Abstract
Neuroblastomas (NBs) with favorable outcome usually express TrkA, whereas unfavorable NBs frequently express TrkB and its cognate ligand BDNF. P75 (p75LNTR, NGFR, TNFRSF16) binds NGF-related neurotrophins with low affinity and usually is co-expressed with Trk receptors in NBs. Here, we investigated the importance of p75 coexpression with Trk receptors in NBs. We transfected p75 into two Trk-null NB cell lines, SH-SY5Y and NLF that were also engineered to stably express TrkA or TrkB. Cell numbers were compared between single (Trk alone) and double (Trk+p75) transfectants, and proliferation was assessed by flow cytometry. P75 coexpression had little effect on cell growth in Trk NB cells in the absence of ligand, but it increased sensitivity and greatly enhanced the effect of cognate ligand. Exogenous NGF induced greater phosphorylation of TrkA and AKT. This was associated with increased cell number in TrkA/p75 cells compared to TrkA cells (p<0.01), which was due to increased proliferation in TrkA/p75 cells (p<0.05), followed by differentiation. Exogenous BDNF also increased cell number in TrkB/p75 compared to TrkB cells (p<0.01), due to an increase in proliferation, but without differentiation. Co-expression of p75 also increased specificity of Trk-expressing cells to ligand. NT3-induced phosphorylation of TrkA and AKT was reduced in TrkA/p75 cells. NT3-induced phosphorylation of TrkB (as well as AKT and MAPK) was also reduced with p75 coexpression. Our results suggest that p75 plays an important role in enhancing both the sensitivity of Trk receptors to low levels of ligand, as well as increasing the specificity of Trks to their cognate ligands. It also enhances ligand-induced differentiation in TrkA/p75 but not TrkB/p75 cells.
Keywords: TrkA, TrkB, p75, neuroblastoma, neurotrophin, sensitivity, specificity, proliferation, differentiation
1. Introduction
Neuroblastoma (NB) is the most common extracranial solid tumor in children. It is derived from the neural crest and usually arises in the adrenal medulla or along the sympathetic chain [1]. Neurotrophins bind to Trk receptors and initiate signaling cascades that promote cell proliferation and differentiation. TrkA, the biological receptor for nerve growth factor (NGF), is commonly expressed in the most favorable NBs and leads to differentiation of NB cells upon ligand activation [2; 3; 4]. Conversely, TrkB leads to proliferation of NB cells upon binding its ligand, brain-derived neurotrophic factor (BDNF). TrkB and BDNF are coexpressed in more aggressive tumors, particularly those with MYCN amplification [5]. The coexpression of receptor and ligand suggests an autocrine survival pathway in these tumors [5; 6; 7; 8]. TrkC, the receptor for neurotrophin 3 (NT3), is expressed in a subset of TrkA expressing tumors, and it is similarly associated with favorable clinical features and outcome [9; 10]. Overall, these findings suggest that the Trk family of neurotrophin receptors plays an important role in the behavior of both favorable and unfavorable NBs.
All neurotrophins also bind to p75 (p75LNTR, NGFR), a member of the tumor necrosis factor receptor superfamily (TNFRSF16). P75 binds NGF and related neurotrophins with low affinity, but its effect on the function of Trk receptor signaling in NBs is less clear. Transfection with p75 increases the number of high- and low-affinity NGF binding sites in TrkA-expressing PC12 cells [11], and p75 expression may increase the sensitivity of TrkA to low concentrations of NGF [12; 13; 14]. Furthermore, p75 expression in the absence of TrkA may induce apoptosis in response to NGF [15; 16; 17; 18], but this apoptosis is inhibited by the presence of TrkA receptors [19]. Nevertheless, the effect of p75 on the cellular response to neurotrophins is complex, and may depend on the concentration of ligand, the ratio of receptors, the cell type in which it is expressed, and its stage of differentiation [20; 21; 22; 23].
Several investigators have addressed the prevalence and clinical significance of p75 expression in NBs. Suzuki and coworkers analyzed 80 NBs for the expression of TrkA and p75 mRNA [4], but p75 expression did not correlate with TrkA expression, histological differentiation, stage, or survival. In contrast, Kogner and colleagues examined 45 NBs and three benign ganglioneuromas for expression of TrkA and p75 mRNA, and they found that both correlated with younger age, favorable clinical stages, and absence of MYCN amplification [2]. They concluded that NBs co-expressing both TrkA and p75 mRNAs are favorable tumors likely to differentiate, regress spontaneously or respond to conventional therapy. Bunone demonstrated that p75 expression mediates apoptosis in NBs in the absence of NGF [16], and we have previously shown that coexpression of TrkA inhibits the apoptosis associated with p75 expression [19].
Most primary NBs express at least one of the Trk family genes (usually TrkA or TrkB), and many also express p75, but the functional consequences of p75 coexpression with either TrkA or TrkB in NBs has not been studied. Therefore, we have examined the effect of p75 coexpression on the sensitivity and specificity of ligand binding in TrkA- or TrkB-expressing NBs. Activation of the PI3 kinase/AKT and Ras/MAPK pathways play important roles in the survival, proliferation and differentiation of NB cells [24; 25]. Therefore, we also assessed the effect of p75 coexpression on intracellular signaling, proliferation and differentiation.
2. Materials and Methods
2.1. Cell culture and transfection of p75
We used the SH-SY5Y (SY5Y) and NLF human NB cell lines, which had the lowest endogenous expression of Trk family genes of all NB cell lines tested. Cells were maintained in an atmosphere of 5% CO2 in RPMI 1640 supplemented with 10% FBS, 1% glutamate, and 50 μg/ml gentamicin. Clones of the SY5Y parental cell line were established to stably express either TrkA (SY5Y-TrkA) or TrkB (SY5Y-TrkB) using the pLNCX retroviral expression vector (Clontech, Palo Alto, CA). Similarly, TrkA- and TrkB-expressing clones were established in NLF using pLNCX (NLF-TrkA and NLF-TrkB). We transfected full-length p75 cDNA (using the pLPCX vector) into the Trk-expressing SY5Y and NLF clonal lines by electroporation. Stably expressing, double-transfected cells were selected in 400 μg/ml geneticin and 0.5 μg/ml puromycin. The double-resistant cells were further subcloned and expanded. SY5Y-TrkA/p75 (clone #7) and SY5Y-TrkB/p75 (clone #8) were used for subsequent experiments, but additional clones of SY5Y and NLF were tested in parallel experiments to determine consistency of results. The expression of Trk and p75 protein was characterized by Western blotting using anti-Trk and anti-p75 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).
2.2. Cell morphology and immunofluorescence
We assessed cell morphology using phase-contrast microscopy, and captured images using digital photomicrography. NGF can induce cell differentiation in TrkA-expressing NB cells [26], so we assessed for neuronal differentiation by changes in cell shape, and by measuring neurite outgrowth. Cells that were three or more times the size of undifferentiated cells, with development of euchromatin and prominent nucleoli, were considered differentiated. Furthermore, neurite outgrowth was assessed by counting the number of cells that had neurites extending 3 or more times the longest diameter of the cell, as assessed by ocular micrometer measurement. Only cells exhibiting one or the other (or both) of these features were considered differentiated. Furthermore, to determine whether cells were undergoing neuronal differentiation, we assessed the relative expression of tyrosine hydroxylase (Leica Microsystems, Inc, Bancockburn, IL), and synaptophysin (Invitrogen, Carlsbad, CA) using immunofluorescence.
