Abstract
Background
The URECA study was established to investigate the immunologic and environmental causes of asthma in inner-city children.
Objective
To evaluate potential atopic outcomes in the first12 months and their relationships to environmental exposures and immune development.
Methods
A birth cohort of 560 children with at least one parent with allergy or asthma was established in Baltimore, Boston, New York, and St. Louis. Wheezing is assessed every 3 months, allergen-specific IgE yearly, mononuclear cell cytokine responses at birth and yearly; environmental assessments include dust allergen and endotoxin, maternal stress, and indoor nicotine and nitrogen dioxide.
Results
Key outcomes in the first year include wheeze in 49%, ≥2 episodes of wheeze 23%, eczema 30%, and detectable IgE to milk, egg, and/or peanut in 32% and to cockroach in 4%. Household dust revealed levels >2mcg/g to cockroach in 40%, mite 19%, cat 25%, and mouse 29%, and 66% of homes housed at least one smoker. Positive associations were detected between multiple wheeze and cotinine, maternal stress, and maternal depression, while cytokine responses to a variety of innate, adaptive, and mitogenic stimuli were inversely related to eczema.
Conclusions
This high risk cohort of inner-city infants is exhibiting high rates of wheeze, eczema, and allergic sensitization. Low cytokine responses at birth may be a risk factor for eczema, while a variety of adverse environmental exposures contribute to the risk of wheezing in infancy. These findings provide evidence of specificity in the interactions between immune development, environmental exposures, and the development of early features that may predict future asthma.
Keywords: Immune development, birth cohort, atopy, asthma, cytokines, allergen exposure, inner-city
Introduction
Inner-city children and adults have disproportionately high asthma prevalence; in addition, those with asthma have greater morbidity and are more likely to die of their asthma compared to their non-inner-city peers 1-4 A number of studies over the past two decades have identified risk factors for asthma morbidity in inner-city patients, and a few have sought to identify reasons for increased asthma prevalence in these populations.5-8 Most have focused on environmental exposures that are increased or even unique to inner-city areas. The urban environment presents a unique collection of potentially harmful exposures, such as rodent and cockroach allergens, air pollution, increased stressful life events, infections, and microbial exposures.9-12 While much has been learned about how these environmental exposures affect asthma morbidity among inner-city children, there is relatively little information about the role that allergens and other environmental exposures encountered in the inner city play in the initiation of asthma.
It is likely that a complex interplay of genetic and environmental factors lead to the development of asthma and other allergic diseases through effects on both the airways and immune development, not only during early childhood but during fetal life. Although published data are not completely consistent in this regard,13 the most common immunologic abnormalities detected early in life among children that go on to develop asthma include diminished IFN-γ production, and surprisingly, reduced Th2 responses that are followed by skewing of immune responses towards a Th2 phenotype.14-17
In an effort to better understand the pathogenesis of asthma and allergy in children living in urban environments, the Urban Environment and Childhood Asthma (URECA) study was established by the Inner-City Asthma Consortium in 2005. This birth cohort study is designed to assess the influence of a multitude of environmental factors on immune development and the development of allergy and asthma. In this paper, we report data from the prenatal period through 12 months of age with regard to environmental exposures, immune development, and early outcomes that may be predictive of future asthma.
Methods
The design, methods, and study population of the URECA study have been previously reported in detail.18 This is a prospective, observational birth-cohort study examining environmental, lifestyle, and genetic factors that may influence early childhood immunologic development and subsequent asthma risk. The study was approved by Institutional Review Boards at each participating institution. Expectant families were recruited during pregnancy in 4 cities: Baltimore, Boston, New York, and St. Louis; written informed consent was obtained prior to enrollment. Selection criteria for the main cohort included residence in an area with >20% residents below the poverty level, mother or father with allergic rhinitis, eczema, and/or asthma, birth at ≥ 34 weeks gestation, and collection of a suitable cord blood sample. Exclusion criteria included conditions and congenital anomalies that could potentially affect lung or immune system development or function. Between February 2005 and March 2007, 1850 families were screened, 889 met eligibility criteria, and 560 were enrolled.
