Abstract
Fibromyalgia (FM) is a chronic widespread musculoskeletal pain disorder that is very prevalent in the general population (approx. 5%). Accumulating evidence suggests that FM is associated with central pain processing abnormalities, i.e. central sensitization. Several previous studies of chronic pain patients, including FM, have shown gray matter atrophy of brain areas associated with sensory and affective pain processing. These findings, however, have not been confirmed in all FM studies. In this study, we investigated gray matter volumes of brain areas associated with pain-related areas of FM patients identified by functional brain imaging. Using voxel-based morphometric (VBM) analysis of magnetic resonance brain images, we compared 19 pain related brain areas of 14 female FM patients and 11 healthy controls (NC). We found that FM patients had significantly less gray matter volumes than NC in three of these brain regions, including the anterior and mid-cingulate, as well as mid-insular cortices. Importantly, FM patients neither demonstrated global gray matter atrophy nor gray matter changes associated with depression, as shown in some studies. Using a more stringent analysis than other VBM studies, we provide evidence for decreased gray matter volumes in a number of pain related brain areas in FM. Although the mechanisms for these gray matter changes are presently unclear, they may contribute to some of the core features of this chronic disorder including affective disturbances and chronic widespread pain
Keywords: VBM, fibromyalgia, pain
Introduction
Clinical symptoms of chronic musculoskeletal conditions like fibromyalgia (FM) include pain, stiffness, subjective weakness, and muscle fatigue. Pain in FM is usually described as fluctuating and always associated with local or generalized tenderness (hyperalgesia and/or allodynia). FM-related tenderness depends on increased peripheral and central nervous system responsiveness to stimulation of muscle and other deep tissues and is manifested as hyperalgesia or allodynia. The pathogenesis of such peripheral and central nervous system changes in FM is unclear, but peripheral, spinal and supra-spinal changes have been implicated 10,40.
Parallel lines of research suggest that chronic pain disorders, including FM are associated with not only functional neuronal plasticity, but also changes in brain morphology 25. The impact of chronic pain on nervous system structures has primarily been studied in animal models 17,19,52. These studies not only showed reorganization of nociceptive coding but also evidence for atrophy of dorsal horn neurons 9,26,48. The large majority of these studies demonstrated that chronic pain and stress-related disorders, including chronic low back pain, headache, post-traumatic stress disorder, and FM, may be accompanied by reductions in gray matter of various brain regions 3,22,35,46. These brain areas have included regions within the thalamus, frontal cortex, anterior cingulate, insula, and parahippocampal gryus (May, A. 2008 for review). Although most of these studies report gray matter reductions, the implications and causal relationships associated with them remain uncertain. Nevertheless, voxel based morphometry (VBM) estimates of global gray matter decrease have been validated by close agreement with other MRI measures of local gray matter changes 24,31,45. However, only direct histological evidence can ultimately confirm tissue atrophy.
The clinical relevance of gray matter atrophy is uncertain because studies are lacking that examine brain function and structure within the same patient population or across chronic pain and control populations. Most critically, it is entirely unclear whether changes in gray matter occur in relationship to pain-processing. Consequently, the purpose of the current study was to determine whether differences in VBM volumes exist in pain-related brain regions of FM patients and normal controls (NC). Nineteen such regions were activated in both FM and NC subjects during temporal summation of second pain (TSSP or windup) in a recent fMRI study 41. This follow-up analysis allowed us to address three questions: 1) Are there reductions in gray matter in these 19 pain processing areas of FM patients? 2) Are these reductions associated with the magnitudes of neural responses in these regions? 3) Are these reductions associated with clinical pain and levels of negative affect?
We selected voxels of interest (VOIs) that were activated during TSSP in both FM and NC 41 and compared the gray matter volumes of these brain regions between groups. This strategy provided a basis for determining whether morphological changes in FM patients are related to afferent processing within the “pain matrix”.
Methods
Subjects
The study participants consisted of 11 middle-aged healthy pain-free female subjects [mean age (SD): 42.4 (9.8) years) and 14 female FM patients (43.1 (6.9) years] from the local community or FM support groups (for more details see 41). All FM patients fulfilled the 1990 American College of Rheumatology Criteria for FM 50. . Informed consent was obtained from all participants and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. The University of Florida Institutional Review Board approved the procedures and protocol for this study.
Experimental design
Using fMRI we have previously identified 19 brain areas of FM and NC subjects that become activated during TSSP 41. Briefly, we elicited TSSP by applying sensitivity adjusted repetitive heat pulses to the glabrous surface of the right foot of study subjects. During such repetitive heat pulses all subjects reported moderate pain which displayed latencies consistent with C-fiber activation 42,43.
