Abstract
The neuronal mechanism underlying the phantom auditory perception of tinnitus remains at present elusive. For over 25 years, temporary tinnitus following acute salicylate intoxication in rats has been used as a model to understand how a phantom sound can be generated. Behavioral studies have indicated the pitch of salicylate-induced tinnitus in the rat is approximately 16 kHz. In order to better understand the origin of the tinnitus pitch, in the present study, measurements were made at the levels of auditory input and output; both cochlear and cortical physiological recordings were performed in ketamine/xylazine anesthetized rats. Both compound action potentials and distortion product otoacoustic emission measurements revealed a salicylate-induced band-pass-like cochlear deficit in which the reduction of cochlear input was least at 16 kHz and significantly greater at high and low frequencies. In a separate group of rats, frequency receptive fields of primary auditory cortex neurons were tracked using multichannel microelectrodes before and after systemic salicylate treatment. Tracking frequency receptive fields following salicylate revealed a population of neurons that shifted their frequency of maximum sensitivity (i.e., characteristic frequency) towards the tinnitus frequency region of the tonotopic axis (~16 kHz). The data presented here supports the hypothesis that salicylateinduced tinnitus results from an expanded cortical representation of the tinnitus pitch determined by an altered profile of input from the cochlea. Moreover, the pliability of cortical frequency receptive fields during salicylate-induced tinnitus is likely due to salicylate’s direct action on intracortical inhibitory networks. Such a disproportionate representation of middle frequencies in the auditory cortex following salicylate may result in a finer analysis of signals within this region which may pathologically enhance the functional importance of spurious neuronal activity concentrated at tinnitus frequencies.
Keywords: tinnitus, salicylate, auditory cortex, cochlea, rat
INTRODUCTION
Subjective tinnitus is a disorder characterized by the perception of sound in the absence of an acoustic source in the environment. This disorder is surprisingly prevalent in the general population. A recent assessment indicates approximately 25.3% of the United States adult population has experienced some tinnitus, while 7.9% reported experiencing tinnitus frequently (Shargorodsky et al., 2010). Tinnitus affects an estimated 1 – 3% of the population to a degree which negatively impacts many aspects of their lives (Dobie, 2003). Despite increased attention in recent years, a cohesive physiological framework underlying tinnitus generation remains elusive.
The current state of tinnitus research suggests that peripheral deafferentation – i.e., altered peripheral input – is the most common event that triggers central mechanisms of neuronal plasticity which generates and perpetuates the phantom sound (Weisz et al., 2006, Eggermont, 2008). The relationship between this altered profile of peripheral input and the perceived pitch of tinnitus, however, remains unclear. In order to facilitate investigation into the physiological basis of phantom sound generation, a pharmacologically-induced model of tinnitus in rats was developed (Jastreboff et al., 1988). In this model, a single systemic injection of a high-dose of salicylate, the active component in aspirin, reliably induces behavioral evidence of temporary tinnitus in rats (Jastreboff and Sasaki, 1994, Lobarinas et al., 2004, Yang et al., 2007, Turner and Parrish, 2008, Ralli et al., 2010). Using various behavioral assays, our laboratory and others have approximated the pitch of acute salicylate-induced tinnitus to be experienced at approximately 16 kHz in the Sprague-Dawley rat (Lobarinas et al., 2004, Yang et al., 2007, Kizawa et al., 2010). Although several investigations have observed salicylateinduced alterations in both peripheral and central auditory structures, evidence unifying these phenomena in relation to the animal’s behaviorally-reported tinnitus pitch is lacking.
Salicylate’s effects on the peripheral auditory system tend to manifest as a general decrease in signal transduction to the brain (i.e., increased auditory thresholds; for review see Cazals, 2000). Changes in cochlear sensitivity can be assessed by recording the sound-evoked compound action potential (CAP) from a low impedance electrode placed on the round window of the cochlea. This elevated auditory threshold has been attributed to a reduction in outer hair cell (OHC) electromotility as measured using distortion product otoacoustic emissions (DPOAE) (Cazals, 2000, Chen et al., 2010). Despite this decrease in peripheral sensitivity, acute systemic salicylate exposure has been shown to paradoxically enhance sound-evoked AC responses (Yang et al., 2007, Sun et al., 2009). This enhancement has been attributed to salicylate’s ability to directly reduce inhibition in AC (Wang et al., 2008, Su et al., 2009, Sun et al., 2009).
In the present study we investigated the relationship between peripheral and central changes following systemic salicylate administration at doses known to reliably induce tinnitus at ~16 kHz in rats. We report the results of series of experiments on anesthetized Sprague-Dawley rats in which we used high frequency DPOAE to assess OHC function in the previously uninvestigated high frequency base of the basilar membrane, CAP to assess the state of sensorineural input to the brain, and used multichannel electrodes to track the tone-evoked receptive fields of AC neurons before and after systemic salicylate administration. Since our previous studies showed that acute salicylate exposure decreased DPOAE at low frequencies but seemed to spare function at 16 kHz (Ralli et al., 2010), and long-term salicylate administration in young F344 rats decreased CAP amplitude at low and high, but not mid (~12 kHz) frequencies (Chen et al., 2010), we predicted that OHC function would be differentially affected at apical (low frequency), middle (mid frequency), and basal (high frequency) regions of the cochlea resulting in an altered profile of peripheral input related to the behaviorally assessed 16 kHz pitch. In addition, recent reports of cortical disinhibition following salicylate application led us to predict that the tuning of AC neurons may be released from their normal excitatory frequency receptive fields, leading to an overrepresentation of the tinnitus pitch dictated by the periphery. Gaining a more granular understanding of the neurophysiological changes associated with salicylate-induced tinnitus will help us better understand how the brain can change to produce forms of subjective tinnitus more common in the general population.