Each mother underwent a prenatal study visit (60% in the third trimester of pregnancy, most of the remainder in the second) at which nine stress-related questionnaires were administered. These included the Pregnancy Anxiety Scale (PAS), which was developed and validated as a measure of situation-specific stress related to pregnancy;19 the Edinburgh Postnatal Depression Scale (EPDS), developed in England to assist primary care health professionals in detecting those mothers suffering from postnatal depression (http://www.hfs.illinois.gov/mch/edinburgh.html); and the Perceived Stress Scale (PSS), a four-item scale that measures the degree to which the respondents felt their lives were unpredictable, uncontrollable and overwhelming in the preceding one month (reliability = 0.85).20 These measures can all be considered to represent internal stress. We have also created a composite measure of external stressors, which includes Difficult Life Circumstances (i.e. stressful life events),21 financial strain, neighborhood violence,22 and housing problems.23
Cord blood samples were collected at the time of the child's birth using sterile procedures; they were transported to the center's laboratory on the day of collection and kept at room temperature. Blood mononuclear cells were stimulated and tested for cytokine responses.24 After incubation for 24 hrs (phytohemagglutinin, LPS, poly-IC, CpG, peptidoglycan, respiratory syncytial virus, medium alone) or 5 days (cockroach extract, dust mite [D. pteronyssinus] extract, tetanus toxoid, medium alone), supernatants were collected, divided into aliquots, frozen at -80°C, and shipped to a central laboratory for analysis. Supernatants were analyzed for cytokines with a bead-based multiplex assay (Beadlyte, Upstate Biotechnology, Lake Placid, NY), producing a panel of 46 stimulant-cytokine outcomes (plus controls) per child. Cytokines were selected based on involvement with specific innate and adaptive immune responses previously related to allergic inflammation and immune response to respiratory viruses.24
Postnatally, telephone surveys were administered every 3 months to assess the child's respiratory and allergy symptoms, medications, tobacco smoke exposure, and diet. In addition, at 3 months of age, URECA staff visited the child's home where a home environment questionnaire was administered, and household dust samples were collected and assayed for the allergens Bla g 1 (German cockroach), Can f 1 (dog), Fel d 1(cat), Der f 1 Dermatophagoides farinae), Der p 1 (Dermatophagoides pteronyssinus), and Mus m 1 (mouse) by two-site monoclonal antibody ELISA (Indoor Biotechnologies, Inc., Charlottesville, VA). Another aliquot was analyzed for endotoxin by the recombinant factor C assay25 and for ergosterol, a cell wall component of fungi, using GC-mass spectroscopy. Finally, at 12 months of age all infants were seen by a study physician in the research clinic, during which a physical examination was performed, an eczema assessment was completed, and a blood sample was collected to assess sensitization to common allergens.
Primary outcomes in the first 12 months include wheeze (categorized as zero episodes, a single reported episode, or two or more episodes), eczema / atopic dermatitis, and allergic sensitization. The primary source of information on the occurrence and frequency of wheezing was the Respiratory and Allergy Symptoms questionnaire administered via telephone every three months. Information on wheezing was also collected during phone calls at the time of illnesses, from records of hospitalizations due to respiratory illnesses, and from physical examinations at scheduled study visits. Atopic dermatitis was assessed by questionnaire every 3 months by asking if a health care provider ever diagnosed eczema and whether medication was prescribed for the condition. In addition, at the 3-month home visit and the child's 12-month physical examination, a nurse or physician completed the Eczema Area and Severity Index (EASI) form.26 We report both self-reported eczema and that diagnosed by physical exam, but for all analyses limit the eczema diagnoses only to those infants with an EASI score greater than or equal to 1.0 at either the 3 or 12 month exam. Finally, allergic sensitization at age 12 months was defined as the presence of one or more positive (≥0.35 kU/L) serologic tests for allergen-specific IgE, which was measured by ImmunoCAP (Phadia, Uppsala Sweden) to milk, egg, peanut, and cockroach.
Statistical Analysis
Univariate and multivariate analyses were performed using logistic regression for binary outcomes, and both unadjusted and adjusted odds ratios were calculated. For continuous outcomes, univariate and multivariate analyses were performed using linear regression and parameter estimates were calculated. Multivariate models were adjusted for season of birth, site, and gender of child.27 These variables were selected as confounders as they were found in earlier work to be associated with cytokine responses.28 The figures present results from adjusted models. All statistical analyses were carried out using R 2.8.1 and SAS 9.1.
Factor analysis with an orthogonal (varimax) rotation was used to reduce the 46-item cytokine panel to 11 composite factor scores, or linear combinations of the original, correlated, stimulant-specific cytokine responses. The factors were identified separately for the innate and adaptive panels of stimulant-cytokine combinations and without including unstimulated responses. After examination of eigenvalues and factor loadings, we identified seven factors for the innate panel, some of which clustered by cytokine and some of which clustered by stimulant (see Table E1 in the online repository). Four factors were identified for the adaptive panel. One factor was created for the IFN-γ, IL-10 and IL-13 responses to PHA, while 3 others clustered according to cytokine (e.g. IFN-γ response across the adaptive stimulants [cockroach, dust mite, and tetanus toxoid]).
In order to avoid making all the means equal (the default in factor analysis) where there were important differences across cytokine responses (e.g. IFN-γ responses to LPS were qualitatively lower than IFN-γ responses to CpG), we rescaled the responses for each cytokine to a 0-5 scale where all non-detectable responses were 0 and the range of detectable responses across all stimulants was calculated and divided into 4 levels of equal size in absolute pg/ml (on the log scale). A score was calculated for each of the 7 innate and 4 adaptive factors by averaging the values of the stimulant/cytokine responses that were included in each factor. Due to the rescaling, each child gets a score for each factor that ranges from 0 to 5.
Results
Clinical Outcomes
Five hundred fifteen of the original 560 infants (92%) completed the 12 month clinic visit. Demographics of the entire study population have been reported previously and those pertinent to the infants followed through 12 months are summarized in Table 1. The population was predominantly black (71%) or Hispanic (19%), and a majority of the mothers were young, with a median age of 23 years (range 13 – 42 years), unmarried (85%), and had a high school education or less (75%).
