Abstract
Many chronic trigeminal pain conditions, such as migraine or temporo-mandibular disorders, are associated with inflammation within peripheral endings of trigeminal ganglion (TG) sensory neurons. A critical role in mechanisms of neuroinflammation is attributed to proinflammatory cytokines, such as interleukin-1β and tumor necrosis factor-α (TNFα) that also contribute to mechanisms of persistent neuropathic pain resulting from nerve injury. However, the mechanisms of cytokine-mediated synaptic plasticity and nociceptor sensitization are not completely understood. In the present study, we examined the effects of TNFα on neuronal expression of brain-derived neurotrophic factor (BDNF), whose role in synaptic plasticity and sensitization of nociceptive pathways is well documented. We show that 4- and 24-hr treatment with TNFα increases BDNF mRNA and protein, respectively, in neuron-enriched dissociated cultures of rat TG. TNFα increases the phosphorylated form of the cyclic adenosine monophosphate-responsive element binding protein (CREB), a transcription factor involved in regulation of BDNF expression in neurons, and activates transcription of BDNF exon IV (former exon III) and, to a lesser extent, exon VI (former exon IV), but not exon I. TNFα-mediated increase in BDNF expression was accompanied by increase in calcitonin gene-related peptide (CGRP), which is consistent with previously published studies, and indicates that both peptides are similarly regulated in TG neurons by inflammatory mediators. The effect of TNFα on BDNF expression is dependent on sodium influx through TTX-sensitive channels and on p38-mitogen-activated protein kinase. Moreover, electrical stimulation and forskolin, known to increase intracellular cAMP, potentiate the TNFα-mediated upregulation of BDNF expression. This study provides new evidence for a direct action of proinflammatory cytokines on TG primary sensory neurons, and reveals a mechanism through which TNFα stimulates de novo synthesis of BDNF in these neurons. Thus, TNFα should be considered in mechanisms of BDNF-dependent neuronal plasticity.
Keywords: Activity, BDNF, Inflammation, p38-MAPK, TNFα, Trigeminal
Recent studies provide several lines of evidence for a cross-talk between the nervous and immune systems. Many disorders of the central and peripheral nervous system, including Alzheimer's and Parkinson's disease, and a variety of pain conditions, are accompanied by inflammation of the neural tissue which is affected by the disease process (‘neuroinflammation’; McAllister and van de Water, 2009). Moreover, there is increasing evidence that the immune system contributes to normal neuronal development and plasticity (Vitkovic et al., 2000; Boulanger, 2009; Carpentier and Palmer, 2009). Thus, identifying the mechanisms that underlie neuro-immune interactions could become fundamental to understanding how the nervous system functions under physiological conditions and what goes awry in the disorders whose pathogenesis involves an immunological component.
Cytokines, first discovered as signaling molecules within the immune system, are also produced by the nervous system. Two prototypic proinflammatory cytokines, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα), are released from glia and regulate development and plasticity of neuronal circuits (Deverman and Patterson, 2009), including nociceptive pathways (Schäfers and Sorkin, 2008; McMahon and Malcangio, 2009; Ren and Torres, 2009). Cytokines and other inflammatory mediators have been shown to alter ion currents in the peripheral endings of nociceptors (Binshtok et al., 2008), including trigeminal neurons (Flake and Gold, 2005; Vaughn and Gold, 2010), leading to increased excitability and sensitization. However, the role of cytokines in inflammatory and neuropathic pain is not completely understood (Schäfers and Sorkin, 2008; Basbaum et al., 2009; Costigan et al., 2009; Latremoliere and Woolf, 2009).
The neurotrophin brain-derived neurotrophic factor (BDNF) is a mediator of plasticity at central synapses (Huang and Reichardt, 2001; Poo, 2001; Cohen and Greenberg, 2008), and modulator of nociceptive signaling (Pezet et al., 2002; Malcangio and Lessman, 2003; Merighi et al., 2008; Latremoliere and Woolf, 2009). Previous studies by our group indicate that BDNF is a candidate mediator of plasticity at first-order synapses in trigeminal nociceptive pathways (Buldyrev et al., 2006), with implications for pathophysiology of migraine and other primary headaches (Latremoliere and Woolf, 2009). Our recent studies show that inflammation within peripheral endings of trigeminal nociceptors dramatically upregulates BDNF in trigeminal ganglion (TG) neurons in vivo (Tarsa et al., 2010). However, the cellular mechanisms underlying the inflammation-induced BDNF upregulation remain unknown. Notably, several pain conditions, including migraine, are accompanied by inflammation within endings of TG neurons.
The goal of the present study was to examine the effects of the proinflammatory cytokine TNFα on BDNF expression, using sensory neurons isolated from the rat TG as a model. Our data indicate that TNFα increases BDNF mRNA and protein expression in TG neurons in a promoter-selective manner. The effect of TNFα depends on TTX-sensitive sodium currents and the p38-MAPK pathway, and is potentiated by neuronal activity and intracellular cAMP. Our findings identify a novel mechanism of regulation of BDNF expression in TG neurons by TNFα. Portions of this work have previously been published in abstract form (Balkowiec-Iskra and Balkowiec, 2009).
EXPERIMENTAL PROCEDURES
Animals and preparation of TG cultures
Postnatal day 1-3 Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used for this study. All procedures were approved by the Institutional Animal Care and Use Committee of the Oregon Health and Science University, and conformed to the Policies on the Use of Animals and Humans in Neuroscience Research approved by the Society for Neuroscience. Neuron-enriched TG cultures were prepared as previously described by our laboratory (Buldyrev et al., 2006; Tarsa and Balkowiec, 2009), and grown in Neurobasal-A medium (Invitrogen, Carlsbad, CA) supplemented with B-27 (Invitrogen), 0.05 mg/ml penicillin- 0.05 mg/ml streptomycin-0.1 mg/ml neomycin (Invitrogen), 0.5 mM L-glutamine (Invitrogen), and 2.5% fetal bovine serum (HyClone, Logan, UT) for 4-6 days in 48-well tissue culture-treated plates (Nalge Nunc Int., Naperville, IL) pre-coated with poly-D-lysine (0.1 mg/ml; Sigma, St. Louis, MO) and laminin (0.4 μg/ml; Sigma; for quantitative RT-PCR and ELISA), or on poly-D-lysine/laminin-coated glass coverslips (for immunocytochemistry). The medium was replaced with fresh medium every 2-3 days. In some experiments, the medium was supplemented with the mitotic inhibitor cytosine β-D-arabinofuranoside (AraC; 5 μM, Sigma) approximately 24 hrs after cell plating to prevent non-neuronal cell proliferation. Since the results of experiments using AraC-treated cultures were not significantly different from cultures not treated with AraC, the data were pooled.
Electrical field stimulation of TG neurons
TG neurons were stimulated as previously described (Buldyrev et al., 2006), except that pairs of platinum electrodes (0.25 mm wire diameter) were fitted into 48-well plates, the pulse duration was 0.2 ms, and the current amplitude was 100 mA/well. All non-stimulated control wells were fitted with electrodes which were not connected to the stimulator.
Pharmacological treatment
Cultures were treated with tumor necrosis factor-α (TNFα) for 4 hrs (for BDNF mRNA by quantitative RT-PCR) or 24 hrs (for BDNF, CGRP and NGF protein by ELISA). Other drugs were applied 30 min prior to the electrical stimulation or cytokine treatment, and remained present until the end of culture. Each drug (or drug combination) and each vehicle (or vehicle combination) were added to both stimulated and non-stimulated cultures. Stock solutions of TNFα (100 μg/ml in phosphate-buffered saline [PBS]; R&D Systems, Minneapolis, MN), tetrodotoxin (TTX; 3 mM in citrate buffer, pH=4.8; Tocris Bioscience, Ellisville, MO), 3-Isobutyl-1-methylxanthine (IBMX; 25 mM in ethanol; Sigma), and SB 203580 hydrochloride (10 mM in water; Tocris Bioscience) were stored at -80°C; forskolin stock (100 mM in ethanol; Sigma) was prepared fresh just before use.