2.3. Cell viability assay
We measured cell viability using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. This assay specifically detects living but not dead cells. The signal generated is dependent on the degree of activation of the tetrazolium salt by the cells. It can therefore be used to measure cytotoxicity and proliferation. The absorbance is correlated with cell number [27]. Cells were seeded into 24-well or 96-well plates at a density of 2×104 cells/well. The MTT assay was performed at day 1, day 2 and day 5. A multiple wavelength scanner was used to measure the absorbance at 570 nm and 630 nm dual wavelengths. The experiments were performed in duplicate and repeated 4 to 5 times. Additional MTT experiments were performed in RT-CES plates (see below) at the end point of an RT-CES experiment, and this was used to correlate with viable cell number.
2.4. Real-Time Cell-Electronic Sensing (RT-CES) measurements
The RT-CES system (ACEA Biosciences, San Diego, CA) was used to monitor changes in cell number under varying conditions over time. RT-CES produces an electronic readout of impedance to non-invasively quantify adherent cell proliferation and viability in real-time. Interaction of cells with the electronic biosensors leads to the generation of a cell-electrode impedance response (cell index, or CI), which reflects the state of cell viability, and correlates well with the number of cells seeded [28]. This technology allows us to compare the effect of neurotrophin on different cell lines by normalizing CI to the time point when neurotrophin is added. To study the effect of neurotrophin on different cell lines, 100 μl of medium was added to wells of the ACEA 16x E-plates to obtain background readings, followed by the addition of 100 μl of cell suspension containing 40,000 cells (for SY5Y) or 5,000 cells (for NLF). The E-plate containing cells is incubated at room temperature for 10 min prior to placement in the device station at 37°C for continuous recording of CI. Cells were allowed to attach and spread for 6–12 hours prior to neurotrophin addition. Cell morphology can also influence CI, which is recorded every 5 minutes. Transient changes (within minutes) in CI immediately following neurotrophin addition represent cytoskeletal modulation that may occur following receptor phosphorylation [28]. However, sustained or progressive changes in CI recorded every 30 minutes generally represent changes in cell number. The results are expressed as normalized CI, unless indicated otherwise, which is derived from the ratio of CI before and after addition of the compounds.
2.5. Flow cytometry
Flow cytometric analysis of DNA content was used to determine whether NGF or BDNF enhances growth by increasing proliferation (increase in S + G2/M phase) or decreasing apoptosis (decrease in sub-G0/G1). Cells were grown with or without NGF or BDNF (100 ng/ml) for 18 hrs. DNA content was measured as described previously [25].
2.6. Immunoblotting
Trk and Trk/p75 NB cells were grown to 70–80% confluency in standard culture medium, and serum deprived overnight prior to ligand treatment. To study receptor sensitivity to ligands, a series of concentrations (0.01, 0.1, 1, 10, 100 ng/ml) of NGF or BDNF was added to cells for 10 minutes prior to assessing receptor autophosphorylation. To study receptor specificity for ligand, 100 ng/ml of either NGF, BDNF, NT3 or NT4 was added for 10 minutes. Whole cell lysates were prepared as described previously [25], and 50–100 μg of total protein was resolved by SDS-PAGE, transferred to nitrocellulose, and proteins detected with specific antibodies. Activation of the PI3 kinase/AKT and Ras/MAPK pathways play a role in the survival, proliferation and differentiation of NB cells. We probed blots with anti-phospho-Trk (Tyr-490), anti-phospho-AKT (Ser-473) or anti-phospho-p42/p44 MAPK (Cell Signaling, Beverly, MA)— using an ECL system (Amersham Corp., Arlington Heights, IL). Corresponding membranes were reprobed with anti-Trk (Santa Cruz), anti-AKT (Cell Signaling) or anti-MAPK (Santa Cruz) antibody to ensure equal loading of protein.
2.7. Statistical analysis
The results of the RT-CES and MTT assay (two group comparisons) were analyzed by paired Student’s t test. The results of flow cytometry were analyzed by two-sample t-test, assuming Trk and Trk/p75 groups are independent and samples are normally distributed.
3. Results
3.1. Protein expression in Trk and Trk/p75 transfectants
To understand the effect of p75 on Trk-mediated signaling in NB, we used SY5Y cells expressing TrkA or TrkB (SY5Y-TrkA or SY5Y-TrkB) and stably transfected full-length p75 into the these Trk-expressing clones in order to generate stable double transfectants. We confirmed the expression of Trk and p75 protein in single and double transfectants by Western blotting (Fig. 1). We used single- and double-transfectant clones of SY5Y in these studies, but additional cell clones, as well as corresponding clones of NLF, were analyzed to validate consistency of biological responses.
Fig. 1.
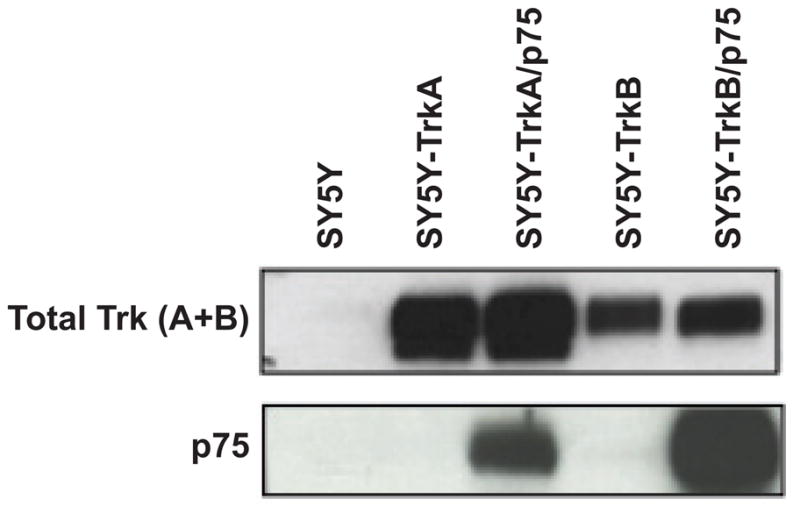
Expression of Trk and p75 receptors in NB single and double transfectants. (A) Immunoblotting demonstrates protein expression of total Trk (TrkA or TrkB) and p75 receptors in all four cell lines used in this study: SY5Y-TrkA, SY5Y-TrkA/p75, SY5Y-TrkB, and SY5Y-TrkB/p75.
