Abstract
Nitric oxide (NO)-based therapies decrease neointimal hyperplasia; however, studies have only been performed in male animal models. Thus, we sought to evaluate the effect of NO on vascular smooth muscle cells (VSMC) in vitro and neointimal hyperplasia in vivo based on sex and hormone status. In hormone-replete media, male VSMC proliferated at greater rates than female VSMC. In hormone-deplete media, female VSMC proliferated at greater rates than male VSMC. However, in both hormone environments, NO inhibited proliferation and migration to a greater extent in male versus female VSMC. These findings correlated with greater G0/G1 cell cycle arrest and changes in cell cycle protein expression in male versus female VSMC following exposure to NO. Next, the rat carotid artery injury model was performed to assess the effect of NO on neointimal hyperplasia in vivo. Consistent with the in vitro data, NO was significantly more effective at inhibiting neointimal hyperplasia in hormonally intact males versus females using weight-based dosing. An increased weight-based dose of NO in females was able to achieve efficacy equal to that in males. Surprisingly, NO was less effective at inhibiting neointimal hyperplasia in both sexes in castrated animals. In conclusion, these data suggest that NO inhibits neointimal hyperplasia more effectively in males than females and in hormonally-intact compared to castrated rats, indicating that the effect of NO in the vasculature may be sex- and hormone-dependent.
Keywords: neointimal hyperplasia, vascular smooth muscle proliferation, nitric oxide, hormones, cell cycle
INTRODUCTION
Neointimal hyperplasia is caused by injury to the vascular wall, which occurs during every vascular intervention. Nitric oxide (NO) is a small gaseous molecule normally produced by endothelial cells that has many different vasoprotective properties, including inhibition of platelet aggregation, leukocyte adherence, vascular smooth muscle cell (VSMC) proliferation, VSMC migration, stimulation of VSMC apoptosis, and endothelial cell growth.1-3 These vasoprotective properties have led many researchers to demonstrate the efficacy of NO at inhibiting neointimal hyperplasia in numerous small and large animal models of arterial injury and vein bypass grafting.2 However, all of these studies have been conducted in males. The efficacy of NO in female animal models remains unknown.
Women are a distinct subset of patients with cardiovascular disease (CVD). It has been shown that pre-menopausal women have a lower incidence of coronary artery disease, hyperlipidemia and hypertension compared to age-matched males and post-menopausal females.4, 5 In women with peripheral arterial disease (PAD), the prevalence increases with age and in post-menopausal women is equal to or greater than the prevalence in men.6 Women are also more likely to present with more advanced PAD and to have worse lower extremity function and worse outcomes following intervention for PAD than men.6-8 In spite of these findings, few large studies have adequately addressed potential gender disparities in response to medical or procedural interventions for PAD. Much research has evaluated the effect of estrogen on the cardiovascular system in a clinical arena9-11 as well as in cell culture12, 13 and animal models.14-17 While many vasoprotective properties of estrogen have been identified,12, 13, 15 systemic therapy has fallen out of favor following the release of the Women’s Health Initiative trial data.18-20 Clearly hormones play a significant role in vasoprotection in the local, if not systemic environment.
Therefore, it is unclear, given the obvious sex and hormone differences that exist with respect to the cardiovascular system, if NO-based therapies will be equally effective in these two diverse populations. Since our overall goal is to develop a NO-based therapy that can be used in the clinical arena to improve outcomes following vascular interventions, and it is not yet established whether NO-based therapies will be efficacious in a female model, the purpose of this study is to evaluate the efficacy of NO at inhibiting neointimal hyperplasia in vivo as well as vascular smooth muscle cell (VSMC) proliferation, migration, and cell cycle progression in vitro based on sex and hormone status. We hypothesize that NO will be effective at inhibiting neointimal hyperplasia in a female rodent model of arterial injury, regardless of hormone status.
EXPERIMENTAL PROCEDURES
NO-releasing donors
The diazeniumdiolates evaluated in this study include: 1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate (DETA/NO) for in vitro experiments and disodium 1-[(2-carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO) for in vivo experiments. Diazeniumdiolates were chosen as NO donors due to their predictable and spontaneous NO release rates of 2 moles of NO per mole of compound.21-23 DETA/NO was chosen for the in vitro studies based on its half-life of 20 hours in an aqueous environment at 37°C and pH7.4, which is ideal for a 24-hour assay. PROLI/NO was chosen for the in vivo studies based on our prior publications demonstrating efficacy at inhibiting neointimal hyperplasia.23-26 It is not possible to use PROLI/NO for the in vitro studies given that in an aqueous environment at pH 7.4 and temperature 37°C it has a half-life of approximately 30 seconds.27 For the in vitro experiments, a 10 mmol/L stock solution of DETA/NO was prepared fresh immediately before use for each experiment. For in vivo experiments, the PROLI/NO powder was applied directly to the periadventitial surface of the artery after injury and restoration of flow.
Cell culture
VSMC were isolated and cultured from the aortas of 8-week male and female Sprague-Dawley rats (Harlan, Indianapolis, IN) using the collagenase method and maintained as previously described.3, 28, 29 Three different lots of VSMC were harvested for each sex on three different dates. Each lot was harvested from the aortas of three different animals for each sex. Purity of each lot of VSMC was confirmed using anti-smooth muscle α-actin monoclonal antibodies (Sigma; St. Louis, MO) and by specific morphology for VSMC. Cells were maintained in hormone replete medium (HRM) containing equal volumes of Dulbecco’s Modified Eagle’s Medium-low glucose (SAFC Biosciences; Lenexa, KS) and Ham’s F12 (JRH; Lenexa, KS) supplemented with 10% fetal bovine serum (FBS, Invitrogen; Carlsbad, CA), 100 U/mL penicillin (Invitrogen), 100 μg/mL streptomycin (Invitrogen) and 4 mmol/L L-glutamine (VWR; West Chester, PA) and incubated at 37°C, 95% air an d 5% CO2. In designated experiments, hormone deplete medium (HDM) was used containing 10% dextran-charcoal-stripped FBS (DCS-FBS, Invitrogen), a 1:1 mixture of DMEM-low glucose and Ham’s F12 without phenol red (Invitrogen), which has been shown to be an estrogen receptor agonist, and penicillin, streptomycin, and L-glutamine as above.30 VSMC were used between passages 3-8 for all experiments. Every experiment was conducted in male and female VSMC simultaneously under identifical conditions.
ELISA
Estrogen and testosterone levels were measured from FBS, DCS-FBS, HRM, HDM, and rat serum using commercially available ELISA kits (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions.
Proliferation assay
Tritiated (3H)-thymidine incorporation was assessed as a surrogate for cellular proliferation as previously described.24 Male and female VSMC were plated in 12-well plates (4×104 cells/well) and were growth-arrested for 24 hours with media containing no FBS. Cells were then exposed to HRM and HDM ± DETA/NO (125 - 1000 mol/L) in the presence of 3H-thymidine (5 mCi/L, PerkinElmer, Wellesley, MA) for an additional 24 hours. 3H-thymidine incorporation into trichloroacetic acid–precipitated DNA was quantified by scintillation counting.
Migration
Male and female VSMC were plated in 6-well plates (1×105 cells/well) and were growth-arrested x 24 hours. Adherent cell monolayers were injured by a single scrape with a 1000 μL pipette tip along the transverse diameter of the plate then immediately photographed. Following treatment with media ± DETA/NO for 24 hours the cells were photographed again. Blinded counting of cell nuclei that migrated into the empty space created by the scrape was performed at both time points using Adobe Photoshop 8.0 (Adobe Systems Inc., San Jose, CA), and quantification was performed using ImageJ software (National Institutes of Health [NIH], Bethesda, MD).