Table 1. Participant Characteristics.
| Total | 515 | |
|---|---|---|
| Mother's age at child's birth (years median, range) | 23 | 13-42 |
| Race or ethnicity of mother (n, %) | ||
| Hispanic of any race | 99 | 19.3 |
| Black alone | 363 | 70.9 |
| White alone | 20 | 3.9 |
| More than one race | 20 | 3.9 |
| All others | 10 | 2.0 |
| Missing | 3 | 0.01 |
| Maternal education (n, %) | ||
| Less than high school | 212 | 41.4 |
| High school | 175 | 34.2 |
| More than high school | 125 | 24.4 |
| Missing | 3 | 0.01 |
| Mother Married (n, %) | 63 | 14.8 |
| Household income <$15,000/yr (n, %) | 334 | 68.3 |
| Type of delivery (n, %) | ||
| Vaginal | 352 | 68.3 |
| C-section | 163 | 31.7 |
| Sex is Male (n, %) | 271 | 52.6 |
| Gestational age (weeks median, range) | 39 | 34-42 |
| Breastfed at Birth (n, %) | 289 | 57.2 |
| Breastfed at 3 Months (n, %) | 121 | 24.0 |
| Maternal Smoking (n, %) | ||
| Pregnancy | 91 | 17.8 |
| First year of life | 206 | 40.0 |
| Cotinine Level (ng/ml median, range) | 2 | 2-197 |
| Maternal Stress (median, range) | ||
| Difficult Life Circumstances Score | 4 | 0-16 |
| Edinburgh Postnatal Depression Score | 7 | 0-30 |
| Pregnancy Anxiety Score | 1.7 | 1-4 |
| Perceived Stress Score | 6 | 0-15 |
| Any wheeze (n, %) | 254 | 49.3 |
| Single wheeze | 134 | 26.0 |
| Multiple wheeze (≥ 2 wheezing episodes) | 120 | 23.3 |
| EASI ≥ 1 at 3 or 12 month clinic visit (N=392) (n, %) | 118 | 30.1 |
| Eczema reported or diagnosed – physical exam (n, %) | 241 | 46.8 |
| Positive allergen-specific IgE (N=354) (n, %) | ||
| Any allergen | 115 | 32.5 |
| Any food | 112 | 31.6 |
| Milk | 78 | 22.1 |
| Egg | 70 | 19.8 |
| Peanut | 44 | 12.5 |
| Cockroach | 15 | 4.3 |
Assessment of the primary outcomes during the first 12 months revealed a report of wheeze in 49%, with 26% reporting only a single episode of wheeze and 23% 2 or more episodes of wheeze (maximum = 4). Eczema was reported or diagnosed on physical exam in 47% with mean and maximum EASI scores at age 12 months of 0.9 and 16.2, respectively, while 30% had evidence of eczema at the 3-month home visit or the 12-month clinic visit together with an EASI score ≥ 1.0. Allergic sensitization, defined as an allergen specific IgE ≥0.35 kU/L to milk, egg, peanut, and/or cockroach, was detected in 33% of the 354 infants who had sufficient blood drawn at their 12 month visit (milk 22%, egg 20%, peanut 13%, any food 32%, and cockroach 4%). In those with detectable results, median and maximum specific IgE levels were 1.4 and 32.9 kU/L for milk, 1.6 and 34.5 kU/L for egg, 1.8 and 55.7 kU/L for peanut, and 0.7 and 28.6 for cockroach.
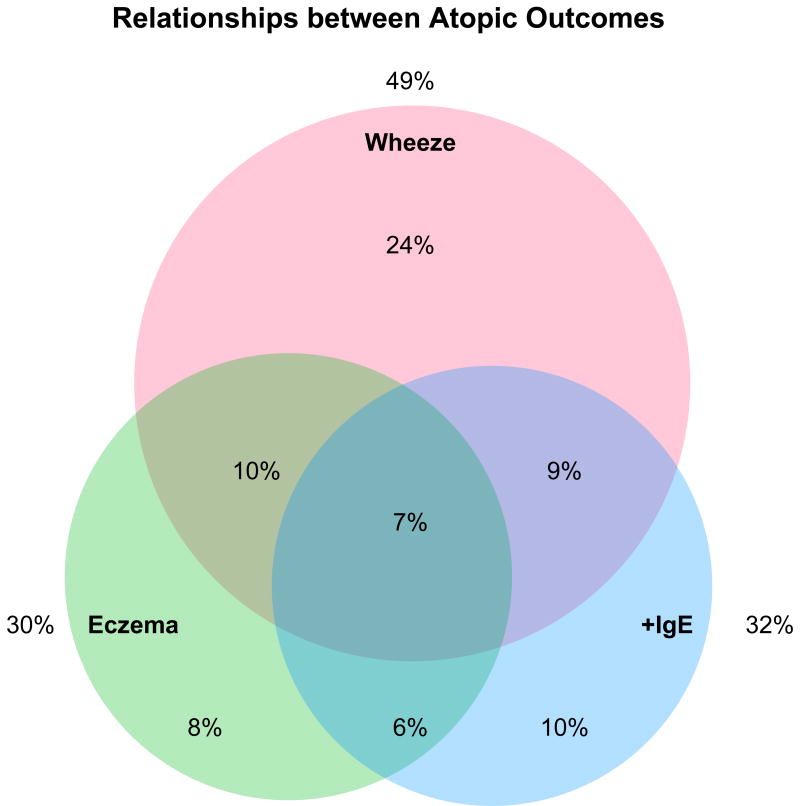
Analysis of overlap between the three primary outcomes found that, of those who had complete data (n=332), 10% had both wheeze and eczema, 9% had wheeze plus allergic sensitization, 6% had sensitization plus eczema, and 7% had wheeze, sensitization, and eczema (Figure 1). In assessing the relationships between these three outcomes, significant associations were detected between eczema and sensitization (p=0.005).No associations were found between eczema and any, single, or multiple episodes of wheeze or between sensitization and any, single, or multiple episodes of wheeze.