EXPERIMENTAL PROCEDURES
AC recordings (n = 15) were performed before (baseline), 0.5, 1.5, and 2.5 h post-salicylate injection. The AC experiment data are presented up to 2.5 h post-salicylate for three reasons: 1) Behavioral evidence of salicylate-induced tinnitus plateaus between approximately 1 and 2 h following IP injection. 2) Preliminary recordings in the AC reflected this time course. 3) The general wellness of the animal following repeated dosing of ketamine/xylazine for several hours (including surgery) tends to deteriorate over time. DPOAE (n = 6) and CAP (n = 5) recordings were performed before (baseline), 1 and 2 h post-salicylate for similar reasons. All experimental protocols were approved by the University at Buffalo Institutional Animal Care and Use Committee and carried out according with the National Institutes of Health guidelines.
Distortion Product Otoacoustic Emissions (DPOAE)
DPOAEs were measured for the right ear of adult male Sprague-Dawley rats (n = 6) under ketamine/xylazine (50/6 mg/kg) anesthesia. Briefly, two primary tones, f1 and f2, were presented at a ratio of 1.2, with f1 intensity (L1) 10 dB higher than the f2 level (L2). Input/Output (I/O) functions were generated using the magnitude of the distortion product (DP) spectral peak (2•f1 - f2) for f2 = 6, 8, 12, 16, 24, 30 kHz and L1 from 70 to 25 dB SPL in -5 dB steps (Intelligent Hearing Systems, Miami, FL, USA). Following a baseline recording, sodium salicylate (Sigma-Aldrich) was administered systemically (dose: 300 mg/kg IP; concentration: 50 mg/mL normal saline). DPOAE measurements were performed before and 1 and 2 h following salicylate injection. Results in figure 1 are plotted for each DP frequency, 2• f 1 - f 2, in order to indicate which frequency region of the basilar membrane is presumably being measured by DPOAE. Group results (at 50 dB SPL) in figure 3A are plotted against the DP frequency on the abscissa. DPOAE I/O functions were analyzed for significant changes following salicylate using two-way repeated measures ANOVA (Prism GraphPad v5).
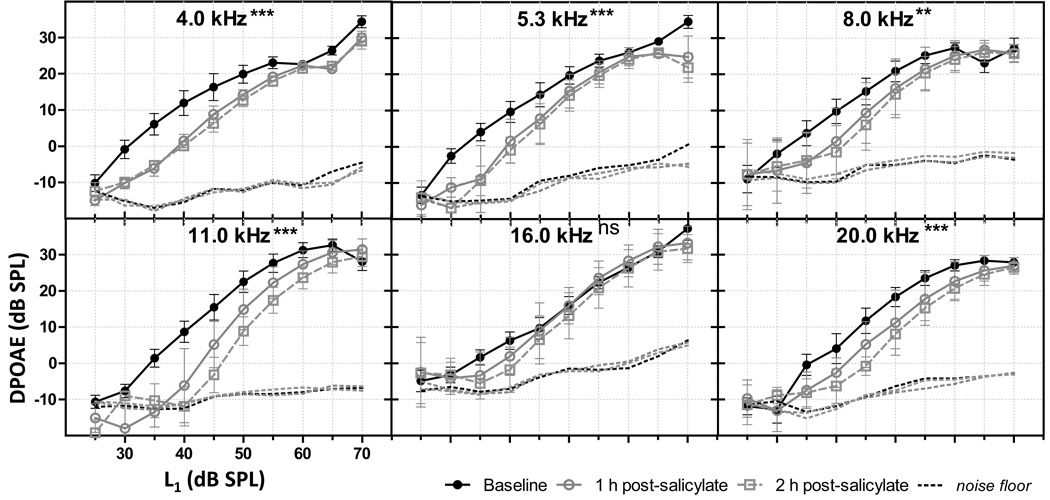
Fig 1.
Salicylate’s effects on DPOAE I/O functions. Acute systemic salicylate administration significantly decreased the DPOAE I/O functions for DP (2•f1-f2) below (4.0, 5.3, 8.0, and 11.0 kHz) and above (20.0 kHz) the tinnitus pitch (16.0 kHz). Error bars indicate standard error of the mean (SEM). (p < 0.05*, p< 0.01**, p < 0.001***, ns = not significant).
Fig 3.
Mean effects of acute systemic salicylate administration on the profile of cochlear frequency responsiveness to tones. (a) Mean DPOAE at L1 = 50 dB. At both 1 and 2 h following salicylate, DPOAE at all frequencies were significantly decreased except at 16 kHz which is the behaviorally assed pitch of tinnitus. (b) Mean CAP threshold shift following salicylate was between ~20 and 30 dB at 1 h post-salicylate; between ~25 and 40 dB at 2 h post-salicylate, being more pronounced at higher frequencies. (c) Mean change in cochlear amplification reflected an altered profile with a peak at 16 kHz 2 h post-salicylate. All error bars indicate SEM. (p < 0.05*, p< 0.01**, p < 0.001***, ns = not significant).