Using painful stimuli several studies have shown increased activation of pain related brain areas in FM subjects compared to NC 7,14. However, when sensitivity adjusted stimuli were used to induce perceptually equivalent levels of pain 41, activations of pain-related brain regions were not statistically different across NC and FM groups for any of the 19 regions activated, including the thalamus. However, these sensitivity adjusted activations required lower stimulus intensities in FM as compared to NC. Thus, the causal relationships between neural output of the 19 regions and reported pain intensity were similar across both groups [equivalent magnitudes of response do not necessitate equivalent processing mechanisms]. To clarify whether the same amount of gray matter is used to generate these causal relationships, our current study compared the gray matter volumes among the 19 previously identified voxels of interest (VOIs) across FM and NC subjects 41.
Image acquisition
For more detailed information, see Staud et al., 2008. Briefly, MRI data were acquired on a research-dedicated head scanner (Siemens Allegra, 3.0 T) using a standard head RF coil. High-resolution 3D anatomical images were acquired using a T1- weighted MP-RAGE protocol (128 1-mm axial slices; TR = 2000 ms, TE = 4.13 ms, FA = 8°, matrix = 256 × 256 mm, FOV = 24 cm). Data were analyzed with a Xeon dual-processor 3.4 GHz workstation using BrainVoyager (BVQX 2.1 – Brain Innovation, Maastricht, the Netherlands; www.brainvoyager.com).
Volumes of interest (VOIs)
We have previously reported on windup-related brain activity in discrete volumes of interest (VOIs) in healthy pain-free and FM subjects 41,42. VOIs were determined to be actively involved in TSSP if the voxel clusters of the resultant SPMs (statistical parameter maps) met the following criteria: (a) cluster volumes were at least 100 µL (i.e., 100 contiguous voxels); (b) the voxel cluster maintained integrity for both individual (Level 1) and group (Level 2) contrasts; (c) all of the voxels exceeded the significance test threshold of FDR < 0.02, p < 0.0005; and (d) the center-of-mass gravity for the voxel cluster was in an identifiable, conceptually meaningful brain region.
Because the neural activity in these brain regions was found to be functionally equivalent in both the FM and NC groups, the geographical boundaries of the VOIs was determined by the analyses of the fMRI data (data not shown here). Hence, the volumetric analyses were performed in a common standardized 3D (i.e., Talairach) space for all individuals.
Tissue Segmentation and Volumetric Analyses
To avoid any measurement bias, all of the 3D volumetric scans from NC and FM subjects were renamed by one of the authors. Subsequent volumetric measurements were performed by a different author who was blind to the renaming scheme. Using BrainVoyager QX the first step in the volumetric analyses was to prepare the 3D anatomical images for automatic tissue segmentation. This included checks for inhomogeneity, pre-segmentation, and inhomogeneity correction. The next step involved the visualization and manual correction of errors. Detailed information about these procedures can be found online at <http://wiki.brainvoyager.net/Segmentation_Guide>. The result of these steps was new 3D datasets for each subject representing the gray and white matter of each hemisphere. Once these new datasets were created, the number of gray matter voxels within the boundaries of each previously identified VOI were counted using Matlab R2008a and a toolbox created specifically for BrainVoyager. As each voxel is 1mm3 the total number of non-zero voxels in each VOI is equivalent to the volume (µL) of gray matter in that VOI. This procedure was replicated for all the VOIs across all the subjects in the study. As an additional control against the potential confound of systematic variation introduced during the warping (into standardized space) procedure the total cortical gray matter volume was calculated and used as a covariate during subsequent analyses.
Questionnaires
Medical College of Virginia Pain Questionnaire
All subjects were asked to complete the Medical College of Virginia (MCV) Pain Questionnaire 32,47. This questionnaire was used to characterize the study subjects. The MCV Pain Questionnaire has three domains, consisting of ratings of pain (VAS), negative emotions related to chronic pain (VAS), and the impact of pain on subjects’ lives (VAS). In addition, they were asked to complete Beck’s Depression Inventory (BDI) 4 and the Spielberger State/Trait Anxiety Questionnaires 39. The BDI is a self-administered 21 item self-report rating inventory measuring characteristic attitudes and symptoms of depression. Scores can range from 0 – 63. A score of 19 and higher is indicative of clinical depression. Spielberger's State/Trait Anxiety Inventory consists of 20 items each that ask how a person feels now, and reflects situational factors that may influence anxiety levels. Scores range from 20 to 80 and the higher the score the greater the level of anxiety.
