Abstract
A remarkable feature of prion biology is that the same prion protein can misfold into more than one infectious conformation and these conformations, in turn, lead to distinct heritable prion strains with different phenotypes. The yeast prion [PSI+] is a powerful system for studying how changes in the strain conformation affect cross-species transmission. We have previously established that a chimera of the Saccharomyces cerevisiae (SC) and Candida albicans (CA) Sup35 prion domains can cross the SC/CA species barrier in a strain-dependent manner. In vitro, the conversion of the monomeric chimera to the prion (amyloid) form can be seeded by either SC or CA Sup35 amyloid fibers, resulting in two strains: Chim[SC] and Chim[CA]. These strains have a “molecular memory” of their originating species in that Chim[SC] preferentially seeds conversion of SC Sup35, and vice versa. To investigate how this species specificity is conformationally encoded, we used amide exchange and limited proteolysis to probe the structures of these two strains. We found that the amyloid cores of Chim[SC] and Chim[CA] are predominantly confined to the SC- and CA-derived residues respectively. In addition, the chimera is able to propagate the Chim[CA] conformation even when the SC residues that comprise the Chim[SC] core were deleted. Thus the two strains have non-overlapping and modular amyloid cores that determine whether SC or CA residues are presented on the growing face of the prion seed. These observations establish how conformations determine the specificity of prion transmission and demonstrate a remarkable plasticity to amyloid misfolding.
Keywords: [PSI+], prion strain, prion species barrier, protein misfolding, hydrogen/deuterium exchange
Prions, originally postulated to explain transmissible spongiform encephalopathies1, also underlie a number of epigenetic elements in fungi and perhaps in higher organisms2. Arguably the best studied of these elements is the yeast prion [PSI+], which results from self-propagating aggregates of the Sup35 translation termination factor3. Although Sup35 and the mammalian prion protein PrP have unrelated amino acid sequences, both proteins misfold into ordered, beta-sheet rich amyloid fibers. The self-templating nature of amyloid fibers allows the prion state to be stably propagated through the continual binding and conversion of newly-synthesized soluble proteins to the prion form4–7. Sup35 amyloid fibers, when introduced into yeast cells via protein transformation, induce cells to convert to the [PSI+] state with high efficiency8–10. These studies provided direct demonstration of the “protein-only” prion hypothesis and established amyloid as the infectious form of the Sup35 protein.
Remarkably, a single prion protein can adopt a spectrum of amyloid conformations that lead to heritable strain variants11,12. These strain variants manifest as distinct pathological symptoms in mammalian prions and as differences in the strength and stability of heritable phenotypes in yeast prions including [PSI+]. Beyond causing phenotypic differences, strains can also have different propensities for crossing “species barriers”12–15, which inhibit the transmission of prions between species, even those with closely related prion proteins16–18. The central relationship between strains and species barriers has been underscored by “new variant” Creutzfeld-Jacob disease (“mad cow disease”), which is attributed to a strain of bovine spongiform encephalopathy with an enhanced ability to cross the species barrier to humans12,19,20. In the case of [PSI+], distinct conformations of Sup35 can be formed in vitro by altering the polymerization conditions such as temperature. When introduced into yeast, these conformations can induce different prion strains, establishing that heritable differences in prion strain variants are enciphered within the conformation of the infectious protein9,10. The ability to relate the physical properties of synthetic prions to their biological effects provides a critical tool for exploring basic principles of prion inheritance, including how changes in a prion's conformation alter its ability to template or “seed” the polymerization of Sup35 from other species. As a result, [PSI+] has been particularly valuable for exploring the relationship between prion strains and species barriers13,14,21.
