Abstract
Regional elevations in cerebral blood flow (CBF) often occur in response to localized increases in cerebral neuronal activity. An ever expanding literature has linked this neurovascular coupling process to specific signaling pathways involving neuronal synapses, astrocytes and cerebral arteries and arterioles. Collectively, these structures are termed the “neurovascular unit” (NVU). Astrocytes are thought to be the cornerstone of the NVU. Thus, not only do astrocytes “detect” increased synaptic activity, they can transmit that information to proximal and remote astrocytic sites often through a Ca2+-and ATP-related signaling process. At the vascular end of the NVU, a Ca2+-dependent formation and release of vasodilators, or substances linked to vasodilation, can occur. The latter category includes ATP, which upon its appearance in the extracellular compartment, can be rapidly converted to the potent vasodilator, adenosine, via the action of ecto-nucleotidases. In the present review, we give consideration to experimental model-specific variations in purinergic influences on gliovascular signaling mechanisms, focusing on the cerebral cortex. In that discussion, we compare findings obtained using in vitro (rodent brain slice) models and multiple in vivo models (2-photon imaging; somatosensory stimulation–evoked cortical hyperemia; and sciatic nerve stimulation-evoked pial arteriolar dilation). Additional attention is given to the importance of upstream (remote) vasodilation; the key role played by extracellular ATP hydrolysis (via ecto-nucleotidases) in gliovascular coupling; and interactions among multiple signaling pathways.
Keywords: adenosine, neurovascular unit, astrocyte, glia limitans, cerebral blood flow
1. Introduction
Coupling between neuronal activity and blood flow is fundamental to brain function. When a specific brain region is activated, cerebral blood flow (CBF) increases in a temporally and spatially coordinated manner. At the core of the coupling process is the neurovascular unit (NVU). In the context of CBF regulation, the three key components of the NVU are neurons, astrocytes, and arteries/arterioles. The general organization of the NVU reflects its function. That is, on one end of the structure, astrocytic endfeet extensively ensheath cerebral microvessels, such as arterioles. On the other end, astrocytes contact both pre- and post-synaptic elements (the “tripartite” synapse—see ref. [1]). The latter arrangement permits astrocytes to “sample” synaptic function. However, the NVU, and its ability to couple blood flow to synaptic activity, is far more complex than an organization consisting of “one synapse-one astrocyte-one arteriole”. In fact, astrocytes are thought to be arranged in physically non-overlapping domains [2]. As such, the astrocyte in each domain can have multiple contacts with neuronal synapses, with more than 105 (rodents) or 106 (humans) synaptic contacts per domain, in addition to an indeterminate number of arteriolar contacts. Furthermore, increased synaptic activity may promote vasodilation that can occur within a single astrocytic domain (local parenchymal arteriolar dilation), or may involve multiple domains resulting in upstream or “remote” vasodilation. Another regulatory function arising from remote astrocytic signaling is heterosynaptic modulation. Related to the common theme of this special issue,“Purinergic Signaling in Neurones and Glia”, purinergic factors play key roles in this remote signaling process, irrespective of whether the distal target of the astrocytic signaling conduit is an arteriole or a synapse [3] (see also fig. 1).
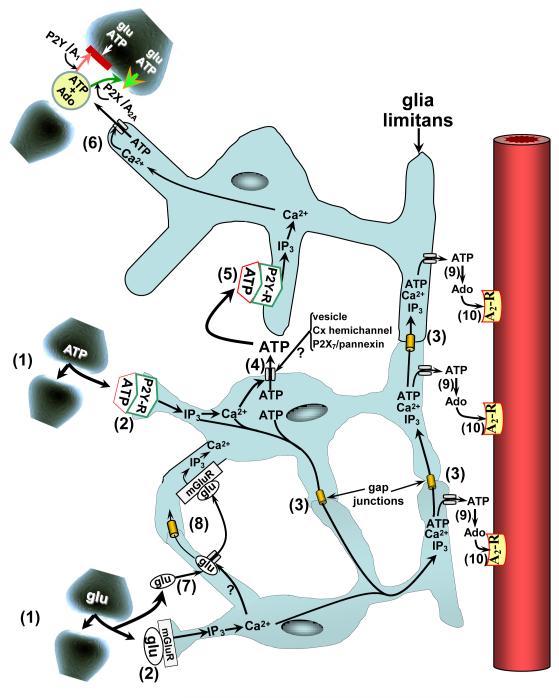
FIGURE 1.
Purine-linked coupling among components of the neurovascular unit (NVU). Neurovascular/gliovascular coupling begins with increased synaptic activity, represented by release of the neurotransmitters (gliotransmitters), ATP and glutamate (glu) (1). Engagement of metabotropic purinergic and glutamatergic receptors (P2Y-R and mGluR, respectively) on nearby astrocytes promotes PLC-mediated formation of IP3 and leads to Ca2+ release from intracellular stores (2). The Ca2+ and IP3 may be distributed among adjacent astrocytes and their processes either directly, via diffusion through intercellular gap junctional channels (3); or, indirectly, by Ca2+-dependent ATP release into the extracellular milieu via specialized release processes, including vesicles, connexin-linked hemichannels, and pannexin/P2X7-linked hemichannels (4). That ATP is then able to engage P2Y receptors on nearby astrocytes (5) resulting in elevations in cytosolic IP3 and Ca2+ levels in those cells. ATP also may move from astrocyte to astrocyte following the same gap junction diffusional routes as IP3 and Ca2+ (3). ATP efflux occurring in the vicinity of synapses (6) may lead to prevention (red bar) or enhancement (green arrowhead) of neurotransmitter release. This may occur via ATP interactions with P2Y or P2X receptors or, following ecto-nucleotidase-mediated conversion to adenosine (Ado), engagement of presynaptic A1 or A2A receptors [56]. Synaptic depression is thought to occur when P2Y and/or A1 receptors are involved; while facilitation may occur in association with P2X [57] and/or A2A [3] engagement. Following transporter-mediated uptake into astrocytes (7), glutamate may follow pathways similar to those of ATP (8). Efflux of ATP from the glia limitans should be accompanied by rapid ecto-nucleotidase-mediated formation of AMP, then Ado (9). Upon subsequent activation of pial arteriolar A2 receptors (10), vasodilation ensues (see fig. 2).