Figure 1.
Relationships between the major atopic outcomes. Includes those who have IgE data and EASI scores recorded at 3 or 12 months (n=332). Those who have no atopic outcomes (n=87, 26%) are included in the calculation of the percentages, but are not displayed in the Venn diagram. Significant associations were detected between eczema and sensitization (p=0.005).No associations were found between eczema and any, single, or multiple wheeze or between positive IgE and any, single or multiple episodes of wheeze.
Environmental Exposures
Environmental assessment conducted at 3 months of age revealed that most homes had detectable levels of cockroach, mouse, mite, cat, and dog allergens, and that significant exposure (>2 U or mcg/g) was found to cockroach in 40%, mite in 19%, cat in 25%, and mouse in 29% (Table 2). Similarly, both endotoxin and ergosterol were detected in every home with median levels of 36.7 and 3.6 ng/mg, respectively. Assessment of environmental tobacco smoke exposure revealed that the mother reported smoking in pregnancy in 18%, cord blood cotinine was detectable in 17% of cases (median of those detectable 47 ng/ml, range 3.4 to 197), and 66% of homes had at least one smoker during the child's first 12 months. Other relevant exposures included a household N02 >40 ppb in 13%.
Table 2. Environmental exposures (bedroom dust sample age 3 months).
| N | % detected | Range | Median | % >2 | % > 8 | |
|---|---|---|---|---|---|---|
| Bla g 1 (U/g) | 481 | 54.9 | BD – 183 | 0.7 | 39.5 | 23.7 |
| Mus m 1 (ng/g) | 488 | 98.6 | BD – 554,907 | 403.5 | 29.1 | 11.5 |
| Der f 1 (ng/g) | 486 | 68.9 | BD – 129,571 | 181.0 | 19.1 | 9.9 |
| Der p 1 (ng/g) | 482 | 16.0 | BD – 65839 | BD | 1.7 | 0.4 |
| Fel d 1 (ng/g) | 484 | 84.9 | BD – 837,935 | 419.0 | 25.4 | 16.7 |
| Can f 1 (ng/g) | 481 | 71.3 | BD – 424769 | 154.0 | 13.3 | 7.3 |
| rFC (endotoxin) | 443 | 100.0 | 0.12 – 2842 | 36.7 | NA | NA |
| Ergosterol (ng/mg) | 442 | 99.9 | 0.1 – 154 | 3.6 | NA | NA |
BD=Below detection
NA=Not applicable
Key measures of stress and depression collected prenatally revealed a median Edinburgh Postnatal Depression Score (EPDS) of 7 (range 0-30 with 25% >12), a median Pregnancy Anxiety Score (PAS) of 1.71 (range 1-4), and a median Perceived Stress Scale (PSS) of 6 (0-15). The composite External Stress Score had a median of 6 (3-9).
Relationships between Environmental Exposures and Outcomes
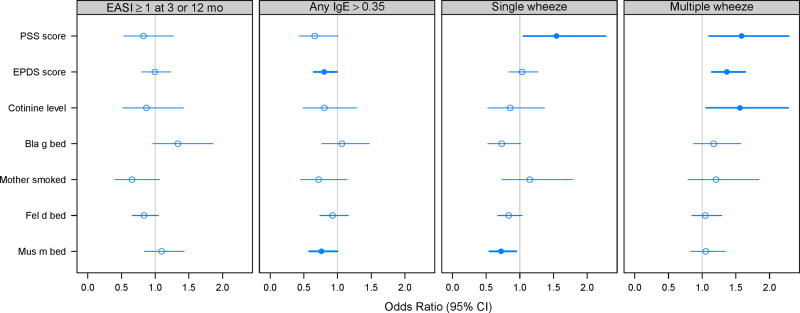
Analyses were performed to determine whether environmental exposures were related to the clinical outcomes (Figure 2). For single report of wheeze, a weak but significant inverse association was found with mouse allergen exposure and a significant positive association was found with maternal stress. For multiple (≥2) reports of wheeze, significant positive associations were found for cotinine, maternal stress, and maternal depression. Allergic sensitization was negatively associated with mouse allergen exposure and maternal depression (Figure 2 and Table E2 in the online repository). There were no significant relationships between environmental exposures and eczema.
Figure 2.
Relationships between atopic outcomes and environmental exposures. Bold lines and filled circles represent statistical significance in models adjusted for site, season of birth, and gender of child. PSS=Perceived Stress Score, EPDS=Edinburgh Postnatal Depression Scale (mean of first-year measurements; OR for 5-level increase). ORs for cotinine and allergens are given for a 1-log increase.