Flow Cytometry
Aortic VSMC plated in 4 cm plates (9×105 cells/well) were assessed using flow cytometry to determine the percent of cells in each phase of the cell cycle, as previously described.24 Cells were growth-arrested for 24 hours, after which they were exposed to media ± the DETA/NO (125-1000 μmol/L) for 24 hours. Trypsinized cells were re-suspended in 50 μL PBS and fixed with 450 μL of ice-cold 70% ethanol, followed by re-suspension in a propidium iodide staining solution [1X PBS (pH 7.4), 50 mg/L PI (Invitrogen), 204 mg/L RNase A (Sigma), 0.1% Triton X-100 (Fisher Biotech; Fair Lakes, NJ)]. Samples were analyzed on a Coulter Epic XL flow cytometer gated to include debris. Analysis was performed using ModFit 3.1 LT (Verity; Topsham, ME).
Western blot analysis
VSMC were collected after 24 hours of exposure to the different treatment groups by scraping and were resuspended in 20 mmol/L Tris with 100 μmol/L phenylmethylsulfonylflouride (Sigma), 1 μmol/L leupeptin (Sigma), and 1 μmol/L sodium orthovanadate (Sigma). Protein was quantified with the bicinchoninic acid protein assay according to manufacturer’s instructions (Pierce, Rockford, IL). Whole cell samples (20 μg) were subjected to sodium dodecylsulfate-polyacrylamide gel electrophoresis on 8-13% gels and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Membranes were hybridized using antibodies to p21 (mouse monoclonal, 1:500), p27 (mouse monoclonal, 1:1000), cdc2 (rabbit polyclonal, 1:500), cyclin-dependent kinase (cdk) 2 (mouse monoclonal, 1:500), cdk4 (mouse monoclonal 1:500), cdk6 (mouse monoclonal, 1:750), cyclin D1 (mouse monoclonal, 1:500), cyclin D2 (rat monoclonal, 1:500), and cyclin D3 (rat monoclonal, 1:500) all from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The antibodies to cyclin A (mouse monoclonal 1:500) and cyclin B1 (mouse monoclonal, 1:500) were from Abcam (Cambridge, MA) for 1-2 hours followed by horseradish peroxidase-linked goat anti-rabbit secondary antibodies (1:10,000; Pierce) for 1 hour. Proteins were visualized using chemiluminescent reagents according to the manufacturer’s instructions (Supersignal Substrate; Pierce), and the membranes were exposed to film and developed. Western blot films were scanned to JPEG images, and densitometry was performed on representative images using ImageJ software. Densitometries were calculated as a fold-induction standardized to male controls, after normalizing the data to the respective beta-actin loading control.
Animal surgery
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the NIH (NIH Publication 85-23, 1996) and approved by the Northwestern University Animal Care and Use Committee. For all surgeries, rats were anesthetized with inhaled isoflurane (0.5-3%), and weights were documented. Two weeks prior to conducting the rat carotid artery injury model, adult 10-week male and female Sprague-Dawley rats first underwent castration and ovariectomy, or a sham operation for the hormonally intact group. The rat carotid artery injury model was then performed as previously described.23-25 Atropine was administered subcutaneously (0.1 mg/kg) to decrease airway secretions. Rimadyl (0.15 mg/kg) was given for pain control. Equaline eye ointment was applied to prevent dryness while anesthetized. Following a sterile prep and midline neck incision, the left common, internal and external carotid arteries were dissected and the internal and common carotid arteries were occluded with non-crushing vascular clamps. A 2 French arterial embolectomy catheter (generously provided by Edwards Lifesciences, Irvine, CA) was inserted into the external carotid artery and advanced into the common carotid artery. Uniform injury was created by inflating the balloon to 5 atmospheres of pressure for 5 minutes. After removal of the balloon, the external carotid artery was ligated and blood flow was restored. After injury and restoration of flow, PROLI/NO powder was applied to the external surface of the injured common carotid artery and the neck incision was closed. Because male rats weigh more than 100 grams heavier than the female rats (46% greater, P<0.001) and the carotid artery is larger at baseline in males than females (lumen area 122% greater, medial area 115% greater, circumference 115% greater, P<0.05 for all measurements), the animals were given weight-based dosing. Male intact and castrated rats received 26 mg/kg of PROLI/NO, while female intact and castrated rats received 26 or 39 mg/kg of PROLI/NO. Alternatively, calculation of the absolute dose received according to the mean weight for the intact animals was 9.7 mg (males) and 6.6 and 10.0 mg (females). Absolute dose received for the castrated animals was 9.2 mg (males) and 6.9 and 10.4 mg (females). Rats were sacrificed at 14-day (n=6 per group) time points, and blood was collected to measure serum estrogen and testosterone levels using ELISA kits from Cayman Chemical (Ann Arbor, Michigan) according to manufacturer’s instructions. One castrated female injured rat had an estrogen level > 300 pg/ml. This rat was excluded from morphometric analysis. All procedures were performed by the same surgeon.
Tissue processing
Carotid arteries were harvested following in-situ perfusion-fixation with PBS (300 mL) and 2% paraformaldehyde (300 mL). Tissue was processed as previously described.24 Vessels were placed in paraformaldehyde at 4°C fo r 1 hour, then 30% sucrose in PBS at 4°C for cryo-protection. The tissue was qui ck-frozen in Optimal Cutting Temperature compound (Tissue Tek, Hatfield, PA) and 5-μm sections were cut throughout the entire injured segment of the common carotid artery.
Morphometric analysis
Carotid arteries harvested at 14 days were examined histologically for evidence of neointimal hyperplasia using routine hematoxylin and eosin staining. Digital images were collected with light microscopy using an Olympus BHT microscope (Melville, NY) with 4X, 10X and 40X objectives. Six evenly-spaced sections through each injured carotid artery were morphometrically analyzed. Luminal area and arterial circumference were measured. Intima area was measured as the area between the lumen and the internal elastic lamina. Media area was measured as the area between the internal and external elastic lamina. These values were measured using ImageJ software. All analysis was done by the same person. Because age-matched male rats weighed 46% more than female rats (P<0.001), and male arterial circumference, lumen area, and medial area were statistically significantly larger than female arterial circumference, lumen area, and medial area (P<0.001), morphometric measurements were normalized to arterial circumference to account for these differences in weight and arterial size.
Statistical analysis
Results are expressed as mean ± standard error of the mean. For baseline properties between male and female VSMC, the male control was compared to the female control. Each DETA/NO group was compared to the same-sex control group. In order to compare the effects of NO between the male and female groups, the male group was calculated as a percent of its control and the female group was calculated as a percent of its control. The percent reduction of each male DETA/NO group was compared to the female counterpart. Differences between two groups were analyzed using t-tests. Differences between multiple groups were analyzed using one-way analysis of variance with the Student-Newman-Keuls post hoc test for all pairwise comparisons (SigmaStat; SPSS, Chicago, IL). Statistical significance was assumed when P<0.05.
RESULTS
A hormone deficient environment was created using DCS-FBS
ELISA was performed to measure estrogen and testosterone concentrations in serum and media. DCS-FBS had a 24-fold decrease in estrogen levels (19.6 ± 0.33 pg/ml) and a 20-fold decrease in testosterone levels (10.1 ± 0.9 pg/ml) compared to standard FBS (476.6 ± 29.7 pg/ml and 202.0 ± 7.2 pg/ml, respectively, P<0.05). HDM with 10% DCS-FBS had a 4-fold reduction in estrogen (14.8 ± 6.1 pg/ml) and a 5-fold reduction in testosterone (5.4 ± 0.1 pg/ml) compared to HRM with 10% FBS (56.6 ± 5.4 pg/ml and 23.1 ± 0.5 pg/ml, respectively, P<0.05).