Statistical Analysis
Using SPSS 17.0 (Chicago, USA) group differences of mood measures and gray matter volumes were examined utilizing independent t-tests
Results
Ratings of negative affect
The mean (SD) score on the Beck Depression Inventory (BDI) [range 0–63] for the control subjects was 2.6 (3.9) and their Spielberger State/Trait Anxiety scores were 29.7 (9.1) and 45.6 (6.6), respectively. The mean BDI score for patients with FM was 13.2 (9.5) and their state/trait anxiety scores were 33.9 (3.4) and 43.1 (3.4), respectively. Compared to control subjects, independent sample t-test of the BDI scores revealed significantly higher scores among patients with FM (t(27) = 2.9; p < .05). The elevated BDI scores suggests that patients with FM may experience low levels of depression, however, the scores overall are well below the cut-off for major depressive episodes [i.e., scores > 20] 13. The Spielberger State/Trait anxiety scores were low and statistically equivalent in both groups (i.e., p > .05).
Ratings of somatic and experimental pain
Healthy control subjects did not report somatic pain prior, or after the fMRI scans. Conversely, patients with FM rated their baseline pain as 2.9 (1.2) VAS units. Their pain ratings significantly increased after the fMRI scans to 3.7 (1.4) (t(22) = 1.24; p < .05).
As reported previously 41, all subjects rated their level of pain for the last stimulus of either 2-pulse or 6-pulse trains of thermal stimuli presented at 0.33 Hz and 0.17 Hz. Because sensitivity adjusted stimuli were used for each subject, no main effect for group was found (p > .05). The results identified the presence of a frequency-by-stimulus number interaction (p < .001), indicating that in both groups greater pain was associated with the 6-pulse train presented at 0.33 Hz compared to the 6-pulse train at 0.17 Hz..
Voxel Based Morphometry (VBM)
Global Gray Matter Analysis
The total gray matter volume of NC and FM subjects was 628,680 µL and 576,022 µL respectively. There was no statistically significant difference between the total volumes of gray matter of NC and FM subjects (p > .05).
Gray matter volumes of TSSP activated Volumes of Interest (VOIs) as determined by fMRI
As shown above, TSSP resulted in robust activation of pain related brain areas in NC and FM subjects 41. Those analyses resulted in the identification of 19 VOIs with similar magnitude of activation in both groups (Table 1).
Table 1.
TSSP-Related VOIs for Pain-Free NC and FM Subjects
| Talairach Coordinates | Cluster Volume | ||||
|---|---|---|---|---|---|
| Hemisphere | Structure | X | Y | Z | (µL) |
| Left | THAL, Lateral Posterior Nucleus | −15 | −21 | 13 | 2325 |
| Right | THAL, Lateral Posterior Nucleus | 16 | −20 | 13 | 1651 |
| Left | THAL, Medial Dorsal Nucleus | 0 | −16 | 9 | 396 |
| Right | S1, BA 2,3,5 | 3 | −38 | 62 | 101 |
| Left | S1, BA 3,5 | −4 | −33 | 70 | 171 |
| Right | S2, BA 40 | 50 | −37 | 26 | 2092 |
| Left | S2, BA 40 | −51 | −44 | 29 | 1077 |
| Left | S2, BA 40 | −51 | −29 | 24 | 846 |
| Left | Inferior Parietal Lobule, BA 40 | −51 | −30 | 25 | 911 |
| Left | Post INS | −33 | −18 | 8 | 770 |
| Left | Mid INS | −34 | −2 | 8 | 984 |
| Left | Dorsal ACC, BA 31 | −12 | −31 | 44 | 1984 |
| Left | Rostral ACC, BA 24 | −3 | 11 | 31 | 2060 |
| Left | Mid ACC, BA 24 | −3 | −12 | 42 | 1589 |
| Right | Precentral Gyrus, BA 13 | 48 | −10 | 12 | 307 |
| Right | Inferior Frontal Gyrus, BA 47 | 45 | 19 | −4 | 156 |
| Left | Medial Frontal Gyrus, BA 9 | −8 | 43 | 17 | 295 |
| Right | Superior Temporal Gyrus, BA 13 | 47 | −42 | 25 | 1436 |
| Midline | Cerebellum | 3 | −55 | −22 | 473 |
BA: Brodman’s area; THAL: thalamus; S1: somato-sensory cortex 1; S2: somato-sensory cortex 2; INS: insula; ACC: anterior cingulate cortex
Thus we examined these 19 brain regions for potential structural differences between NC and patients with FM. More specifically, for this study, we used voxel-based morphometry (VBM) analyses to determine whether there were significant group differences in the gray matter volume of the 19 VOIs.