Compound Action Potential (CAP)
For CAP, adult male Sprague-Dawley rats (n = 6) were anesthetized with ketamine/xylazine (50/6 mg/kg, IM). A silver wire electrode was placed on the round window to record the response to tone bursts. A silver chloride reference electrode was inserted into the neck muscles. Tone bursts (4, 6, 8, 12, 16, 20, 24, 30, 35, and 40 kHz) were generated by a real-time processor (RP2.1, Tucker-Davis Technologies (TDT), Alachua, FL) and presented to the tympanic membrane via high frequency earphone (constructed from an ACO 1/2" microphone, 7013) placed within a speculum. The cochlear response was amplified (1000x) with a Grass AC preamplifier (Model P15; West Warwick, RI) and filtered online (0.1 – 3.0 kHz). Stimuli were repeated 50x and the filtered/amplified CAP response was recorded and stored on hard disk drive using a MATLAB software interface with digitizing hardware (TDT RP2.1).
The voltage of the first negative peak (N1) of CAP was measured and called the “CAP amplitude”. I/O functions were generated and threshold was defined as the stimulation intensity which elicited a response amplitude greater than 1 µV (approximate noise floor). A measure of cochlear amplification was used to describe the non-linearity of the cochlear response (Chen and Zhao, 2007). Cochlear amplification was defined as the difference between the CAP I/O function at 2µV and a linear line (i.e. a relationship of 1-dB stimulation level versus 1-dB CAP amplitude) passing through the CAP amplitude at 90 dB stimulus level. Following baseline measures, sodium salicylate (dose: 300 mg/kg IP; concentration: 50 mg/mL normal saline) was systemically administered via a pediatric intravenous catheter placed in the IP prior to recordings. Changes in CAP I/O functions following systemic salicylate were tested for significance using two-way ANOVA (Prism GraphPad v5).
Auditory Cortex Recordings
Adult male Sprague-Dawley rats were anesthetized with ketamine/xylazine (50/6 mg/kg IM), placed on a homoeothermic heating pad (maintained at ~37°C body temperature) and fixed in a stereotaxic frame with blunted ear bars. The skull was surgically exposed revealing both bregma and lambda. A head post was fixed to the skull near bregma with dental cement so the head of the animal could be held in a fixed position for free-field sound stimulation of the ear contralateral to the craniotomy. A small window (~2×1 mm) was cut into the temporal bone between approximately -4.5 to -6 mm caudal to bregma using a pointed scalpel blade (#11) to expose temporal cortex. Once the brain was exposed, the animal was transferred to a platform and custom head post holder for free-field sound stimulation of the contralateral (right) ear. During this transfer, a pediatric intravenous catheter with sodium salicylate (n = 10; 300 mg/kg IP, 50 mg/mL normal saline), or saline alone as a vehicle control (n = 4), was inserted into the intraperitoneal cavity of the rat and taped in place. The dura overlying the AC was carefully resected before electrode insertion.
In 14 rats, a 16 channel silicon microelectrode with 4-shanks (spaced 125 µm), each with 4 electrodes (spaced 100 µm; NeuroNexus Technologies, Ann Arbor, MI) was slowly inserted into the exposed temporal cortex. Multi-unit (MU) responses to broadband noise bursts were monitored online to ensure placement of the electrode grid in primary AC according to descriptions from previous reports: 1) Short-latency MU response onset latency (Polley et al., 2007); 2) Characteristic averaged evoked local-field potential response with sharply defined, short-latency positive-negative peaks (Di and Barth, 1992); 3) Canonical shapes of preliminary frequency-response area maps (Polley et al., 2007). If an insertion site met these criteria, then the electrode grid was slowly advanced until all 16 sites received MU responses. Once a recording site was accepted the electrode grid was not moved for the duration of the experiment. The rat was maintained during recordings at a light plane of anesthesia with ketamine (~5 mg/kg/h, IM).
Since the electrode used in the cortical recordings described above had a relatively dense site mapping, an additional experiment was performed (n = 1) in order to track changes across the tonotopy of primary AC simultaneously (see Fig 5). In one subject, the orientation and extent of the tonotopic axis of the primary AC was initially estimated from quick recordings using single tungsten microelectrodes (Z ≈ 1MΩ; FHC, Bowdoin, ME). Once the tonotopic axis was confirmed, dura was resected and an 8-shank electrode (shanks spaced 200 µm covering 1400 µm of cortex; NeuroNexus Technologies) was oriented to the tonotopy of primary AC by preliminary recordings. This subject received a slightly lower dose of salicylate at 250 mg/kg (50 mg/mL) via IP catheter as described above. This lower dose of salicylate has also been shown to reliably induce behavioral evidence of tinnitus at ~16 kHz (Yang et al., 2007).
Fig 5.
Tuning curves of receptive fields from eight MU sites recorded from across the tonotopy of primary AC simultaneously. Receptive fields of low (CF < 10 kHz) and high (CF ≥ 20 kHz) frequency sensitive units retuned to mid frequencies (10 – 20 kHz) following systemic salicylate administration (250 mg/kg IP).