Immuno- and DAPI-staining of TG cultures, and image analysis
Cultures were fixed with 4% (for NGF staining) or 2% (for BDNF staining) paraformaldehyde in 0.1 M sodium phosphate buffer, pH=7.4, for 30 min, and stained according to our previously published protocols (Buldyrev et al., 2006; Martin et al., 2009). The following primary and secondary antibody pairs were used: i) chicken anti-BDNF (1:50; Promega, Madison, WI) with either goat anti-chicken-biotinylated IgG (1:200; Vector Laboratories, Burlingame, CA) or donkey anti-chicken IgG-Cy3 (1:200; Jackson Immunoresearch, West Grove, PA), ii) rabbit anti-NGF (1:1000; Millipore, Temecula, CA) with goat anti-rabbit IgG-Alexa 488 (1:200; Invitrogen), iii) mouse anti-Neurofilament 68 and 160 (1:100; Sigma) with goat anti-mouse IgG-Cy3 (1:200; Jackson Immunoresearch), and iv) rabbit anti-phospho-CREB (1:1000; Millipore) with goat anti-rabbit IgG-Alexa 488 (1:200; Invitrogen). Following secondary antibody application, the DNA stain 4',6-diamidino-2-phenylindole (DAPI; 300 nM in PBS, Invitrogen) was applied to some coverslips for 10 min at room temperature to label all cell nuclei. Control cultures, in which primary antibodies were omitted, were completely devoid of staining.
Stained TG cultures were imaged as recently described (Martin et al., 2009; Tarsa and Balkowiec, 2009). To determine the ‘neuron to non-neuronal cell’ ratio, the total number of cells with DAPI-positive nuclei and the total number of neurofilament-immunoreactive cells (putative neurons) were counted in at least 10 randomly chosen fields per coverslip (200x total magnification), in 6 independent vehicle-treated cultures. To determine the percentage of NGF-immunoreactive cells, all NGF- and all neurofilament-immunoreactive cells were counted in 12 randomly chosen fields per coverslip (200x total magnification), in 6 vehicle-treated and 6 TNFα-treated cultures.
Measurement of BDNF and CGRP cell content, and NGF release
At the end of treatment, the culture medium was collected from each well and replaced with 75 μl of pre-chilled lysis buffer (Hsieh et al., 2010), followed by rinsing with 150 μl of pre-chilled Block & Sample buffer (1x; BDNF Emax™ ImmunoAssay System, Promega). The content of two identically treated culture wells (the total volume of 450 μl) was sonicated as recently described (Hsieh et al., 2010). BDNF and CGRP ELISAs were performed using BDNF Emax™ ImmunoAssay System (Promega), and Rat CGRP EIA (SPI-BIO; Montigny-le-Bretonneux, France), respectively, according to the manufacturers’ protocols. In some experiments, the medium collected from each culture well was immediately processed for detection of NGF by ELISA, using NGF Emax™ ImmunoAssay System (Promega) according to the manufacturer's instructions. BDNF, CGRP, and NGF levels were calculated from the standard curves prepared for each plate, using SOFTmax PRO® vs. 4.3 software (Molecular Devices, Sunnyvale, CA). The standard curves were linear within the range used (0-500 pg/ml for BDNF and CGRP; 0-250 pg/ml for NGF) and the quantities of BDNF, CGRP, and NGF in experimental samples were always within the linear range of the standard curve. The specificity of the BDNF ELISA has been confirmed by our laboratory using BDNF siRNA approach (Hsieh et al., 2010).
RNA extraction and quantitative RT-PCR
Quantitative RT-PCR was performed as recently described by our laboratory (Hsieh et al., 2010). Briefly, total RNA was extracted using TRIzol (Invitrogen), followed by re-suspension in 10 mM Tris pH 8.5. To make cDNA, 1 μg of total RNA was reverse transcribed (20 μl) using Tetro cDNA synthesis kit (Bioline, Taunton, MA) following the manufacturer's instructions. Quantitative PCR was performed in triplicates following the MIQE guidelines (Bustin et al., 2009) using the MX3000P real time PCR system (Stratagene; Cedar Creek, TX) in a final volume of 10 μl using Sensimix Plus SYBR master mix (Quantace, Taunton, MA), 2 μl of cDNA (diluted 1:4), and 3μM of each forward and reverse primers. All primer sequences were designed using Primer3 online software (http://frodo.wi.mit.edu/primer3/) and synthesized by Integrated DNA Technologies (Coralville, IA), for sequences see below. To check for primer specificity, DNA products were amplified by PCR using Taq polymerase (Invitrogen, Carlsbad, CA). Single PCR products were gel-purified using QIAquick Gel Extraction Kit (Qiagen, Valencia, CA), sequenced (OHSU DNA sequencing facilities) and quantified spectrophotometrically. Quantitative RT-PCR was performed with DNA standard dilutions ranging from 10pg to 10 ag. The real time amplification data were collected continuously using the software supplied with the MX3000P real time PCR system. Cq values for standard curves were between 10 and 30, with Cq values for samples falling within this range. RT-PCR efficiencies were all above 97%. The forward primers for BDNF are 5’-aaagccgaacttctcacatgat-3’ (BDNF-I), 5’-ctcccccttttaactgaagagaa-3’ (BDNF-IV), ctttggggcagacgagaaag-3’ (BDNF-VI), with a common reverse primer (5’-attcacgctctccagagtcc-3’). The PCR products were 273, 280, and 275 bp, respectively. Data normalization was carried out against the endogenous reference gene transcript hypoxanthine-guanidine phosphoribotransferase (HPRT, forward: 5’-caggccagactttgttggat-3, reverse: 5’- ggctgcctacaggctcatag-3’; PCR product of 328 bp in size). Genbank accession numbers are EF125675.1 (BDNF-I), EF125679.1 (BDNF-IV/former BDNF-III), EF125680.1 (BDNF-VI/former BDNF-IV), and NM012583.2 (HPRT).
Calculations and statistical analysis
Data are expressed as mean ± standard error, and the sample size (n) represents the number of individual cultures. Samples were compared using Student's t-test or ANOVA followed by Duncan's multiple comparison procedure, and P<0.05 was considered significant.
RESULTS
Tumor necrosis factor-α (TNFα) increases BDNF expression in trigeminal ganglion (TG) neurons in vitro
Proinflammatory cytokines have previously been shown to directly affect nociceptive neurons in a variety of ways (Schäfers and Sorkin, 2008; Basbaum et al., 2009; Costigan et al., 2009; Latremoliere and Woolf, 2009). However, it has not been established whether these influences include changes in expression of BDNF, a major player in mechanisms of synaptic plasticity.
To address this issue, we used our previously developed model of neuron-enriched rat TG cultures (Buldyrev et al., 2006), in which the ratio of neurons to non-neuronal cells is approximately 1:3 (2.99 ± 0.24 non-neuronal cells identified by DAPI-stained nuclei per neuron identified by neurofilament immunoreactivity; n= 6 cultures not treated with AraC). We examined BDNF mRNA and protein expression, along with the cell content of calcitonin gene-related peptide (CGRP) as a control, in response to TNFα applied in the presence of different levels of neuronal activity and modulators of signaling pathways. We focused on TNFα because of its established role in mechanisms of pain hypersensitivity.
In the first set of experiments, we treated TG cultures with TNFα (1, 10 and 50 ng/ml) for 24 hrs in order to determine if this cytokine regulates BDNF protein synthesis. The range of TNFα concentrations was chosen based on previous studies of TNFα levels in the inflamed tooth pulp (Ataoğlu et al., 2002; Pezelj-Ribaric et al., 2002; Bletsa et al., 2006), one of the major targets of trigeminal nociceptors and a source of BDNF upregulation by trigeminal neurons in vivo (Tarsa et al., 2010).