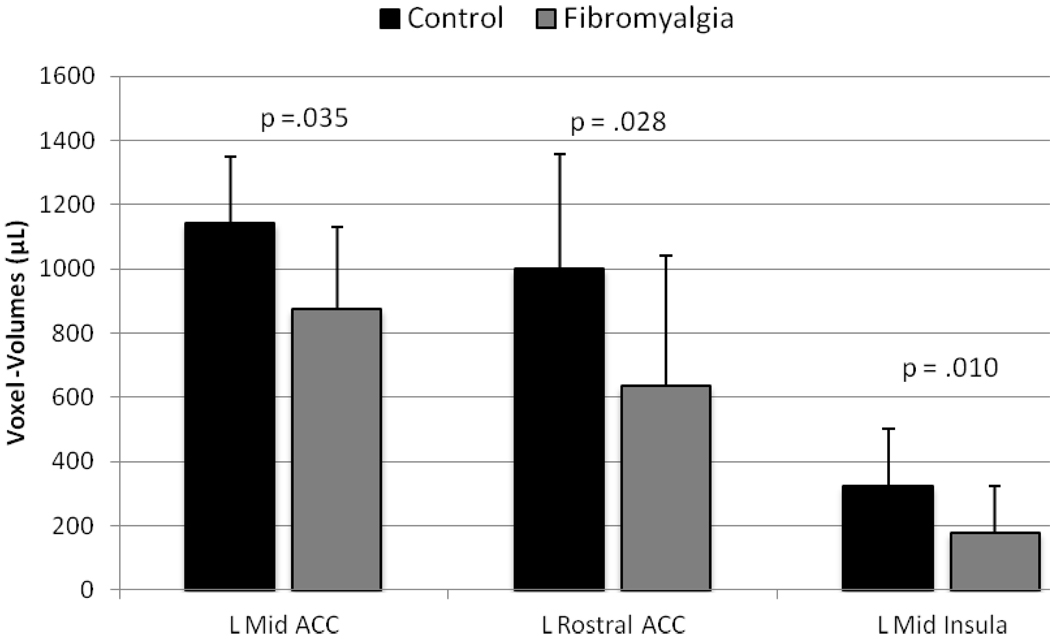
Using independent samples t-tests three of the 19 VOIs showed significant lower gray matter volumes in FM subjects compared to NC (left mid insula, left dorsal anterior cingulate cortex, and left rostral cingulate cortex) (Table 2). In all three areas, subjects with FM had significantly less gray matter compared to control participants (Figure 1).
Table 2.
Independent Samples t-Test for Gray Matter
| t | df | Sig. (2-tailed) | Cohen’s d | N to detect | |
|---|---|---|---|---|---|
| (power = .8, alpha = .05) | |||||
| L_THAL | .660 | 23 | .516 | .27 | 420 |
| R_THAL | −.031 | 23 | .976 | .017 | 109,792 |
| L_THAL_Medial | −.507 | 23 | .617 | .19 | 792 |
| R_S1_BA3 | −1.601 | 23 | .123 | .66 | 74 |
| R_S2_BA40 | −1.711 | 23 | .101 | .75 | 58 |
| L_S2_BA40a | −1.005 | 23 | .325 | .41 | 94 |
| L_S2_BA40b | −.575 | 23 | .571 | .23 | 562 |
| L_Inf_ParietLob | −.707 | 23 | .487 | .29 | 378 |
| L_Post_INS | −1.945 | 23 | .064 | .77 | 56 |
| L_Mid_INS | −2.244 | 23 | .035* | .89 | 42 |
| L_Dorsal_ACC | −1.103 | 23 | .281 | .44 | 164 |
| L Rostral ACC | −2.347 | 23 | .028* | .95 | 38 |
| L_Mid_ACC | −2.796 | 23 | .010* | 1.4 | 28 |
| R_PreCenGyrus | −1.840 | 23 | .079 | .7 | 60 |
| R_Inf_FrntGyrus | −1.187 | 23 | .248 | .48 | 136 |
| L_Med_FrntGyrus | −1.496 | 23 | .148 | .6 | 90 |
| R_Sup_TempGyrus | −1.045 | 23 | .307 | .42 | 182 |
| Cerebellum | −1.589 | 23 | .126 | .65 | 78 |
Figure 1.