2. Signaling mechanisms within the NVU
Multiple mechanisms have been linked to astrocytes in their capacity to orchestrate cerebral vasodilation during increased synaptic activity (reviewed in refs. [2,4,5]). Vasodilation in response to enhanced synaptic activity can be attributed, either directly or indirectly, to release of paracrine substances from astrocytes arising in response to transient elevations in astrocyte intracellular Ca2+ concentrations. Those Ca2+-dependent paracrine substances have been found to include products of arachidonic acid metabolism via cyclooxygenases (e.g., PGE2) [6] or epoxygenases (e.g., epoxyeicosatrienoic acid regioisomers [EETs] [4,7,8]); K+ release through large conductance, Ca2+-operated K+ (BKCa) channels [9]; and Ca2+-dependent release of glutamate and ATP from astrocytes [10,11]. Glutamate and ATP also are released into the synaptic cleft during increased neural activity. Subsequently, these gliotransmitters, by virtue of their interactions with astrocytic glutamatergic and purinergic metabotropic receptors (fig. 1), may act as a primary trigger for the endoplasmic reticulum (ER)-derived Ca2+ increases in astrocytes.
3. In vitro models: Acute brain slice
The acute cortical brain slice often has been used in studies examining neurovascular coupling. Despite their limitations (reviewed in ref. [12]), much useful information has been derived using slice preparations. Slice studies most commonly involve examinations of the direct influence of astrocytes on intraparenchymal arteriolar diameters. In many cases, this entails “endfoot delimited” astrocytic signaling elicited by photolytic uncaging of endfoot Ca2+ [13] or inositol trisphosphate (IP3), with the latter yielding localized Ca2+ increases [14]. Other paradigms used in generating endfoot Ca2+ increases involved applications of a glutamate metabotropic receptor (mGluR) agonist [15] or direct electrical stimulation of neurons [15,16]. In the overwhelming majority of cases, those astrocytic Ca2+ elevations were accompanied by dilation of adjacent parenchymal arteriolar segments. In a few instances, the astrocytic Ca+2 elevations were associated with a vasoconstriction response [13]. It was suggested that the constriction was caused by a product of arachidonic acid metabolism via cytochrome P450 ω-hydroxylase (i.e., 20-HETE—see ref. [13]). However, an alternative explanation was that the vessels in the slice preparation were deficient in physiologic tone and, thus, were impaired in their ability to dilate further. It is important to emphasize that, in their “normal” physiologic state, these resistance vessels are pressurized, which imparts myogenic tone. In a subsequent report, it was shown that the normal dilating response of brain slice parenchymal arterioles to applications of a mGluR agonist, in the presence of added tone, were converted to vasoconstrictions in the absence of tone [17].
Purinergic ATP receptors (especially the P2Y2 and P2Y4 subtypes) are richly expressed in rat perivascular astrocytic endfeet [18]. In rat cortical brain slices, local application of ATP was accompanied by substantial mobilization of cytosolic Ca2+ in astrocytic endfeet at the gliovascular interface [18]. Although the arteriolar responses were not measured, based upon evidence from other laboratories (see above), one might anticipate that such Ca2+ increases would have elicited vasodilation.
Recently, Gordon et al [6] reported that reducing brain slice bath O2 concentrations from 95% to 20% prevented the vasoconstrictions that occur in response to the mGluR agonist, trans-1-aminocyclopentane-1,3-dicarboxylic acid (t-ACPD), endfoot Ca2+ uncaging, or electrical stimulation. The prevention of the vasoconstrictions in the lower O2 environment was, in part, attributable to the presence of adenosine acting via its A2A receptor. Interestingly, the 20% O2 bath solution yielded slice tissue PO2 levels (12-20 mmHg) that fell within the normal physiologic range (~10-30 mmHg) for rat cortical tissue in vivo [19]. Thus, 20% O2 may represent a normoxic environment, where increased formation of adenosine extracellularly reflects the normal physiologic response to the ATP release into the extracellular milieu arising from the stimuli listed above. However, there is another confounding factor that may have influenced findings, and that relates to the fact that these experiments were performed at room temperature. Electrical stimulation-evoked adenosine release in brain slices is profoundly temperature-sensitive [20]. Accordingly, caution is warranted in the interpretation of such findings.
Above, we touched upon a gliovascular coupling process that involves generation of PGE2 [15] and EETs [7] via the action of cyclooxygenase (COX) and epoxygenase, respectively. Additionally, K+-related mechanisms play a vital role. These processes rely on BKCa-linked K+ release from astrocytic endfeet at the gliovascular interface [21] coupled with the interaction of the released K+ with inward rectifier K+ (Kir) channels on arteriolar vascular smooth muscle. This leads to smooth muscle cell hyperpolarization and relaxation [9]. It is of interest to note that interactions may occur among gliovascular coupling processes. As one example, EETs may possess an autocrine influence in astrocytic endfeet, via promotion of increased BKCa function [7]. Based upon the presence of purinergic P2Y receptors in astrocytic endfeet, as well as a robust endfoot Ca2+ response to ATP application, purinergic influences on vascular tone in brain slice preparations seem likely.
4. In vivo models
4.1. In vivo models:Two-photon imaging
Takano et al. [22] employed in vivo two-photon laser-scanning microscopy in anesthetized mice to explore the vascular effects of Ca2+ uncaging in astrocyte endfeet directly adjacent to cortical penetrating arterioles. Within seconds of the photolytic event, arteriolar dilation was observed. The only agents which prevented the vasodilating response were blockers of arachidonic acid synthesis and COX-1, consistent with a primary role for arachidonic acid-derived PGE2. In contradistinction with results obtained in brain slices (see above) and multiple in vivo studies (see below), the response was not affected by epoxygenase, COX-2, or adenosine receptor blockade. Factors related to differences in experimental models (e.g., unique “qualities” of penetrating arterioles in vivo) and/or selectivity of pharmacologic agents used (see ref. [4]) could account for some of these disparate findings. Nevertheless, a more definitive explanation must await further study.