NO-mediated inhibition of VSMC proliferation based on sex and hormone status
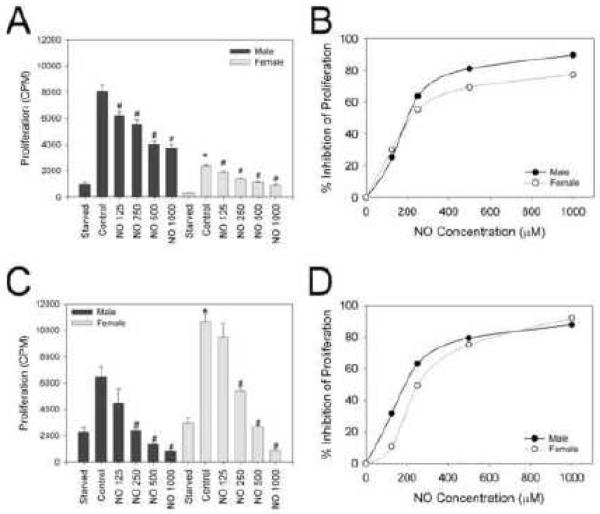
In an effort to characterize the effects of DETA/NO on proliferation of VSMC based on sex and hormone status, both before and after exposure to NO, a 3H-thymidine proliferation assay was conducted [Figure 1A-D]. In HRM, Male VSMC (8.1×103±433 counts per minute [cpm]) proliferated more than female VSMC (2.3×103±86 cpm, *P<0.05). Male and female VSMC demonstrated a dose-dependent inhibition of VSMC proliferation upon exposure to DETA/NO (125-1000 μmol/L; #P<0.05). However, at equivalent dosing, male VSMC exhibited a statistically significant greater reduction in proliferation upon exposure to similar concentrations of NO than female VSMC as can be seen by a shift in the dose-response curve to the right for females [Figure 1B, P<0.05 between the two curves]. In HDM, an opposite pattern was observed for baseline proliferation. Female VSMC (10.6×103 ± 609 cpm) proliferated more than male VSMC (6.5×103 ± 743 cpm, *P<0.05). Similar to that observed with HRM, male and female VSMC demonstrated a dose-dependent inhibition of VSMC proliferation with DETA/NO 125-1000 μM (#P<0.05) in HDM. Surprisingly, at equivalent dosing, male VSMC still exhibited a statistically significant greater reduction in proliferation upon exposure to NO even though baseline proliferation rates were lower [Figure 1D, P<0.05 between the two curves].
Figure 1.
Inhibition of proliferation by nitric oxide (NO) in male versus female vascular smooth muscle cells (VSMC) in hormone replete medium (HRM) and hormone deplete medium (HDM). VSMC proliferation was assessed using 3H-thymidine incorporation at 24 hours ± exposure (CPM=counts per minute) to the NO donor DETA/NO (NO, units are mol/L). A) Male VSMC proliferated faster than female VSMC in HRM (*P<0.05 vs. male control). NO caused a dose-dependent inhibition of VSMC proliferation in both sexes (#P<0.05 vs. same-sex control). B) Dose response curve demonstrating the percent reduction of proliferation by a given concentration of NO in male and female VSMC (P<0.05 for male vs. female curve). C) Female VSMC proliferated faster than male VSMC in HDM (*P<0.05, compared to male control). NO caused a dose-dependent inhibition of VSMC cell proliferation in males and females (#P<0.05, compared to same-sex control). D) Dose response curve demonstrating the percent reduction of proliferation by a given concentration of NO in male and female VSMC in HDM (P<0.05 for male vs. female curve). Data shown are representative of more than five separate experiments; n = 3/treatment group.
NO-mediated inhibition of migration in male and female VSMC
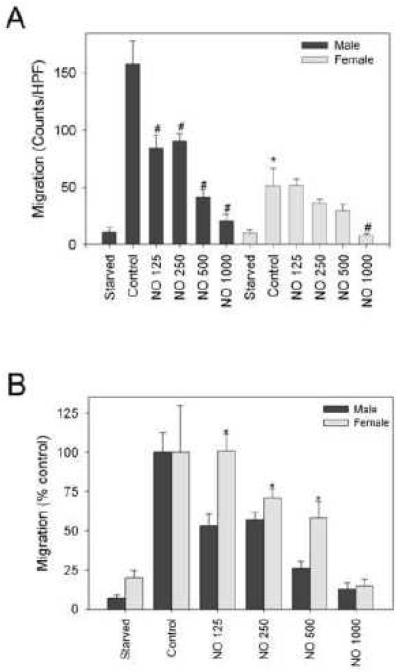
The scrape assay was used to delineate the effects of sex on VSMC migration at baseline and following exposure to NO in HRM [Figure2A-B]. Male VSMC migrated more (158±20 counts per high-power field [chpf]) than female VSMC (51±14 chpf, *P<0.05)[Figure 2A]. Male VSMC showed a statistically significant dose-dependent inhibition of migration with increasing concentrations of DETA/NO (125–1000 μmol/L). However, only the highest concentration of DETA/NO (1000 μmol/L) inhibited migration in female VSMC (#P<0.05). At equivalent dosing of DETA/NO, male VSMC exhibited greater reduction in migration than female VSMC (74% vs. 42% at 500 μmol/L; *P<0.05; Figure 2B).
Figure 2.
Inhibition of migration by nitric oxide (NO) in male versus female vascular smooth muscle cells (VSMC). Migration was assessed using the scrape assay at 24 hours ± exposure (HPF=high power field) to the NO donor DETA/NO (NO, units are mol/L). A) Male VSMC migrate faster than female VSMC (*P<0.05, vs. male control). NO caused a dose-dependent inhibition of VSMC migration in males and decreased VSMC migration in females at the highest concentration (#P<0.05, vs. same-sex control). B) Graphical representation of the percent inhibition of migration by a given concentration of NO after adjusting the data to the control group (*P<0.05, vs. male NO counterpart). Data shown are representative of more than five separate experiments; n = 3/treatment group.
NO-induced alterations in cell cycle progression in male and female VSMC
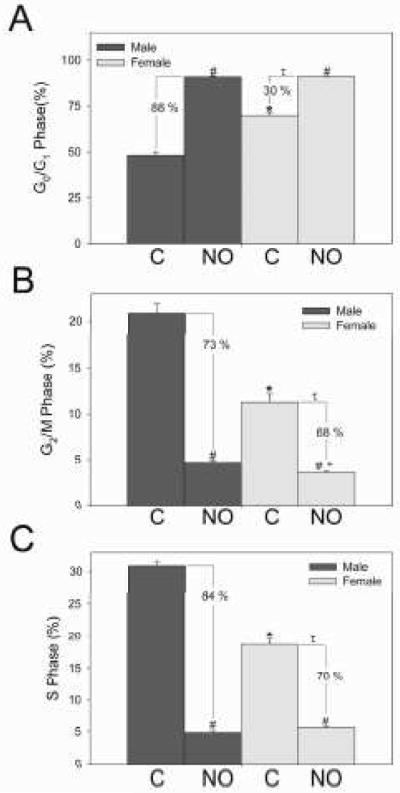
In order to further characterize the differences we observed between the sexes, the cell cycle was analyzed using flow cytometry with propidium iodide staining in HRM [Figures 3A-C]. All NO values for cell cycle analysis represent DETA/NO 500 μmol/L. More female VSMC were in G0/G1 than male VSMC under control conditions (70%±1.8% vs. 48%±1.7% respectively; *P<0.001). NO increased G0/G1 cell cycle arrest in male (90%±0.5%) and female (91%±0.2%) VSMC (#P<0.001). The percent increase was greater in males compared to females (88% vs. 30%, respectively, tP<0.001). More male VSMC were in G2/M than female VSMC under control conditions (21%±1.8% and 11%±1.6% respectively; *P=0.002). NO decreased G2/M in male and female VSMC (4.7%±0.2% vs. 3.5%±0.1% respectively; #P<0.001). After exposure to NO, more male VSMC were in G2/M than female VSMC (+P=0.008) and the percent decrease was greater in males compared to females (73% vs. 68%, respectively; τP<0.05). More male VSMC were also in the S phase than female VSMC under control conditions (31%±1.3% vs. 19%±1.5% respectively; *P<0.001). NO decreased synthesis in male and female VSMC (4.8%±0.4% vs. 5.6%±0.3%, respectively; #P<0.001). However, the percent decrease was greater in males (84%) compared to females (70%, τP<0.05). In summary, these differences between the sexes in cell cycle progression may in part explain the proliferation differences observed in male and female VSMC at baseline and following exposure to NO.