Average (SD) group differences in gray matter volumes of pain related brain areas; L = left; ACC = anterior cingulated cortex.
Effects of Negative Affect on Gray Matter Volume
Individuals suffering from chronic pain often report elevated ratings on measures of negative affect and mood. Moreover, the presence of affective spectrum disorders has been reported to account for the observed gray matter loss reported in FM subjects 16. Thus we analyzed measures of depression, anxiety, frustration, anger, and fear for group differences. As can be seen in Table 3, individuals with FM had significantly higher scores on all measures of mood. However, no significant correlation between the measures of negative affect and the gray matter volume was identified for any VOI. (all p >.05)
Table 3.
Independent Samples t-Test for Group Differences on Mood Measures
| Group Statistics | |||||
|---|---|---|---|---|---|
| Group | N | Mean | Std. Deviation | p-value | |
| BDI total score | FM | 14 | 13.5000 | 7.99760 | |
| .001 | |||||
| NC | 10 | 2.7000 | 3.16403 | ||
| Depression | FM | 13 | 34.3846 | 23.85560 | |
| .001 | |||||
| NC | 10 | 4.0000 | 9.66092 | ||
| Anxiety | FM | 13 | 37.6154 | 15.21260 | |
| .001 | |||||
| NC | 10 | 8.5000 | 18.86355 | ||
| Frustration | FM | 13 | 50.3077 | 21.55375 | |
| .000 | |||||
| NC | 10 | 8.5000 | 18.86355 | ||
| Anger | FM | 13 | 33.4615 | 25.93137 | |
| .001 | |||||
| NC | 10 | .0000 | .00000 | ||
| Fear | FM | 13 | 34.0769 | 26.65977 | |
| .001 | |||||
| NC | 10 | 1.0000 | 3.16228 | ||
Mood measures were obtained by visual analogue scale ratings (0–100) using the MCV
Discussion
Previous VBM analyses of gray matter changes in chronic pain patients have shown inconsistent results. Whereas most studies have demonstrated gray matter atrophy of the prefrontal cortex, ACC, insula, and thalamus 5,22, others reported increased gray matter in the basal ganglia and other brain areas 36. These inconsistencies may be related to functional differences between pain conditions, but also to subject variability and analytical technique. To control for inter-individual variability of pain related brain activation we compared only FM and NC subjects whose functional pain related brain areas had been mapped in a previous fMRI study. These functional pain-related brain areas were subsequently selected for gray matter comparisons unlike most other VBM studies which compared brain areas solely according to stereotactic coordinates. Using such stringent criteria, this study provided three important and novel findings. First, three of the 19 brain regions (left mid insula, left rostral ACC, and left mid ACC) had less gray matter in FM as compared to NC subjects, despite no difference in overall brain gray matter (Figure 2). Second, group differences in gray matter volumes of these three brain areas were not associated with group differences in neural responses to painful stimuli as shown by Staud et al., 2008. Finally, reductions in gray matter could not be accounted for by differences in pain related negative affect, such as depression. VBM comparison between groups, however, were not statistically different for the majority of areas of the “pain matrix”, including thalamus, S-1, S-2, and posterior insular cortex.
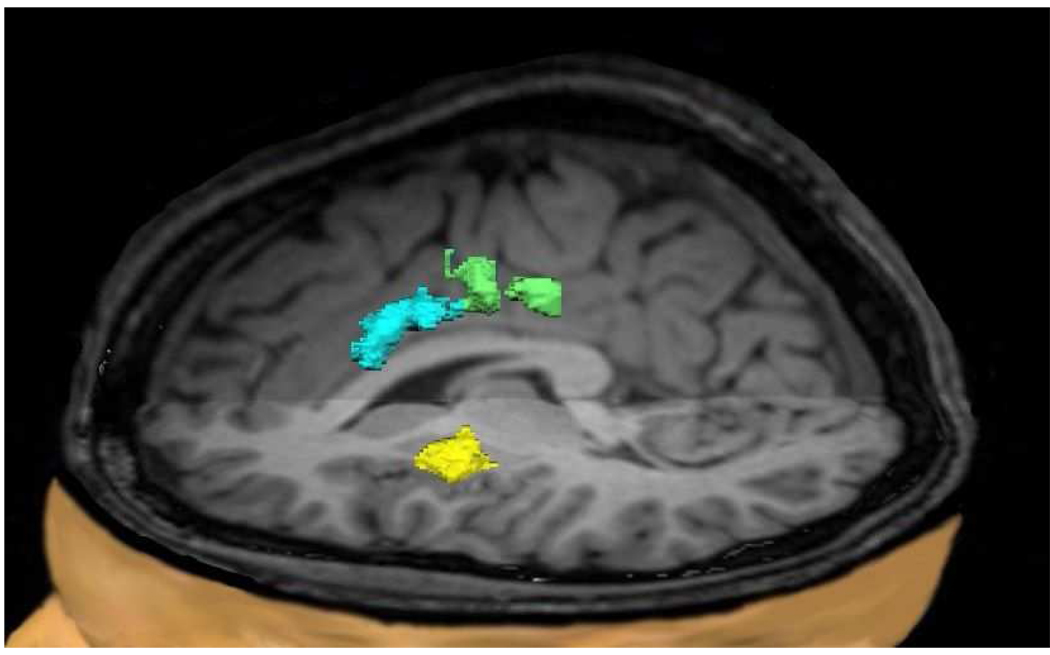
Figure 2.