Relationships between Cord Blood Cytokine Responses and Atopic Outcomes
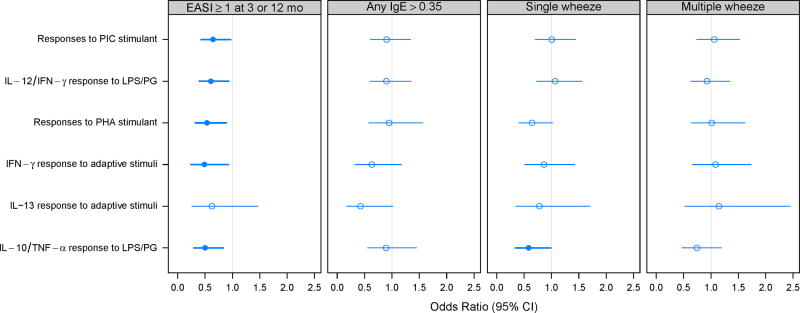
Cord blood cytokine profiles were most strongly predictive of eczema. In the univariate analysis, eczema was inversely related to a number of cytokine responses (Table E3). After adjustment for confounders, there were persistent inverse relationships between eczema and responses to LPS/PG (IL-12/IFN-γ and IL-10/TNF-α), PIC, and PHA, along with IFN-γ responses to adaptive stimuli (Figure 3). For single wheeze, there was a significant negative association with IL-10/TNF-α responses to LPS/PG, and a similar trend for PHA-induced responses. There were no significant relationships between cord blood cytokine responses and the risk of developing allergic sensitization or multiple wheeze.
Figure 3.
Relationships between atopic outcomes and cord blood cytokine profiles. Bold lines and filled circles represent statistical significance in models adjusted for site, season of birth, and gender of child. Cytokine factors are scaled to 5 levels where 0=non-dectectable, and 1-4 represent quartiles of the detectable levels. ORs are given for a 1-step increase.
Relationships between Cord Blood Cytokine Responses and Environmental Exposures
Lastly, to determine whether there was evidence that maternal exposures during pregnancy influenced prenatal immune development, we tested for associations between environmental exposures and cord blood cytokine profiles (see Table E4 in the online supplement). Both endotoxin and ergosterol exposures were associated with reduced IL-8 responses, and endotoxin exposure was also negatively associated with IL-10/TNF-α responses to LPS/PG. In addition, ergosterol was positively associated with response to CpG and with IFN-γ responses to adaptive stimuli. Other than ergosterol and endotoxin, there were few significant associations.
Discussion
The URECA study provides a unique opportunity to study early immune development in inner-city infants at risk for the development of asthma, and most importantly to assess the relationships between environmental exposures, immune development, and the development of asthma and allergy. At 12 months of age, we detected high rates of potential atopic disease in these infants, with relatively little overlap among the three main outcomes. Although a broader allergen panel may have produced different results, this finding is consistent with the fact that many of those with wheeze will not go on to develop asthma, and that not all eczema, especially when mild, is a true atopic manifestation. Panels of cytokine responses were analyzed at the time of birth as an indicator of immune development; innate responses were well developed, and nascent responses to adaptive stimuli were also detected. Notably, cytokine responses were inversely related to the risk of eczema, while environmental factors such as exposure to mice and maternal stress were related to allergic sensitization and wheezing. Furthermore, there was evidence that maternal exposure to measures of microbial exposure (endotoxin and ergosterol) influenced the development of cytokine responses even before the time of birth.
While the rates of wheeze are similar to those reported in other studies, the incidence of both eczema and allergic sensitization are considerably higher than those reported in most prior studies.10, 29-37 Differences in definition of eczema limit the comparison to previous reports. For example, in another study of inner-city children by Perzanowski et al, while 42% of infants wheezed in the first year, a parental report of doctor diagnosed eczema was found in 22%.38 Although no study of inner-city children has reported sensitization this early in life, one study did report that 28% of at risk infants had positive skin tests to food or inhalant allergens at 12 months,29 while in a large German study of high risk children the prevalence of serologic sensitization at 12 months was only 11%.30, 31 These results suggest that rates of persistent asthma and allergy will be quite high in the URECA population later on in childhood.
Adverse exposures detected in the participants' households were common and are typical of those found in other inner city studies such as the National Cooperative Inner-City Asthma Study (NCICAS) where cockroach allergen was detected in 85% of homes and mouse allergen was detectable in 95% of the homes.39 Tobacco exposure was high among the URECA cohort with 18% of the URECA mothers reporting smoking during pregnancy versus 11% reported on birth certificates in 2002.40 In addition, the measures of stressful life events were high. More importantly, however, are the unique opportunities provided by this study to assess the relationships of these environmental exposures to both clinical and immunologic outcomes from birth onward.
Of the environmental factors assessed, exposure to mice, smoking, and maternal stress or depression were most closely associated with clinical outcomes at 12 months of age. Mouse exposure was nearly ubiquitous, and was associated with both single wheeze and allergic sensitization. These findings suggest that mouse exposure is one of the most important urban exposures relative to early, potentially atopic outcomes, although these results contradict those of a Boston birth cohort in which mouse exposure was associated with wheeze in the first year.5 Notably, cockroach exposure was not associated with early outcomes. Our findings contrast with published findings linking cockroach exposure to wheezing in infancy,41 and it will important to reassess this relationship over time. Tobacco exposure and measures of both maternal depression and stress were associated with wheeze in both the univariate and multivariate analyses. While the effects of tobacco smoke exposure on asthma development have long been recognized, the potential effects of the mother's psychological state on the developing immune system clearly deserves further study.