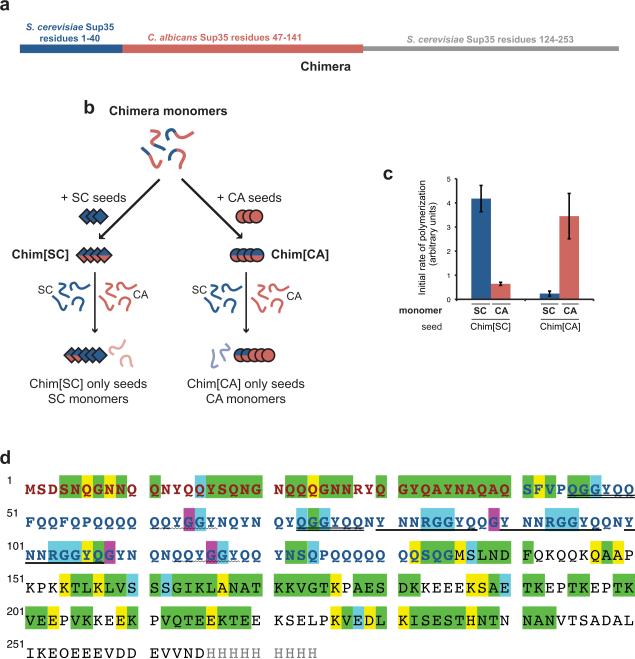
Previously we found that a chimeric Sup35 (Chimera; Fig. 1a) constructed by replacing residues 41–123 of Saccharomyces cerevisiae (SC) Sup35 with the corresponding residues 47–141 from Candida albicans (CA) could cross the SC/CA species barrier in a strain-dependent manner13,14. Even though Chimera was originally created to identify a minimal region of SC Sup35 required for self-recognition18, the conversion of Chimera to the prion form could be seeded by both SC and CA Sup35. When seeded by SC Sup35, Chimera forms a strain we call Chim[SC] and this strain readily seeds SC but not CA Sup35 (Fig. 1b,c). In contrast, seeding Chimera with CA Sup35 results in the Chim[CA] strain, which preferentially seeds conversion of CA Sup35. Thus, the Chim[SC] and Chim[CA] strains retain a molecular memory of their seed. Elegant peptide array experiments by Tessier and Lindquist revealed that short peptides of Sup35 could drive polymerization of Chimera into the amyloid form21. Chimera preferentially interacted with peptides derived from SC or CA Sup35 at temperatures14 that favored Chim[SC] or Chim[CA] polymerization, respectively. The above studies established that the conformation of each Chimera prion form dictates seeding specificity, presumably by presenting different regions of the protein on the growing amyloid face. Nonetheless, the nature and extent of these conformational differences and how they alter seeding specificity remain largely unexplored.
Figure 1.
Chimera sequence and assignments
(a) Schematic diagram of Chimera. Chimera and all other proteins in this study are expressed as the prion domain indicated followed by the SC Sup35 middle domain and a C-terminal 7xhistidine or 9xhistidine tag not shown in the schematic13,18. Proteins were purified as described29. These proteins do not include the Sup35 C-terminal domain that confers the translation termination function because it is not required for amyloid formation in vitro. (b) Cartoon representation of the seeding specficity of Chim[SC] and Chim[CA]. (c) Species specificity of Chim[SC] and Chim[CA]. Amyloid fibers were formed and assayed for species specificity as previously described14. Chim[SC] and Chim[CA] were formed by two rounds of Chimera polymerization, with 5% seeding (w/w) by preformed SC or CA fiber seeds as indicated. Resulting fibers contain no more than 0.25% of SC or CA seed. To take advantage of previous work characterizing structural features of defined SC strains23, Chim[SC] was seeded by SC fibers formed at 4°C. Chim[CA] was formed at 37°C to optimize stability of species specificity. Error bars represent s.e.m. for 3 or 5 replicates. (d) Chimera assignments. SC Sup35 residues 1–40 are red; CA 47–141 are blue; SC 124–253 are black. Exact repeats of 5 or more residues are underlined, except the two polyglutamine repeats at residues 57–62 and 126–132. Green highlighting indicates assigned residues with distinct NMR peaks, blue indicates assigned residues that exactly overlap another peak, and yellow indicates assigned residues that are in crowded areas and thus cannot be distinguished. G64/G116 and G89/108 could be mapped ambiguously to pairs of residues respectively but not further resolved; these residues have been highlighted in purple.
Hydrogen exchange reveals reciprocal regions of protection
To address these questions, we used amide hydrogen/deuterium exchange (HX) coupled to multidimensional NMR22,23 to probe the conformations of Chim[SC] and Chim[CA]. HX NMR can provide atomic-level information about which residues are involved in stable hydrogen bonds, including those that comprise the structural core of amyloid fibers. Here, uniformly 15N-labeled fibers are placed in a D2O-containing buffer to allow exchange of solvent-accessible hydrogens. After quenching, the fibers are dissolved in DMSO to a monomeric form amenable to the collection of high-resolution spectra. Exchange of the backbone amide hydrogen of a given residue results in a decrease in the signal of the corresponding peak in the 2D 15N-HSQC spectrum.