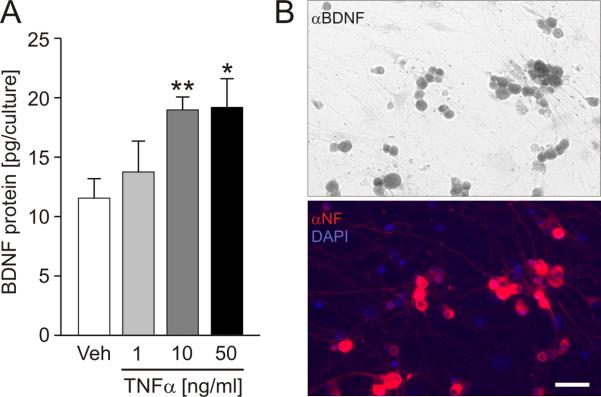
Treatment with 10 ng/ml of TNFα resulted in a highly significant, over 60% increase in BDNF cell content compared to vehicle (Fig. 1 A). A 10-fold lower concentration of TNFα, i.e. 1 ng/ml, did not have a significant effect on BDNF protein expression. On the other hand, the highest concentration of TNFα examined, i.e. 50 ng/ml, resulted in the increase in BDNF protein expression that was not significantly different from the effect of 10 ng/ml (Fig. 1A). Based on these results, in subsequent experiments of this study we used TNFα at the concentration of 10 ng/ml.
Figure 1. Tumor necrosis factor-α (TNFα) increases BDNF protein expression in cultured newborn rat trigeminal ganglion (TG) neurons.
A, Mean BDNF cell content (pg/culture) of TG cultures treated for 24 hrs with vehicle (Veh) or TNFα (1, 10 and 50 ng/ml); n=5 experiments; at least 2 cultures per experiment per group; * p<0.05; ** p<0.01. B, Representative neuron-enriched dissociate culture of newborn rat TG treated for 24 hrs with TNFα (10 ng/ml), followed by double-immunostaining for BDNF (αBDNF, top panel) and neurofilament (red) with DAPI (blue) counterstain (αNF/DAPI, bottom panel); scale bar, 50 μm.
Our previous studies using the same culture model indicate that BDNF is not expressed by non-neuronal cells under resting conditions (Buldyrev et al., 2006). However, TNFα has been shown to stimulate BDNF expression in astrocytes (Saha et al., 2006). Thus, to rule out the possibility that the TNFα-induced increase in BDNF cell content is a result of BDNF expression by non-neuronal cells, we next examined the cellular pattern of BDNF immunoreactivity in our neuron-enriched cultures. Our data show that non-neuronal cells remain BDNF-non-immunoreactive following treatment with TNFα (Fig. 1B). This result indicates that the observed upregulation of BDNF expression in response to TNFα occurs in TG neurons.
We next addressed the possibility that the TNFα-induced upregulation of BDNF involves nerve growth factor (NGF), whose role in neuroinflammation is well established (Woolf et al., 1994, 1997), including regulation of BDNF expression (Mannion et al., 1999). We first examined immunoreactivity for NGF in cultures treated with 10 ng/ml of TNFα for 24 hrs, compared to vehicle-treated control cultures. The results of this analysis indicate that NGF is abundantly expressed by only a small subset of TG neurons under both vehicle- and TNFα-treated conditions (Vehicle, 7.34 ± 0.59 %, TNFα, 8.38 ± 1.98 %, n= 6 cultures, P=0.6537; Fig. 2). Moreover, non-neuronal cells are completely lacking NGF immunoreactivity in our culture model, vehicle- or TNFα-treated (Fig. 2). We also quantified levels of NGF in the medium from TG cultures treated with 10 ng/ml of TNFα for 24 hrs, compared to vehicle-treated cultures. Consistent with the results of the immunocytochemical analysis, levels of NGF in our cultures are very low, and not affected by TNFα treatment (vehicle, 0.45 ± 0.04 pg/culture, TNFα, 0.46 ± 0.19 pg/culture, n=12 cultures, P=0.9466). Together, these data suggest that NGF does not play a role in mechanisms of TNFα-mediated upregulation of BDNF expression in TG neurons in our model.
Figure 2. Immunoreactivity for nerve growth factor (NGF), which is expressed by a small fraction of TG neurons and none of non-neuronal cells, is not affected by TNFα.

Representative neuron-enriched dissociate cultures of newborn rat TG treated for 24 hrs with vehicle (Veh) or TNFα (10 ng/ml). The cultures were double-immunostained for nerve growth factor (αNGF, green, top panel) and neurofilament (red) with DAPI (blue) counterstain (Overlay, bottom panel). Arrows indicate cells that express double NGF/neurofilament immunoreactivity; scale bar 50 μm.
Previous studies from our laboratory demonstrate that BDNF is significantly co-localized with CGRP (Buldyrev et al., 2006) whose role in neuroinflammation and trigeminal pain is well established. Thus, we examined the effects of TNFα on the cell content of CGRP in extracts of the same cultures that were examined for changes in BDNF cell content. Consistent with studies by Bowen and colleagues (2006), 24-hr application of TNFα (10 ng/ml) significantly increased CGRP expression (Vehicle: 125.71 ± 34.93 pg/culture vs. TNFα: 164.69 ± 49.68 pg/culture, n=4; 1.28 ± 0.05 increase relative to vehicle; P<0.01). These results indicate that BDNF and CGRP are upregulated in parallel by TNFα in TG neurons. Together with our previous data showing CGRP-dependent upregulation of BDNF release from these neurons (Buldyrev et al., 2006), the present result suggests that the two peptides interact in mechanisms of TG neuroinflammation in vivo.
To begin examining the molecular mechanisms of TNFα-induced upregulation of BDNF protein, we stained our TNFα-treated cultures with an antibody against the phosphorylated form of the cyclic adenosine monophosphate-responsive element binding protein (CREB), a marker of neuronal activation (Ghosh et al., 1994; Moore et al., 1996) and a transcription factor involved in regulation of BDNF expression in neurons (Tao et al., 1998). The analysis of double BDNF/phospho-CREB immunoreactivity indicates that treatment with TNFα leads to marked increases in phospho-CREB staining in the nuclei of TG neurons that are immunoreactive for BDNF (Fig. 3A). This result suggests that the mechanism of the TNFα-mediated upregulation of BDNF occurs at the transcriptional level.
Figure 3. Tumor necrosis factor-α (TNFα) stimulates BDNF expression at the transcriptional level.
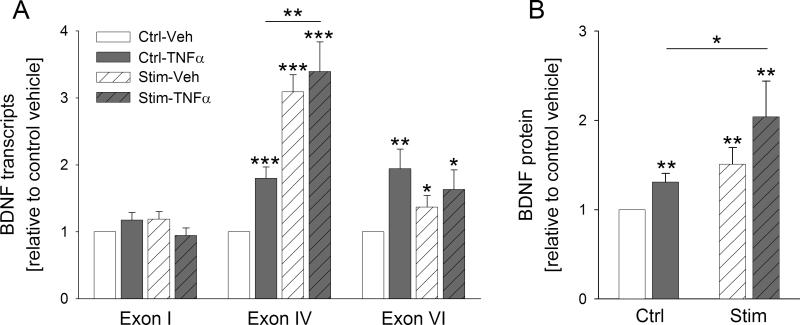
A, Representative neuron-enriched dissociate cultures of newborn rat TG treated for 24 hrs with vehicle (Veh) or TNFα (10 ng/ml), followed by double-immunostaining for the phosphorylated form of the cyclic adenosine monophosphate-responsive element binding protein (αpCREB, green, top panel) and brain-derived neurotrophic factor (αBDNF, red, bottom panel). Arrows indicate cells that show strong pCREB immunoreactivity in the nucleus (top panel) and BDNF immunoreactivity in the cytoplasm (bottom panel); scale bar 50 μm. B, Levels of three activity-dependent BDNF transcripts (I, IV, VI) in response to 4-hr treatment of sister cultures with increasing concentrations of TNFα (1, 10 or 50 ng/ml), normalized in relation to levels of the endogenous reference gene transcript hypoxanthine-guanidine phosphoribotransferase (HPRT), and presented relative to vehicle (Veh); n=4 experiments. *p<0.05, **p<0.01.