3.2. P75 enhanced NGF-induced proliferation and increased sensitivity of TrkA cells
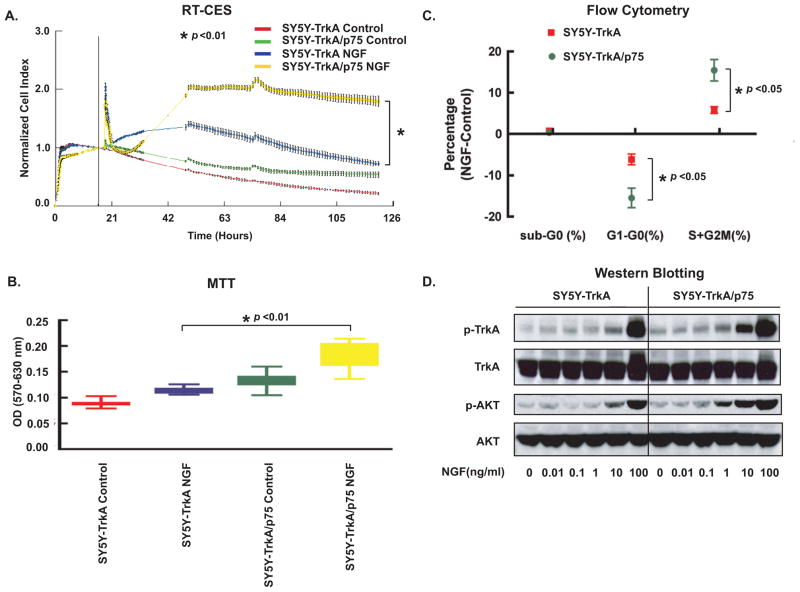
To determine the effect of p75 on TrkA cells after cognate ligand binding, we examined the response of SY5Y-TrkA and SY5Y-TrkA/p75 to exogenous NGF. By RT-CES monitoring, we observed that higher concentrations of NGF 25–100 ng/ml) increased the CI of both TrkA and TrkA/p75 cells in serum-free medium. NGF caused greater increase in CI in TrkA/p75 cells than in TrkA cells (p<0.01) (Fig. 2A). However, lower doses (<25 ng/ml) did not have a significant effect. Changes in CI may reflect changes in cell number and/or changes in cell morphology. Because NGF can induce cell differentiation in TrkA-expressing NB cells, we compared NGF-induced differentiation between SY5Y-TrkA and SY5Y-TrkA/p75 cells. Initially, there was increased CI in TrkA/p75 NB cells compared to TrkA alone which was due in part to increased cell number after NGF treatment, and this was confirmed by MTT assay (Fig. 2B). To determine if the increase in cell number was due to increased cell proliferation or decreased apoptosis, cells were analyzed by flow cytometry to measure DNA content (Fig. 2C). Compared to control, NGF did not alter the number of cells in the sub-G0 phase in either TrkA or TrkA/p75 NB cells (Fig. 2C). However, NGF-treated cells at 18 hr had significantly fewer cells in the G1 phase than in the S + G2/M phase for both TrkA and TrkA/p75 cells (Fig. 2C). This suggests that NGF treatment initially increases proliferation (at 1–2 days), which is significantly greater in cells expressing TrkA/p75 compared to TrkA alone at 18 hr (p<0.05).
Fig. 2.
P75 increases sensitivity of TrkA cells. (A) Cells were seeded in serum-free medium, and NGF (100 ng/ml) was added after overnight incubation. Cells were continuously monitored by the RT-CES system. Cell Index, which reflects cell number in the plates, was recorded every 5–30 min. The vertical black line (in 2A) indicates the time point when NGF was added. Transient changes (within minutes) in CI immediately following neurotrophin addition represent cytoskeletal modulation that may occur following receptor phosphorylation [28]. Points are the averaged normalized CI of six replicate wells, (bars=SD). The difference in normalized CI between SY5Y-TrkA and SY5Y-TrkA/p75 treated with NGF is significant. (*p<0.01). The difference of normalized CI between TrkA and TrkA/p75 without added NGF is not significant. Graph represents a single experiment (repeated at least three times with similar results). (B) MTT assay performed at end point of RT-CES experiment, average OD of triplicate wells measured (error bars, ±SD). The difference in OD between SY5Y-TrkA and SY5Y-TrkA/p75 treated with NGF is significant (*p<0.01). (C) Flow cytometry analysis shows effect of NGF on cell cycle after 18 h treatment. The difference of G1 and S+G2/M phase percent changes by NGF between TrkA and TrkA/p75 cells is significant (p<0.05). ). Graph represents mean of five experiments (bars=SD). (D) Phosphorylation of TrkA and AKT (Ser473) was examined by immunoblotting after treatment with indicated NGF concentrations. The blots were stripped and re-probed with anti-Trk and anti-AKT antibodies.
However, this early proliferative response was followed by a significant increase in the extent of neurite outgrowth between TrkA and TrkA/p75-expressing cells after 4–5 days of NGF treatment (data not shown). We also assessed the expression of two markers of differentiation, tyrosine hydroxylase and synaptophysin in SY5Y-TrkA and SY5Y-TrkA/p75 cells without and with ligand. There was no significant difference in the level of expression of either marker in the absence of ligand, but expression was substantially higher at 4–5 days in the cells coexpressing TrkA and p75 compared to those expressing TrkA alone (Fig. 3A, 3B). We obtained similar results using other SY5Y-TrkA/p75 clones. Thus, the co-expression of p75 with TrkA in NB cells results in an early increase in proliferation (1–2 days), followed by significantly increased differentiation after NGF treatment (4–5 days) that is greater in the TrkA/p75 NB cells compared to cells expressing TrkA alone.
Fig. 3.
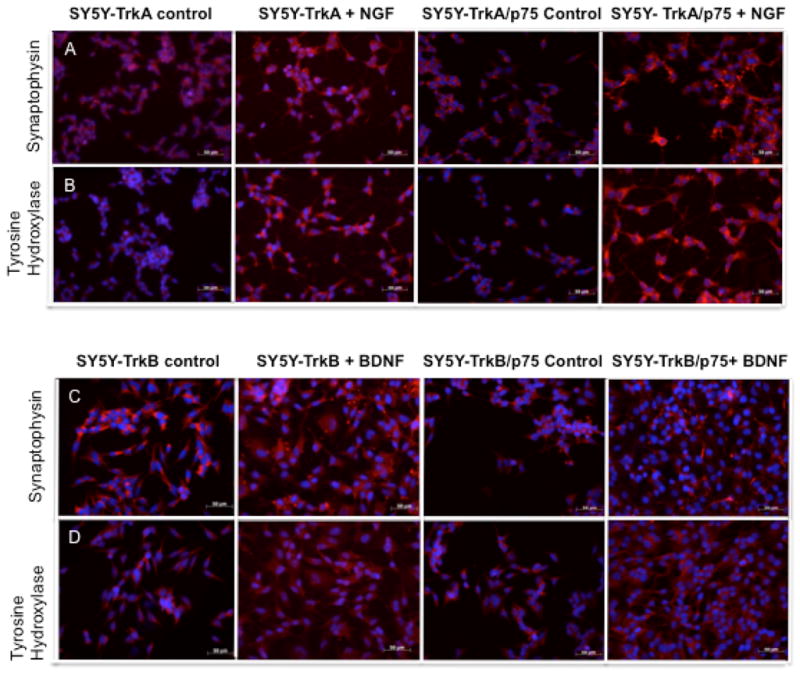
Immunohistochemistry to detect the expression of tyrosine hydroxylase (A, C) or synaptophysin (B, D) in NB cells as markers of neuronal differentiation. We assessed expression in SY5Y-TrkA and SY5Y-TrkA/p75 NB cells in the absence and presence of NGF (A, B), as well as in SY5Y-TrkB, and SY5Y-TrkB/p75 NB cells in the absence and presence of BDNF (C, D).