Figure 3.
Effect of nitric oxide (NO) on cell cycle progression in male and female vascular smooth muscle cells (VSMC). NO=DETA/NO 500 μmol/L and C=control. A-C) NO induced G0/G1 cell cycle arrest and decreased G2/M and S phase in male and female VSMC (#P<0.05 vs. same sex control; *P<0.05 vs. male control; +P<0.05 vs. male NO). However, the percent change is greater in males compared to females (τP<0.05 vs. percent reduction in males). Data shown are representative of five separate experiments; n = 3/treatment group.
Cell cycle protein expression in male and female VSMC
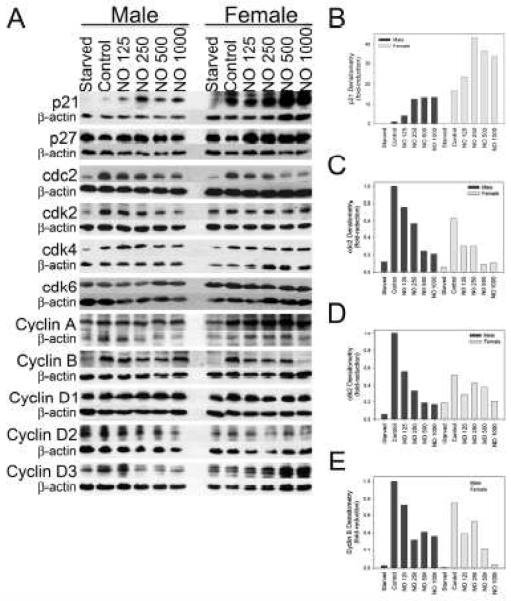
Given the differences observed in the cell cycle based on sex and NO exposure, expression of cell cycle proteins was compared based on sex and after NO exposure using Western blot analysis in HRM [Figure 4A-E]. No differences were found in the protein expression of the cyclin-dependent kinase inhibitor p27 between male and female VSMC, but a small increase in expression was noted after NO exposure in females more than in males. Female VSMC exhibited a 6.5-fold increase in p21 expression compared to male VSMC at baseline [Figure 4B] but NO exposure resulted in a larger increase in p21 expression in males (13.4-fold, DETA/NO 500 μmol/L) compared to females (2.2-fold, DETA/NO 500 μmol/L). This increase in p21 expression at baseline in females, and greater increase in p21 expression following NO exposure in males, correlates with the proliferation and cell cycle analysis data, suggesting that female VSMC at baseline experience more arrest, but after NO exposure male VSMC are more responsive to cell cycle arrest than female VSMC.
Figure 4.
Nitric oxide (NO) differentially effects the expression of cell cycle proteins based on sex. A) NO differentially effects the expression of cell cycle proteins based on sex by Western blot analysis. B-E) Densitometries of Western blots normalized by beta-actin loading controls and graphed as a percent of the male control. Female vascular smooth muscle cells (VSMC) expressed more p21 at baseline than male VSMC and NO increased p21 expression in males and females. NO decreased cdc2 expression in males and females. Male VSMC had a greater expression of cdk2 at baseline than female VSMC and that NO decreased cdk2 in male VSMC but not in female VSMC. NO decreased the expression of cyclin B in male and female VSMC, but to a slightly greater extent in females. NO=DETA/NO in mol/L. Data are representative of at least five separate experiments.
Differences were observed between the sexes for cdc2 and cdk2. Males had a negligible increase in expression of cdc2 at baseline and following exposure to NO compared to females [Figure 4C]. NO caused a dose-dependent decrease in cdc2 expression in male (75% reduction, DETA/NO 500 μmol/L) and female VSMC (86% reduction, DETA/NO 500 μmol/L). Males exhibited a 2-fold greater expression of cdk2 than females [Figure 4D]. NO caused a dose-dependent decrease in cdk2 in males (80% reduction, DETA/NO 500 μmol/L), but no significant reduction was observed in females (26% reduction, DETA/NO 500 μmol/L). No differences based on sex or following NO exposure were found in the expression of cdk4; but, at baseline, female VSMC had a 1.5-fold greater expression of cdk6 compared to the male VSMC. However, no difference was observed in protein expression of cdk6 following NO exposure in male or female VSMC.
No differences based on sex or following NO exposure were found in the expression of cyclin A, cyclin D1, cyclin D2, and cyclin D3 in VSMC. Males had a negligible increase in expression of cyclin B compared to females [Figure 4E]. NO caused a dose-dependent decrease in cyclin B expression in males (58% reduction, DETA/NO 500 μmol/L) and females (71% reduction, DETA/NO 500 μmol/L). In summary, the expression of these cell cycle proteins correlated with the cell cycle data showing more males in G2/M and S phases at baseline, and that NO caused a greater reduction in mitosis and synthesis phases in males compared to females. Cdk2 partners with cyclin E in the G1 phase to regulate entry into the S phase. Cyclin B forms a complex with cdc2 to regulate entry into the M phase of the cell cycle. Thus, these subtle differences in expression of cdks may explain why male VSMC have a greater percentage of cells in G2/M and S phases than females and why these cells proliferate and migrate more than female VSMC.
Inhibition of neointimal hyperplasia in hormonally intact and castrated male and female rodents
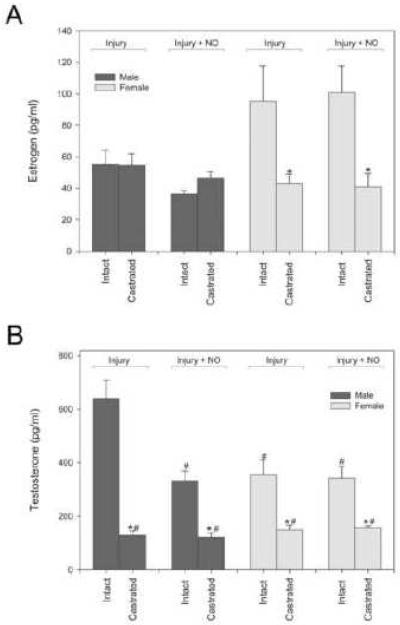
In an effort to characterize the effect of a NO-based therapy based on sex and hormone status, the rat carotid artery injury model was performed in intact and castrated male and female Sprague-Dawley rats. Serum was collected prior to sacrifice from all animals and estrogen and testosterone levels were measured to confirm the model [Figure 5]. In female rats, castration significantly decreased estrogen levels compared to intact animals in the injury [Figure 5A] (43.3±5.8 vs. 95.3±22.3 pg/ml, P=0.05) and injury plus NO treatment groups (40.9±8.6 vs. 100.8±16.8 pg/ml, P<0.05), but it did not affect estrogen levels in male rats. In male rats, castration significantly decreased testosterone levels compared to hormonally-intact animals in the injury [Figure 5B] (128±16.7 vs. 641±67.3 pg/ml, *P<0.05) and injury plus NO treatment groups (121±16.1 vs. 332±37.1 pg/ml, *P<0.05). In female rats, castration also reduced testosterone compared to intact animals in both the injury (149±18.4 vs. 355±54.6 pg/ml, *P<0.05), and injury plus NO treatment groups (156±8.5 vs. 343±44.3 pg/ml, *P<0.05). Thus, castration was effective at significantly reducing estrogen and testosterone levels in females and testosterone levels in males.