Frequency receptive field maps were generated by summing the MU response for 50 ms following the onset of tone-bursts (25 ms duration, cos2 gate, 5 ms rise/fall, presented at 3 Hz) with 40 logarithmically spaced steps between 1 and 48 kHz at sound levels between 0 and 90 dB SPL in 5 dB steps, presented in a pseudorandomized fashion (5 repetitions, 3800 tones in total). Sound stimuli were generated (TDT RX6-2, ~100 kHz sampling rate) presented with a free-field magnetic speaker (Fostex FT28D) to the ear contralateral to the AC recording site. Results for AC recordings are presented as estimated times of 0.5, 1.5, and 2.5 h post-treatment due to the time required to present all stimuli.
The characteristic frequency (CF) of each frequency receptive field was defined as the tone frequency which elicited a response at the unit’s minimum threshold (i.e. the unit’s maximum sensitivity to tone stimuli). Units were categorized based on baseline frequency sensitivity, where their baseline CFs were low: CF < 10 kHz, mid: 10 kHz ≤ CF < 20 kHz, or high: CF ≥ 20 kHz, respectively. Significance of CF shifts over time was determined using the Kruskal-Wallis one-way ANOVA and a post test to determine the effect of treatment on each CF category (Prism GraphPad v5).
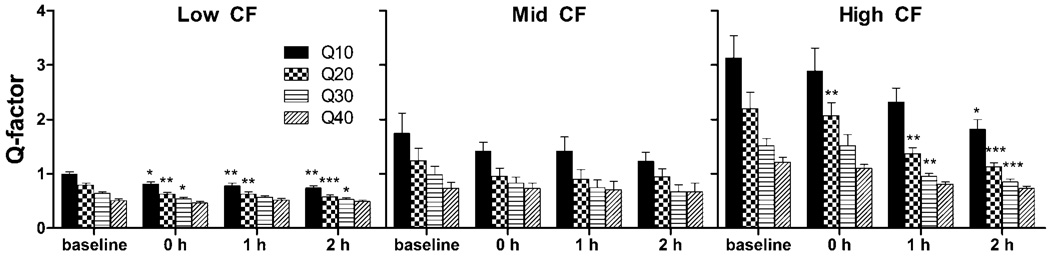
The bandwidths of frequency receptive fields were also analyzed in using the Q-factor. The Q-factor is defined as the CF divided by the frequency receptive field bandwidth at some sound intensity above the minimum threshold. Q-factors are presented here for 10, 20, 30, and 40 dB above the unit’s minimum threshold. Significance for the effect of salicylate on Q-factors was tested using a one-way ANOVA with Bonferroni’s post test against baseline Q-factor for low, mid, and high baseline CF units (Prism GraphPad v5).
RESULTS
Peripheral Measures: Distortion Product Otoacoustic Emissions
Acute high doses of salicylate are known to decrease OHC function (for review see Cazals, 2000). DPOAE I/O functions (Fig. 1) were recorded at baseline, 1 and 2 h post-salicylate injection (300 mg/kg IP). Significant decreases in DPOAE I/O function amplitude were observed at all DP frequencies tested following salicylate injection with the exception at 16.0 kHz (Fig 1 & 3A). Interestingly, DPOAE response to a stimulus with frequency above the tinnitus pitch (20 kHz) decreased significantly following systemic salicylate (4.0 kHz: F = 36.33***; 5.4 kHz, F = 35.84***; 8.0 kHz, F = 5.86**; 11.0 kHz, F = 47.68***; 16.0 kHz, F = 3.03, p = 0.0513; 20.0 kHz, F = 15.29***; where *** p < 0.001, ** p<0.01). Figure 3A shows a frequency cross-section of the reduction in DPOAE amplitude following salicylate at moderate stimulus intensity (50 dB SPL). Salicylate had an effect on DPOAE with significant reductions in response amplitudes above and below the tinnitus pitch of 16 kHz.