TrkA transduces cell signals through two major signaling cascades: Ras/MAPK and PI3K/AKT. We studied the impact of p75 coexpression on NGF induced AKT phosphorylation in TrkA NB cells. TrkA and TrkA/p75 cells were treated with a range of NGF concentrations and whole cell lysates collected for immunoblotting. Comparable levels of phospho-AKT (p-AKT473) were demonstrated in TrkA cells at 10 ng/ml of NGF and in TrkA/p75 cells at 1 ng/ml of NGF. At a concentration of 10 ng/ml, NGF induced greater phosphorylation of TrkA (p-TrkA) and AKT in TrkA/p75 cells than in TrkA alone (Fig. 2D). Phosphorylation levels of TrkA and AKT are similar for TrkA and TrkA/p75 at 100 ng/ml NGF, suggesting receptor saturation. Thus, p75 coexpression sensitized TrkA receptors to NGF and resulted in signaling pathway activation/phosphorylation (and enhanced survival) at lower ligand concentrations.
3.3. P75 increases the specificity of TrkA for ligand
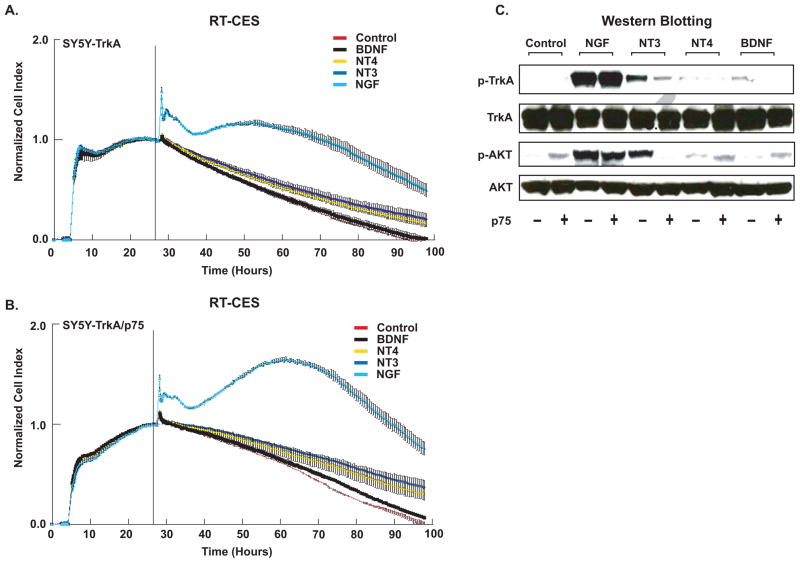
We examined SY5Y-TrkA and SY5Y-TrkA/p75 cells to determine if p75 coexpression affected the response of TrkA to related neurotrophins: BDNF, NT3, and NT4. For both TrkA and TrkA/p75 cells, only NGF significantly increased CI as measured by RT-CES (Fig. 4A, 4B). CI was significantly greater in TrkA/p75 than in TrkA after treatment with NGF, as was seen previously (compare to Fig. 2A). Similar results were obtained by MTT assay (data not shown). We also compared the ability of related neurotrophins to induce phosphorylation of TrkA and the downstream effector protein, AKT, in TrkA and TrkA/p75 cells (Fig. 4C). NGF treatment induced equal phosphorylation of TrkA and AKT in TrkA and TrkA/p75 cells at higher concentrations (100 ng/ml). Phosphorylation of TrkA and AKT with NT4 and BDNF treatment was nearly undetectable in both TrkA and TrkA/p75 cells. NT3 induced less phosphorylation of TrkA and AKT than NGF in TrkA-expressing cells. However, when p75 was co-expressed, the phosphorylation of TrkA and AKT in response to NT3 was almost completely abrogated (Fig. 4C). Thus, p75 increased the specificity of TrkA to its cognate ligand NGF, in terms of receptor phosphorylation and downstream pathway activation.
Fig. 4.
P75 increases specificity of TrkA. (A) TrkA and (B) TrkA/p75 cells were seeded in serum-free RPMI medium and treated with NT3, NT4, NGF, BDNF (100 ng/ml), or media alone (Control), and continuously monitored using RT-CES. CI was recorded every 5–30 min. The vertical black line indicates the time point when a neurotrophin was added Transient changes (within minutes) in CI immediately following neurotrophin addition represent cytoskeletal modulation that may occur following receptor phosphorylation [28]. Points are averaged normalized CI of duplicate wells (bars=SD). Graphs are a representative result, (experiment was repeated at least three times with similar results). (C) Cells (TrkA +/− p75) were treated with the indicated neurotrophin for 10 minutes, and phosphorylation of TrkA, AKT (Ser473) was examined by immunoblotting. The blots were stripped and reprobed with anti-Trk, anti-AKT antibodies.
3.4. P75 enhances BDNF-induced proliferation and increases sensitivity of TrkB cells
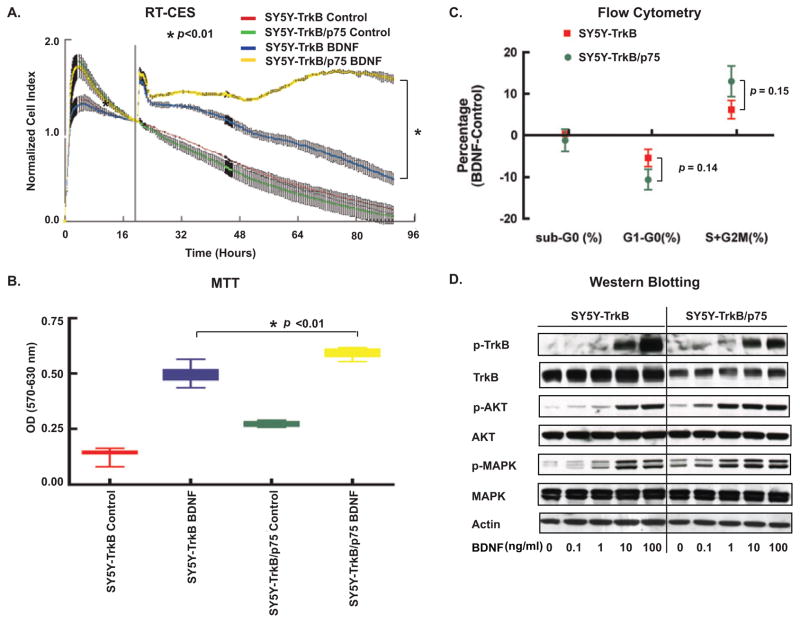
To determine the effect of p75 on TrkB cells after cognate ligand binding, we examined the response of SY5Y-TrkB and SY5Y-TrkB/p75 to exogenous BDNF (Fig. 5). BDNF addition increased the CI of both TrkB and TrkB/p75 cells in serum-free medium, as detected by RT-CES. BDNF produced a significantly greater increase in CI (over 60 hrs) in cells coexpressing TrkB/p75 compared to TrkB alone (p<0.01) (Fig. 5A). Similar results were obtained by adding different concentrations (10, 25, 50, 100 ng/ml) of BDNF. Low concentration of BDNF (under 10 ng/ml) did not have a significant effect on either TrkB or TrkB/p75 cells. BDNF treatment produced only marginal neurite outgrowth (less than 1 × cell body) over 5 days of treatment, so we observed no significant morphological difference between TrkB and TrkB/p75 cell lines (Fig. 3). This suggests that the increased CI results from an increase in cell number, which was confirmed by MTT assay (Fig. 5B). We also performed flow cytometry analysis to measure DNA content of TrkB and TrkB/p75 cells. BDNF treatment decreased the percentage of cells in G1 phase and increased the percentage of cells in S+G2/M phases both in TrkB and TrkB/p75 cells (Fig. 5C). In the presence of p75 co-expression, the decrease of cells in G1 and increase number of cells in the S + G2/M phases after BDNF was greater in TrkB/p75 than TrkB cells (p=0.14) (Fig. 5C). Thus, p75 coexpression enhanced BDNF-induced cell proliferation in TrkB expressing cells.