4.2. In vivo models: Somatosensory stimulation-evoked cortical hyperemia
Many studies have been published where neurovascular coupling mechanisms were identified based upon the reductions in cortical hyperemic responses to somatosensory activation (measured using laser-Doppler flowmetry) that occurred in the presence of selective pharmacologic blockers. Evidence from those investigations pointed to contributions from a number of neurovascular coupling pathways. This included arachidonic acid-linked pathways, namely, COX and epoxygenase [23,24]; metabotropic receptor-linked pathways (activated by glutamate or ATP [25]); pathways involving coupled participation of astrocyte BKCa and smooth muscle Kir channels [23]; adenosinergic mechanisms [25]; and NO-related influences [8].
Phospholipase A2, is well-expressed in astrocytes [26]. The protein or mRNA expression of arachidonic acid metabolizing enzymes, such as COX-1 [22] or the P4502C11 epoxygenase [27], respectively, is concentrated in perivascular astrocytic structures. However, despite COX-1 presence in perivascular astrocytic elements, neither selective inhibition of COX-1, nor COX-1 gene deletion, had any effect on whisker stimulation evoked increases in cortical blood flow in mice [28]. On the other hand, COX-2 inhibition and COX-2 gene deletion were both associated with an attenuated CBF response [24]. Constitutive COX-2 expression appears to be non-astrocytic, limited to neurons [22]. Multiple G-protein-coupled metabotropic purinergic P2Y receptor subtypes are well-represented in astrocytes (see ref. [18]), and appear to respond to the presence of ATP with increased arachidonic acid generation [26]. Metabotropic glutamate receptors (in particular, the mGluR5 isoform [29]) also display a strong expression in astrocytes and, like P2Y receptors, have been linked to increased PLA2 activity [30], as well as IP3 generation and increased Ca2+ mobilization [31]. The presence of BKCa channels within astrocytic endfeet [21], in association with Kir2.1 expression in cerebral vascular smooth muscle [32], is consistent with the “coupled K+ channel” model proposed by Nelson and co-workers (e.g., [9]). Furthermore, in rats studied in vivo, BKCa channel inhibition [33] and Kir-channel inhibition [23], in separate studies, were found to be associated with attenuated cortical blood flow increases during somatosensory activation.
A role for adenosine in the cortical CBF increases accompanying somatosensory activation (whisker stimulation) was reported many years ago [34]. Since that time, several publications (reviewed in refs. [4,8]) have confirmed these early results and have provided additional information regarding the important role of Gs-protein- linked adenylyl cyclase (AC)-activating adenosine A2A and A2B receptors in the coupling process. Both receptor subtypes are generally well-expressed in astrocytes and cerebral blood vessels [3]. However, compared to the A2A receptor, the A2B receptor shows a 30-40-fold lesser affinity toward adenosine [35]. Yet, pharmacologic blockade of the A2A receptor, with ZM 241385 (which shows an ~30-80-fold greater A2A vs A2B selectivity [36,37]), had no effect on whisker stimulation-evoked cortical hyperemia. On the other hand, in the presence of alloxazine, a blocker with modest A2B over A2A selectivity (<10-fold [35]), a significant attenuation of the CBF response to whisker stimulation was observed [25]. An explanation for the apparently greater role of A2B over A2A receptors in somatosensory stimulation-evoked cortical hyperemia could, perhaps, relate to greater A2B vs A2A influence in astrocytes [38].
Multiple pathways participate in cortical hyperemic responses to somatosensory activation (e.g., [2,4,23,34]). To a large degree, the influences of those pathways are non-additive and likely interactive. Overlapping effects of NOS inhibition, blockade of adenosine receptors, and epoxygenase inhibition have been widely observed [25,27,34]. In a recent paper, Leithner et al. [23] reported a reduction in forepaw stimulation-induced cortical hyperemia, in the presence of Kir blockade with Ba2+ alone, that was similar to the attenuation seen when Ba2+ was combined with inhibitors of nNOS, epoxygenase, adenosine receptors, and COX (non-selectively). This finding hinted at possible interactions between the targets of the inhibitor “cocktail” and Kir channels. Additional sites of interaction were revealed by Shi et al. [25] who found similar 40-60% reductions in whisker stimulation-evoked cortical hyperemia in the presence of EET, A2B, or mGluR blockade, either alone or in paired combinations. The non-additive nature of combined treatments with selective pharmacologic antagonists is also seen in studies involving sciatic nerve stimulation-induced pial arteriolar dilation. This is discussed in the following section.
5. Sciatic nerve stimulation (SNS)-evoked pial arteriolar dilation
5.1. Model for upstream vasodilation and gliovascular signaling
Upstream vasodilation is an important component of neurovascular coupling. One example of remote, upstream dilation would be the pial arteriolar dilation that occurs in the hindlimb region of the cerebral cortex during sciatic nerve stimulation (SNS) [39,40]. Pial arterioles represent the upstream segments of the parenchymal arterioles that lie in the vicinity of activated synapses [9]. In recent publications (e.g., ref. [40]), we reported astrocytes were a major conduit for transmitting vasodilating signals upstream to pial arterioles, with the glia limitans, the mantle of astrocytic processes overlying the cerebral cortex, representing the principal structural contact with the pial arterioles. A key strategy employed in investigations involving the model of SNS-evoked dilation of pial arterioles involved cortical surface applications of a highly specific astrocytic toxin, L-α-aminoadipic acid (L-AAA). It has been established that 5-24h exposure to topically-applied L-AAA [40-42]) selectively ablates the superficial glia limitans without directly damaging neuronal or pial vascular functions.