Peripheral Measures: Compound Action Potential
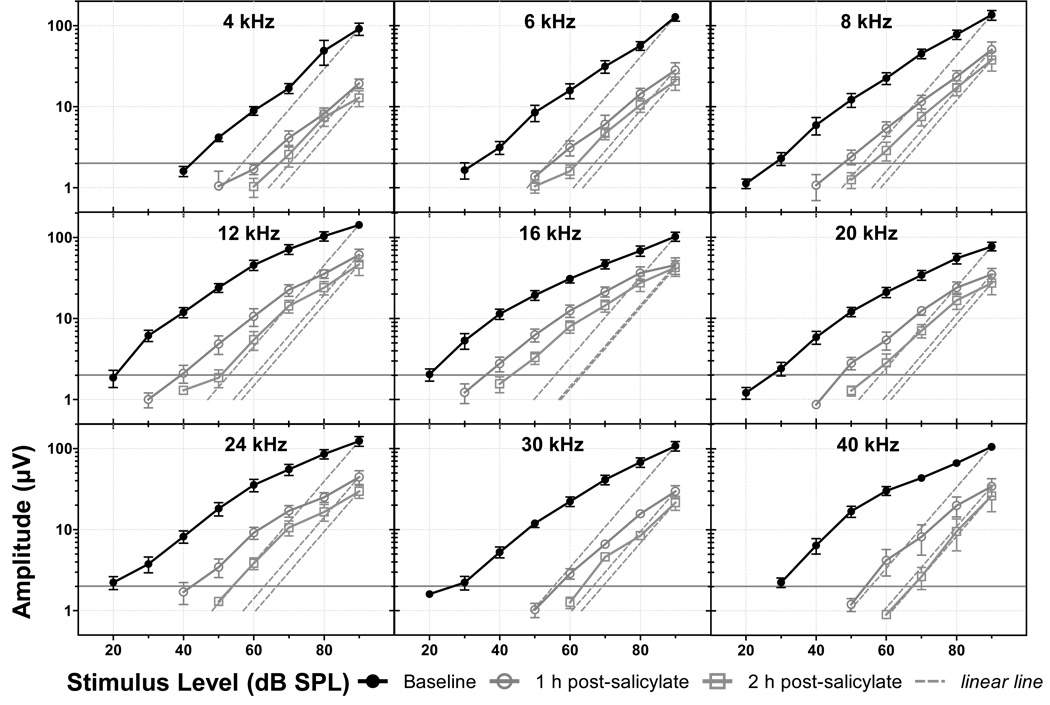
Similar to the effects of salicylate on DPOAE, systemic administration of salicylate exhibited strong effects on the CAP measured at the round window. CAP I/O functions were generated before, 1 and 2 h post-salicylate injection (Fig 2). Salicylate induced a clear increase in CAP threshold (i.e. a right- and downward shift in the I/O function) at all frequencies tested. In addition, cochlear amplification was measured at 2 µV (Fig 2). Cochlear amplification is a measure of the nonlinearity of the CAP response; i.e. the difference between the CAP amplitude and a linear line fit through the response at a 90 dB stimulus level (Chen and Zhao, 2007). CAP amplitude was significantly reduced at all measured frequencies (p < 0.001); least at 16 kHz. The greatest reduction in cochlear amplification occurred at frequencies above 16 kHz.
Fig 2.
Salicylate’s effects on CAP I/O functions and the cochlear amplification measure. CAP I/O functions reflect the amplitude of the CAP response as measured from the round window of the cochlea with a low impedance electrode in response to tones at sound levels from 90 dB down to threshold (noise floor ≈1 µV). Cochlear amplification is a measure of the non-linearity of the I/O functions. Cochlear amplification is defined as the distance (in dB) between each CAP I/O function and its associated linear line (dashed lines intersecting the maximum stimulus level) at 2 µV.
Auditory Cortex Recordings
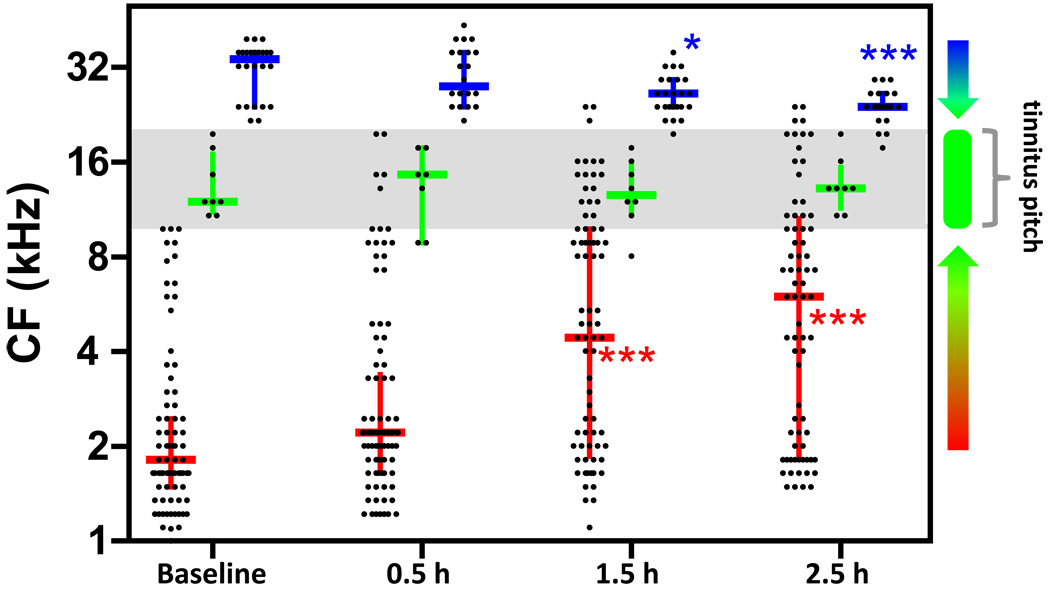
In these experiments, electrodes were inserted into the primary AC and maintained in place for the duration of the recording. MU receptive fields were tracked over time before and after systemic administration of either salicylate (300 mg/kg) or saline. AC units were binned into low (CF ≤ 10 kHz), mid (10 kHz < CF ≤ 20 kHz), or high (CF > 20 kHz) categories based on their CFs before salicylate injection (Fig 4). Following systemic salicylate administration, the median CF increased for low CF units (p < 0.0001 for 1.5 and 2.5 h post vs. baseline) and decreased for high CF units (p < 0.05 1.5 h post vs. baseline, p < 0.01 2.5 h post vs. baseline), but remained stable for mid CF units (p > 0.05 at all time points re baseline). The median of receptive field CFs in saline control subjects remained stable for the duration of the recording (p > 0.05). This result indicates that the frequency sensitivity of cortical neurons rapidly migrate towards the tinnitus pitch following systemic salicylate administration.