Analysis of the data requires assignment of the spectrum of Chimera, which is challenging due to the extensive glutamine stretches and multiple sequence repeats in the CA domain. By transferring existing assignments from SC Sup3523 and assigning additional peaks with seven three-dimensional NMR experiments on uniformly 13C-,15N-labeled Chimera, we succeeded in assigning 142 residues (Fig. 1d). This includes 30 of the first 40 SC-derived residues (Ch1–40) and 33 of the CA-derived residues (Ch41–135). With these assignments, we were able to monitor protection from exchange at many residues throughout the prion domain on Chim[SC] and Chim[CA] fibers after 1 minute, 1 hour, and 1 day of exchange.
The regions most protected from exchange in Chim[SC] and Chim[CA] were nearly perfectly reciprocal (Figs. 2 and 3a). The residues most strongly protected in Chim[SC] were those derived from the SC Sup35 prion domain (Ch1–40), while the residues most strongly protected in Chim[CA] were CA-derived. This immediately suggested that the amyloid cores of Chim[SC] and Chim[CA] were radically different. Furthermore, the protected region in Chim[SC] resembled that of the seeding SC conformation (see legend to Fig. 1a) which has a compact core limited to the first 40 residues23. Consistent with previous studies9,23,24, the Sup35 middle domain, Ch136–265, which is not essential for prion behavior25, showed minimal protection from exchange indicating that it was easily solvent-accessible and thus relatively unstructured (Fig. S1).
Figure 2.
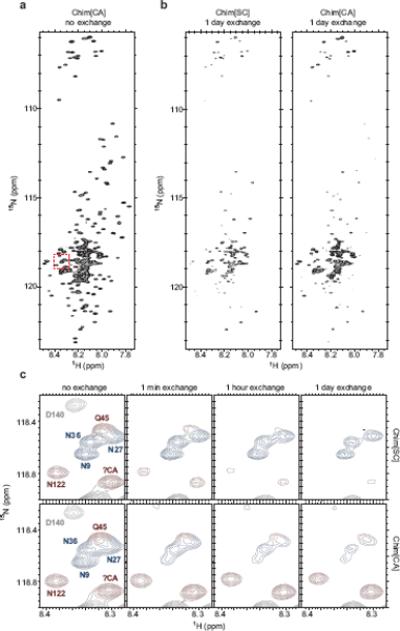
Hydrogen/deuterium exchange of Chimera fibers
Hydrogen/deuterium exchange and NMR on 15N-labeled Chim[SC] and Chim[CA] fibers was performed as previously described23 with the following modification. Rather than pelleting fibers by ultracentrifugation and resuspending fibers into D2O-containing buffer to start the exchange, freshly prepared 15N-Chimera fibers were first concentrated using an Amicon Ultra-15 centrifugal filter unit with Ultracel-30 membrane (Millipore), then diluted 12.5-fold into the equivalent buffer in D2O at pH 7.0 to start the exchange. Time points were taken at 0 minutes, 1 minute, 1 hour, and 1 day of exchange. Exchange was quenched by adjusting pH to 2.5 with DCl. (a) 15N-HSQC spectrum for Chim[CA], no exchange. Chim[SC] spectrum appears very similar. Red dashed box indicates residues shown in (c). (b) Spectra for Chim[SC] and Chim[CA], 1 day exchange. After 1 day of exchange, the same spectra for Chim[SC] and Chim[CA] qualitatively reveal large differences from the no exchange spectra and from each other. (c) A subset of residues (indicated by red box in (a)) from Chim[SC] and Chim[CA] after no exchange and 1 minute, 1 hour, and 1 day of exchange. Peaks are colored according to assignments: blue, Ch1–40 (SC-derived); red, Ch41–135 (CA-derived); gray, Ch136–253 (middle domain). Because most residues at the boundaries of the SC and CA segments of Chimera were assigned, a number of unassigned peaks (including the peak denoted as ?CA) could be identified as originating from CA residues due to the lack of a corresponding peak in SC spectra (see Supplementary methods for details on peak assignments).