Based on these data, we further explored the TNFα-mediated upregulation of BDNF protein and the underlying molecular mechanisms. Previous studies of activity-dependent regulation of the BDNF gene have demonstrated that production of specific transcripts is dependent on the type of stimulus and the signaling pathways that are activated in response to that stimulus (reviewed by Cohen and Greenberg, 2008). Thus far, the most thoroughly characterized activity-dependent promoters of the BDNF gene are I, IV (formerly known as III), and VI (formerly known as IV; Aid et al., 2007). For example, promoters I and IV show different sensitivity of response to activation of NMDA glutamate receptors and L-type calcium channels (Tabuchi et al., 2000). Therefore, we next asked whether TNFα regulates transcription of the BDNF gene and, if so, which promoters are sensitive to this regulation.
A 4-hour application of TNFα resulted in a significant upregulation of BDNF transcripts IV and VI. Interestingly, the 1 ng/ml concentration of TNFα was sufficient to significantly increase the amount of transcript IV, but not transcript VI. This suggests a higher sensitivity of BDNF promoter IV to TNFα-mediated activation compared with promoter VI. Moreover, TNFα had no effect on transcript I (Fig. 3B). Together, these data indicate that stimulation of TG neurons with TNFα leads to an upregulation of transcription of the BDNF gene in a promoter-specific manner.
Electrical stimulation and forskolin potentiate the TNFα-mediated upregulation of BDNF expression
Inflammatory pain is associated with nociceptor sensitization and a resulting increased neuronal activity. Therefore, we used electrical field stimulation in order to mimic an increased activity of TG neurons and examine TNFα-mediated regulation of BDNF expression under those conditions.
TG neurons were electrically stimulated for 24 hrs and next treated with TNFα for either 4 hrs (BDNF mRNA) or 24 hrs (BDNF protein). Consistent with the effects of TNFα alone on BDNF transcripts, electrical stimulation (1 Hz, 0.2-ms pulses, 100 mA/culture well) followed by a 4-hr treatment with TNFα (10 ng/ml) exerted the strongest effect on the levels of BDNF transcript IV, and BDNF transcript I remained unaffected. Out of 22 experiments, TNFα-induced upregulation of BDNF transcript IV was significantly potentiated by the electrical stimulation in 8 experiments. In the remaining 14 experiments, the potentiating effect was absent. Since we were not able to identify the difference in experimental conditions between these two groups, the data were combined and, consequently, the average effect of TNFα following the electrical field stimulation was very similar to the effect of the electrical stimulation alone (Fig. 4A). This result suggests that TNFα and electrical activation utilize overlapping signaling pathways to elicit expression of BDNF mRNA.
Figure 4. Electrical stimulation of TG neurons results in potentiation of TNFα-induced BDNF protein expression.
A, Levels of three BDNF transcripts (I, IV and VI; relative to unstimulated vehicle; Ctrl-Veh, white solid bar) in response to 4-hr treatment of cultures with 10 ng/ml TNFα (Ctrl-TNFα, grey solid bar), 24-hr electrical field stimulation at 1 Hz followed by 4-hr treatment with vehicle (Stim-Veh, white hatched bar), or 24-hr electrical stimulation followed by 4-hr treatment with 10 ng/ml TNFα (Stim-TNFα, grey hatched bar). Individual transcript levels were calculated using the reference gene HPRT; n=22 experiments. B, BDNF cell content (relative to unstimulated vehicle whose value was made equal to one; Ctrl-Veh, white solid bar) in response to 24-hr treatment with 10 ng/ml TNFα alone (Ctrl-TNFα, grey solid bar), 24-hr electrical field stimulation at 1 Hz followed by 24-hr treatment with vehicle (Stim-Veh, white hatched bar), or 24-hr stimulation followed by 24-hr treatment with 10 ng/ml TNFα (grey hatched bar); n=5 experiments. *p<0.05, **p<0.01, ***p<0.001.
A 24-hr application of TNFα following a 24-hr electrical stimulation resulted in a 2-fold increase in BDNF protein synthesis, compared to a 1.3-fold increase by TNFα in unstimulated sister cultures (Fig. 4B). Given that electrical stimulation itself caused only a 1.5-fold increase in BDNF synthesis, these data indicate that neuronal activation potentiates the effect of TNFα on BDNF protein synthesis. It is worth noting that electrical stimulation applied prior to the TNFα treatment was more effective than electrical stimulation delivered during the treatment (data not shown).
Neuronal depolarization leads to increased intracellular content of cAMP (Meyer-Franke et al., 1995), and forskolin, an activator of adenylate cyclase, enhances activity-dependent BDNF mRNA expression (Zafra et al., 1992). Moreover, forskolin and/or cAMP analogs have been shown to increase nociceptor sensitivity (Ferreira and Nakamura, 1979). Therefore, one potential mechanism through which electrical stimulation promotes TNFα-mediated BDNF expression in TG neurons is an increased intracellular cAMP. To begin addressing this possibility, here we examined the effects of the adenylate cyclase activator forskolin on TNFα-mediated BDNF expression.
Previous studies have indicated the need for continuous exposure to foskolin and resulting continuous activation of signal transduction pathways in mechanisms of sensory neuron sensitization (Bolyard et al., 2000). For that reason, forskolin (5 μM) was applied to TG neuron cultures simultaneously with TNFα (10 ng/ml). A 4-hr treatment with forskolin and TNFα resulted in a strong upregulation of BDNF transcript IV that was more than three times larger than the upregulation caused by TNFα alone (TNFα + Forskolin: 1.9-fold increase vs. TNFα alone: 0.6-fold increase above vehicle; Fig. 5A). On the other hand, when forskolin alone was applied to sister cultures, it showed a strong trend toward increasing BDNF expression, but the effect was not statistically significant (Fig. 5A). Similarly to electrical stimulation, forskolin failed to increase BDNF transcript I and its effects on transcript VI were markedly weaker compared to transcript IV (data not shown). The effect of forskolin on BDNF transcripts was mimicked by 3-Isobutyl-1-methylxanthine (IBMX; 100 μM), a non-selective inhibitor of cAMP and cGMP phosphodiesterases (data not shown). Consistent with the effects of forskolin on BDNF transcripts, a 24-hr treatment with 5 μM forskolin and 10 ng/ml of TNFα resulted in an upregulation of BDNF protein that was more than two-thirds larger than the effect of TNFα alone (Fig. 5B). Together, these results identify a novel phenomenon of activity- and cAMP-dependent potentiation of BDNF expression induced by TNFα.
Figure 5. Forskolin, an activator of adenylate cyclase, potentiates the TNFα-mediated increase in BDNF transcription.
A, Levels of BDNF transcript IV (relative to vehicle whose value was made equal to one, white solid bar) in response to 4-hr treatment with 10 ng/ml TNFα alone (grey solid bar), 5 μM forskolin alone (white striped bar) or 10 ng/ml TNFα combined with 5 μM forskolin (grey striped bar). Transcript levels were calculated using levels of the reference gene HPRT; n=7 experiments. B, BDNF cell content (relative to vehicle whose value was made equal to one; white solid bar) in response to 24-hr treatment with 10 ng/ml TNFα alone (grey solid bar), 5 μM forskolin alone (white striped bar) or 10 ng/ml TNFα combined with 5 μM forskolin (grey striped bar); n=3 experiments. *p<0.05, **p<0.01, ***p<0.001.
TNFα-mediated increase in BDNF mRNA expression requires tetrodotoxin (TTX)-sensitive sodium currents and p38-mitogen-activated protein kinase (MAPK) signaling
We next sought to examine the underlying cellular mechanisms of TNFα-induced BDNF expression in TG neurons. As demonstrated above, the TNFα effects on BDNF expression are sensitive to changes in neuronal activity. The level of neuronal activity is determined to a large extent by activity of voltage-dependent sodium channels, and primary sensory neurons express both TTX-sensitive and TTX-resistant sodium channels. Also, there is evidence that proinflammatory cytokines can enhance TTX-resistant currents in nociceptors, leading to their increased excitability (Binshtok et al., 2008). Therefore, we first examined the effects of TTX on TNFα-induced BDNF protein synthesis.