Fig. 5.
P75 increases sensitivity of TrkB cells. (A) Cells were seeded in serum-free medium, and BDNF (100 ng/ml) was added after overnight incubation. Cells were continuously monitored by the RT-CES system and CI was recorded every 5–30 min. The vertical black line (in 4A) indicates the time point when BDNF was added. Transient changes (within minutes) in CI immediately following neurotrophin addition represent cytoskeletal modulation that may occur following receptor phosphorylation [28]. Points=average of normalized CI in duplicated wells. Bars=SD at recorded time. The differences in normalized CI between SY5Y-TrkB and SY5Y-TrkB/p75 treated with BDNF is significant (*p<0.01). (B) MTT assay performed on TrkB and TrkB/p75 cells growing in 0% serum, treated with BDNF (100 ng/ml) or medium alone (Control). (Data points=average OD of quadruplicates, bars=SD). The difference between SY5Y-TrkB and SY5Y-TrkB/p75 cells treated with BDNF is significant (*p<0.01). (C) Flow cytometry analysis shows effect of BDNF (100 ng/ml) on cell cycle after 18 h treatment. The difference of G1 and S+G2/M phase percent changes by BDNF between TrkB and TrkB/p75 cells is not significant (p=0.14 and p=0.15). Graph represents mean of five experiments. (Bars=SD). (D) Phosphorylation of TrkB and MAPK was examined by immunoblotting after treatment with indicated BDNF concentrations. The blots were stripped and reprobed with anti-Trk and anti-MAPK antibodies.
We compared BDNF-induced differentiation between SY5Y-TrkB and SY5Y-TrkB/p75 cells. There was no significant difference in the extent of neurite outgrowth and cell shape between TrkB and TrkB/p75-expressing cells during 5 days of BDNF treatment (data not shown). We also assessed the expression of tyrosine hydroxylase and synaptophysin in SY5Y-TrkB and SY5Y-TrkB/p75 cells without and with ligand. There was no significant difference in the level of expression of either neuronal differentiation marker in the absence or presence of ligand, regardless of whether or not p75 was coexpressed with TrkB (Fig. 3C, 3D). Thus, p75 had no effect on ligand-induced differentiation in TrkB-expressing cells.
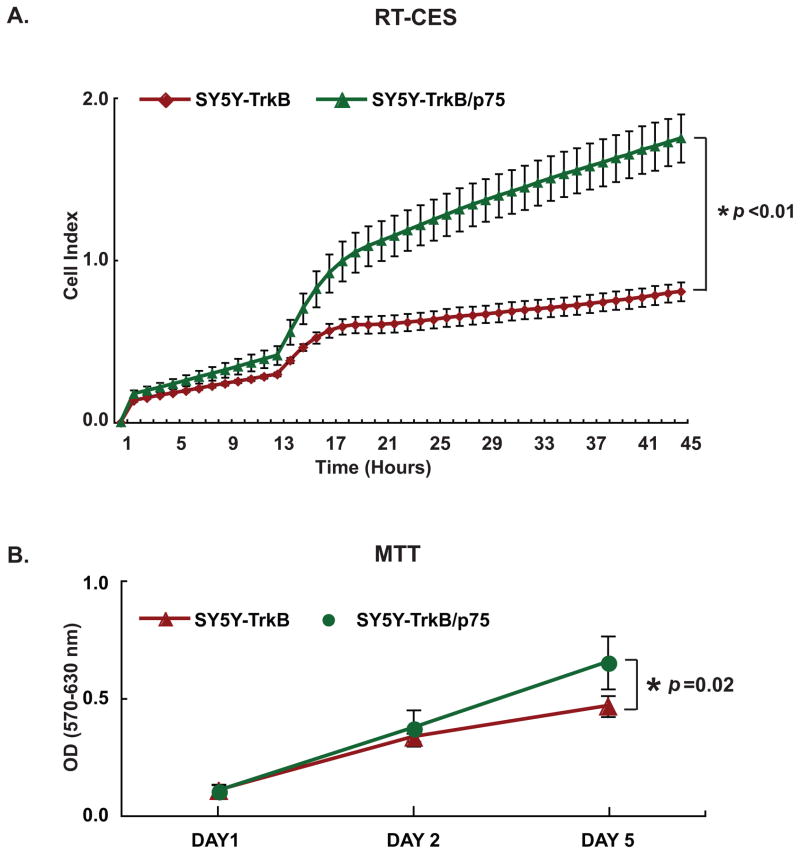
We studied the impact of p75 coexpression on BDNF-induced signal transduction pathways in TrkB NB cells. TrkB and TrkB/p75 cells were treated with a range of BDNF concentrations, and whole cell lysates were collected for immunoblotting. Maximum TrkB phosphorylation was induced by 100 ng/ml of BDNF in TrkB cells and by 10 ng/ml in TrkB/p75 cells. Comparable levels of phospho-AKT (p-AKT473) were detected at 10 ng/ml of BDNF in TrkB cells and at 1 ng/ml in TrkB/p75. There was more basal p-MAPK in TrkB/p75 cells, and maximum phosphorylation was achieved at 10 ng/ml for TrkB and 1 ng/ml for TrkB/p75 cells (Fig. 4D). Thus, p75 coexpression sensitized TrkB receptors to BDNF and resulted in signaling pathway activation by phosphorylation at a 10-fold lower ligand concentration (Fig. 5D). This likely reflects increased sensitivity to low-level endogenous BDNF production by SY5Y cells, leading to autocrine activation of TrkB and MAPK [25]. Prolonged treatment with exogenous BDNF (for 6 hrs) results in greater TrkB and MAPK phosphorylation in TrkB/p75 cells (data not shown). We further compared the growth of TrkB and TrkB/p75 cells in medium without exogenous BDNF by both RT-CES system and MTT assay (Fig. 6A and 6B). We found that the growth rate of TrkB/p75 cells was significantly greater than cells expressing TrkB alone.
Fig. 6.