Pain related brain areas showing decreased gray matter densities in FM patients compared to NC subjects. These areas include the left rostral (blue) and mid-ACC (green), as well as left mid-insula (yellow).
Similar to a previous VBM study 16 we were unable to replicate global gray matter difference between FM patients and NC as reported previously 22. In addition, we were unable to confirm reductions in regional gray matter in many previously reported VOIs (left parahippocampal gyrus, left and right mid/posterior cingulated, medial frontal cortex 22; bilateral striatum, and left thalamus 36). One possible reason for these discrepancies may be that our stringent VBM analysis did not provide enough power to detect subtle differences of some pain related brain areas between NC and FM patients.
Altered Gray Matter Volumes in Chronic Pain Patients
Alterations of resting and stimulus-evoked regional cerebral blood flow have been described in a number of functional brain imaging studies of FM patients. These studies provided evidence for altered CNS processing in pain related brain areas such as the thalamus, somatosensory cortex, insula, and anterior cingulate cortex 41,49. In contrast to functional brain imaging, structural neuroimaging techniques – such as VBM – use differences in gray matter volume or density to explore abnormalities of CNS function. VBM has been used to study abnormalities in gray matter associated with many chronic pain conditions, including vulvodynia 37, chronic fatigue syndrome 27, irritable bowel syndrome 38, tension headache 33, chronic back pain 3,34, and FM 5,22,23,51.
Gray Matter Volumes and Depression
Several neuro-imaging studies have reported reduced hippocampal volumes in patients with major depression 1,12,20. Extensive neuromorphologic abnormalities in the hippocampus, DLPFC, and anterior cingulum, particularly during the course of depression, seem to be clinically associated with more severe depression. The VBM results for other brain regions have been inconsistent. For example, enlarged amygdala volumes and reduced volumes of the ACC and the prefrontal cortex have been reported in some investigations using VBM, suggesting alterations in the fronto-limbic network 6. Basal ganglia volumes were frequently reduced in patients with major depression, but this was more likely in late-onset depression 18,21. When FM patients with affective spectrum disorders (AD) were compared to NC a reduction in gray matter volume of the left anterior insula was detected in FM patients 16. However, this difference disappeared when only FM patients without AD were compared to healthy controls. Our study also detected gray matter atrophy of the left anterior insula in FM patients. This result, however, did not change after controlling for depression. Although it appears that decreased gray matter atrophy in the left anterior insula can be attributed to affective disturbance, other factors like chronic pain or stress may also impact this particular brain area.
Association of Chronic Pain with Brain Atrophy
In recent years the representation of pain in the CNS has been extensively studied with various brain imaging techniques, including fMRI, SPECT, and PET (Price & Bushnell, 2004). Despite the use of different techniques, equipment, and statistical criteria, there has been considerable consistency in brain regions involved in pain related processes in NC and pain populations alike 28,30. Similar to our study 41, neuroimaging investigations have consistently identified multiple brain regions (i.e., ACC, insula, somatosensory cortex, thalamus, dorsolateral prefrontal cortex) associated with pain in chronic pain patients 8,29. The findings from imaging and behavioral studies suggest a functional plasticity of the brain circuitry that is consistent with theories of central sensitization, which is likely a key factor in the development and/or maintenance of chronic pain conditions 11,44. A possible explanation for the decreased gray matter volumes in chronic pain disorders might be atrophy secondary to excitotoxicity and/or exposure to inflammatory cytokines 3. It is also noteworthy that gray matter loss of FM patients not only occurred in regions associated with pain processing, i.e. cingulate, insular, and prefrontal cortices 2, but also occurred in brain areas related to stress responding i.e. parahippocampal gyrus 15. Because the ACC has been implicated in pain modulation 2 (i.e., inhibition and facilitation of pain), atrophy of this area could contribute to abnormal pain processing of FM patients. Prospective studies will be necessary to determine whether the observed structural changes are the cause or the consequence of FM.