Analysis of the relationships between cord blood cytokine profiles and early atopic outcomes revealed several significant relationships. One pattern that stood out was that the risk of developing eczema was associated with reduced cytokine responses to a variety of innate, adaptive, and mitogenic stimuli. Several previous studies have linked low-level polyclonal or antigen-specific cord blood IFN-γ responses,16, 42-44 or increases in Th2-like cytokine responses,45, 46 to the subsequent development of atopic dermatitis. Our data provide new evidence to show that reduced cytokine responses to microbial cell wall components (LPS, PG) and nucleic acid (bacterial DNA or viral RNA) increase the risk of eczema. These findings raise the possibility that deficient innate antimicrobial responses (perhaps involving TLR-3, TLR-4, and TLR-9 pathways) contribute to the increased susceptibility of children with eczema to infections with bacteria such as Staphylococcus, and Herpes viruses. These findings also suggest the presence of global factors that influence the expression of many different cytokines at birth, and also contribute to the risk for eczema. In contrast to our findings, Prescott et al. reported that higher IL-6 and TNF-α responses to several TLR were associated with an increased risk for developing allergic manifestations (food allergy, atopic dermatitis, or allergic sensitization) by age 1 year.47 It difficult to compare these studies given the use of a combined outcome, together with differences in cell processing (cryopreservation vs. fresh cells) and specific cytokine outcomes.
The availability of cord blood cytokine analysis on all subjects provided the opportunity to study the potential effects of environmental exposures on in utero immune development. While the environmental assessments, aside from measures of maternal stress and tobacco use, were not undertaken until shortly after birth, we assume they are reasonable surrogates for those occurring during pregnancy. In that regard, there were significant negative associations of exposure to both ergosterol and endotoxin with specific innate immune responses. Prenatal exposures to farming environments and pets have previously been associated with enhanced cord blood responses to mitogen and TLR stimulation.47, 48 Our findings are more suggestive of impaired innate immune responses, but still lend support to the concept that environmental exposures during pregnancy can influence immune development in the fetus.28, 47 The clinical significance of these associations, if any, will become clearer as we follow these infants into childhood.
We recognize a number of limitations, especially at this early stage of the study. The population is unique in being high risk, so the results may not be generalizable, although the exposures we have detected are likely very representative of other inner-city environments. More importantly, we have conducted a very large number of tests for multiple correlated exposures and outcomes and recognize that some of the associations we have detected could be due to chance. Considering the immunologic responses, the cytokine responses to dust mite and cockroach extracts were of low magnitude, and since the extracts are complex mixtures of proteins and contaminants such as endotoxin, these data should be interpreted with caution. In summary, we have established a high risk birth cohort of inner-city children who in the first year have exhibited high rates of wheeze, eczema, and allergic sensitization. Multiple cord blood cytokine responses at birth were inversely related to eczema, while environmental exposures influenced some cytokine responses at birth, and were important influences for wheezing in the first year of life. At present the URECA participants are young, and the atopic outcomes are still evolving. Retention in the study remains high, which presents the opportunity to carry these studies forward to determine the impact of these early life events on the development of persistent asthma and other atopic disorders in urban environments.
Supplementary Material
Acknowledgments
Funding: This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, under Contracts number NO1-AI-25496 and NO1-AI-25482. Additional funds were provided by the National Center for Research Resources, National Institutes of Health, under grants RR00052, M01RR00533, 1UL1RR025771, M01RR00071, 1UL1RR024156, and 5UL1RR024992-02.
Abbreviations
- AIC
Akaike Information Criterion
- EASI
Eczema Area and Severity Index
- EPDS
Edinburgh Postnatal Depression Scale
- NCICAS
National Cooperative Inner-City Asthma Study
- PAS
Pregnancy Anxiety Scale
- PSS
Perceived Stress Scale
- URECA
Urban Environment and Childhood Asthma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert A. Wood, Johns Hopkins University School of Medicine.
Gordon R. Bloomberg, Washington University School of Medicine.
Meyer Kattan, Columbia University College of Physicians and Surgeons.
Kathleen Conroy, Boston University School of Medicine.
Megan T. Sandel, Boston University School of Medicine.
Amy Dresen, University of Wisconsin School of Medicine and Public Health.
Peter J. Gergen, Division of Allergy, Immunology, and Transplantation – NIH.
Diane R. Gold, Channing Laboratory, Brigham and Women's Hospital.
John C. Schwarz, Rho Federal Systems Division, Inc..
Cynthia M. Visness, Rho Federal Systems Division, Inc..
James E. Gern, University of Wisconsin School of Medicine and Public Health.