Fig 4.
Salicylate-induced retuning of AC neurons resulting in overrepresentation of tinnitus pitch. CF of units (each point is one unit) categorized by baseline CF (low: CF < 10 kHz, red; mid: 10 kHz ≤ CF < 20 kHz, green; high: CF ≥ 20 kHz, blue) and tracked over time. Horizontal lines are the median, up/down bars are the interquartile ranges. Frequency sensitivity of cortical units tended to aggregate towards the mid frequencies (10 – 20 kHz) surrounding the tinnitus pitch following salicylate. (p < 0.05*, p< 0.01**, p < 0.001***).
To further investigate this finding, we performed an additional experiment in which recordings of MU activity were made from eight sites along the tonotopic axis of the primary auditory cortex simultaneously (Fig 5). Figure 5 shows the tuning curves of frequency receptive fields of 8 units from a single recording from across the tonotopic axis of the primary AC before and 2.5 h after systemic salicylate injection. The low frequency tuning curves (maroon, red, orange, and yellow lines in Fig 5) shifted to represent the tinnitus pitch approximately 3 octaves above the baseline CF. Higher frequency tuning curves (dark blue) increased its sensitivity to frequencies near the tinnitus pitch. Such large and rapid retuning of receptive fields in primary AC have been observed in the guinea pig following tone paired with shock classical conditioning (Bakin and Weinberger, 1990).
In addition to CF shifts, frequency receptive fields of AC neurons were also observed to expand following salicylate. The Q-factor was calculated, , for the tuning of frequency receptive fields at 10, 20, 30, and 40 dB above each unit’s minimum threshold. Figure 6 shows the Q-factor of units with baseline CFs categorized as low, mid, or high (same as above). Following salicylate, significant drops in Q-factors (i.e. increased bandwidth) were observed for low (Q10, Q20, and Q30) and high (Q20 and Q30) baseline CF units, but not for mid CF units near the salicylate-induced pitch of tinnitus. Frequency receptive fields of mammalian AC neurons have turned out to be far more pliable than expected (Weinberger, 1995). AC receptive fields are not entirely determined by ascending thalamic innervations. Rather, the extent to which neurons respond to sound frequency in the AC can in large part be attributed to cortical inhibition (Kaur et al., 2004). Strong cortical inhibition masks intracortical inputs from neurons responding sound frequencies distant to the direct input from the thalamus (Wang et al., 2000, Kaur et al., 2004).
Fig 6.
Q-factor of low and high CF units is altered by systemic salicylate administration. The Q-factor for units with low baseline CFs (CF < 10 kHz) and high baseline CFs (CF ≥ 20 kHz) at 10, 20 and 30 dB above minimum threshold significantly increased as compared to baseline (see significance for each case on graph). Units with baseline CFs near the tinnitus pitch (10 kHz ≤ CF < 20 kHz) remained relatively stable during the time period recording. (p < 0.05*, p< 0.01**, p < 0.001***).
DISCUSSION
The present study investigated changes in both cochlear and AC function in ketamine anesthetized rats before and on a time course following systemic salicylate injection at a dose (300 mg/kg IP) known to reliably induce behavioral evidence of tinnitus in Sprague-Dawley rats at a pitch near 16 kHz (Yang et al., 2007, Kizawa et al., 2010). Peripheral measures of OHC function (DPOAE) and neural output of the cochlea (cochlear amplification in CAP) revealed evidence of a salicylate-induced cochlear band-pass filter with a frequency center near 16 kHz. In addition, a large number of AC neurons shifted their frequency sensitivities towards the center frequency of the cochlear band-pass filter near the tinnitus pitch, ~16 kHz. This evidence supports the hypothesis that the pitch of salicylate-induced tinnitus results from the salicylate’s direct actions on peripheral input; however, the large shifts and expansion of frequency receptive fields of cortical neurons moreover implies a critical role for salicylate’s direct action on the AC.
Peripheral Effects
The major effect of systemic salicylate injection on cochlear function in rats observed in this study was a significant alteration in the profile of peripheral input; low and high frequencies became less sensitive following salicylate administration leaving 16 kHz as the dominant input frequency (Fig 1). Salicylate’s effects on OHC function have been evaluated following cochlear perfusion in the guinea pig (Kujawa et al., 1992) as well as systemic administration in the rat (Wang et al., 1984, Ralli et al., 2010). In agreement with the results presented here, both of these studies showed a decrease in DPOAE following salicylate administration at low to moderate intensities (Figs 1 and 3a). The disproportionate effect of salicylate on OHC function across frequencies, sparing 2•f1-f2 = 16 kHz (Fig 3a) has not to our knowledge been reported previously. While the effects of salicylate toxicity on various biochemical and morphological features of OHCs have been described previously (Dieler et al., 1991, Lue and Brownell, 1999, Cazals, 2000, Guitton et al., 2003, Puel, 2007, Ruel et al., 2008, Kizawa et al., 2010, Wu et al., 2010), the relative resilience of sensory cells within the middle frequency region of the basilar membrane following salicylate administration is not easily explained by the available data and deserves further investigation.