P75 increases growth rate of TrkB Cells. (A) Cells were seeded in serum-free medium and continuously monitored by the RT-CES. The difference in CI between SY5Y-TrkB and SY5Y-TrkB/p75 without BDNF treatment was significant. (*p<0.01). (Points=average CI of duplicate wells, bars=SD). (B) Cells were seeded in 96-well plates in 10% FBS-RPMI medium. An MTT assay was performed at day 1, day 2 and day 5 to measure cell number. The difference in OD between SY5Y-TrkB and SY5Y-TrkB/p75 without BDNF treatment was significant, (*p=0.02). (Points=average OD of five wells, bars=SD).
3.5. P75 increases the specificity of TrkB for ligand
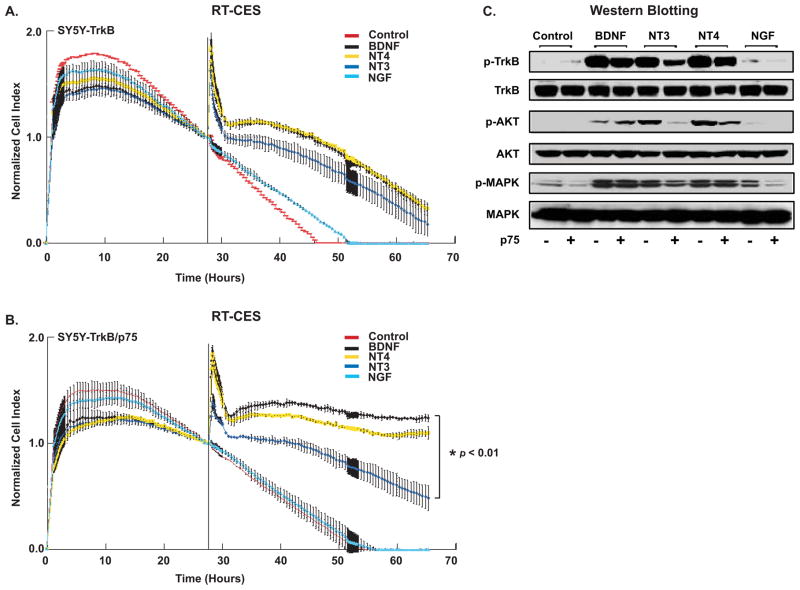
We examined the response of TrkB- and TrkB/p75-expressing NB cells to related neurotrophins by RT-CES and immunoblotting to determine if p75 increases the specificity of TrkB receptor for ligand binding. BDNF, NT4 and NT3 all had a similar ability to increase proliferation of TrkB-expressing cells, whereas in the TrkB/p75-expressing cells, NT3 treatment had significantly less effect than either BDNF or NT4. Indeed, both BDNF and NT4 exposure produced greater proliferation of TrkB/p75 cells than observed in cells expressing TrkB alone (Fig. 7A and 7B). When p75 was co-expressed, there was much less NT3-induced phosphorylation of TrkB, AKT and MAPK (Fig. 7C). P75 coexpression also significantly reduced AKT phosphorylation, but not phosphorylation of TrkB, by NT4 treatment. Thus, p75 increased the specificity of TrkB for ligand activation, largely blocking NT3-induced responses, and it also reduced the NT4 response in cotransfected cells.
Fig. 7.
P75 increases specificity of TrkB. (A) TrkB and (B) TrkB/p75 cells were seeded in serum-free medium and treated with BDNF, NT3, NT4, NGF (100 ng/ml), or media alone (Control), and continuously monitored using RT-CES. Cells index was recorded every 5–30 min. The vertical black line indicates the time point when a neurotrophin was added. Transient changes (within minutes) in CI immediately following neurotrophin addition represent cytoskeletal modulation that may occur following receptor phosphorylation [28]. (B) The difference in normalized CI of SY5Y-TrkB/p75 between BDNF (black) and NT3 (light blue) treatment is significant (*p<0.01). Points are average of duplicate wells, bars=SD. Graph represents one experiment, (experiment repeated twice with similar results). (C) Cells were treated with the indicated neurotrophins and phosphorylation of TrkB, AKT (Ser473) and MAPK was examined by immunoblotting. The blots were stripped and reprobed with anti-Trk, anti-AKT, and anti-MAPK antibodies, respectively, to detect total TrkB, AKT and MAPK.
4. Discussion
NB is a common pediatric cancer and contributes disproportionately to morbidity and mortality from cancer in children [1]. The expression and function of Trk receptors likely contributes to the heterogeneous clinical behavior of this tumor. The expression of TrkA possibly plays a role in the differentiation or regression of favorable NBs. TrkA-expressing tumors may regress because of delayed activation of developmentally programmed cell death in the absence of NGF in their microenvironment [29]. Alternatively, these tumors may differentiate into benign ganglioneuromas in the presence of NGF, which may come from reactive, invading Schwann cells [30]. In contrast, the TrkB/BDNF autocrine pathway confers a more aggressive behavior on unfavorable NBs. TrkB expression clearly contributes to enhanced angiogenesis, drug resistance and tumorigenicity [25; 31; 32; 33; 34]. P75 is frequently expressed in primary NBs, and high p75 expression is generally associated with favorable behavior and outcome [2; 4]. However, because p75 is rarely expressed in NBs in the absence of Trk expression, it is unclear if p75 has an independent role. Miller and colleagues reported that p75 might facilitate the process of TrkA-induced differentiation or regression of favorable NBs [35]. Thus, it was important to examine the effect of p75 on NBs expressing either TrkA or TrkB, as modulating their response to ligand may be an important role of p75 in these tumors.
Trk and p75 receptors frequently are co-expressed in neuronal precursors, particularly in the vertebrate peripheral nervous system [36]. Indeed, p75 is not expressed in neurons of dorsal root ganglia independent of Trk expression [37]. There is evidence in normal neurons that p75 can increase sensitivity to lower concentrations of NGF, leading to enhanced survival under adverse conditions [22; 38]. In addition, p75 coexpression reduces responsiveness of TrkB-expressing cells to NT3 [39]. On the other hand, reducing the level of p75 expression leads to increased response of TrkB to NT3 [40]. In the absence of p75 expression in sympathetic neurons, NT3 substitutes for NGF and leads to enhanced survival [41]. There is some evidence that p75 also may alter the sensitivity and specificity of the TrkB response to BDNF and related ligands in explants of neuronal cells from rodents [42; 43]. However, the effect of p75 coexpression has not been examined in human NBs.
Here we demonstrate that p75 coexpression enhances NGF-induced cell differentiation in vitro for TrkA cells under serum-deprived conditions, and it leads to increased sensitivity of TrkA to NGF. The autophosphorylation of TrkA by NGF that was seen at 10 ng/ml in TrkA/p75-expressing cells was much higher compared to that seen in cells expressing TrkA alone. Phosphorylation of AKT by 1 ng/ml NGF in TrkA/p75 expressing cells was higher than that induced by 10 ng/ml NGF in TrkA cells. Furthermore, p75 coexpression greatly reduced the phosphorylation of TrkA and AKT in response to NT3, whereas NT3-induced phosphorylation was consistently seen in TrkA-expressing cells in the absence of p75. Thus, p75 increased both the sensitivity and specificity of TrkA to NGF. Physiologically, concentrations of NGF and NT3 are low in primary NBs (unpublished observations). But continuous activation of downstream cell signaling effectors by lower concentrations of ligand when p75 is coexpressed may have a similar effect as what is seen with higher concentrations of ligand. Thus, the major biological effect of p75 on NB is likely to enhance response to lower concentrations of NGF in the microenvironment, leading to initial proliferation followed by enhanced neuronal differentiation.