5.2. Inter-astrocytic communication in SNS-evoked pial arteriolar dilation
The presence of nerve fibers extending from the underlying neuropil to the vicinity of pial vessels is sparse. This “intrinsic innervation” may be limited to a few vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) nerve fibers [43]. However, that intrinsic innervation may have little direct influence on pial arteriolar responses to neural activation. Thus, in rats exposed to the gliotoxin, L-AAA, SNS-evoked dilations were essentially eliminated [40], without any effects on cerebral cortical electrical responses evoked by SNS [44]. These findings reflect evidence that L-AAA is only taken up into astrocytes, causing extensive damage [45], while being excluded from neurons and producing no changes in neuronal appearance or function [46]. L-AAA also has been shown to have no direct effects on pial arteriolar function [41]. The substantial repression of SNS-evoked pial arteriolar dilation in the presence of L-AAA, therefore, can only be attributed to loss of communication between the glia limitans and pial vessels.
In figure 1, we depict an astrocytic signaling pathway, where communication between astrocytes relies on ATP efflux from one cell and interaction with purinergic P2Y receptors on neighboring cells and passage of signaling molecules through intercellular gap junctions. Although some of the ATP-related signaling pathways may also apply to glutamate [47], we have chosen to limit the discussion to purinergic mechanisms. The signaling process is initiated by increased synaptic activity. The released ATP can interact with P2Y receptors on nearby astrocytes, leading to the mobilization of Ca2+ from intracellular stores. The intracellular Ca2+ rise can regenerate the Ca2+ signal in neighboring astrocytes by facilitating ATP cellular efflux via a variety of Ca2+-dependent ATP release processes. We show three possible release mechanisms. The first is vesicular release of ATP [48,49]. A second mechanism involves cellular ATP efflux via Cx43 hemichannels [50,51]. However, those findings were recently challenged by Iglesias et al. [52]. A third mechanism of ATP release from astrocytes is thought to involve pannexin-1 hemichannels, coupled with ionotropic purinergic P2X7 receptors. Thus, in conjunction with P2X7 activation, pannexin-1 is postulated to form large conductance ATP permeable channels [52,53]. However, whether this mechanism participates in neurovascular coupling in vivo is not entirely clear. In a preliminary study [54], we found no effect of topical applications of the selective P2X7 blocker, brilliant blue G (BBG, 100 nM), on SNS-evoked pial arteriolar dilations. But, with more intense synaptic activation (bicuculline-induced seizure), we noted >50% reductions in pial arteriolar dilations in the presence of BBG. One implication of these findings is that, if there is a P2X7/pannexin role in inter-astrocytic communication and gliovascular coupling, it may only contribute during states of excessively high levels of synaptic activity and extracellular ATP [55].
We recently reported [40], that Cx43 expression was particularly concentrated in the glia limitans, with limited expression in pial vascular endothelium, and no detectable expression in smooth muscle cells. The role of Cx43 in SNS-evoked pial arteriolar dilations was revealed through the use of topical applications of selective inhibitory peptides. Because Cx43 hemichannels may also be affected by these blocking peptides, one cannot completely ignore their possible contributions [51]. However, whether or not there is a role for Cx43 hemichannels in inter-astrocytic communication in vivo remains unsettled (see ref. [52]). These findings, at the least, indicate the participation of Cx43-related gap junctional communication in pial arteriolar responses to neuronal activation. Moreover, the finding that glial injury completely prevented the pial arteriolar response, but endothelial injury had no effect [40], suggests that the Cx43-containing sites involved in the pial response (gap junctions?) are more likely to be on astrocytes than blood vessels.
Calcium and IP3, along with ATP, can move from astrocyte-to-astrocyte via gap junctions. This can spread the ATP (and Ca2+) signals among astrocytic elements, including perivascular endfeet. As discussed earlier, the ATP may also exit the astrocyte via several Ca2+-dependent mechanisms. The released ATP has several fates. This includes interactions with G-protein-coupled P2Y receptors on adjacent astrocytes, contributing to the “Ca2+/ATP wave”. Second, ATP released in the vicinity of a synapse can interact with P2Y or ionotropic P2X receptors on presynaptic elements, or be hydrolyzed to adenosine by ecto-nucleotidases, with the adenosine interacting with presynaptic A1 or A2A receptors. Engagement of A1 and/or selected P2Y receptors (e.g., P2Y1, P2Y2, P2Y4) is often accompanied by diminished synaptic activity (sometimes labeled “heterosynaptic depression” [56]); while engagement of A2A, and/or a subset of P2X receptors (e.g., P2X1, P2X2/3, P2X3), can facilitate synaptic function [3,57]. As a third possibility, ATP and Ca2+ may diffuse into the glial endfeet comprising the glia limitans. The ATP may then undergo Ca2+-dependent efflux followed by rapid hydrolysis to adenosine, resulting in pial arteriolar dilation mediated by smooth muscle A2 receptors.
5.3. Glia limitans to pial arteriole communication: The key role of ATP “ecto-hydrolysis”
Ecto-nucleotidases are widely distributed in brain tissue and include: ectopyrophosphatases/phosphodiesterases (e-NPPs), ecto-nucleoside triphosphate diphosphohydrolases (e-NTPDases), and ecto-5′-nucleotidase [58] (see also contribution by Zimmermann in this issue). There is even evidence that ecto-nucleotidases may be concentrated at ATP release sites on astrocytes [59]. The reader should also be aware of potential influences arising from the intracerebral presence of the ectophosphatase, tissue non-specific alkaline phosphatase (TNAP). However, for the sake of brevity, no further discussion of the implications of documented expression of TNAP in cerebral neurons, vessels, and even glial cells [58,60] will be entertained. A consequence of this abundance of cell surface ecto-nucleotidases is that the appearance of ATP in the brain extracellular space may be short-lived, on the order of several hundred milliseconds (e.g., [61]). A rapid hydrolysis might explain why it has been difficult to detect extracellular ATP in brain tissue [57]. To mimic the effects of ATP release from the glia limitans that is postulated to occur in association with SNS, we examined, in a preliminary study, pial arteriolar responses to topical applications of ATP. It was found that ATP elicited a marked dose-dependent dilation that was substantially attenuated in the presence of a blocker of adenosine A2 receptors (ZM 241385) [62]. This finding is in accord with data published by Meno et al. who showed the importance of adenosine in SNS-evoked pial arteriolar dilations [63].