We also report that acute systemic salicylate administration had significant effects on the neural output of the cochlea as measured by the CAP recorded from the round window. CAP thresholds increased following the acute salicylate treatment across frequencies (Fig 3b); however, there was a disproportionate effect across frequencies on the non-linearity of the CAP I/O functions termed cochlear amplification (Fig 3c; Chen and Zhao, 2007). This result corroborates the DPOAE data in that the resulting profile of cochlear amplification had a pronounced peak at 16 kHz at 2 h following systemic salicylate administration. Such a rapidly altered profile of cochleoneural output to the brain would be incongruous with established tonotopic maps in central auditory structures providing an impetus for central mechanisms of neuronal plasticity to accommodate the new profile of input. In the present study, the retuning of frequency receptive fields of AC neurons only occurred towards the frequency center of the band-pass shape of the peripheral input at 16 kHz. The band-pass profile of auditory input we observed here following salicylate is seemingly the inversion of the band-reject profile resulting from deafferentation associated with hearing loss commonly associated with tinnitus (Weisz et al., 2006).
Cortical Effects
Inhibitory networks in the AC have been described as a regulator of the extent of spectral integration occurring via intracortical horizontal projections (Metherate et al., 2005). Salicylate’s direct reduction of AC inhibition may result in the unmasking of normally subthreshold non-CF signals (Kaur et al., 2004). Here we show that systemic salicylate had a profound shifting (Figs 4 and 5) and expanding (Fig 6) effect on the tuning of AC neurons. As predicted, we observed an overrepresentation of the mid frequencies (10 – 20 kHz) following salicylate. This finding, as well as recent reports of salicylate-induced disinhibition in vitro, supports the hypothesis that cortical disinhibition plays a critical role in salicylate, and likely noise-induced, tinnitus.
Interestingly, it was recently found that salicylate application to AC slices in vitro selectively suppressed activity and increased activation thresholds of fast-spiking inhibitory interneurons, but did not affect pyramidal cell activity (Su et al., 2009). An earlier study which applied the γ-aminobutyric acid subtype-A (GABAA) antagonist bicuculline directly to the AC of anesthetized chinchillas resulted in expanded frequency tuning of AC neurons (Wang et al., 2000), indicating that inhibition plays a critical role in shaping and maintaining the frequency receptive fields of AC neurons. It is plausible that a salicylate-induced cortical disinhibition (Su et al., 2009) results in an invigorated cortical plasticity resulting in CF shifts towards the frequency center of the altered cochlear input which we observed to correspond with the tinnitus pitch of ~16 kHz.
A similar overrepresentation of the tinnitus pitch has been observed in humans (Muhlnickel et al., 1998); however, the relationship to the peripheral sensorineural hearing deficit is unknown. The functional significance of the increased representational area to the tinnitus frequency may be analogous to the observation of increased cortical representation to a conditioning tone stimulus. Expanded sensitivity to specific frequencies at the cortical level have been reported previously in the literature for paired tone/shock classical conditioning paradigms (Bakin and Weinberger, 1990, Edeline et al., 1993, Weinberger et al., 1993, Rutkowski and Weinberger, 2005) as well as for paired tone/electrical stimulation of neuromodulatory areas of the basal forebrain such as the nucleus basalis (Kilgard and Merzenich, 1998, Kilgard et al., 2001, Weinberger et al., 2006). Whether salicylate has any significant effects directly on neuromodulatory areas remains to be investigated.
Conclusion
The salicylate model of tinnitus in rats has accelerated our understanding of the tinnitus phenomenon in general. Here, we presented evidence that the behaviorally assessed pitch of salicylate-induced tinnitus has physiological correlates in the altered profile of peripheral input and enlarged representational area by neurons in primary AC. We observed a profound retuning of AC neurons normally sensitive to frequencies above and below the tinnitus pitch towards the tinnitus pitch.
It is likely the case that any persistent alteration of sensorineural input to the brain results in some level of cortical plasticity. While the physiological factor determining the extent of plasticity required to result in tinnitus remains unclear, we believe our data supports the hypothesis that the overrepresentation of a specific frequency at the cortical level is necessary, but perhaps not sufficient, to perceive tinnitus (see Rauschecker et al., 2010). Understanding how salicylate directly affects AC permitting such dramatic shifts in frequency sensitivity of neurons may yield plausible mechanisms underlying more common forms of subjective tinnitus. Salicylate’s effects on the profile of peripheral input observed here, its direct effects on cortical inhibition and retuning of receptive fields, as well as its reliability of inducing behavioral evidence of tinnitus, may prove the wherewithal of the salicylate model for understanding noise-trauma induced tinnitus.