Figure 5.
A) Castration decreased estrogen levels in all female groups but did not affect estrogen levels in male rats (*P<0.05 compared to intact counterpart). B) Intact male injured rats had higher testosterone levels than any other rat groups (#P<0.05 compared to intact male injury). Castration significantly reduced testosterone levels in every group (*P<0.05 compared to intact counterpart). n=6/group.
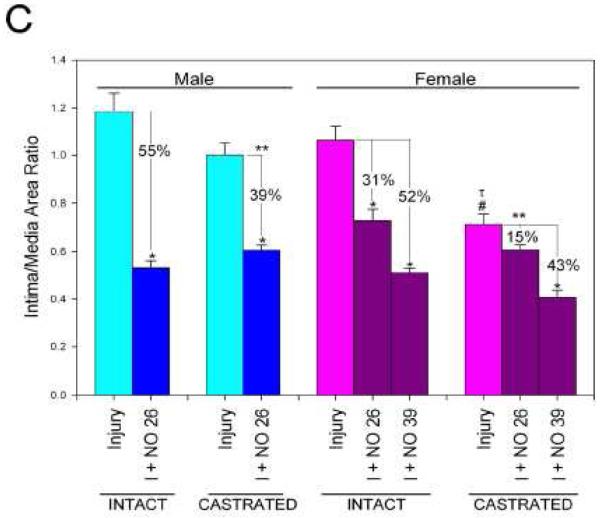
In hormonally-intact animals, balloon injury produced reproducible neointimal hyperplasia at 14 days in both sexes [Table 1, Figure 6A and 6C]. With respect to neointimal hyperplasia, the intimal area and the intima/media area ratio (I/M) showed no differences between male and female rodents in the injury-alone treatment groups. In both sexes, NO significantly reduced intimal area and the I/M (P<0.05). To compare the efficacy of NO between the sexes, the percent reduction of neointima was compared. NO reduced intimal area to a greater extent in males versus females using equivalent weight-based dosing (65% vs. 53%, respectively, 26 mg/kg, P<0.05). NO resulted in a greater reduction in the I/M area ratio in males versus females at equivalent weight-based dosing (55% vs. 31%, respectively, 26 mg/kg, P<0.001) [Figure 6A and 6C]. However, when females received a higher weight-based dose of NO (39 mg/kg), they exhibited reductions similar to males in intimal area (68%) and I/M (52%).
Table 1.
Carotid Artery Injury Model Morphometric Analysis in Hormonally Intact Rats
| Males |
Females |
||||
|---|---|---|---|---|---|
| Injury | Injury + PROLI/NO 26 mg/kg |
Injury | Injury + PROLI/NO 26 mg/kg |
Injury + PROLI/NO 39 mg/kg |
|
|
Weight (grams) |
371 ± 3 | 374 ± 4 | 269 ± 14 b |
239 ± 7 c | 257 ± 3 c |
|
Circumference |
1.71 ± 0.02 |
1.60 ± 0.02 | 1.44 ± 0.03 b |
1.37 ± 0.02 c | 1.41 ± 0.01 c |
|
Luminal Area |
8.33 ± 0.14 |
8.35 ± 0.20 | 5.82 ± 0.29 b |
7.63 ± 0.17 a,c | 7.90 ± 0.17* |
|
Intima Area |
21.96 ± 1.15 |
7.79 ± 0.44a | 19.12 ± 0.97 |
9.76 ± 0.71 a | 6.10 ± 0.23 a |
|
Media Area |
20.29 ± 0.80 |
14.69 ± 0.47a | 18.00 ± 0.38 |
12.89 ± 0.37 a | 12.37 ± 0.31 a |
|
Intima/Media |
1.18 ± 0.08 |
0.53 ± 0.03a | 1.06 ± 0.06 |
0.73 ± 0.05 a | 0.51 ± 0.02 a |
|
Intima/(Intima +
Media) |
0.53 ± 0.01 |
0.35 ± 0.01a | 0.51 ± 0.01 |
0.41 ± 0.02 a,c | 0.33 ± 0.01 a |
P < 0.05 compared to same sex injury.
P < 0.05 compared to male injury-alone.
P < 0.05 compared to male injury + NO. n=6 per treatment group. All measured units of the artery are arbitrary units from ImageJ
Figure 6.
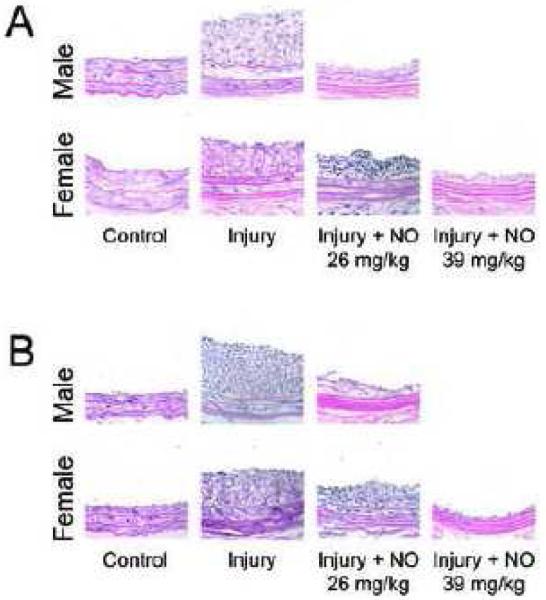
Hematoxylin-eosin stained cross-sections of A) intact and B) castrated rat carotid arteries at 400X magnification. C) Morphometric analysis of the effect of NO on neointimal hyperplasia in male and female hormonally intact and castrated rodents (*P<0.05). NO=PROLI/NO (n=6/group).
With respect to luminal area, female rats had a 30% smaller luminal area than male rats in the injury-alone groups (P<0.05) [Table 1]. After NO therapy, female rodents experienced a significant enlargement in their luminal area for both NO doses, whereas males did not have enlargement in luminal area after NO therapy. With respect to medial remodeling, no differences were observed in the medial area between male and female rodents in the injury-alone groups. In both sexes, NO significantly reduced media area (P<0.05). However, no difference in percent reduction by NO was found in the medial area between male (28%) and female (28%) rodents.
In castrated animals, balloon injury produced reproducible neointimal hyperplasia at 14 days in both sexes [Table 2, Figure 6B and 6C]. Following arterial injury, males had a larger intimal area than females (17.6±0.92 vs. 13.0±0.68, P<0.05). In both sexes, NO significantly reduced intima area (7.3±0.25 males, 8.5±0.27 females [26 mg/kg], and 4.4±0.39 females [39 mg/kg], P<0.05). However, NO resulted in a greater reduction in intimal area in males than females at equivalent weight-based dosing (59% vs. 31%, 26 mg/kg, P<0.05). Females that received a higher weight-based dose of NO (39 mg/kg) experienced a greater reduction than males in intimal area (66%). Following arterial injury, males had a larger I/M than females (1.00±0.05 vs. 0.71±0.04, P<0.05). In male and high dose NO-treated female groups (39 mg/kg), NO significantly reduced the I/M (0.61±0.02 and 0.41±0.03, respectively, P<0.05). No reduction was observed in the I/M in the equivalent weight-based dosed female group (0.61±0.02, P=NS, 26 mg/kg). Thus, NO resulted in a significantly greater reduction in the I/M in males compared to females at equivalent weight-based dosing (39% vs. 15%, P<0.05). Females that received the higher dose of NO (39 mg/kg) achieved a reduction in the I/M equal to males that received 26 mg/kg.
Table 2.