One implication of these findings is that ecto-ATPase activity (ATP → ADP conversion), at least at the cortical surface, is weak. In addition, ecto-ADPase function at the brain surface would appear to be negligible, in view of our findings showing that pial arteriolar dilations elicited by topical ADP suffusion were unaffected by administration of a non-selective adenosine receptor blocker [64]. This leaves us, then, with a mechanism that involves diphosphohydrolase-mediated ATP → AMP conversion, coupled with formation of adenosine from AMP via ecto-5′-nucleotidase. The two principal ecto-nucleotidases that seem to mediate direct ATP → AMP conversions are e-NTPDase-1 and e-NPP. Both are well-expressed in rodent brain blood vessels [58,65]. In contrast, ecto-5′-nucleotidase expression was reported to be negligible in rodent cerebral vascular tissue [58], but richly expressed in astrocytes [66]. Another experimental strategy one can use to examine ecto-nucleotidase pathways at the cortical surface involves monitoring pial arteriolar dilating responses to SNS in the presence of topically-applied ARL-67156, a purported blocker of ecto-apyrase (diphosphohydrolase) function [67]. As reported in a preliminary study [62], we observed an 80% reduction in pial arteriolar dilating response to SNS following ARL-67156 application. The rather marked effect of ARL-67156 on SNS-evoked pial arteriolar dilation suggested that a substantial portion of the adenosine contributing to that response derived from ecto-nucleotidase action, allowing little room for contributions from pre-formed adenosine released from cellular sources. To illustrate the above, figure 2 focuses on purinergic mechanisms at the astrocyte endfoot (glia limitans)-pial arteriole interface. Figure 2 also considers the possibility that, in addition to adenosine, AMP itself is an A2 receptor agonist, as previously reported [68], and more recently confirmed for pial arterioles in our laboratory (unpublished observations).
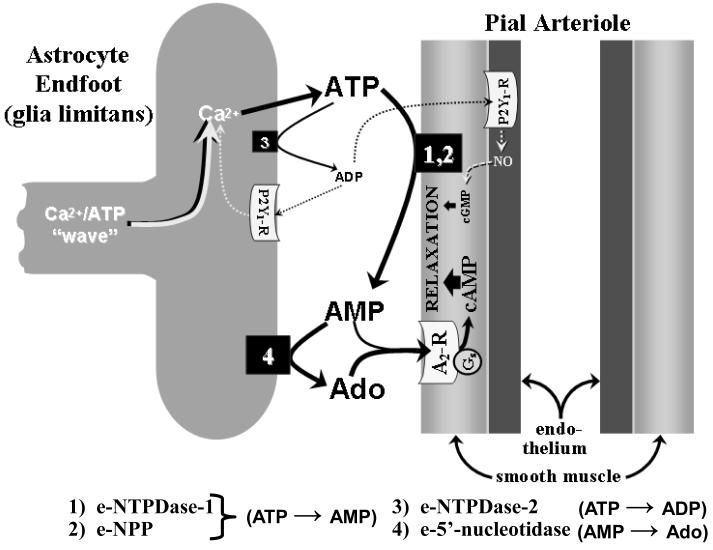
FIGURE 2.
Postulated events thought to occur following the arrival of a Ca2+/ATP “wave” at the glia limitans and the subsequent Ca2+-dependent ATP release from the glia limitans. Preliminary findings indicated that, when ATP is topically applied to the cortical surface (mimicking SNS-associated increased ATP release from the glia limitans [see fig. 1]), the ensuing dilation of pial arterioles is mediated by products of ATP hydrolysis, the first step being a rapid ATP conversion to AMP, mediated through the actions of ecto-nucleoside triphosphate dihosphohydrolase-1 (e-NTPDase-1 (1) and ecto-pyrophosphatase/phosphodiesterase (e-NPP) (2), which are found on the surfaces of vascular cells and, perhaps, astrocytes as well [72,73]. Most of the AMP is converted to adenosine (Ado) via ecto-5′-nucleotidase (4), mainly found on astrocytes [73]. Pial arteriolar dilation arises from the cAMP generated following activation of vascular smooth muscle Gs-linked A2 receptors (A2-R). The principal A2-R ligands are Ado and (to a lesser degree) AMP. If extracellular ATP elevations are sufficiently high, modest generation of ADP may occur, allowing some ATP→ADP (i.e., ecto-ATPase) activity to be manifested [74], for example, via astrocytic e-NTPDase-2 (3). ADP is known to dilate pial arterioles through activation of purinergic P2Y1 receptors found both on the glia limitans and on vascular endothelium [75], with the latter representing a minor pathway linked to NO and cGMP generation.
5.4. Glia limitans to pial arteriole communication: Multiple, interacting pathways
In a recent investigation [44], we examined potential interactions among adenosine A2 receptors, Kir channels and BKCa channels in SNS-induced pial arteriolar dilations. Similar to findings obtained in studies monitoring somatosensory-stimulation-evoked CBF responses, all three of these targets exhibited roughly equivalent (~60%), but non-additive, reductions in SNS-evoked pial arteriolar dilations in the presence of their respective inhibitors.
We then examined some potential mechanisms that might have contributed to the interdependence suggested by the above findings. First, we addressed an hypothesis whereby adenosine A2 receptor-linked AC activation gives rise to cAMP-dependent protein kinase (PKA)-mediated phosphorylations of BKCa channels and Kir channels, enhancing their function (see ref. [69]). In accord with that postulate, in the presence of selective PKA or AC blockade, we observed marked reductions in pial arteriolar responses to suffusions of adenosine, a Kir channel opener (6-12 mM K+), and a BKCa opener (NS-1619), as well as SNS. Thus, in what might be labelled as a “permissive” function, a basal level of adenosine-linked phosphorylation may need to be present for the Kir and BKCa channels to open in response to their activators, and to facilitate pial arteriolar reactivity to SNS. Second, only Kir channel blockade (with 100 μM Ba2+) was able to attenuate pial arteriolar dilations to suffusions of 6-12 mM K+, NS-1619, and adenosine. The sensitivity of the three dilators to Ba2+ implies a central role for Kir channels in the vasodilations arising from both BKCa channel opening and increased adenosine. The interactions between BKCa and Kir channels in our model are consistent with the relationships outlined by Nelson and co-workers [9], with K+ released through astrocytic BKCa channels on the glia limitans activating Kir channels on overlying pial arteriolar smooth muscle. The validity of the above pathway was bolstered by experiments involving L-AAA exposure. Thus, glia limitans ablation was associated with a substantial loss of NS-1619 (but not adenosine or K+ reactivity), implying the presence and importance of glia limitans BKCa channels, [44].