TTX (1.5 μM) was added to a half of TG neuron cultures 30 min before the application of TNFα (10 ng/ml), and both agents stayed until the end of the 24-hr incubation period. The remaining sister cultures were treated with vehicle solutions and served as controls. The magnitude of the TNFα-mediated upregulation of BDNF protein was decreased in the presence of TTX. Even so, the effect of TNFα remained highly significant (relative to vehicle: TNFα, 1.40 ± 0.11, TTX alone, 0.98 ± 0.1, TNFα+TTX, 1.24 ± 0.11; n=5; P<0.01 between ‘TTX alone’ and ‘TNFα+TTX’). These data suggest that a significant proportion of the sodium current involved in the mechanisms of TNFα-induced BDNF protein synthesis over the 24-hr period is mediated by TTX-resistant channels. Alternatively, TNFα-mediated increase in BDNF protein cell content may not directly require generation of action potentials.
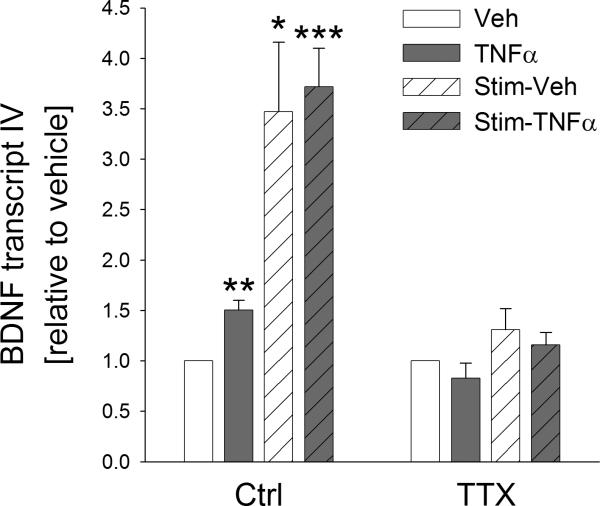
Since our data presented in the previous chapter reveal potential differences in the mechanisms underlying TNFα-induced expression of BDNF protein compared to mRNA, we next examined the role of TTX-sensitive sodium channels in TNFα-dependent upregulation of BDNF mRNA, including the effects of electrical stimulation.
One half of TG neuron cultures was pre-treated with TTX (1.5 μM) for 30 min prior to the beginning of a 24-hr electrical field stimulation, which was followed by a 4-hr treatment with TNFα (10 ng/ml) in the presence or absence of TTX. Control cultures were treated with corresponding vehicle solutions. Unlike TNFα-dependent upregulation of BDNF protein synthesis, TTX completely abolished the TNFα-induced upregulation of BDNF mRNA, both in the presence and absence of the preceding electrical stimulation (Fig. 6). Together, these data show that de novo synthesis of BDNF in response to TNFα requires TTX-sensitive sodium currents.
Figure 6. Tetrodotoxin (TTX), an inhibitor of voltage-activated sodium channels, abolishes the TNFα- and electrical stimulation- mediated upregulation of BDNF transcription in TG neurons.
Levels of BDNF transcript IV (relative to unstimulated vehicle whose value was made equal to one, Veh, white solid bars) in response to 4-hr treatment of cultures with 10 ng/ml TNFα (TNFα, grey solid bars), 24-hr electrical field stimulation at 1 Hz followed by 4-hr treatment with vehicle (Stim-Veh, white hatched bars), or 24-hr electrical stimulation followed by 4-hr treatment with 10 ng/ml TNFα (Stim-TNFα, grey hatched bars), in the absence (Ctrl) and presence of 1.5 μM TTX (TTX). Individual transcript levels were calculated using the reference gene HPRT; n=4 experiments. *p<0.05, **p<0.01, ***p<0.001.
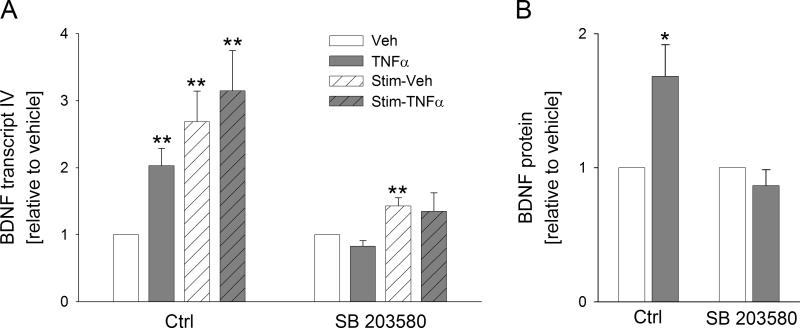
Next, we investigated the intracellular signaling pathways through which stimulation of TG neurons with TNFα leads to de novo synthesis of BDNF. TNFα has previously been shown to act through various members of the MAPK family, which includes two stress-activated protein kinase sub-families, p38 and c-jun N-terminal kinase (JNK; Thalhamer et al., 2008). There are several lines of evidence that TNFα signaling in sensory neurons is mediated through p38-MAPK (Schäfers et al., 2003b; Jin and Gereau, 2006). Therefore, we considered p38-MAPK as a likely intracellular mediator of the TNFα-induced upregulation of BDNF expression. To this end, we examined the effects of the pharmacological blockade of p38-MAPK with SB203580 on TNFα-induced BDNF mRNA and protein expression in the presence and absence of the prior electrical field stimulation.
Sister cultures of TG neurons were either electrically stimulated (according to the same parameters as those used in experiments described in the previous chapter) or only incubated for 24 hrs, followed by addition of SB203580 (1μM) 30 min before the beginning of a 4-hr (BDNF mRNA) or 24-hr (BDNF protein) treatment with TNFα (10 ng/ml). We found that SB203580 completely abolished both BDNF mRNA and protein expression caused by TNFα (Fig. 7A,B). In addition, SB203580 significantly inhibited the increase in BDNF mRNA due to electrical field stimulation alone (Fig. 7A). This is interesting because the p38-MAPK inhibitor was applied after the cessation of the electrical stimulation. The latter result suggests that electrical field stimulation leads to a prolonged increase in the overall activity of neurons (please see the TTX data above) and a resulting activation of intracellular signaling pathways, including the p38-MAPK pathway (Fig. 8). Together, these data show that p38-MAPK intracellular signaling pathway appears to be necessary for de novo synthesis of BDNF in TG neurons in response to TNFα.
Figure 7. Inhibition of the p38 MAPK pathway abolishes the TNFα-induced upregulation of BDNF transcription in TG neurons, in the absence or presence of electrical stimulation.
A, Levels of BDNF transcript IV (relative to unstimulated vehicle whose value was made equal to one, Veh, white solid bars) in response to a 4-hr treatment of cultures with 10 ng/ml TNFα (TNFα, grey solid bars), 24-hr electrical field stimulation at 1 Hz followed by 4-hr treatment with vehicle (Stim-Veh, white hatched bars), or 24-hr electrical stimulation followed by 4-hr treatment with 10 ng/ml TNFα (Stim-TNFα, grey hatched bars), in the absence (Ctrl) or presence of an inhibitor of p38 MAPK (SB 203580, 1μM). Individual transcript levels were calculated using the reference gene HPRT; n= 6 experiments. B, BDNF cell content (relative to vehicle whose value was made equal to one; white solid bars) in response to a 24-hr treatment with 10 ng/ml TNFα (grey solid bar) in the absence (Ctrl) or presence of an inhibitor of p38 MAPK (SB 203580, 1μM); n=4 experiments.*p<0.05, **p<0.01.
Figure 8. Proposed model of TNFα-mediated regulation of BDNF expression in neurons.