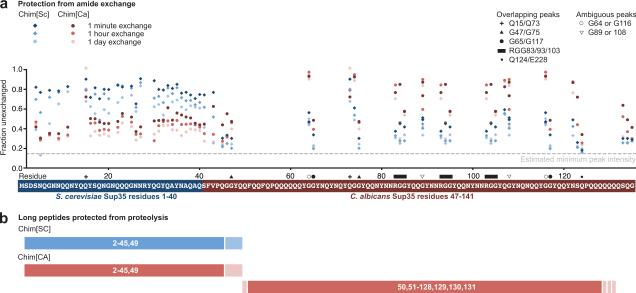
Figure 3.
Dramatically different regions of Chim[SC] and Chim[CA] are protected from amide exchange and proteolysis
(a) Amide exchange data for Chim[SC] and Chim[CA] mapped to residue location. Intensities for all assigned and distinct peaks in the Chimera prion domain are plotted as a fraction of the unexchanged intensity. Estimated minimum peak intensity (dotted line) is calculated based on maximum exchange observed in the middle domain (Fig. S1). For overlapping peaks, values represent the combined intensities. For ambiguous peaks, intensities of both peaks are plotted. (b) Limited proteolysis of Chim[SC] and Chim[CA]. Chimera amyloid fibers (5 μM, 1 ml) in 5 mM potassium phosphate, 150 mM NaCl, pH 7.5 were digested with proteinase K (1.5 μg/ml) at room temperature for 2 hours. After the amyloid solution was ultracentrifuged at 214,000g for 30 min, the supernatant was removed and the pellet was washed with 1 ml of buffer and ultracentrifuged again. The pellet was dissolved in 100 μl DMSO (similar results were found with 6 M guanidine HCl, 25 mM Tris, pH 7.5). For the MALDI-TOF MS measurement, the dissolved peptides were desalted by NuTip C4 (Glygen) and analyzed with Microflex (Bruker Daltonics). As a matrix, we used 3,5-dimethoxy-4-hydroxycinnamic acid. Identification of peptides was performed using the program PAWS (ProteoMetrics). Summary of peptides identified are schematically diagrammed here.
Limited proteolysis confirms differences in amyloid cores
We also used limited proteolysis to identify the protease-resistant core26 of Chim[SC] and Chim[CA]. Long protected peptides that persist during digestion with proteinase K were found to be a reliable method for distinguishing distinct amyloid strain conformations of SC Sup35 (M.T., unpublished observations). Chim[SC] and Chim[CA] were digested, pelleted by ultracentrifugation, and dissolved in DMSO. The resulting peptides were identified by MALDITOF mass spectrometry. Consistent with our amide exchange results, the only long peptides observed in Chim[SC] following proteinase K treatment spanned residues 2–45 and 2–49 (Figs. 3b and S2). In contrast, digestion of Chim[CA] revealed long protected peptides derived from residues 50–131 in addition to peptides spanning residues 2–49.
Proteolysis confirmed the differences observed by HX in the protected regions of Chim[SC] and Chim[CA], but both conformations had protease-resistant peptides spanning Ch1–40. This protease protection could indicate that Chim[CA] has an expanded amyloid core that comprises almost the entire prion domain. Alternatively, these residues could be protected from proteolysis due to other factors such as secondary aggregation or partial structure while not being essential for the structural integrity of the amyloid core.
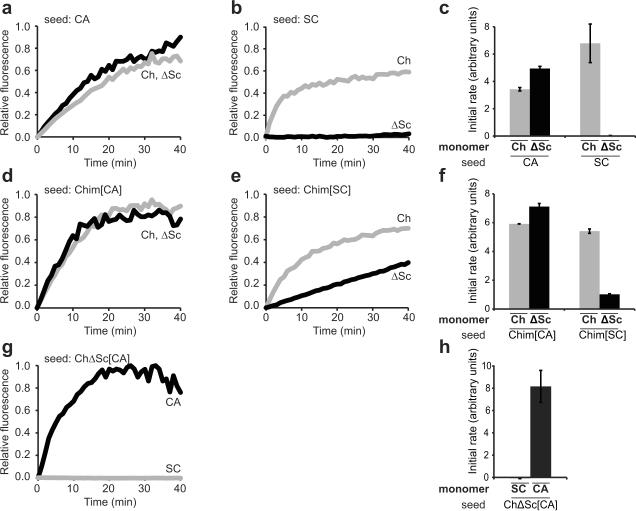
Chim[CA] does not require SC-derived residues 1–40
To test whether the protection observed in Ch1–40 was structurally critical for the Chim[CA] core, we created a mutant Chimera without these SC-derived residues (ChΔSc). ChΔSc retained the ability to polymerize into an amyloid form and this polymerization was efficiently seeded by CA and Chim[CA] but not by SC or Chim[SC] (Figs. 4a–c and 4d–f). Moreover, when seeded by CA, the resulting amyloid conformation (ChΔSc[CA]) exhibited the same species specificity as Chim[CA] (Fig. 4g,h). Taken together, the above studies argue that the minimal structural core required for propagation of the Chim[CA] conformation is composed solely of CA residues and is thus distinct from that of Chim[SC].