Limitations
Our interpretation of observed differences in ACC and insula between NC and FM patients entailed the use of rather liberal interpretations of statistical significance. If we had employed conservative adjustments for multiple comparisons (ie. Bonferroni), it is likely that our results would not have reached statistical reliability. However, examination of statistical effect sizes of both the significant (p < .05) and non-significant comparisons (Table 2), showed the magnitude of the group differences ranging from very small (.017) to large (1.4), with the majority of the effects in the moderate range (.4 to .7). Our analyses represented a priori hypotheses about specific regions of interest, rather than a whole brain analysis approach which would have capitalized on chance findings. Moreover, several previous reports also described decreased gray matter volumes in the ACC and insula of FM patients, thus confirming our analysis 22,23,51. Because a larger sample size may demonstrate a more definitive association of brain plasticity with chronic pain, further investigations are needed.
Conclusions
Our results extend and corroborate those of previous studies in demonstrating decreased gray matter volumes in 3 of 19 brain areas involved in pain processing in FM, on the basis of previously acquired fMRI data. However, these reductions in gray matter were neither distributed throughout the “pain matrix” nor associated with depression. Further research is needed to determine whether they reflect mechanisms of reduced pain inhibition or selective alterations in later stages of pain processing. The lack of gray matter differences at 16 of the 19 pain-related areas, especially those reflecting earlier stages of processing (e.g., thalamus, S-1) suggest that additional mechanistic factors are involved in abnormal afferent pain processing in FM.
Perspective
Increasing evidence supports the association of chronic pain with accelerated gray matter atrophy in pain disorders like low back pain, IBS, and FM syndrome. However, cause-effect relationships between chronic pain and decreased gray matter volumes have not been established yet and will require future prospective studies.
Acknowledgments
This work was supported by NIH grants NS041670 and AR053541. The expert technical assistance of Amber M. Schwier, Elena M. Moran, and Tyler Robinson is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors had any conflicts of interest.
References
- 1.Andreescu C, Butters MA, Begley A, Rajji T, Wu MJ, Meltzer CC, Reynolds CF, Aizenstein H. Gray matter changes in late life depression - a structural MRI analysis. Neuropsychopharmacology. 2008;33:2566–2572. doi: 10.1038/sj.npp.1301655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. In: Pichot P, editor. Psychological measurements in psychopharmacology. Basel: Karger; 1974. pp. 151–169. [Google Scholar]
- 5.Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, Pfleiderer B. Decreased Gray Matter Volumes in the Cingulo-Frontal Cortex and the Amygdala in Patients With Fibromyalgia. Psychosom Med. 2009;71:566–573. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- 6.Campbell TS, Hughes JW, Girdler SS, Maixner W, Sherwood A. Relationship of ethnicity, gender, and ambulatory blood pressure to pain sensitivity: Effects of individualized pain rating scales. J Pain. 2004;5:183–191. doi: 10.1016/j.jpain.2004.02.305. [DOI] [PubMed] [Google Scholar]
- 7.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–378. [PubMed] [Google Scholar]
- 8.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Novellis V, Siniscalco D, Galderisi U, Fuccio C, Nolano M, Santoro L, Cascino A, Roth KA, Rossi F, Maione S. Blockade of glutamate mGlu5 receptors in a rat model of neuropathic pain prevents early over-expression of pro-apoptotic genes and morphological changes in dorsal horn lamina II. Neuropharmacology. 2004;46:468–479. doi: 10.1016/j.neuropharm.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48:1420–1429. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 11.Eriksen HR, Ursin H. Sensitization and subjective health complaints. Scand J Psychol. 2002;43:189–196. doi: 10.1111/1467-9450.00286. [DOI] [PubMed] [Google Scholar]
- 12.Frodl TS, Koutsouleris N, Bottlender R, Born C, Jager M, Scupin I, Reiser M, Moller HJ, Meisenzahl EM. Depression-related variation in brain morphology over 3 years - Effects of stress? Archives of General Psychiatry. 2008;65:1156–1165. doi: 10.1001/archpsyc.65.10.1156. [DOI] [PubMed] [Google Scholar]