Carotid Artery Injury Model Morphometric Analysis in Hormonally Castrated Rats
| Males |
Females |
||||
|---|---|---|---|---|---|
| Injury | Injury + PROLI/NO 26 mg/kg |
Injury | Injury + PROLI/NO 26 mg/kg |
Injury + PROLI/NO 39 mg/kg |
|
| Weight (grams) | 347 ± 5 | 357 ± 4 | 285 ± 5 b | 243 ± 7 c | 271 ± 6 c |
|
Circumference |
1.61 ± 0.0 1 |
1.56 ± 0.02 | 1.50 ± 0. 0 2 b |
1.51 ± 0.02 | 1.42 ± 0.02 c |
|
Luminal Area |
8.42 ± 0.1 4 |
8.86 ± 0.23 | 8.02 ± 0. 2 4 |
7.97 ± 0.19 c | 7.89 ± 0.24 c |
|
Intima Area |
17.6 ± 0.9 2 |
7.3 ± 0.25 a | 13.0 ± 0. 6 8 b |
8.5 ± 0.27 a | 4.4 ± 0.39 a,c |
|
Media Area |
17.6 ± 0.6 1 |
12.3 ± 0.43 a | 17.7 ± 0. 8 1 |
14.6 ± 0.33 | 10.8 ± 0.38 a |
|
Intima/Media |
1.00 ± 0.0 5 |
0.61 ± 0.02 a | 0.71 ± 0. 0 4 b |
0.61 ± 0.02 | 0.41 ± 0.03 a,c |
|
Intima/(Intima + Media) |
0.50 ± 0.0 1 |
0.37 ± 0.01 a | 0.44 ± 0. 0 b |
0.38 ± 0.01 | 0.27 ± 0.02 a,c |
P < 0.05 compared to same sex injury.
P < 0.05 compared to male injury alone.
P < 0.05 compared to male injury + NO. n=6 per treatment group. All measured units of the artery are arbitrary units from ImageJ
With respect to luminal area, no significant differences were noted in the castrated rats between the sexes or after treatment with NO [Table 2]. With respect to medial area, male and female rats had equal media areas following arterial injury (17.6±0.61 and 17.7±0.81 respectively). In male and high dose NO-treated female groups, NO significantly reduced medial area (12.3±0.43 and 10.8±0.38 respectively, P<0.05). No reduction was observed in the equivalent-dosed female group (14.6±0.33). Thus, NO brought greater reduction in medial area in males than females at equivalent weight-based dosing (30% vs. 17%, P<0.05). Females with higher dosed NO had reduction equal to males in media area (39%) and greater than the other NO-treated female group (P<0.05).
Lastly, significant differences were noted between the hormonally-intact and castrated animals. Overall, castrated animals developed significantly less intimal area and I/M compared to hormonally-intact animals (Tables 1 and 2, Figure 6C). Furthermore, NO was found to be more effective at reducing neointimal hyperplasia in both sexes in hormonally-intact rats compared to castrated rats when evaluating intimal area and the I/M (Figure 6C).
DISCUSSION
In our experiments, we examined the effects of NO on VSMC proliferation, migration, and cell cycle regulation in vitro and neointimal hyperplasia in vivo based on sex and hormone status of the animal. As predicted by our hypothesis, the results of this study show that NO is effective at inhibiting these vascular processes in both sexes, however we found that NO appears to be more effective in males versus females and more effective in a hormonally intact versus deficient environment. At baseline in a hormone replete environment, male VSMC proliferated and migrated more and had less cell cycle arrest compared to female VSMC. After exposure to NO, male VSMC experienced greater inhibition of proliferation, migration, and cell cycle arrest than female VSMC. In a hormone deplete environment, while females proliferated more than males, NO was still more effective at inhibiting VSMC proliferation in males compared to females. In our in vivo model, when using weight-based dosing, NO therapy resulted in a greater reduction in neointimal hyperplasia in males than females, and in hormone intact versus castrated rats. In summary, this study suggests that VSMC growth and the efficacy of NO at inhibiting neointimal hyperplasia is sex- and hormone-dependent.
While we are unaware of any studies that have investigated the efficacy of NO-based therapies to inhibit neointimal hyperplasia in a female environment, our findings on the effects of NO on female VSMC are consistent with published literature for male systems. Inhibition of VSMC migration and proliferation and induction of cell cycle arrest after NO therapy have been well-established in previous publications.3, 31, 32 Additionally, Ling et al evaluated mouse VSMC proliferation in male and female wild-type and aromatase-knockout mice.33 While they did not evaluate the effects of NO, they found that in both wild-type and aromatase knockout mice male VSMC proliferated more than female VSMC.33 Other studies exist that examine VSMC proliferation to assess sex-differences for alternative therapies, but frequently data were represented as a percentage of control, making comparisons between the sexes impossible.10
Multiple investigators have examined the effects of β-estradiol on VSMC migration and showed that this therapy inhibits migration of VSMC.11-14 However, Dai-Do et al only examined cells from post-menopausal females and Kolodgie et al only examined female rat VSMC.11, 12 Akishita et al examined male and female rat VSMC, but published results only include data on males, and Geraldes et al did not indicate the sex of the porcine VSMC evaluated for migration.13, 14 Clearly, more sex-specific investigation into these processes is warranted given that VSMC proliferation and migration are the cornerstone of neointimal hyperplasia and this process occurs in both male and female patients.
Sex differences in the development of neointimal hyperplasia following arterial injury have been described. Chen et al in 1996 were the first to compare male and female neointimal hyperplasia after carotid balloon angioplasty.15 Using the rat carotid artery injury model, they evaluated intact and castrated male and female rats, and administered back either estrogen or testosterone. Neointimal proliferation after injury was reduced in intact females compared to age-matched males. This did not hold true in castrated females, which developed equal injury compared to castrated male rats. Exogenous estrogen prevented neointimal formation in gonadectomized rats of both sexes, but greater in females compared to males. We were surprised to find that our castrated female rats did not have greater injury than intact female rats or equal injury to male rats based on this previous study. Our data may be different for several reasons. First, the Sprague-Dawley rodents from the Chen et al study were obtained from Charles River Laboratories, which have been shown to develop a different injury pattern than Sprague-Dawley rats obtained from Harlan Laboratories.34 Second, their injury technique was different from ours. Instead of advancing the balloon into the common carotid artery and inflating it once to a constant and controlled 5 atmospheres of pressure for 5 minutes, the balloon was inflated to unknown atmospheres, advanced into the thoracic aorta, and passed 6 times. Third, their rats were age-matched and the females weighed significantly less, and measurements were not controlled for the differences in arterial size due to weight differences15. In 2005, Kurumazuka et al compared male and female neointimal hyperplasia in Japanese rodents after carotid injury.16 These authors evaluated atorvastatin as a therapy but found equivalent neointimal hyperplasia between male and female injury groups, similar to our study. Thus, studies have been conducted that demonstrate a difference in the development of neointimal hyperplasia based on sex and hormone status.
Our study does indicate a vasoprotective role for estrogen and NO in that the NO therapy reduced neointimal hyperplasia to a greater extent in hormonally intact versus castrated female rodents. The exact role, however, of estrogen and NO in this process is not fully understood and further experiments supplementing back estrogen and administering NO therapy need to be performed. Furthermore, it has been well-established that estrogen stimulates NO release in the vasculature.35 But, it is not clear if the vasoprotective actions of NO are mediated through the estrogen-estrogen receptor signaling pathway. There is evidence to suggest the later may be the case. Both estrogen and NO share many similarities. They both inhibit VSMC proliferation, VSMC migration, leukocyte chemotaxis, and endothelial cell apoptosis, and stimulate endothelial cell proliferation and vasodilation.36, 37 Thus, it is possible that NO may in fact function to stimulate or potentiate the estrogen-estrogen receptor signaling pathway to exert its vasoprotective effects and that when estrogen is deficient, the effects of NO are less potent. Alternatively, our in vivo data also show that NO is more effective at inhibiting neointimal hyperplasia in the presence of higher serum concentration of testosterone. This could potentially establish a role of testosterone mediating NO-induced vasoprotection. However, since testosterone is converted to estrogen locally via aromatase, serum testosterone levels may ultimately affect local estrogen levels. Neointimal hyperplasia and hormone pathways are complex, multifactorial processes. These data suggest that hormone status plays a role in mediating the efficacy of NO at inhibiting neointimal hyperplasia and VSMC proliferation, but more work needs to be conducted to evaluate the exact mechanisms.