TNFα, released from macrophages or microglial cells, stimulates the p38 mitogen-activated protein kinase (p38 MAPK) pathway to increase phosphorylation (P) of cAMP response element (CRE)-binding (CREB), a transcription factor known to regulate the BDNF gene. In turn, CREB phosphorylation leads to activation of exon IV of the BDNF gene, and subsequent BDNF mRNA and protein synthesis. Neuronal depolarization, dependent on tetrodotoxin-sensitive (TTX-S) sodium channels, increases intracellular cAMP, which acts through several putative mechanisms to potentiate the TNFα regulation of BDNF transcription. For example, cAMP activates protein kinase A (PKA), a well-established mechanism of CREB phosphorylation. In addition, cAMP is a likely mediator of depolarization-induced upregulation of p38 MAPK activity.
DISCUSSION
The present study demonstrates for the first time that TNFα stimulates de novo synthesis of BDNF in TG neurons, acting at the transcriptional level in a promoter-specific manner. Moreover, electrical activity and forskolin, known to stimulate synthesis of cAMP, potentiate the TNFα-induced BDNF expression, whereas TTX and a selective antagonist of p38-MAPK abolish it.
The proinflammatory cytokines, such as TNFα and IL-1β, play an essential role in the development and maintenance of inflammatory and neuropathic pain (Schäfers and Sorkin, 2008; Basbaum et al., 2009; Costigan et al., 2009; Latremoliere and Woolf, 2009; McMahon and Malcangio, 2009). The role of TNFα in pain sensitization has been known for almost 15 years (Sorkin et al., 1997; Woolf et al., 1997). Studies have shown that TNFα is involved in thermal and mechanical hypersensitivity (Junger and Sorkin, 2000; Opree and Kress, 2000) by modulating TTX-resistant sodium channels in primary sensory neurons (Jin and Gereau, 2006) and by promoting insertion of calcium-permeable glutamatergic AMPA receptors into membranes of spinal cord neurons (Choi et al., 2010). TNFα also induces expression of pronociceptive proteins, including cyclooxygenase-2 (Fehrenbacher et al., 2005) and the transient receptor potential vanilloid type 1 (TRPV1; Constantin et al., 2008; Khan et al., 2008). Moreover, TNFα-mediated upregulation of TRPV1 can take place at both transcriptional (Khan et al., 2008) and posttranscriptional (Constantin et al., 2008) level.
The present study identifies a novel transcriptional target of TNFα in sensory neurons, the neurotrophin BDNF (Fig. 8) whose role as a modulator of nociceptive transmission is established (Malcangio and Lessman, 2003; Merighi et al., 2008; Latremoliere and Woolf, 2009). Previous evidence for effects of cytokines on BDNF expression is scarce. In neurons, TNFα and IL-1β increase BDNF mRNA in hypothalamic neurons in vitro (Rage et al., 2006; Taishi et al., 2008) and intrathecal infusion of interleukin-6 increases BDNF mRNA in dorsal root ganglia (DRG; Murphy et al., 2000). Another study showed that inhibiting TNFα activity attenuates an increase in BDNF immunoreactivity in DRG neurons induced by nucleus pulposus in vivo (Onda et al., 2004).
We and others have previously shown that BDNF is upregulated in primary sensory neurons by inflammation (Cho et al., 1997a,b; Kim et al., 2001; Obata et al., 2003; Qiao et al., 2008; Tarsa et al., 2010). Based on the work by Mannion and colleagues (1999), the inflammation-induced upregulation of BDNF expression has commonly thought to be mediated by nerve growth factor (NGF). In our neuron-enriched cultures, NGF was expressed by only a small fraction of TG neurons and no non-neuronal cells. Moreover, neither NGF expression nor release were significantly affected by treatment with TNFα. Furthermore, evidence from other laboratories indicates that NGF strongly upregulates BDNF transcript I (Matsuoka et al., 2007), which was not affected by TNFα in the current study. Together, these data strongly suggest that TNFα upregulates BDNF expression in an NGF-independent manner. Consistent with this notion, TNFα increased BDNF expression via p38-MAPK (Fig. 8), which has previously been shown to be critical for direct effects of TNFα on sensory neurons (Schäfers et al., 2003b; Jin and Gereau, 2006; Constantin et al., 2008).
Our data indicate that TNFα and electrical stimulation lead to a selective upregulation of BDNF transcripts, with transcript IV being most strongly upregulated and transcript I completely unaffected. Production of distinct BDNF transcripts may determine the function of the BDNF protein or, alternatively, provide means to regulate the stimulus-dependence of BDNF synthesis (Cohen and Greenberg, 2008). The current findings contribute to our understanding of the role of different BDNF transcripts in determining the functional specificity of the BDNF protein, including the TNFα-mediated upregulation.
Peripheral inflammation and injury are associated with the release of inflammatory mediators, including TNFα, from immune and non-neuronal cells, and TNFα activates a cascade of other cytokines and growth factors (Schäfers and Sorkin, 2008). Many studies point to the role of glia in nociceptive sensitization and in development of chronic pain (Tsuda et al., 2005; Scholz and Woolf, 2007). They indicate that a key mediator is IL-1β. For example, release of IL-1β from activated microglia in spinal cord inflammation is associated with an enhanced nociceptive transmission (Clark et al, 2006, 2010). In trigeminal models of inflammatory pain hypersensitivity, astroglia-derived IL-1β induces phosphorylation of NMDA receptors (Guo et al., 2007), and glial IL-1β released within the trigeminal ganglion modulates the excitability of Aδ trigeminal nociceptors leading to a decreased mechanical hyperalgesia (Takeda et al., 2008). There is also evidence for gap junction and paracrine signaling within the trigeminal ganglion (Thalakoti et al., 2007). In our neuron-enriched TG culture model, exogenous IL-1β does not increase BDNF expression (Balkowiec-Iskra and Balkowiec, unpublished observations). This fact rules out the possibility that the effects of TNFα are indirect, through release of IL-1β from non-neuronal cells. Moreover, our current and previous (Buldyrev et al., 2006) studies indicate that BDNF is not expressed by non-neuronal cells, thus, the increase in BDNF expression is unlikely to be attributed to the non-neuronal source.
This study reveals the previously unknown role of cAMP in regulating cytokine-mediated BDNF expression. These data are consistent with the previously shown effects of activating adenylate cyclase on neuronal expression of neurotrophin mRNAs induced by conventional transmitters and neuropeptides (Zafra et al., 1992). It has previously been demonstrated that central, but not peripheral, neurons require activity or increased intracellular cAMP for their responsiveness to trophic factors (Meyer-Franke et al., 1995, 1998; Shen et al., 1999; Goldberg et al., 2002), in part through an increased surface expression of the BDNF receptor TrkB (Meyer-Franke et al., 1998). Our current data indicate that neuronal activity and cAMP enhance responsiveness of sensory neurons to cytokines that, in turn, leads to an augmented BDNF expression (Fig. 8). This mechanism could play an important role in axonal regeneration after injury, which is associated with inflammation and release of proinflammatory cytokines, such as TNFα.
TG neurons express both types of TNFα receptors and the role of TNFα as a neuromodulator in the trigeminal system has been proposed several years ago (Cunningham et al., 1997). Studies in DRG show that expression of TNFα and its receptors can be strongly upregulated by inflammation and injury in both neurons and non-neuronal cells (Schäfers et al., 2003a; Li et al., 2004; Schäfers et al., 2008), and the neuronal expression of TNFα increases after nerve injury (Shubayev and Myers, 2001). These data suggest that neuron- and non-neuronal cell-derived TNFα could act in an auto- or paracrine fashion to upregulate BDNF expression in TG neurons. For example, our previous study showed that inflammation within just two teeth leads to a widespread upregulation of BDNF expression in TG neurons (Tarsa et al., 2010), the phenomenon which could potentially be explained by upregulation of TNFα receptors in not only inflamed, but also non-inflamed neurons and/or paracrine action of TNFα on these neurons. In support, spinal nerve ligation leads to sensitization of both injured and adjacent uninjured DRG neurons to TNFα (Schäfers et al., 2003a).