References
- 1.Akinbami L. Asthma prevalence, health care use and mortality: United States, 2003-05. In: Services DoHaH, editor. National Center for Health Statistics. National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 2.Weiss KB, Gergen PJ, Crain EF. Inner-city asthma. The epidemiology of an emerging US public health concern. Chest. 1992;101:362S–7S. doi: 10.1378/chest.101.6.362s. [DOI] [PubMed] [Google Scholar]
- 3.Carr W, Zeitel L, Weiss K. Variations in asthma hospitalizations and deaths in New York City. Am J Public Health. 1992;82:59–65. doi: 10.2105/ajph.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lilly CM. Diversity of asthma: evolving concepts of pathophysiology and lessons from genetics. J Allergy Clin Immunol. 2005;115:S526–31. doi: 10.1016/j.jaci.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Phipatanakul W, Gold DR, Muilenberg M, Sredl DL, Weiss ST, Celedon JC. Predictors of indoor exposure to mouse allergen in urban and suburban homes in Boston. Allergy. 2005;60:697–701. doi: 10.1111/j.1398-9995.2005.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew GL, Carlton EJ, Kass D, Hernandez M, Clarke B, Tiven J, et al. Determinants of cockroach and mouse exposure and associations with asthma in families and elderly individuals living in New York City public housing. Ann Allergy Asthma Immunol. 2006;97:502–13. doi: 10.1016/S1081-1206(10)60942-8. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Jaffe A, Dixon G. Immunomodulatory effects of macrolide antibiotics in respiratory disease: therapeutic implications for asthma and cystic fibrosis. Paediatr Drugs. 2007;9:107–18. doi: 10.2165/00148581-200709020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–63. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 9.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–80. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- 10.Phipatanakul W, Celedon JC, Sredl DL, Weiss ST, Gold DR. Mouse exposure and wheeze in the first year of life. Ann Allergy Asthma Immunol. 2005;94:593–9. doi: 10.1016/S1081-1206(10)61139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry T, Matsui E, Merriman B, Duong T, Eggleston P. The prevalence of rat allergen in inner-city homes and its relationship to sensitization and asthma morbidity. J Allergy Clin Immunol. 2003;112:346–52. doi: 10.1067/mai.2003.1640. [DOI] [PubMed] [Google Scholar]
- 12.Gruchalla RS, Pongracic J, Plaut M, Evans R, 3rd, Visness CM, Walter M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–85. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Halonen M, Lohman IC, Stern DA, Spangenberg A, Anderson D, Mobley S, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol. 2009;182:3285–93. doi: 10.4049/jimmunol.0711996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr Opin Immunol. 2006;18:727–32. doi: 10.1016/j.coi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Prescott SL, Macaubas C, Holt BJ, Smallacombe TB, Loh R, Sly PD, et al. Transplacental priming of the human immune system to environmental allergens: universal skewing of initial T cell responses toward the Th2 cytokine profile. J Immunol. 1998;160:4730–7. [PubMed] [Google Scholar]
- 16.Prescott SL, Holt PG. Abnormalities in cord blood mononuclear cytokine production as a predictor of later atopic disease in childhood. Clin Exp Allergy. 1998;28:1313–6. doi: 10.1046/j.1365-2222.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 17.Williams TJ, Jones CA, Miles EA, Warner JO, Warner JA. Fetal and neonatal IL-13 production during pregnancy and at birth and subsequent development of atopic symptoms. J Allergy Clin Immunol. 2000;105:951–9. doi: 10.1067/mai.2000.106211. [DOI] [PubMed] [Google Scholar]
- 18.Gern JE, Visness CM, Gergen PJ, Wood RA, Bloomberg GR, O'Connor GT, et al. The Urban Environment and Childhood Asthma (URECA) birth cohort study: design, methods, and study population. BMC Pulm Med. 2009;9:17. doi: 10.1186/1471-2466-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lobel M, Dunkel-Schetter C, Scrimshaw SC. Prenatal maternal stress and prematurity: a prospective study of socioeconomically disadvantaged women. Health Psychol. 1992;11:32–40. doi: 10.1037//0278-6133.11.1.32. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 21.Booth CL, Mitchell SK, Barnard KE, Spieker SJ. Development of maternal social skills in multiproblem families: Effects on the mother-child relationship. Developmental Psychology. 1989;25:403–12. [Google Scholar]
- 22.Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health. 2004;94:625–32. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright RJ, Visness CM, Calatroni A, Grayson MH, Gold DR, Sandel MT, et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. Am J Respir Crit Care Med. doi: 10.1164/rccm.200904-0637OC. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shreffler WG, Visness CM, Burger M, Cruikshank WW, Lederman HM, de la Morena M, et al. Standardization and performance evaluation of mononuclear cell cytokine secretion assays in a multicenter study. BMC Immunol. 2006;7:29. doi: 10.1186/1471-2172-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alwis KU, Milton DK. Recombinant factor C assay for measuring endotoxin in house dust: comparison with LAL, and (1 --> 3)-beta-D-glucans. Am J Ind Med. 2006;49:296–300. doi: 10.1002/ajim.20264. [DOI] [PubMed] [Google Scholar]