This study has limitations. One inherent limitation is that controlling for every aspect of rodent characteristics other than sex is not possible. In order to mirror what has been demonstrated in the literature as well as the rat carotid artery model used in our laboratory, 12-week age-matched Sprague-Dawley rodents from Harlan were used. However, except in rare cases of retired breeders, age-matched female rodents are significantly smaller than male rodents. In our study, male rodents weighed on average 46% more than females, and the male carotid artery circumference, lumen area, and medial area were larger than in the females. Given this, we controlled dosing of PROLI/NO by weight, as is commonly done for many patient administered drugs. Analysis of the weight-based data suggests that NO inhibits VSMC proliferation and neointimal hyperplasia more in males than females. However, if analysis is conducted based on absolute dosing, few differences are observed between the sexes. For example, intact males that received 9.7 mg PROLI/NO (i.e. 26 mg/kg) experienced 55% reduction in neointimal hyperplasia while intact females that received 10.0 mg PROLI/NO (i.e. 39 mg/kg) experienced 52% reduction in neointimal hyperplasia. A similar pattern is observed for the castrated male and female rodents that received 9.1 mg and 10.4 mg PROLI/NO (39% and 43% inhibition, respectively). Yet, the differences observed in the efficacy of NO based on hormone status persist, regardless of the type of analysis (i.e. weight-based vs. absolute dose). Even at the highest doses administered (ranging from 9.1-10.4 mg PROLI/NO), the NO-based therapy is more effective in hormonally intact males and females (55% and 52% inhibition, respectively) compared to hormonally castrated males and females (39% and 43% inhibition, respectively). With weight-based dosing, the differences are even greater. Thus, while NO may or may not have different efficacies based on sex, NO is clearly more effective in hormonally intact hormonally deprived environments.
Another inherent limitation of this study is that, in terms of castration, the animals were not deprived of their hormones chronically. Furthermore, the rats were not aged, thus making an exact comparison to post-menopausal females not possible. Also, the hormonally intact female group’s estrous cycle was not controlled for, thus exact hormone levels were not controlled. Despite these limitations we wanted to simulate a hormone-deprived and hormone-rich environment for comparison purposes and this was achieved. Lastly, we did use different diazeniumdiolates for the in vitro work versus the in vivo work. PROLI/NO was chosen for the in vivo work due to our earlier work that has shown that PROLI/NO is the most efficacious diazeniumdiolate in this animal model.23-26 Unfortunately, PROLI/NO cannot be used for the in vitro studies that were conducted over 24 hours due to its very short half-life in an aqueous environment at physiologic temperature and pH. However, given that the class of diazeniumdiolates release 2 moles of NO per mole of compound, we felt it was valid to use the diazeniumdiolate with the half-life idea for the assay being conducted, i.e. DETA/NO. This should have no bearing on the interpretation of our data, as our purpose was not to compare the in vitro studies to the in vivo studies, but to determine the effect of NO on VSMC in vitro, and also to determine the effect on neointimal hyperplasia in vivo, based on sex and hormone statues. We believe we accomplished this.
In conclusion, periadventitial application of NO inhibits neointimal hyperplasia in female rodents in a dose-dependent manner. The efficacy of NO at inhibiting neointimal hyperplasia is hormone-dependent, inhibiting neointimal hyperplasia to a greater extent in hormonally-intact versus castrated animals. Depending upon the method of analysis, the efficacy of NO at inhibiting neointimal hyperplasia may or may not be sex dependent. Since most female vascular patients are post-menopausal and most studies have shown that with regards to PAD women have an increased prevalence as they age, advanced disease at presentation, worse function from their disease, and worse outcomes after intervention for PAD than males, it is critical that specific attention be paid to this patient population when developing new therapies. Ultimately our goal is to develop a therapy that can be used in all patients to prevent restenosis following vascular interventions, and to be able to translate this therapy to every patient population that will benefit. Overall, NO-based therapies have promising clinical potential for an ever-growing population of patients with vascular disease, but more specific evaluation into mechanisms and efficacy of NO-based therapies is warranted.
ACKNOWLEDGMENTS
The authors would like to express their thanks to the Feinberg Cardiovascular Research Institute and to Edwards Lifesciences for generously providing the Fogarty balloon catheters. This work was supported in part by funding from the Institute for Women’s Health Research at the Feinberg School of Medicine, Northwestern University, Chicago, Illinois through the Pioneer Award competitive funding mechanism. This work was also supported in part by funding from the National Institutes of Health (K08HL084203); Department of Veterans Affairs Merit Review award; the American Vascular Association, and an American Medical Association Seed Grant. It was supported in part by the generosity of Mrs. Hilda Rosenbloom and Ms. Eleanor Baldwin. Lastly, this work was supported in part by funding from the National Cancer Institute, NIH, under contract NO1-CO-12400 with SAIC-Frederick Inc. and by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The authors would also like to express their thanks to the Institute for BioNanotechnology in Medicine and Lynnette Dangerfield for her “tireless” hours.
ABBREVIATIONS
- NO
nitric oxide
- VSMC
vascular smooth muscle cells
- CVD
cardiovascular disease
- PAD
peripheral arterial disease
- DETA/NO
1-[N-(2-aminoethyl)-N-(2-ammonioethyl)amino]diazen-1-ium-1,2-diolate
- PROLI/NO
disodium 1-[(2-carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate
- HRM
hormone replete media
- HDM
hormone deplete media
- DCS-FBS
dextran-charcoal stripped FBS
- 3H
tritiated
- NIH
National Institutes of Health
- H&E
hematoxylin-eosin
- CPM
counts per minute
- HPF
high power field
- I/M
intimal to medial ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- (1).Kibbe M, Billiar T, Tzeng E. Inducible nitric oxide synthase and vascular injury. Cardiovasc Res. 1999 Aug 15;43(3):650–7. doi: 10.1016/s0008-6363(99)00130-3. [DOI] [PubMed] [Google Scholar]
- (2).Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007 Jun;45(Suppl A):A64–A73. doi: 10.1016/j.jvs.2007.02.027. A64-73. [DOI] [PubMed] [Google Scholar]
- (3).Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, et al. Inducible nitric oxide synthase (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg. 2000 Jun;31(6):1214–28. doi: 10.1067/mva.2000.105006. [DOI] [PubMed] [Google Scholar]
- (4).Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, et al. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Dec 15; doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- (5).Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991 Apr 10;265(14):1861–7. [PubMed] [Google Scholar]
- (6).Sigvant B, Wiberg-Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg J Vasc Surg. 2007 Jun;45(6):1185–91. doi: 10.1016/j.jvs.2007.02.004. [DOI] [PubMed] [Google Scholar]