For SY5Y-TrkB cells, we observed enhanced cell growth with p75 coexpression even in the absence of exogenous ligands. This may mimic what is seen biologically in primary NBs in which TrkB and BDNF are frequently coexpressed. Furthermore, greater phosphorylation of AKT and MAPK were observed in TrkB/p75 cells at low levels of BDNF. We also observed enhanced proliferation of cells coexpressing TrkB and p75 compared to TrkB alone. This suggests that p75 coexpression enhances the adverse effects of TrkB expression in NBs. In addition, TrkB is the primary receptor for BDNF and NT4, but it can also respond to NT3 by TrkB autophosphorylation and activation of downstream signaling pathways such as RAS/MAPK and PI3K/AKT [44]. However, the growth enhancement and phosphorylation responses to NT3, as well as the AKT phosphorylation in response to NT3 or NT4, were markedly reduced when p75 was coexpressed with TrkB. This is similar to the finding of Bibel and colleagues [45] that suggests p75 increased the specificity of ligand-dependent behavior of TrkB. Physiologically, NT3 and NT4 are rarely expressed in NBs, so the major biological effect of p75 on TrkB is to sensitize its response to BDNF, leading to enhanced survival and proliferation.
Following Trk receptor activation, there are two major signaling pathways that are associated with enhanced survival and proliferation: the RAS/MAPK pathway and the PI3K/AKT pathway [25; 46; 47]. In addition, both Trk and p75 receptors can activate the NFkB signaling pathway, which is an alternative survival pathway for neuronal cells [36; 48; 49]. From our Western analysis, we observed increased phosphorylation of both MAPK and AKT even at low concentrations of NGF, but we did not observe activation of NFkB in our system (data not shown). P75 coexpression and activation also enhanced NGF-induced neurite outgrowth and neuronal differentiationin TrkA-expressing cells, as detected by increased expression of tyrosine hydroxylase and synaptophysin [50]. However, no such effect was seen on cells coexpressing TrkB and p75.
In summary, p75 coexpression enhanced differentiation of TrkA expressing cells and proliferation of TrkB-expressing cells by their cognate ligands. It also increased sensitivity of both TrkA and TrkB to low concentrations of their cognate ligands, NGF and BDNF, and essentially blocked the response to the more promiscuous TrkC ligand, NT3. Given that p75 coexpression can increase responsiveness of both TrkA and TrkB to low concentrations of ligand, it is likely that it enhances the effects of expressing these respective Trk receptors on both favorable and unfavorable NBs, contributing to these disparate clinical behaviors.
Acknowledgments
This work was supported in part by NIH grants CA094194 and CA097323 (GMB), by a Mentored Research Scholar Grant from the American Cancer Society (JEM), by the Richard and Nancy Wolfson Young Investigator Award (RH, JEM) and by the Audrey E. Evans Endowed Chair (GMB).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CI
cell index
- FBS
fetal bovine serum
- MAPK
mitogen-activated protein kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NFkB
nuclear factor-kB
- NGF
nerve growth factor
- NT3
neurotrophin-3
- NT4
neurotrophin-4
- OPI
oxaloacetae-pyruvate-insulin
- PI3K
phosphatidylinositol 3′-kinase
- P75
p75 low affinity neurotrophin receptor
- RT-CES
real-time cell electronic sensing system
- RT-PCR
reverse transcription-polymerase chain reaction
- SY5Y
SH-SY5Y
- TNFR
tumor necrosis factor receptor
- TNFRSF
tumor necrosis factor receptor superfamily
- Trk
tropomyosin-related kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement
There are no conflicts of interest for this article. If accepted, we agree to transfer all copyright ownership of the article to the Elsevier, Inc.
References
- 1.Brodeur GM, Maris JM. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 933–970. [Google Scholar]
- 2.Kogner P, Barbany G, Dominici C, Castello MA, Raschella G, Persson H. Coexpression of messenger RNA for TRK protooncogene and low affinity nerve growth factor receptor in neuroblastoma with favorable prognosis. Cancer Res. 1993;53:2044–50. [PubMed] [Google Scholar]
- 3.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328:847–54. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki T, Bogenmann E, Shimada H, Stram D, Seeger RC. Lack of high-affinity nerve growth factor receptors in aggressive neuroblastomas. J Natl Cancer Inst. 1993;85:377–84. doi: 10.1093/jnci/85.5.377. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawara A, Azar CG, Scavarda NJ, Brodeur GM. Expression and function of TRK-B and BDNF in human neuroblastomas. Mol Cell Biol. 1994;14:759–67. doi: 10.1128/mcb.14.1.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Acheson A, Conover JC, Fandl JP, DeChiara TM, Russell M, Thadani A, Squinto SP, Yancopoulos GD, Lindsay RM. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–3. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ. Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Eukaryotic Signal Transduction Group. Neuron. 1993;11:321–31. doi: 10.1016/0896-6273(93)90187-v. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto K, Wada RK, Yamashiro JM, Kaplan DR, Thiele CJ. Expression of brain-derived neurotrophic factor and p145TrkB affects survival, differentiation, and invasiveness of human neuroblastoma cells. Cancer Res. 1995;55:1798–806. [PubMed] [Google Scholar]
- 9.Ryden M, Sehgal R, Dominici C, Schilling FH, Ibanez CF, Kogner P. Expression of mRNA for the neurotrophin receptor trkC in neuroblastomas with favourable tumour stage and good prognosis. Br J Cancer. 1996;74:773–9. doi: 10.1038/bjc.1996.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashiro DJ, Nakagawara A, Ikegaki N, Liu XG, Brodeur GM. Expression of TrkC in favorable human neuroblastomas. Oncogene. 1996;12:37–41. [PubMed] [Google Scholar]
- 11.Hempstead BL, Rabin SJ, Kaplan L, Reid S, Parada LF, Kaplan DR. Overexpression of the trk tyrosine kinase rapidly accelerates nerve growth factor-induced differentiation. Neuron. 1992;9:883–96. doi: 10.1016/0896-6273(92)90241-5. [DOI] [PubMed] [Google Scholar]
- 12.Birren SJ, Verdi JM, Anderson DJ. Membrane depolarization induces p140trk and NGF responsiveness, but not p75LNGFR, in MAH cells. Science. 1992;257:395–7. doi: 10.1126/science.1321502. [DOI] [PubMed] [Google Scholar]
- 13.Davies AM, Lee KF, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–74. doi: 10.1016/0896-6273(93)90069-4. [DOI] [PubMed] [Google Scholar]
- 14.Lee KF, Davies AM, Jaenisch R. p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development. 1994;120:1027–33. doi: 10.1242/dev.120.4.1027. [DOI] [PubMed] [Google Scholar]
- 15.Bono F, Lamarche I, Bornia J, Savi P, Della Valle G, Herbert JM. Nerve growth factor (NGF) exerts its pro-apoptotic effect via the P75NTR receptor in a cell cycle-dependent manner. FEBS Lett. 1999;457:93–7. doi: 10.1016/s0014-5793(99)01006-6. [DOI] [PubMed] [Google Scholar]