The pathways involved in the SNS-evoked pial arteriolar dilation model did not entirely recapitulate the somatosensory activation/cortical hyperemia model pathways discussed earlier. For example, we did not detect any role for NO in SNS-evoked pial arteriolar dilations [44], consistent with findings reported by Ngai et al. [70]. Also, we obtained preliminary data in 4 rats (not shown) indicating that epoxygenase blockade with miconazole (20 μM, given topically) does not alter SNS-evoked pial arteriolar dilations. It may be noteworthy that Ohata et al. [71] reported an absence of any effects of NOS or epoxygenase inhibition on pial arteriolar dilations elicited by AMPA suffusion. On the other hand, like SNS-evoked pial arteriolar relaxation, that arteriolar response exhibited a significant adenosine A2 receptor dependence.
6. Summary
Adenosine triphosphate plays a central role in coupling increased synaptic activity in the brain to increases in CBF. One key aspect of that signaling is inter-astrocytic communication, where ATP released from one astrocyte can activate metabotropic purinergic receptors on neighboring astrocytes, thereby promoting IP3-mediated Ca2+ release from cellular stores. Through repeating that process, or via diffusion of IP3 and Ca2+ through inter-astrocytic (gap junction) channels, local and remote increases in cytosolic Ca2+ levels in astrocytes can occur. This may then promote the formation and release of vasodilating substances at the interface between astrocytic endfeet and the arterioles that they contact. In addition, ATP may also diffuse from astrocyte-to-astrocyte via gap junctions, where it can emerge, via a Ca2+-dependent process, at the gliovascular interface, but instead of interacting with P2 receptors, the ATP is rapidly hydrolyzed by ecto-nucleotidases. These enzymes are widespread in the brain, but are differentially expressed on vascular cells and astrocytes. Each hydrolysis product of ATP (i.e., ADP, AMP, and adenosine) possesses strong vasodilating properties. However, evidence indicates that ATP ecto-hydrolysis occurs predominantly through ATP to AMP conversions mediated by e-NTPDase-1 and e-NPP. The AMP formed in this reaction is then dephosphorylated (via ecto-5′nucleotidase) to adenosine, which (along with any remaining AMP) interacts with arteriolar smooth muscle adenosine A2 receptors, promoting vasodilation. Monophosphohydrolase reactions, mediated by e-ATPases and e-ADPases, appear to play only minor roles in ATP hydrolysis and, by extension, the coupling between astrocytes and cerebral arterioles, at least at the cortical surface. Finally, a number of interactive pathways have been identified as participants in the process of neurovascular coupling. Which pathways predominate often is a function of the experimental model one uses. Nevertheless, with limited exceptions, an important role for purinergic factors has been indicated in multiple models of neurovascular/gliovascular coupling. This includes in vivo somatosensory stimulation-evoked cortical hyperemia or pial arteriolar dilation, in addition to gliovascular coupling in brain slice models. However, many more details of purinergic involvement need to be delineated. For example, one conundrum that should be addressed relates to the relative contributions to the intercellular signaling process that can be assigned to metabotropric P2 vs adenosinergic P1 receptors. That is, in light of the apparently rapid ecto-hydrolysis that occurs when ATP is released to the extracellular fluid, it is difficult to envisage any major role for intercellular communication based upon ATP release and interaction with P2 receptors. Perhaps there are regional variations in P2 and ecto-nucleotidase expression that permit both pathways to co-exist. This and many other issues remain to be explored in future studies.
ACKNOWLEDGMENTS
This work was supported by a grant from the American Heart Association (AHA-0635337N) and National Institutes of Health Grants, HL-088259 and NS-063279.
