothalamic networks. Arch Ital Biol. 1995;134:5–20. [PubMed] [Google Scholar]
- 11.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165:830–843. doi: 10.1176/appi.ajp.2008.08010077. [DOI] [PubMed] [Google Scholar]
- 12.Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. J Neurosci. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tononi G, Cirelli C. Sleep and synaptic homeostasis: a hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Born J. Slow-wave sleep and the consolidation of long-term memory. World J Biol Psychiatry. 11 Suppl 1:16–21. doi: 10.3109/15622971003637637. [DOI] [PubMed] [Google Scholar]
- 15.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, et al. Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron. 2009;61:213–219. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci. 2009;32:421–431. doi: 10.1016/j.tins.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Araque A, Carmignoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Annu Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 18.Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 19.Fellin T, Halassa MM, Terunuma M, Succol F, Takano H, Frank M, et al. Endogenous nonneuronal modulators of synaptic transmission control cortical slow oscillations in vivo. Proc Natl Acad Sci U S A. 2009;106:15037–15042. doi: 10.1073/pnas.0906419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 21.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 22.Davidson TJ, Kloosterman F, Wilson MA. Hippocampal replay of extended experience. Neuron. 2009;63:497–507. doi: 10.1016/j.neuron.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Girardeau G, Benchenane K, Wiener SI, Buzsaki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
- 25.Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11:200–208. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 27.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science. 2009;324:109–112. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Medina L, Abellan A. Development and evolution of the pallium. Semin Cell Dev Biol. 2009;20:698–711. doi: 10.1016/j.semcdb.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Mikula S, Manger PR, Jones EG. The thalamus of the monotremes: cyto- and myeloarchitecture and chemical neuroanatomy. Philos Trans R Soc Lond B Biol Sci. 2008;363:2415–2440. doi: 10.1098/rstb.2007.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Funke K, Worgotter F, Eysel UT. Correlated variations in EEG pattern and visual responsiveness of cat lateral geniculate relay cells. J Physiol. 1999;514(Pt 3):857–874. doi: 10.1111/j.1469-7793.1999.857ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirata A, Castro-Alamancos MA. Neocortex network activation and deactivation states controlled by the thalamus. J Neurophysiol. 103:1147–1157. doi: 10.1152/jn.00955.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick DA, Bal T. Sleep and arousal: thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 34.Contreras D. The role of T-channels in the generation of thalamocortical rhythms. CNS Neurol Disord Drug Targets. 2006;5:571–585. doi: 10.2174/187152706779025526. [DOI] [PubMed] [Google Scholar]
- 35.Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- 37.Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 20:R626–R627. doi: 10.1016/j.cub.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 38.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007;164:483–492. doi: 10.1176/ajp.2007.164.3.483. [DOI] [PubMed] [Google Scholar]
- 39.Eschenko O, Molle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006;26:12914–12920. doi: 10.1523/JNEUROSCI.3175-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009;29:1071–1081. doi: 10.1111/j.1460-9568.2009.06654.x. [DOI] [PubMed] [Google Scholar]
- 41.Sirota A, Csicsvari J, Buhl D, Buzsaki G. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci U S A. 2003;100:2065–2069. doi: 10.1073/pnas.0437938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 43.Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 1998;107:69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 44.Dossi RC, Nunez A, Steriade M. Electrophysiology of a slow (0.5–4 Hz) intrinsic oscillation of cat thalamocortical neurones in vivo. J Physiol. 1992;447:215–234. doi: 10.1113/jphysiol.1992.sp018999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol. 1995;483(Pt 3):641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espinosa F, Torres-Vega MA, Marks GA, Joho RH. Ablation of Kv3.1 and Kv3.3 potassium channels disrupts thalamocortical oscillations in vitro and in vivo. J Neurosci. 2008;28:5570–5581. doi: 10.1523/JNEUROSCI.0747-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cueni L, Canepari M, Lujan R, Emmenegger Y, Watanabe M, Bond CT, et al. T-type Ca2+ channels, SK2 channels and SERCAs gate sleep-related oscillations in thalamic dendrites. Nat Neurosci. 2008;11:683–692. doi: 10.1038/nn.2124. [DOI] [PubMed] [Google Scholar]
- 48.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 49.Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–6870. doi: 10.1523/JNEUROSCI.1318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luczak A, Bartho P, Marguet SL, Buzsaki G, Harris KD. Sequential structure of neocortical spontaneous activity in vivo. Proc Natl Acad Sci U S A. 2007;104:347–352. doi: 10.1073/pnas.0605643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 54.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–473. [PubMed] [Google Scholar]
- 55.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 56.Jones BE. Arousal systems. Front Biosci. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- 57.McCormick DA. Cholinergic and noradrenergic modulation of thalamocortical processing. Trends Neurosci. 1989;12:215–221. doi: 10.1016/0166-2236(89)90125-2. [DOI] [PubMed] [Google Scholar]
- 58.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 59.Siegel JM. The neurobiology of sleep. Semin Neurol. 2009;29:277–296. doi: 10.1055/s-0029-1237118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 61.Rector DM, Schei JL, Van Dongen HP, Belenky G, Krueger JM. Physiological markers of local sleep. Eur J Neurosci. 2009;29:1771–1778. doi: 10.1111/j.1460-9568.2009.06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 63.Poulet JF, Petersen CC. Internal brain state regulates membrane potential synchrony in barrel cortex of behaving mice. Nature. 2008;454:881–885. doi: 10.1038/nature07150. [DOI] [PubMed] [Google Scholar]