Figure 4.
Chim[CA] does not require SC-derived Ch1–40 residues
Sonicated fibers were added to monomers as indicated, and polymerization was monitored by an increase in ThioflavinT fluorescence. Data were normalized to initial and final intensities, initial time points were fit to a line, and the slope was calculated as the initial rate of polymerization. Representative normalized kinetic traces are shown. Error bars represent s.e.m. for 2–5 replicates. (a, b, c) Chimera and ChΔSc monomers were seeded by CA and SC fibers. ChΔSC, constructed with standard molecular cloning techniques and verified by sequencing, is identical to the His-tagged Chimera with the exception of deleted residues 2–40. (d, e, f) Chimera and ChΔSc monomers were seeded by Chim[SC] and Chim[CA] fibers. (g, h) SC and CA monomers were seeded by ChΔSC[CA] fibers (compare to Chim[CA] in Fig. 1b).
Conclusions
Here we provide a structural explanation for earlier studies that established that the strain conformation of Chimera determines its seeding specificity for SC or CA Sup35. Specifically, we show that Chimera adopts two radically different conformations depending on the templating species. These two conformations have largely non-overlapping amyloid cores that are restricted to the species-specific region of Chimera, and are consistent with the locations of the short nucleating sequences identified by peptide array21. Although both the SC- and CA-derived segments of Chimera are amyloidogenic, templating Chimera in one region appears to prevent amyloid formation in the other. Our observations provide a model for how the “molecular memory” of the species origin of the templating seed is recorded in the amyloid conformation of Chimera.
That a single polypeptide can form such radically different prion conformations substantially extends our view of the plasticity of protein misfolding. This together with related work showing structural diversity in amyloid formation27,28, emphasizes the inherent challenges of structural studies of prions and other amyloids, where subtle differences in polymerization conditions or the underlying peptide can result in dramatic changes in the resulting conformation. In addition, it also underscores the challenges in preventing the polymerization of such fibers. Given the multiplicity of nucleating sequences in amyloidogenic proteins, therapeutic strategies that selectively direct the energy landscape to favor less toxic amyloid conformations may be more successful than those that seek to abolish polymerization altogether.
Supplementary Material
Acknowledgements
We would like to thank B. Toyama for insightful discussions and experimental advice, and K. Tipton, L. Goins, A. Robinson, E. Chow and members of the Weissman lab for critical reading of the manuscript. This work was funded by the Howard Hughes Medical Institute and the National Institutes of Health (J.S.W.) and a National Science Foundation Graduate Research Fellowship (C.K.F.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement The authors declare no competing financial interests.