Abbreviations
- AC
adenylyl cyclase
- ASM
arteriolar smooth muscle
- BBG
brilliant blue G
- BKCa
large-conductance, Ca2+-operated K+ channel
- CBF
cerebral blood flow
- COX
cyclo-oxygenase
- Cx43
connexin 43
- EET
epoxyeicosatrienoic acid
- e-NPP
ecto-pyrophosphatase/phosphodiesterase
- ER
endoplasmic reticulum
- e-NTPDase
ecto-nucleoside triphosphate diphosphohydrolase
- GFAP
glial fibrillary acidic protein
- HETE
hydroxyeicosatetraenoic acid
- IP3
inositol trisphosphate
- Kir
inward-rectifier K+ channel
- L-AAA
L-α-aminoadipic acid
- mGluR
metabotropic glutamate receptor
- NOS
nitric oxide synthase
- PACAP
pituitary adenylate cyclase activating polypeptide
- PKA
cAMP-dependent protein kinase
- PKG
cGMP-dependent protein kinase
- PLA2
phsospholipase A2
- PLC
phospholipase C
- SNS
sciatic nerve stimulation
- t-ACPD
trans-1-aminocyclopentane-1,3-dicarboxylic acid
- VIP
vasoactive intestinal polypeptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Halassa MM, Fellin T, Haydon PG. Tripartite synapses: roles for astrocytic purines in the control of synaptic physiology and behavior. Neuropharmacology. 2009;57:343–6. doi: 10.1016/j.neuropharm.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–76. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- [3].Pelligrino DA, Xu HL, Vetri F. Caffeine and the Control of Cerebral Hemodynamics. J Alzheimers Dis. 2010;20(Suppl 1):S51–S62. doi: 10.3233/JAD-2010-091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–9. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- [5].Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–31. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- [6].Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, Macvicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–9. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Higashimori H, Blanco VM, Tuniki VR, Falck JR, Filosa JA. Role of epoxyeicosatrienoic acids as autocrine metabolites in glutamate-mediated K+ signaling in perivascular astrocytes. Am J Physiol Cell Physiol. 2010;299:C1068–C1078. doi: 10.1152/ajpcell.00225.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100:307–17. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dunn KM, Nelson MT. Potassium channels and neurovascular coupling. Circ J. 2010;74:608–16. doi: 10.1253/circj.cj-10-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bezzi P, Gundersen V, Galbete JL, Seifert G, Steinhauser C, Pilati E, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–20. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- [11].Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, et al. Exocytotic release of ATP from cultured astrocytes. J Biol Chem. 2007;282:28749–58. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- [12].Filosa JA. Vascular tone and neurovascular coupling: considerations toward an improved in vitro model. Front Neuroenergetics. 2010;2:1–8. doi: 10.3389/fnene.2010.00016. (article 16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mulligan SJ, Macvicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–9. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- [14].Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–6. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- [16].Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–e81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- [17].Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ndubuizu O, LaManna JC. Brain tissue oxygen concentration measurements. Antioxid Redox Signal. 2007;9:1207–19. doi: 10.1089/ars.2007.1634. [DOI] [PubMed] [Google Scholar]
- [20].Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci. 1999;19:1932–9. doi: 10.1523/JNEUROSCI.19-06-01932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Price DL, Ludwig JW, Mi H, Schwarz TL, Ellisman MH. Distribution of rSlo Ca2+− activated K+ channels in rat astrocyte perivascular endfeet. Brain Res. 2002;956:183–93. doi: 10.1016/s0006-8993(02)03266-3. [DOI] [PubMed] [Google Scholar]
- [22].Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–7. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- [23].Leithner C, Royl G, Offenhauser N, Fuchtemeier M, Kohl-Bareis M, Villringer A, et al. Pharmacological uncoupling of activation induced increases in CBF and CMRO2. J Cereb Blood Flow Metab. 2010;30:311–22. doi: 10.1038/jcbfm.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–70. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab. 2008;28:111–25. doi: 10.1038/sj.jcbfm.9600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, et al. Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol Neurobiol. 2005;31:27–41. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- [27].Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–17. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- [28].Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–8. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- [29].Haydon PG, Blendy J, Moss SJ, Rob JF. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse? Neuropharmacology. 2009;56(Suppl 1):83–90. doi: 10.1016/j.neuropharm.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Byrnes KR, Loane DJ, Faden AI. Metabotropic glutamate receptors as targets for multipotential treatment of neurological disorders. Neurotherapeutics. 2009;6:94–107. doi: 10.1016/j.nurt.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal-glial transmission. J Anat. 2007;210:651–60. doi: 10.1111/j.1469-7580.2007.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87:160–6. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- [33].Gerrits RJ, Stein EA, Greene AS. Ca(2+)-activated potassium (K(Ca)) channel inhibition decreases neuronal activity-blood flow coupling. Brain Res. 2002;948:108–16. doi: 10.1016/s0006-8993(02)02957-8. [DOI] [PubMed] [Google Scholar]
- [34].Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol Heart Circ Physiol. 1994;267:H296–H301. doi: 10.1152/ajpheart.1994.267.1.H296. [DOI] [PubMed] [Google Scholar]
- [35].Hinschen AK, Rose’Meyer RB, Headrick JP. Adenosine receptor subtypes mediating coronary vasodilation in rat hearts. J Cardiovasc Pharmacol. 2003;41:73–80. doi: 10.1097/00005344-200301000-00010. [DOI] [PubMed] [Google Scholar]
- [36].Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB. Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:7–10. doi: 10.1007/pl00005326. [DOI] [PubMed] [Google Scholar]
- [37].Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PWR, Jones G, et al. The in vitro pharmacology of ZM 241385, a potent, non- xanthine, a(2a) selective adenosine receptor antagonist. Br J Pharmacol. 1995;115:1096–102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alloisio S, Cugnoli C, Ferroni S, Nobile M. Differential modulation of ATP-induced calcium signalling by A1 and A2 adenosine receptors in cultured cortical astrocytes. Br J Pharmacol. 2004;141:935–42. doi: 10.1038/sj.bjp.0705707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol. 2001;281:H2018–H2027. doi: 10.1152/ajpheart.2001.281.5.H2018. [DOI] [PubMed] [Google Scholar]
- [40].Xu HL, Mao L, Ye S, Paisansathan C, Vetri F, Pelligrino DA. Astrocytes are a key conduit for upstream signaling of vasodilation during cerebral cortical neuronal activation in vivo. Am J Physiol Heart Circ Physiol. 2008;294:H622–H632. doi: 10.1152/ajpheart.00530.2007. [DOI] [PubMed] [Google Scholar]