- 64.Liao F, Taishi P, Churchill L, Urza MJ, Krueger JM. Localized suppression of cortical growth hormone-releasing hormone receptors state-specifically attenuates electroencephalographic delta waves. J Neurosci. 30:4151–4159. doi: 10.1523/JNEUROSCI.6047-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: a mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basheer R, Porkka-Heiskanen T, Strecker RE, Thakkar MM, McCarley RW. Adenosine as a biological signal mediating sleepiness following prolonged wakefulness. Biol Signals Recept. 2000;9:319–327. doi: 10.1159/000014655. [DOI] [PubMed] [Google Scholar]
- 67.Porkka-Heiskanen T. Adenosine in sleep and wakefulness. Ann Med. 1999;31:125–129. doi: 10.3109/07853899908998788. [DOI] [PubMed] [Google Scholar]
- 68.Haulica I, Ababei L, Branisteanu D, Topoliceanu F. Letter: Preliminary data on the possible hypnogenic role of adenosine. J Neurochem. 1973;21:1019–1020. doi: 10.1111/j.1471-4159.1973.tb07549.x. [DOI] [PubMed] [Google Scholar]
- 69.Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 70.Virus RM, Ticho S, Pilditch M, Radulovacki M. A comparison of the effects of caffeine, 8-cyclopentyltheophylline, and alloxazine on sleep in rats. Possible roles of central nervous system adenosine receptors. Neuropsychopharmacology. 1990;3:243–249. [PubMed] [Google Scholar]
- 71.Radulovacki M, Virus RM. Dose-response effects of 8-cyclopropyltheophylline on sleep and wakefulness in rats. Psychopharmacology (Berl) 1988;94:417–420. doi: 10.1007/BF00174700. [DOI] [PubMed] [Google Scholar]
- 72.Schwierin B, Borbely AA, Tobler I. Effects of N6-cyclopentyladenosine and caffeine on sleep regulation in the rat. Eur J Pharmacol. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- 73.Radulovacki M, Virus RM, Djuricic-Nedelson M, Green RD. Hypnotic effects of deoxycorformycin in rats. Brain Res. 1983;271:392–395. doi: 10.1016/0006-8993(83)90309-8. [DOI] [PubMed] [Google Scholar]
- 74.Retey JV, Adam M, Khatami R, Luhmann UF, Jung HH, Berger W, et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin Pharmacol Ther. 2007;81:692–698. doi: 10.1038/sj.clpt.6100102. [DOI] [PubMed] [Google Scholar]
- 75.Retey JV, Adam M, Gottselig JM, Khatami R, Durr R, Achermann P, et al. Adenosinergic mechanisms contribute to individual differences in sleep deprivation-induced changes in neurobehavioral function and brain rhythmic activity. J Neurosci. 2006;26:10472–10479. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Retey JV, Adam M, Honegger E, Khatami R, Luhmann UF, Jung HH, et al. A functional genetic variation of adenosine deaminase affects the duration and intensity of deep sleep in humans. Proc Natl Acad Sci U S A. 2005;102:15676–15681. doi: 10.1073/pnas.0505414102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, et al. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- 79.Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263:689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pape HC, Mager R. Nitric oxide controls oscillatory activity in thalamocortical neurons. Neuron. 1992;9:441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- 81.Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123:451–457. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 82.Virus RM, Djuricic-Nedelson M, Radulovacki M, Green RD. The effects of adenosine and 2'-deoxycoformycin on sleep and wakefulness in rats. Neuropharmacology. 1983;22:1401–1404. doi: 10.1016/0028-3908(83)90231-9. [DOI] [PubMed] [Google Scholar]
- 83.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci. 2003;23:4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res. 2003;12:283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 85.Kimura M, Saitoh N, Takahashi T. Adenosine A(1) receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. J Physiol. 2003;553:415–426. doi: 10.1113/jphysiol.2003.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Halassa MM, Haydon PG. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol. 72:335–355. doi: 10.1146/annurev-physiol-021909-135843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Verkhratsky A, Parpura V, Rodriguez JJ. Where the thoughts dwell: The physiology of neuronal-glial "diffuse neural net". Brain Res Rev. doi: 10.1016/j.brainresrev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 89.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 90.Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, et al. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- 91.Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, et al. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- 92.Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, et al. Regulated ATP release from astrocytes through lysosome exocytosis. Nat Cell Biol. 2007;9:945–953. doi: 10.1038/ncb1620. [DOI] [PubMed] [Google Scholar]
- 93.Duan S, Anderson CM, Keung EC, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci. 2003;23:1320–1328. doi: 10.1523/JNEUROSCI.23-04-01320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 97.Serrano A, Haddjeri N, Lacaille JC, Robitaille R. GABAergic network activation of glial cells underlies hippocampal heterosynaptic depression. J Neurosci. 2006;26:5370–5382. doi: 10.1523/JNEUROSCI.5255-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scales SJ, Bock JB, Scheller RH. The specifics of membrane fusion. Nature. 2000;407:144–146. doi: 10.1038/35025176. [DOI] [PubMed] [Google Scholar]
- 99.Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH. SNAREs contribute to the specificity of membrane fusion. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 100.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, et al. Astrocytes control breathing through pH-dependent release of ATP. Science. 329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sachdev RN, McCormick DA. Under what states do slow oscillations occur in the mouse cortex? Society for Neuroscience Meeting. 2010 [Google Scholar]