References
- 1.Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shorter J, Lindquist S. Prions as adaptive conduits of memory and inheritance. Nat Rev Genet. 2005;6:435–450. doi: 10.1038/nrg1616. [DOI] [PubMed] [Google Scholar]
- 3.Wickner RB. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 4.Caughey B, Baron GS, Chesebro B, Jeffrey M. Getting a Grip on Prions: Oligomers, Amyloids, and Pathological Membrane Interactions. Annu Rev Biochem. 2009;78:177–204. doi: 10.1146/annurev.biochem.78.082907.145410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 6.Greenwald J, Riek R. Biology of Amyloid: Structure, Function, and Regulation. Structure. 2010;18:1244–1260. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Tuite MF, Cox BS. Propagation of yeast prions. Nat Rev Mol Cell Biol. 2003;4:878–890. doi: 10.1038/nrm1247. [DOI] [PubMed] [Google Scholar]
- 8.Sparrer HE, Santoso A, Szoka FC, Weissman JS. Evidence for the prion hypothesis: induction of the yeast [PSI+] factor by in vitro- converted Sup35 protein. Science. 2000;289:595–599. doi: 10.1126/science.289.5479.595. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 10.King C-Y, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- 11.Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW. Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics. 1996;144:1375–1386. doi: 10.1093/genetics/144.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. doi: 10.1126/science.1138718. [DOI] [PubMed] [Google Scholar]
- 13.Chien P, Weissman JS. Conformational diversity in a yeast prion dictates its seeding specificity. Nature. 2001;410:223–227. doi: 10.1038/35065632. [DOI] [PubMed] [Google Scholar]
- 14.Chien P, DePace AH, Collins SR, Weissman JS. Generation of prion transmission barriers by mutational control of amyloid conformations. Nature. 2003;424:948–951. doi: 10.1038/nature01894. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka M, Chien P, Yonekura K, Weissman JS. Mechanism of cross-species prion transmission: an infectious conformation compatible with two highly divergent yeast prion proteins. Cell. 2005;121:49–62. doi: 10.1016/j.cell.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Chernoff YO, et al. Evolutionary conservation of prion-forming abilities of the yeast Sup35 protein. Mol Microbiol. 2000;35:865–876. doi: 10.1046/j.1365-2958.2000.01761.x. [DOI] [PubMed] [Google Scholar]
- 17.Kushnirov VV, Kochneva-Pervukhova NV, Chechenova MB, Frolova NS, Ter-Avanesyan MD. Prion properties of the Sup35 protein of yeast Pichia methanolica. EMBO J. 2000;19:324–331. doi: 10.1093/emboj/19.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoso A, Chien P, Osherovich LZ, Weissman JS. Molecular basis of a yeast prion species barrier. Cell. 2000;100:277–288. doi: 10.1016/s0092-8674(00)81565-2. [DOI] [PubMed] [Google Scholar]
- 19.Bruce ME, et al. Transmissions to mice indicate that `new variant' CJD is caused by the BSE agent. Nature. 1997;389:498–501. doi: 10.1038/39057. [DOI] [PubMed] [Google Scholar]
- 20.Hill AF, et al. The same prion strain causes vCJD and BSE. Nature. 1997;389:448–450. 526. doi: 10.1038/38925. [DOI] [PubMed] [Google Scholar]
- 21.Tessier PM, Lindquist SL. Prion recognition elements govern nucleation, strain specificity and species barriers. Nature. 2007;447:556–561. doi: 10.1038/nature05848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino M, et al. Mapping the core of the beta(2)-microglobulin amyloid fibril by H/D exchange. Nat Struct Biol. 2002;9:332–336. doi: 10.1038/nsb792. [DOI] [PubMed] [Google Scholar]
- 23.Toyama BH, Kelly MJS, Gross JD, Weissman JS. The structural basis of yeast prion strain variants. Nature. 2007;449:233–237. doi: 10.1038/nature06108. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan R, Lindquist SL. Structural insights into a yeast prion illuminate nucleation and strain diversity. Nature. 2005;435:765–772. doi: 10.1038/nature03679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J-J, Sondheimer N, Lindquist SL. Changes in the middle region of Sup35 profoundly alter the nature of epigenetic inheritance for the yeast prion [PSI+] Proc Natl Acad Sci USA. 2002;99(Suppl 4):16446–16453. doi: 10.1073/pnas.252652099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sajnani G, Pastrana MA, Dynin I, Onisko B, Requena JR. Scrapie prion protein structural constraints obtained by limited proteolysis and mass spectrometry. J Mol Biol. 2008;382:88–98. doi: 10.1016/j.jmb.2008.06.070. [DOI] [PubMed] [Google Scholar]
- 27.Sawaya MR, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 28.Toyama BH, Weissman JS. Amyloid Structure: Conformational Diversity and Consequences. Annu Rev Biochem. in press doi: 10.1146/annurev-biochem-090908-120656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DePace AH, Santoso A, Hillner P, Weissman JS. A critical role for amino-terminal glutamine/asparagine repeats in the formation and propagation of a yeast prion. Cell. 1998;93:1241–1252. doi: 10.1016/s0092-8674(00)81467-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.