- 26.Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group Exp Dermatol. 2001;10:11–8. doi: 10.1034/j.1600-0625.2001.100102.x. [DOI] [PubMed] [Google Scholar]
- 27.Venables WN, Ripley BD. Modern Applied Statistics with S. Fourth. New York: Springer-Verlag; 2002. [Google Scholar]
- 28.Gold DR, Bloomberg GR, Cruikshank WW, Visness CM, Schwarz J, Kattan M, et al. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. J Allergy Clin Immunol. 2009;124:1078–87. doi: 10.1016/j.jaci.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeMasters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, et al. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr. 2006;149:505–11. doi: 10.1016/j.jpeds.2006.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 31.Wahn U, Bergmann R, Kulig M, Forster J, Bauer CP. The natural course of sensitisation and atopic disease in infancy and childhood. Pediatr Allergy Immunol. 1997;8:16–20. [PubMed] [Google Scholar]
- 32.Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet. 2001;358:188–93. doi: 10.1016/S0140-6736(01)05406-X. [DOI] [PubMed] [Google Scholar]
- 33.Koopman LP, Smit HA, Heijnen ML, Wijga A, van Strien RT, Kerkhof M, et al. Respiratory infections in infants: interaction of parental allergy, child care, and siblings--The PIAMA study. Pediatrics. 2001;108:943–8. doi: 10.1542/peds.108.4.943. [DOI] [PubMed] [Google Scholar]
- 34.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163:322–8. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 35.Halmerbauer G, Gartner C, Schier M, Arshad H, Dean T, Koller DY, et al. Study on the prevention of allergy in Children in Europe (SPACE): allergic sensitization in children at 1 year of age in a controlled trial of allergen avoidance from birth. Pediatr Allergy Immunol. 2002;13 15:47–54. doi: 10.1034/j.1399-3038.13.s.15.11.x. [DOI] [PubMed] [Google Scholar]
- 36.Halmerbauer G, Gartner C, Schierl M, Arshad H, Dean T, Koller DY, et al. Study on the Prevention of Allergy in Children in Europe (SPACE): allergic sensitization at 1 year of age in a controlled trial of allergen avoidance from birth. Pediatr Allergy Immunol. 2003;14:10–7. doi: 10.1034/j.1399-3038.2003.02069.x. [DOI] [PubMed] [Google Scholar]
- 37.Hagendorens MM, Bridts CH, Lauwers K, van Nuijs S, Ebo DG, Vellinga A, et al. Perinatal risk factors for sensitization, atopic dermatitis and wheezing during the first year of life (PIPO study) Clin Exp Allergy. 2005;35:733–40. doi: 10.1111/j.1365-2222.2005.02254.x. [DOI] [PubMed] [Google Scholar]
- 38.Perzanowski MS, Miller RL, Thorne PS, Barr RG, Divjan A, Sheares BJ, et al. Endotoxin in inner-city homes: associations with wheeze and eczema in early childhood. J Allergy Clin Immunol. 2006;117:1082–9. doi: 10.1016/j.jaci.2005.12.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. I. The prevalence of mouse allergen in inner-city homes. The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–4. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- 40.Statistics NCfH . In: Health, United States, 2008 with Chartbook. Services DoHaH, editor. Hyattsville, MD: 2009. [Google Scholar]
- 41.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–36. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 42.Herberth G, Heinrich J, Roder S, Figl A, Weiss M, Diez U, et al. Reduced IFN-gamma- and enhanced IL-4-producing CD4+ cord blood T cells are associated with a higher risk for atopic dermatitis during the first 2 yr of life. Pediatr Allergy Immunol. 2009;21:5–13. doi: 10.1111/j.1399-3038.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 43.Kondo N, Kobayashi Y, Shinoda S, Takenaka R, Teramoto T, Kaneko H, et al. Reduced interferon gamma production by antigen-stimulated cord blood mononuclear cells is a risk factor of allergic disorders--6-year follow-up study. Clin Exp Allergy. 1998;28:1340–4. doi: 10.1046/j.1365-2222.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 44.Warner JA, Miles EA, Jones AC, Quint DJ, Colwell BM, Warner JO. Is deficiency of interferon gamma production by allergen triggered cord blood cells a predictor of atopic eczema? Clin Exp Allergy. 1994;24:423–30. doi: 10.1111/j.1365-2222.1994.tb00930.x. [DOI] [PubMed] [Google Scholar]
- 45.Ohshima Y, Yasutomi M, Omata N, Yamada A, Fujisawa K, Kasuga K, et al. Dysregulation of IL-13 production by cord blood CD4+ T cells is associated with the subsequent development of atopic disease in infants. Pediatr Res. 2002;51:195–200. doi: 10.1203/00006450-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 46.Sharp MJ, Rowe J, Kusel M, Sly PD, Holt PG. Specific patterns of responsiveness to microbial antigens staphylococcal enterotoxin B and purified protein derivative by cord blood mononuclear cells are predictive of risk for development of atopic dermatitis. Clin Exp Allergy. 2003;33:435–41. doi: 10.1046/j.1365-2222.2003.01627.x. [DOI] [PubMed] [Google Scholar]
- 47.Prescott SL, Noakes P, Chow BW, Breckler L, Thornton CA, Hollams EM, et al. Presymptomatic differences in Toll-like receptor function in infants who have allergy. J Allergy Clin Immunol. 2008;122:391–9. 9 e1–5. doi: 10.1016/j.jaci.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 48.Pfefferle PI, Buchele G, Blumer N, Roponen M, Ege MJ, Krauss-Etschmann S, et al. Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. J Allergy Clin Immunol. 125:108–15. e1–3. doi: 10.1016/j.jaci.2009.09.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.