Our previous studies point to BDNF as a likely modulator of nociceptive transmission at first-order synapses in trigeminal pathways (Buldyrev et al., 2006; Tarsa et al., 2010). TG neurons constitute the first link of nociceptive information processing in such chronic pain conditions as migraine, trigeminal neuralgia and temporomandibular joint disorders. There is evidence that TNFα is significantly increased in plasma and cerebrospinal fluid of migraineurs and patients with daily persistent headaches (Perini et al., 2005; Rozen and Swidan, 2007). Our current data offer a scenario where TNFα increases BDNF expression in TG neurons, leading to trigeminal hyperalgesia. In support, peripheral inflammation enhances BDNF signaling in central pain modulatory circuitry (Guo et al., 2006; Ren and Dubner, 2007). However, the physiological significance of the TNFα-mediated upregulation of BDNF in TG neurons remains to be determined.
There is increasing evidence that cytokines are involved in normal neuronal development and plasticity. The current data show that TNFα activates the transcription factor cAMP-response element-binding protein (CREB), which is essential for the activity-dependent transcription of the BDNF (Shieh et al., 1998; Tao et al., 1998; West et al., 2001) and other synaptic plasticity genes (Ji et al., 2003). Other studies have identified immune cells and microglia as critical for the maintenance of adult neurogenesis and learning abilities. Moreover, the immune T cells have been shown to be required for the expression of BDNF in the hippocampus (Ziv et al., 2006).
Together, our findings provide a new evidence for a link between the nervous and immune systems through TNFα-evoked de novo synthesis of BDNF in neurons. These data have implications for understanding mechanisms of neuroinflammatory disorders of the central and peripheral nervous system, as well as normal neuronal development and plasticity.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (R01HL076113 to A.B.) and the Medical Research Foundation of Oregon (to A.B.), and by the Medical University of Warsaw and OHSU School of Dentistry. We thank Dr. Leila Tarsa for performing pilot experiments with interleukin-1β, Victoria K. Jenkins for advice regarding quantitative RT-PCR, and Hui-ya Hsieh for the excellent technical assistance.
LIST OF ABBREVIATIONS
- BDNF
brain-derived neurotrophic factor
- CGRP
calcitonin gene-related peptide
- CREB
cAMP response element-binding protein
- pCREB
phosphorylated cAMP response element-binding protein
- DAPI
4',6-diamidino-2-phenylindole
- ELISA
enzyme-linked immunosorbent assay
- IBMX
3-Isobutyl-1-methylxanthine
- IL-1β
interleukin-1β
- MAPK
mitogen-activated protein kinase
- NGF
nerve growth factor
- RT-PCR
reverse-transcription polymerase chain reaction
- TG
trigeminal ganglion
- NF
neurofilament
- TNFα
tumor necrosis factor-α
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- BDNF mRNA and protein are upregulated in trigeminal ganglion neurons by TNF-α
- TNF-α upregulates BDNF expression in a promoter-selective manner
- TNF-α upregulation of BDNF expression is potentiated by neuronal activity and cAMP
- TNF-α upregulation of BDNF expression depends on sodium channels and p38-MAPK
- These data have implications for BDNF-dependent sensory plasticity
REFERENCES
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataoğlu T, Ungör M, Serpek B, Haliloğlu S, Ataoğlu H, Ari H. Interleukin-1beta and tumour necrosis factor-alpha levels in periapical exudates. Int Endod J. 2002;35:181–185. doi: 10.1046/j.1365-2591.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- Bałkowiec-Iskra E, Balkowiec A. Regulation of BDNF expression in trigeminal ganglion neurons by proinflammatory cytokines. Society for Neuroscience Abstracts 710.4. 2009 [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bletsa A, Berggreen E, Fristad I, Tenstad O, Wiig H. Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation. J Physiol. 2006;573:225–236. doi: 10.1113/jphysiol.2006.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyard LA, Van Looy JW, Vasko MR. Sensitization of rat sensory neurons by chronic exposure to forskolin or ‘inflammatory cocktail’ does not downregulate and requires continuous exposure. Pain. 2000;88:277–285. doi: 10.1016/S0304-3959(00)00341-9. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96:65–77. doi: 10.1111/j.1471-4159.2005.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buldyrev I, Tanner NM, Hsieh HY, Dodd EG, Nguyen LT, Balkowiec A. Calcitonin gene-related peptide enhances release of native brain-derived neurotrophic factor from trigeminal ganglion neurons. J Neurochem. 2006;99:1338–1350. doi: 10.1111/j.1471-4159.2006.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CL. The MIQE guidelines: Minimum Information for publication of Quantitative Real-Time PCR Experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Palmer TD. Immune influence on adult neural stem cell regulation and function. Neuron. 2009;64:79–92. doi: 10.1016/j.neuron.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Kim JK, Zhou XF, Rush RA. Increased brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia and spinal cord following peripheral inflammation. Brain Res. 1997a;764:269–272. doi: 10.1016/s0006-8993(97)00597-0. [DOI] [PubMed] [Google Scholar]
- Cho HJ, Kim SY, Park MJ, Kim DS, Kim JK, Chu MY. Expression of mRNA for brain-derived neurotrophic factor in the dorsal root ganglion following peripheral inflammation. Brain Res. 1997b;749:358–362. doi: 10.1016/S0006-8993(97)00048-6. [DOI] [PubMed] [Google Scholar]
- Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, D'Aquisto F, Gentry C, Marchand F, McMahon SB, Malcangio M. Rapid co-release of interleukin 1beta and caspase 1 in spinal cord inflammation. J Neurochem. 2006;99:868–880. doi: 10.1111/j.1471-4159.2006.04126.x. [DOI] [PubMed] [Google Scholar]
- Clark AK, Staniland AA, Marchand F, Kaan TK, McMahon SB, Malcangio M. P2X7-dependent release of interleukin-1beta and nociception in the spinal cord following lipopolysaccharide. J Neurosci. 2010;30:573–582. doi: 10.1523/JNEUROSCI.3295-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28:5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ET, Jr., Stalder AK, Sanna PP, Liu SS, Bloom FE, Howes EL, Jr., Campbell IL, Margolis TP. Distribution of tumor necrosis factor receptor messenger RNA in normal and herpes simplex virus infected trigeminal ganglia in the mouse. Brain Res. 1997;758:99–106. doi: 10.1016/s0006-8993(97)00169-8. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC, Burkey TH, Nicol GD, Vasko MR. Tumor necrosis factor alpha and interleukin-1beta stimulate the expression of cyclooxygenase II but do not alter prostaglandin E2 receptor mRNA levels in cultured dorsal root ganglia cells. Pain. 2005;113:113–122. doi: 10.1016/j.pain.2004.09.031. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Nakamura M. I - Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979;18:179–190. doi: 10.1016/0090-6980(79)90103-5. [DOI] [PubMed] [Google Scholar]
- Flake NM, Gold MS. Inflammation alters sodium currents and excitability of temporomandibular joint afferents. Neurosci Lett. 2005;384:294–299. doi: 10.1016/j.neulet.2005.04.091. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glialcytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HY, Robertson CL, Vermehren-Schmaedick A, Balkowiec A. Nitric oxide regulates BDNF release from nodose ganglion neurons in a pattern-dependent and cGMP-independent manner. J Neurosci Res. 2010;88:1285–1297. doi: 10.1002/jnr.22291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85:145–151. doi: 10.1016/s0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Khan AA, Diogenes A, Jeske NA, Henry MA, Akopian A, Hargreaves KM. Tumor necrosis factor alpha enhances the sensitivity of rat trigeminal neurons to capsaicin. Neuroscience. 2008;155:503–509. doi: 10.1016/j.neuroscience.2008.05.036. [DOI] [PubMed] [Google Scholar]