- 13.Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain. 1997;13:163–170. doi: 10.1097/00002508-199706000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 15.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, Williams DA. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143:262–267. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- 18.Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Na C, Escalona PR, Boyko OB, Nemeroff CB, Krishnan KR. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40:95–99. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 19.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 20.Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Pol HEH, Kahn RS. Brain Volume Abnormalities in Major Depressive Disorder: A Meta-Analysis of Magnetic Resonance Imaging Studies. Hum Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnan KR, Hays JC, Tupler LA, George LK, Blazer DG. Clinical and phenomenological comparisons of late-onset and early-onset depression. Am J Psychiatry. 1995;152:785–788. doi: 10.1176/ajp.152.5.785. [DOI] [PubMed] [Google Scholar]
- 22.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, Beyer A, Stahl R, Zirngibl B, Morhard D, Reiser M, Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: A diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- 24.Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada T, Tanaka M, Kuratsune H, Watanabe Y, Sadato N. Mechanisms underlying fatigue: a voxel-based morphometric study of chronic fatigue syndrome. BMC Neurol. 2004;4:14. doi: 10.1186/1471-2377-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 29.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Price DD, Verne GN, Schwartz JM. Plasticity in brain processing and modulation of pain. In: Moller AR, editor. Reprogramming the Brain. Amsterdam, the Netherlands: Elsevier; 2006. pp. 333–352. [DOI] [PubMed] [Google Scholar]
- 31.Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 32.Riley JL, Robinson ME, Price DD. The stages of pain processing across the adult lifespan. J Pain. 2000;1:162–170. [Google Scholar]
- 33.Schmidt-Hansen PT, Svensson P, Bendtsen L, Graven-Nielsen T, Bach FW. Increased muscle pain sensitivity in patients with tension-type headache. Pain. 2007;129:113–121. doi: 10.1016/j.pain.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt-Wilcke T, Leinisch E, Straube A, Kampfe N, Draganski B, Diener HC, Bogdahn U, May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt-Wilcke T, Luerding R, Weigand T, Jurgens T, Schuierer G, Leinisch E, Bogdahn U. Striatal grey matter increase in patients suffering from fibromyalgia - A voxel-based morphometry study. Pain. 2007;132:116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Schweinhardt P, Kuchinad A, Pukall CF, Bushnell MC. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411–419. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional Gray Matter Density Changes in Brains of Patients with Irritable Bowel Syndrome. Gastroenterology. 2010;139:48–57. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (STAI) (Self Evaluation Questionnaire) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 40.Staud R. Abnormal pain modulation in patients with spatially distributed chronic pain: Fibromyalgia. Rheum Dis Clin North Am. 2009;35:263–274. doi: 10.1016/j.rdc.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12:1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD. Brain activity related to temporal summation of C-fiber evoked pain. Pain. 2007;129:130–142. doi: 10.1016/j.pain.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 44.Ursin H, Eriksen HR. Sensitization, subjective health complaints, and sustained arousal. Ann N Y Acad Sci. 2001;933:119–129. doi: 10.1111/j.1749-6632.2001.tb05819.x. [DOI] [PubMed] [Google Scholar]
- 45.Vargha-Khadem F, Watkins KE, Price CJ, Ashburner J, Alcock KJ, Connelly A, Frackowiak RS, Friston KJ, Pembrey ME, Mishkin M, Gadian DG, Passingham RE. Neural basis of an inherited speech and language disorder. Proc Natl Acad Sci U S A. 1998;95:12695–12700. doi: 10.1073/pnas.95.21.12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- 47.Wade JB, Dougherty LM, Archer CR, Price DD. Assessing the stages of pain processing: a multivariate analytical approach. Pain. 1996;68:157–167. doi: 10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- 48.Whiteside GT, Munglani R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J Neurosci Res. 2001;64:168–173. doi: 10.1002/jnr.1062. [DOI] [PubMed] [Google Scholar]
- 49.Williams DA, Gracely RH. Functional magnetic resonance imaging findings in fibromyalgia. Arthritis Res Ther. 2006:8. doi: 10.1186/ar2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin CM, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 51.Wood PB, Glabus MF, Simpson R, Patterson JC. Changes in Gray Matter Density in Fibromyalgia: Correlation With Dopamine Metabolism. Journal of Pain. 2009;10:609–618. doi: 10.1016/j.jpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 52.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]