- [41].Xu HL, Koenig HM, Ye S, Feinstein DL, Pelligrino DA. Influence of the glia limitans on pial arteriolar relaxation in the rat. Am J Physiol Heart Circ Physiol. 2004;287:H331–H339. doi: 10.1152/ajpheart.00831.2003. [DOI] [PubMed] [Google Scholar]
- [42].Leffler CW, Parfenova H, Fedinec AL, Basuroy S, Tcheranova D. Contributions of astrocytes and CO to pial arteriolar dilation to glutamate in newborn pigs. Am J Physiol Heart Circ Physiol. 2006;291:H2897–H2904. doi: 10.1152/ajpheart.00722.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fahrenkrug J, Hannibal J, Tams J, Georg B. Immunohistochemical localization of the VIP1 receptor (VPAC1R) in rat cerebral blood vessels: relation to PACAP and VIP containing nerves. J Cereb Blood Flow Metab. 2000;20:1205–14. doi: 10.1097/00004647-200008000-00006. [DOI] [PubMed] [Google Scholar]
- [44].Paisansathan C, Xu HL, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and potassium channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol. 2010;299:H2009–17. doi: 10.1152/ajpheart.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Khurgel M, Koo AC, Ivy GO. Selective ablation of astrocytes by intracerebral injections of alpha-aminoadipate. GLIA. 1996;16:351–8. doi: 10.1002/(SICI)1098-1136(199604)16:4<351::AID-GLIA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [46].Pow DV. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. GLIA. 2001;34:27–38. doi: 10.1002/glia.1037. [DOI] [PubMed] [Google Scholar]
- [47].Hamilton NB, Attwell D. Do astrocytes really exocytose neurotransmitters? Nat Rev Neurosci. 2010;11:227–38. doi: 10.1038/nrn2803. [DOI] [PubMed] [Google Scholar]
- [48].Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, et al. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–62. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- [49].Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol. 2007;129:485–91. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–11. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhes. 2003;10:251–7. doi: 10.1080/cac.10.4-6.251.257. [DOI] [PubMed] [Google Scholar]
- [52].Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte “hemichannels”. J Neurosci. 2009;29:7092–7. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Macvicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [54].Xu HL, Pelligrino DA. The role of the ionotropic P2X7 receptor in pial arteriolar dilations elicited by intense (seizure) and physiologic (sciatic nerve stimulation) neuronal activation. Soc Neurosci Abst. 2006 Program No. 117.9. [Google Scholar]
- [55].Sun SH. Roles of P2X7 receptor in glial and neuroblastoma cells: the therapeutic potential of P2X7 receptor antagonists. Mol Neurobiol. 2010;41:351–5. doi: 10.1007/s12035-010-8120-x. [DOI] [PubMed] [Google Scholar]
- [56].Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–82. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- [57].Dale N, Frenguelli BG. Release of adenosine and ATP during ischemia and epilepsy. Curr Neuropharmacol. 2009;7:160–79. doi: 10.2174/157015909789152146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res. 2008;334:199–217. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- [59].Joseph SM, Buchakjian MR, Dubyak GR. Colocalization of ATP release sites and ecto-ATPase activity at the extracellular surface of human astrocytes. J Biol Chem. 2003;278:23331–42. doi: 10.1074/jbc.M302680200. [DOI] [PubMed] [Google Scholar]
- [60].Brun-Heath I, Ermonval M, Chabrol E, Xiao J, Palkovits M, Lyck R, et al. Differential expression of the bone and the liver tissue non-specific alkaline phosphatase isoforms in brain tissues. Cell Tissue Res. 2010 doi: 10.1007/s00441-010-1111-4. DOI 10.1007/s00441-010-1111-4. [DOI] [PubMed] [Google Scholar]
- [61].Dunwiddie TV, Diao LH, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–82. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Xu HL, Pelligrino DA. ATP release and hydrolysis contribute to rat pial arteriolar dilatation elicited by neuronal activation. Exp Physiol. 2007;92:647–51. doi: 10.1113/expphysiol.2006.036863. [DOI] [PubMed] [Google Scholar]
- [63].Meno JR, Nguyen TS, Jensen EM, Alexander WG, Groysman L, Kung DK, et al. Effect of caffeine on cerebral blood flow response to somatosensory stimulation. J Cereb Blood Flow Metab. 2005;25:775–84. doi: 10.1038/sj.jcbfm.9600075. [DOI] [PubMed] [Google Scholar]
- [64].Xu HL, Santizo RA, Koenig HM, Pelligrino DA. Chronic estrogen depletion alters adenosine diphosphate-induced pial arteriolar dilation in female rats. Am J Physiol Heart Circ Physiol. 2001;281:H2105–H2112. doi: 10.1152/ajpheart.2001.281.5.H2105. [DOI] [PubMed] [Google Scholar]
- [65].Braun N, Sevigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, et al. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur J Neurosci. 2000;12:4357–66. [PubMed] [Google Scholar]
- [66].Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol Res. 2005;27:153–60. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]
- [67].Muller CE, Iqbal J, Baqi Y, Zimmermann H, Rollich A, Stephan H. Polyoxometalates--a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg Med Chem Lett. 2006;16:5943–7. doi: 10.1016/j.bmcl.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [68].Ross FM, Brodie MJ, Stone TW. Adenosine monophosphate as a mediator of ATP effects at P1 purinoceptors. Br J Pharmacol. 1998;124:818–24. doi: 10.1038/sj.bjp.0701890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. J Smooth Muscle Res. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- [70].Ngai AC, Meno JR, Winn HR. L-NNA suppresses cerebrovascular response and evoked potentials during somatosensory stimulation in rats. Amer J Physiol Heart Circ Physiol. 1995;38:H1803–H1810. doi: 10.1152/ajpheart.1995.269.5.H1803. [DOI] [PubMed] [Google Scholar]
- [71].Ohata H, Cao S, Koehler RC. Contribution of adenosine A2A and A2B receptors and heme oxygenase to AMPA-induced dilation of pial arterioles in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R728–R735. doi: 10.1152/ajpregu.00757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zimmermann H. Biochemistry, localization and functional roles of ectonucleotidases in the nervous system. Prog Neurobiol. 1996;49:589–618. doi: 10.1016/0301-0082(96)00026-3. [DOI] [PubMed] [Google Scholar]
- [73].Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp. 2006;276:113–28. [PubMed] [Google Scholar]
- [74].Duarte-Araujo M, Nascimento C, Timoteo MA, Magalhaes-Cardoso MT, Correia-de-Sa P. Relative contribution of ecto-ATPase and ecto-ATPDase pathways to the biphasic effect of ATP on acetylcholine release from myenteric motoneurons. Br J Pharmacol. 2009;156:519–33. doi: 10.1111/j.1476-5381.2008.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Xu HL, Ye S, Baughman VL, Feinstein DL, Pelligrino DA. The role of the glia limitans in ADP-induced pial arteriolar relaxation in intact and ovariectomized female rats. Am J Physiol Heart Circ Physiol. 2005;288:H382–H388. doi: 10.1152/ajpheart.00727.2004. [DOI] [PubMed] [Google Scholar]