- Kim DS, Lee SJ, Cho HJ. Differential usage of multiple brain-derived neurotrophic factor promoter in rat dorsal root ganglia following peripheral nerve injuries and inflammation. Brain Res Molecular Brain Research. 2001;92:167–171. doi: 10.1016/s0169-328x(01)00154-1. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J Neurosci. 2004;24:9623–9631. doi: 10.1523/JNEUROSCI.2392-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcangio M, Bowery NG, Flower RJ, Perretti M. Effect of interleukin-1 beta on the release of substance P from rat isolated spinal cord. Eur J Pharmacol. 1996;299:113–118. doi: 10.1016/0014-2999(95)00845-4. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Lessmann V. A common thread for pain and memory synapses? Brain-derived neurotrophic factor and trkB receptors. Trends in Pharmacological Sciences. 2003;24:116–121. doi: 10.1016/S0165-6147(03)00025-7. [DOI] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Jenkins VK, Hsieh HY, Balkowiec A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: implications for activity-dependent plasticity at baroafferent synapses. J Neurochem. 2009;108:450–464. doi: 10.1111/j.1471-4159.2008.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Yokoyama M, Kobayashi H, Omori M, Itano Y, Morita K, Mori H, Nakanishi T. Expression profiles of BDNF splice variants in cultured DRG neurons stimulated with NGF. Biochem Biophys Res Commun. 2007;362:682–688. doi: 10.1016/j.bbrc.2007.08.022. [DOI] [PubMed] [Google Scholar]
- McAllister AK, van de WJ. Breaking boundaries in neural-immune interactions. Neuron. 2009;64:9–12. doi: 10.1016/j.neuron.2009.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MGJ, Reichardt LF, Barres BA. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–693. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem. 1996;271:14214–14220. doi: 10.1074/jbc.271.24.14214. [DOI] [PubMed] [Google Scholar]
- Murphy PG, Borthwick LA, Altares M, Gauldie J, Kaplan D, Richardson PM. Reciprocal actions of interleukin-6 and brain-derived neurotrophic factor on rat and mouse primary sensory neurons. Eur J Neurosci. 2000;12:1891–1899. doi: 10.1046/j.1460-9568.2000.00074.x. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Dai Y, Tachibana T, Fukuoka T, Tokunaga A, Yoshikawa H, Noguchi K. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J Neurosci. 2003;23:4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda A, Murata Y, Rydevik B, Larsson K, Kikuchi S, Olmarker K. Infliximab attenuates immunoreactivity of brain-derived neurotrophic factor in a rat model of herniated nucleus pulposus. Spine. 2004;29:1857–1861. doi: 10.1097/01.brs.0000137054.08788.b2. [DOI] [PubMed] [Google Scholar]
- Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini F, D'Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, Bussone G, Toso V. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45:926–931. doi: 10.1111/j.1526-4610.2005.05135.x. [DOI] [PubMed] [Google Scholar]
- Pezelj-Ribaric S, Anic I, Brekalo I, Miletic I, Hasan M, Simunovic-Soskic M. Detection of tumor necrosis factor alpha in normal and inflamed human dental pulps. Arch Med Res. 2002;33:482–484. doi: 10.1016/s0188-4409(02)00396-x. [DOI] [PubMed] [Google Scholar]
- Pezet S, Malcangio M, McMahon SB. BDNF: a neuromodulator in nociceptive pathways?. Brain Research - Brain Research Reviews. 2002;40:240–249. doi: 10.1016/s0165-0173(02)00206-0. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nature Reviews Neuroscience. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Gulick MA, Bowers J, Kuemmerle JF, Grider JR. Differential changes in brain-derived neurotrophic factor and extracellular signal-regulated kinase in rat primary afferent pathways with colitis. Neurogastroenterol Motil. 2008;20:928–938. doi: 10.1111/j.1365-2982.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- Rage F, Silhol M, Tapia-Arancibia L. IL-1beta regulation of BDNF expression in rat cultured hypothalamic neurons depends on the presence of glial cells. Neurochemistry International. 2006;49:433–441. doi: 10.1016/j.neuint.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: role of BDNF-TrkB signaling and NMDA receptors. Mol Neurobiol. 2007;35:224–235. doi: 10.1007/s12035-007-0028-8. [DOI] [PubMed] [Google Scholar]
- Ren K, Torres R. Role of interleukin-1beta during pain and inflammation. Brain Res Rev. 2009;60:57–64. doi: 10.1016/j.brainresrev.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47:1050–1055. doi: 10.1111/j.1526-4610.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- Saha RN, Liu X, Pahan K. Up-regulation of BDNF in astrocytes by TNF-alpha: a case for the neuroprotective role of cytokine. J Neuroimmune Pharmacol. 2006;1:212–22. doi: 10.1007/s11481-006-9020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003a;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfers M, Sommer C, Geis C, Hagenacker T, Vandenabeele P, Sorkin LS. Selective stimulation of either tumor necrosis factor receptor differentially induces pain behavior in vivo and ectopic activity in sensory neurons in vitro. Neuroscience. 2008;157:414–423. doi: 10.1016/j.neuroscience.2008.08.067. [DOI] [PubMed] [Google Scholar]
- Schäfers M, Sorkin L. Effect of cytokines on neuronal excitability. Neurosci Lett. 2008;437:188–193. doi: 10.1016/j.neulet.2008.03.052. [DOI] [PubMed] [Google Scholar]
- Schäfers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003b;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Shen S, Wiemelt AP, McMorris FA, Barres BA. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron. 1999;23:285–295. doi: 10.1016/s0896-6273(00)80780-1. [DOI] [PubMed] [Google Scholar]
- Shieh PB, Hu SC, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- Shubayev VI, Myers RR. Axonal transport of TNF-alpha in painful neuropathy: distribution of ligand tracer and TNF receptors. J Neuroimmunol. 2001;114:48–56. doi: 10.1016/s0165-5728(00)00453-7. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Tabuchi A, Nakaoka R, Amano K, Yukimine M, Andoh T, Kuraishi Y, Tsuda M. Differential activation of brain-derived neurotrophic factor gene promoters I and III by Ca2+ signals evoked via L-type voltage-dependent and N-methyl-D-aspartate receptor Ca2+ channels. J Biol Chem. 2000;275:17269–17275. doi: 10.1074/jbc.M909538199. [DOI] [PubMed] [Google Scholar]
- Taishi P, Churchill L, De A, Obal F, Jr., Krueger JM. Cytokine mRNA induction by interleukin-1beta or tumor necrosis factor alpha in vitro and in vivo. Brain Res. 2008;1226:89–98. doi: 10.1016/j.brainres.2008.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Matsumoto S. Contribution of activated interleukin receptors in trigeminal ganglion neurons to hyperalgesia via satellite glial interleukin-1beta paracrine mechanism. Brain Behav Immun. 2008;22:1016–1023. doi: 10.1016/j.bbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Tarsa L, Balkowiec A. Nerve growth factor regulates synaptophysin expression in developing trigeminal ganglion neurons in vitro. Neuropeptides. 2009;43:47–52. doi: 10.1016/j.npep.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarsa L, Balkowiec-Iskra E, Kratochvil FJ, III, Jenkins VK, McLean A, Brown AL, Smith JA, Baumgartner JC, Balkowiec A. Tooth pulp inflammation increases brain-derived neurotrophic factor expression in rodent trigeminal ganglion neurons. Neuroscience. 2010;167:1205–1215. doi: 10.1016/j.neuroscience.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:409–414. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Tumor necrosis factor alpha but not interleukin 1 beta mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci. 2006;26:143–151. doi: 10.1523/JNEUROSCI.4032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn AH, Gold MS. Ionic mechanisms underlying inflammatory mediator-induced sensitization of dural afferents. J Neurosci. 2010;30:7878–7888. doi: 10.1523/JNEUROSCI.6053-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovic L, Bockaert J, Jacque C. “Inflammatory” cytokines: neuromodulators in normal brain? J Neurochem. 2000;74:457–471. doi: 10.1046/j.1471-4159.2000.740457.x. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992;12:4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]