- (7).McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning - The Women’s Health and Aging Study. Circulation. 2000 Mar 7;101(9):1007–12. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- (8).Enzler MA, Ruoss M, Seifert B, Berger M. The influence of gender on the outcome of arterial procedures in the lower extremity. Eur J Vasc Endovasc. 1996 May;11(4):446–52. doi: 10.1016/s1078-5884(96)80180-8. Eur J Vasc Endovasc. [DOI] [PubMed] [Google Scholar]
- (9).Smith SC, Jr., Dove JT, Jacobs AK, Kennedy JW, Kereiakes D, Kern MJ, et al. ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines)--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty) J Am Coll Cardiol. 2001 Jun 15;37(8):2215–39. doi: 10.1016/s0735-1097(01)01344-4. [DOI] [PubMed] [Google Scholar]
- (10).Fitzpatrick LA, Ruan M, Anderson J, Moraghan T, Miller V. Gender-related differences in vascular smooth muscle cell proliferation: implications for prevention of atherosclerosis. Lupus. 1999;8(5):397–401. doi: 10.1177/096120339900800514. [DOI] [PubMed] [Google Scholar]
- (11).Do DD, Espinosa E, Liu GZ, Rabelink TJ, Julmy F, Yang ZH, et al. 17 beta-Estradiol inhibits proliferation and migration of human vascular smooth muscle cells: Similar effects in cells from postmenopausal females and in males. Cardiovascular Research. 1996 Nov;32(5):980–5. [PubMed] [Google Scholar]
- (12).Kolodgie FD, Jacob A, Wilson PS, Carlson GC, Farb A, Verma A, et al. Estradiol attenuates directed migration of vascular smooth muscle cells in vitro. American Journal of Pathology. 1996 Mar;148(3):969–76. [PMC free article] [PubMed] [Google Scholar]
- (13).Geraldes P, Sirois MG, Bernatchez PN, Tanguay JF. Estrogen regulation of endothelial and smooth muscle cell migration and proliferation - Role of p38 and p42/44 mitogen-activated protein kinase. Arterioscl Throm Vas. 2002 Oct;22(10):1585–90. doi: 10.1161/01.atv.0000035393.11854.6a. Arterioscl Throm Vas. [DOI] [PubMed] [Google Scholar]
- (14).Akishita M, Ouchi Y, Miyoshi H, Kozaki K, Inoue S, Ishikawa M, et al. Estrogen inhibits cuff-induced intimal thickening of rat femoral artery: Effects on migration and proliferation of vascular smooth muscle cells. Atherosclerosis. 1997 Apr;130(1-2):1–10. doi: 10.1016/s0021-9150(96)06023-6. [DOI] [PubMed] [Google Scholar]
- (15).Chen SJ, Li HB, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996 Feb 1;93(3):577–84. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- (16).Kurumazuka D, Mori T, Matsumura Y. Ovariectomized female rats show more favorable response to atorvastatin than male rats against balloon injury. Journal of Pharmacological Sciences. 2005;97:189P. [Google Scholar]
- (17).Kaneda H, Ako J, Kataoka T, Takahashi T, Terashima M, Shimada Y, et al. Impact of gender on neointimal hyperplasia following coronary artery stenting. Circulation. 2005 Oct 25;112(17):U834. doi: 10.1016/j.amjcard.2006.09.094. [DOI] [PubMed] [Google Scholar]
- (18).Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women - Principal results from the Women’s Health Initiative randomized controlled trial. Jama-Journal of the American Medical Association. 2002 Jul 17;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- (19).Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH. A Prospective-Study of Postmenopausal Estrogen Therapy and Coronary Heart-Disease. New England Journal of Medicine. 1985;313(17):1044–9. doi: 10.1056/NEJM198510243131703. [DOI] [PubMed] [Google Scholar]
- (20).Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, et al. Postmenopausal Estrogen Therapy and Cardiovascular-Disease - 10-Year Follow-Up from the Nurses Health Study. New England Journal of Medicine. 1991 Sep 12;325(11):756–62. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- (21).Keefer LK. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu Rev Pharmacol Toxicol. 2003;43:585–607. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- (22).Hrabie JA, Keefer LK. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem Rev. 2002 Apr;102(4):1135–54. doi: 10.1021/cr000028t. [DOI] [PubMed] [Google Scholar]
- (23).Kapadia MR, Chow LW, Tsihlis ND, Ahanchi SS, Eng JW, Murar J, et al. Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia. J Vasc Surg. 2008 Jan;47(1):173–82. doi: 10.1016/j.jvs.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ahanchi SS, Varu VN, Tsihlis ND, Martinez J, Pearce CG, Kapadia MR, et al. Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2388–H2398. doi: 10.1152/ajpheart.00185.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Pearce CG, Najjar SF, Kapadia MR, Murar J, Eng J, Lyle B, et al. Beneficial effect of a short-acting NO donor for the prevention of neointimal hyperplasia. Free Radic Biol Med. 2008 Jan 1;44(1):73–81. doi: 10.1016/j.freeradbiomed.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Varu VN, Ahanchi SS, Hogg ME, Bhikhapurwala HA, Chen A, Popowich DA, et al. Insulin enhances the effect of nitric oxide at inhibiting neointimal hyperplasia in a rat model of type 1 diabetes. Am J Physiol Heart Circ Physiol. 2010 Sep;299(3):H772–H779. doi: 10.1152/ajpheart.01234.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Waterhouse DJ, Saavedra JE, Davies KM, Citro ML, Xu X, Powell DA, et al. Injectable formulation of disodium 1-[2-(carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2-diolate (PROLI/NO), an ultrafast nitric oxide donor prodrug. J Pharm Sci. 2006 Jan;95(1):108–15. doi: 10.1002/jps.20486. [DOI] [PubMed] [Google Scholar]
- (28).Yu SM, Hung LM, Lin CC. cGMP-elevating agents suppress proliferation of vascular smooth muscle cells by inhibiting the activation of epidermal growth factor signaling pathway. Circulation. 1997 Mar 4;95(5):1269–77. doi: 10.1161/01.cir.95.5.1269. see comments. [DOI] [PubMed] [Google Scholar]
- (29).Gunther S, Alexander RW, Atkinson WJ, Gimbrone MA. Functional angiotensin-Ii receptors in cultured vascular smooth-muscle cells. J Cell Biol. 1982;92(2):289–98. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol Red in Tissue-Culture Media Is A Weak Estrogen - Implications Concerning the Study of Estrogen-Responsive Cells in Culture. P Natl Acad Sci USA. 1986 Apr;83(8):2496–500. doi: 10.1073/pnas.83.8.2496. P Natl Acad Sci USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Sarkar R, Gordon D, Stanley JC, Webb RC. Cell cycle effects of nitric oxide on vascular smooth muscle cells. Am J Physiol. 1997 Apr;272(4 Pt 2):H1810–H1818. doi: 10.1152/ajpheart.1997.272.4.H1810. [DOI] [PubMed] [Google Scholar]
- (32).Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996 Feb;78(2):225–30. doi: 10.1161/01.res.78.2.225. [DOI] [PubMed] [Google Scholar]
- (33).Ling SH, Dai AZ, Dilley RJ, Jones M, Simpson E, Komesaroff PA, et al. Endogenous estrogen deficiency reduces proliferation and enhances apoptosis-related death in vascular smooth muscle cells - Insights from the aromatase-knockout mouse. Circulation. 2004 Feb 3;109(4):537–43. doi: 10.1161/01.CIR.0000109699.45186.30. [DOI] [PubMed] [Google Scholar]
- (34).Mnjoyan ZH, Doan D, Brandon JL, Felix K, Sitter CL, Rege AA, et al. The critical role of the intrinsic VSMC proliferation and death programs in injury-induced neointimal hyperplasia. American Journal of Physiology-Heart and Circulatory Physiology. 2008 May;294(5):H2276–H2284. doi: 10.1152/ajpheart.91527.2007. [DOI] [PubMed] [Google Scholar]
- (35).Mendelsohn ME. Nongenomic, estrogen receptor-mediated activation of endothelial nitric oxide synthase - How does it work? What does it mean? Circulation Research. 2000 Nov 24;87(11):956–60. doi: 10.1161/01.res.87.11.956. [DOI] [PubMed] [Google Scholar]
- (36).Mendelsohn ME. Genomic and nongenomic effects of estrogen in the vasculature. Am J Cardiol. 2002 Jul 3;90(1A):3F–6F. doi: 10.1016/s0002-9149(02)02418-9. [DOI] [PubMed] [Google Scholar]
- (37).Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002 Jun;89(12A):12E–7E. doi: 10.1016/s0002-9149(02)02405-0. %20. [DOI] [PubMed] [Google Scholar]