ACKNOWLEDGEMENTS
This research was supported in part by National Institutes of Health grants R01DC0090910, R01DC009219, and F31DC010931-01. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
ABBREVIATIONS
- AC
auditory cortex
- CAP
compound action potential
- DPOAE
distortion product otoacoustic emission
- OHC
outer hair cell
- MU
multi-unit
- CF
characteristic frequency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Cazals Y. Auditory sensori-neural alterations induced by salicylate. Prog Neurobiol. 2000;62:583–631. doi: 10.1016/s0301-0082(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Chen GD, Kermany MH, D'Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R. Too much of a good thing: Long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010 doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GD, Zhao HB. Effects of intense noise exposure on the outer hair cell plasma membrane fluidity. Hear Res. 2007;226:14–21. doi: 10.1016/j.heares.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Di S, Barth DS. The functional anatomy of middle-latency auditory evoked potentials: thalamocortical connections. J Neurophysiol. 1992;68:425–431. doi: 10.1152/jn.1992.68.2.425. [DOI] [PubMed] [Google Scholar]
- Dieler R, Shehata-Dieler WE, Brownell WE. Concomitant salicylate-induced alterations outer hair cell subsurface cisternae and electromotility. J Neurocytol. 1991;20:637–653. doi: 10.1007/BF01187066. [DOI] [PubMed] [Google Scholar]
- Dobie RA. Depression and tinnitus. Otolaryngol Clin North Am. 2003;36:383–388. doi: 10.1016/s0030-6665(02)00168-8. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Role of auditory cortex in noise- and drug-induced tinnitus. Am J Audiol. 2008;17:S162–S169. doi: 10.1044/1059-0889(2008/07-0025). [DOI] [PubMed] [Google Scholar]
- Guitton MJ, Caston J, Ruel J, Johnson RM, Pujol R, Puel JL. Salicylate induces tinnitus through activation of cochlear NMDA receptors. J Neurosci. 2003;23:3944–3952. doi: 10.1523/JNEUROSCI.23-09-03944.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ, Brennan JF, Coleman JK, Sasaki CT. Phantom auditory sensation in rats: an animal model for tinnitus. Behav Neurosci. 1988;102:811–822. doi: 10.1037//0735-7044.102.6.811. [DOI] [PubMed] [Google Scholar]
- Jastreboff PJ, Sasaki CT. An animal model of tinnitus: a decade of development. Am J Otol. 1994;15:19–27. [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- Kizawa K, Kitahara T, Horii A, Maekawa C, Kuramasu T, Kawashima T, Nishiike S, Doi K, Inohara H. Behavioral assessment and identification of a molecular marker in a salicylate-induced tinnitus in rats. Neuroscience. 2010;165:1323–1332. doi: 10.1016/j.neuroscience.2009.11.048. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Fallon M, Bobbin RP. Intracochlear salicylate reduces low-intensity acoustic and cochlear microphonic distortion products. Hear Res. 1992;64:73–80. doi: 10.1016/0378-5955(92)90169-n. [DOI] [PubMed] [Google Scholar]
- Lobarinas E, Sun W, Cushing R, Salvi R. A novel behavioral paradigm for assessing tinnitus using schedule-induced polydipsia avoidance conditioning (SIP-AC) Hear Res. 2004;190:109–114. doi: 10.1016/S0378-5955(04)00019-X. [DOI] [PubMed] [Google Scholar]
- Lue AJ, Brownell WE. Salicylate induced changes in outer hair cell lateral wall stiffness. Hear Res. 1999;135:163–168. doi: 10.1016/s0378-5955(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: mechanisms and modulation. Hear Res. 2005;206:146–158. doi: 10.1016/j.heares.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Muhlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci U S A. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Puel JL. Cochlear NMDA receptor blockade prevents salicylate-induced tinnitus. B-ENT. 2007;3 Suppl 7:19–22. [PubMed] [Google Scholar]
- Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of salicylate- and quinine-induced tinnitus in rats: development, time course, and evaluation of audiologic correlates. Otol Neurotol. 2010;31:823–831. doi: 10.1097/MAO.0b013e3181de4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP, Leaver AM, Muhlau M. Tuning out the noise: limbic-auditory interactions in tinnitus. Neuron. 2010;66:819–826. doi: 10.1016/j.neuron.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel J, Chabbert C, Nouvian R, Bendris R, Eybalin M, Leger CL, Bourien J, Mersel M, Puel JL. Salicylate enables cochlear arachidonic-acid-sensitive NMDA receptor responses. J Neurosci. 2008;28:7313–7323. doi: 10.1523/JNEUROSCI.5335-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci U S A. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Su YY, Luo B, Wang HT, Chen L. Differential effects of sodium salicylate on current-evoked firing of pyramidal neurons and fast-spiking interneurons in slices of rat auditory cortex. Hear Res. 2009;253:60–66. doi: 10.1016/j.heares.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–334. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–S192. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Wang HT, Luo B, Huang YN, Zhou KQ, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11:1137–1140. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- Wang LH, Tu SC, Lusk RC. Apoenzyme of Pseudomonas cepacia salicylate hydroxylase. Preparation, fluorescence property, and nature of flavin binding. J Biol Chem. 1984;259:1136–1142. [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci U S A. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen JC. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiol Learn Mem. 2006;86:270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Hartmann T, Dohrmann K, Schlee W, Norena A. High-frequency tinnitus without hearing loss does not mean absence of deafferentation. Hear Res. 2006;222:108–114. doi: 10.1016/j.heares.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wu T, Lv P, Kim HJ, Yamoah EN, Nuttall AL. Effect of Salicylate on KCNQ4 of the Guinea Pig Outer Hair Cell. J Neurophysiol. 2010 doi: 10.1152/jn.01057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]