- 16.Bunone G, Mariotti A, Compagni A, Morandi E, Della Valle G. Induction of apoptosis by p75 neurotrophin receptor in human neuroblastoma cells. Oncogene. 1997;14:1463–70. doi: 10.1038/sj.onc.1200972. [DOI] [PubMed] [Google Scholar]
- 17.Kuner P, Hertel C. NGF induces apoptosis in a human neuroblastoma cell line expressing the neurotrophin receptor p75NTR. J Neurosci Res. 1998;54:465–74. doi: 10.1002/(SICI)1097-4547(19981115)54:4<465::AID-JNR4>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, Bredesen DE. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–8. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- 19.Eggert A, Sieverts H, Ikegaki N, Brodeur GM. p75 mediated apoptosis in neuroblastoma cells is inhibited by expression of TrkA. Med Pediatr Oncol. 2000;35:573–6. doi: 10.1002/1096-911x(20001201)35:6<573::aid-mpo17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 20.Chao MV. Neurotrophin receptors: a window into neuronal differentiation. Neuron. 1992;9:583–93. doi: 10.1016/0896-6273(92)90023-7. [DOI] [PubMed] [Google Scholar]
- 21.Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR, Sehgal A. Gene transfer and molecular cloning of the human NGF receptor. Science. 1986;232:518–21. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- 22.Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–6. [PubMed] [Google Scholar]
- 23.Greene LA, Kaplan DR. Early events in neurotrophin signalling via Trk and p75 receptors. Curr Opin Neurobiol. 1995;5:579–87. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 24.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–64. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6. [PubMed] [Google Scholar]
- 26.Eggert A, Ho R, Ikegaki N, Liu XG, Brodeur GM. Different effects of TrkA expression in neuroblastoma cell lines with or without MYCN amplification. Med Pediatr Oncol. 2000;35:623–7. doi: 10.1002/1096-911x(20001201)35:6<623::aid-mpo29>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 28.Solly K, Wang X, Xu X, Strulovici B, Zheng W. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;2:363–72. doi: 10.1089/adt.2004.2.363. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawara A, Brodeur GM. Role of neurotrophins and their receptors in human neuroblastomas: a primary culture study. Eur J Cancer. 1997;33:2050–3. doi: 10.1016/s0959-8049(97)00280-3. [DOI] [PubMed] [Google Scholar]
- 30.Ambros IM, Zellner A, Roald B, Amann G, Ladenstein R, Printz D, Gadner H, Ambros PF. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N Engl J Med. 1996;334:1505–11. doi: 10.1056/NEJM199606063342304. [DOI] [PubMed] [Google Scholar]
- 31.Eggert A, Grotzer MA, Ikegaki N, Liu XG, Evans AE, Brodeur GM. Expression of the neurotrophin receptor TrkA down-regulates expression and function of angiogenic stimulators in SH-SY5Y neuroblastoma cells. Cancer Res. 2002;62:1802–8. [PubMed] [Google Scholar]
- 32.Eggert A, Ikegaki N, Kwiatkowski J, Zhao H, Brodeur GM, Himelstein BP. High-level expression of angiogenic factors is associated with advanced tumor stage in human neuroblastomas. Clin Cancer Res. 2000;6:1900–8. [PubMed] [Google Scholar]
- 33.Eggert A, Ikegaki N, Liu XG, Brodeur GM. Prognostic and biological role of neurotrophin-receptor TrkA and TrkB in neuroblastoma. Klin Padiatr. 2000;212:200–5. doi: 10.1055/s-2000-9677. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–55. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 35.Majdan M, Walsh GS, Aloyz R, Miller FD. TrkA mediates developmental sympathetic neuron survival in vivo by silencing an ongoing p75NTR-mediated death signal. J Cell Biol. 2001;155:1275–85. doi: 10.1083/jcb.200110017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casaccia-Bonnefil P, Gu C, Khursigara G, Chao MV. p75 neurotrophin receptor as a modulator of survival and death decisions. Microsc Res Tech. 1999;45:217–24. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<217::AID-JEMT5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 37.Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J Comp Neurol. 1995;351:329–38. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- 38.Casaccia-Bonnefil P, Kong H, Chao MV. Neurotrophins: the biological paradox of survival factors eliciting apoptosis. Cell Death Differ. 1998;5:357–64. doi: 10.1038/sj.cdd.4400377. [DOI] [PubMed] [Google Scholar]
- 39.Clary DO, Reichardt LF. An alternatively spliced form of the nerve growth factor receptor TrkA confers an enhanced response to neurotrophin 3. Proc Natl Acad Sci U S A. 1994;91:11133–7. doi: 10.1073/pnas.91.23.11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc Natl Acad Sci U S A. 1993;90:7859–63. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan C, Rivas-Plata K, Landis SC. The p75 neurotrophin receptor influences NT-3 responsiveness of sympathetic neurons in vivo. Nat Neurosci. 1999;2:699–705. doi: 10.1038/11158. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J, Geula C, Lu C, Koziel H, Hatcher LM, Roisen FJ. Neurotrophins regulate proliferation and survival of two microglial cell lines in vitro. Exp Neurol. 2003;183:469–81. doi: 10.1016/s0014-4886(03)00222-x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YH, Chi XX, Nicol GD. Brain-derived neurotrophic factor enhances the excitability of rat sensory neurons through activation of the p75 neurotrophin receptor and the sphingomyelin pathway. J Physiol. 2008;586:3113–27. doi: 10.1113/jphysiol.2008.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banfield MJ, Naylor RL, Robertson AG, Allen SJ, Dawbarn D, Brady RL. Specificity in Trk receptor:neurotrophin interactions: the crystal structure of TrkB-d5 in complex with neurotrophin-4/5. Structure. 2001;9:1191–9. doi: 10.1016/s0969-2126(01)00681-5. [DOI] [PubMed] [Google Scholar]
- 45.Bibel M, Hoppe E, Barde YA. Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. Embo J. 1999;18:616–22. doi: 10.1093/emboj/18.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–42. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–91. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 48.Yano H, Chao MV. Neurotrophin receptor structure and interactions. Pharm Acta Helv. 2000;74:253–60. doi: 10.1016/s0031-6865(99)00036-9. [DOI] [PubMed] [Google Scholar]
- 49.Hamanoue M, Middleton G, Wyatt S, Jaffray E, Hay RT, Davies AM. p75-mediated NF-kappaB activation enhances the survival response of developing sensory neurons to nerve growth factor. Mol Cell Neurosci. 1999;14:28–40. doi: 10.1006/mcne.1999.0770. [DOI] [PubMed] [Google Scholar]
- 50.Clary DO, Weskamp G, Austin LR, Reichardt LF. TrkA cross-linking mimics neuronal responses to nerve growth factor. Mol Biol Cell. 1994;5:549–63. doi: 10.1091/mbc.5.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]