Abstract
The 40S ribosomal protein S6 kinase (S6K) acts downstream of the mammalian target of rapamycin (mTOR), which plays important roles in cell proliferation, protein translation and cell survival and is a target for cancer therapy. mTOR inhibitors are, however, of limited success. Although Akt is believed to act upstream of mTOR, persistent inhibition of p70 S6 kinase or S6K1 can activate Akt via a negative feedback loop. S6K exists as two homologs, S6K1 and S6K2 but little is known about the function of S6K2. In the present study, we have examined the effects of S6K2 on Akt activation and cell survival. Silencing of S6K1 caused a modest decrease whereas knockdown of S6K2 caused a substantial increase in tumor necrosis factor-α (TNF)- and TNF-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. In contrast to S6K1, depletion of S6K2 by siRNA decreased basal and TNF-induced Akt phosphorylation. Ectopic expression of constitutively-active Akt in MCF-7 cells restored cell survival in S6K2-depleted cells. We have previously shown that activation of Akt induces downregulation of Bid via p53. Knockdown of S6K2 caused an increase in p53 and downregulation of p53 by siRNA decreased Bid level. Silencing of Bid blunted the ability of S6K2 deficiency to enhance TNF-induced apoptosis. Taken together, our study demonstrates that the two homologs of S6K have distinct effects on Akt activation and cell survival. Thus, targeting S6K2 may be an effective therapeutic strategy to treat cancers.
Keywords: S6K, Akt, Bid, TNF, p53, apoptosis, breast cancer
INTRODUCTION
Akt or protein kinase B (PKB), a serine/threonine kinase, is the cellular homolog of the oncogene product v-Akt (1). It is activated downstream of phosphatidyl inositol-3-kinase (PI3K) in response to growth factors or cytokines. Akt performs diverse cellular functions, including cell growth, proliferation and survival (2). It is deregulated in many cancers, including breast cancer and confers resistance to chemotherapeutic drugs (3). Phosphorylation of Akt at Thr308 and Ser473 sites results in its activation (4).
Tumor necrosis factor-α (TNF) was originally identified as a cytokine that induces necrosis in tumors and regression of cancer in animals (5). It causes selective destruction of tumor tissues but has no effect on normal tissues (6). The presence of antiapoptotic proteins, however, can counteract cell death mediated by TNF. It has been reported that TNF causes activation of Akt through phosphorylation at Ser473 (7). Binding of TNF to its cell surface receptors causes activation of initiator caspase-8 followed by activation of effector caspases, such as caspase-3 and -7, resulting in the cleavage of critical cellular proteins and cell death (8, 9). Although caspase-8 is the apical caspase in the death receptor pathway, there is cross-talk between the receptor-initiated and mitochondrial pathway (10–12). The members of the Bcl-2 family proteins play important roles in regulating the intrinsic or mitochondrial cell death pathway (13, 14). Caspase-8 catalyzes the cleavage of the Bcl-2 family protein Bid (10–12). The truncated Bid (tBid) translocates to mitochondria causing release of cytochrome c and activation of caspase-9 (10–12). It has been reported that Akt can exert its antiapoptotic function by inhibiting the function of proapoptotic Bcl-2 family proteins (15–20).
Several cellular functions of Akt are mediated by the mammalian target of rapamycin (mTOR), which is considered the master controller of protein synthesis and cell proliferation (21). Activated Akt can phosphorylate and inactivate tuberous sclerosis complex 2 (TSC2), which negatively regulates mTOR (22). mTOR interacts with either raptor or rictor to form mTOR complex I (mTORC1) or mTOR complex 2 (mTORC2), respectively (22). While phosphoinositide-dependent kinase 1 (PDK1), which acts downstream of PI3K, phosphorylates Akt at Thr308 site, rictor complexed with mTORC2 can phosphorylate Akt at Ser473 (22). mTORC1 is inhibited by rapamycin, which is currently being tested for use in cancer therapy albeit with limited success (23).
The 40S ribosomal protein S6 kinase (S6K) is a downstream target of mTORC1 (24). S6K is represented by two homologous cellular proteins, S6K1 and S6K2, both of which act downstream of mTOR and phosphorylate S6 (25). Persistent inhibition of S6K1 has been shown to activate Akt via feedback inhibition of the PI3K pathway where S6K1 phosphorylates several sites on insulin receptor substrate-1 (IRS-1) and inhibits it (26–30). The limited therapeutic efficacy of rapamycin and its analogs has been attributed to the activation of Akt via this negative feedback loop due to inhibition of S6K1 (26, 29)and the inability of rapamycin to completely activate 4E-BP, another downstream target of mTORC1 (31–33).
Although there are two homologs of S6K(25, 34), most of the studies have been focused on S6K1 and little is known about the function of S6K2. S6K1-deficient mice phosphorylated S6 but had a small body phenotype (35). S6K1/2 double knockout mice also exhibit normal proliferation and growth reduction (36). Similarly, S6K1/2 double knockout mouse embryo fibroblasts and myoblasts show defects in size but not proliferation (31, 36, 37). These results suggest that these two homologs have redundant as well as non-overlapping functions. It has been reported that S6K2 but not S6K1 was important for FGF2-induced chemoresistance of small cell lung cancer cells (38). A recent study demonstrated that S6K2 but not S6K1 was important for cell proliferation in response to mTOR activation (39).
Since the Akt/mTOR/S6K axis plays a critical role in cell survival yet targeting mTOR has been of limited success due to feedback activation of Akt, we have examined if the two homologs of S6 kinase perform distinct functions in mediating breast cancer cell survival. We report for the first time that S6K2 regulates cell survival via the Akt pathway. We have shown that in contrast to S6K1, silencing of S6K2 inhibits Akt and induces cell death via the proapoptotic Bcl-2 family protein Bid. Thus, selective targeting of S6K2 rather than mTOR or S6K1 may be a more effective therapeutic strategy to treat cancers.
MATERIALS AND METHODS
Materials
Human recombinant TNF and TRAIL were purchased from R&D Systems (Minneapolis, MN). Monoclonal antibodies to PARP and p53, and polyclonal antibody to caspase-9 were obtained from Pharmingen (San Diego, CA). Polyclonal antibody to Akt, phospho-Akt (Ser473), S6K1 and phospho-FOXO3a were obtained from Cell Signaling Technology (Beverly, MA). Polyclonal antibody to S6K2 was from Santa Cruz Biotechnology (Santa Cruz, CA) and Bethyl Laboratories (Montgomery, TX). Polyclonal antibody to Bid and monoclonal antibody to caspase-8 were purchased from BioSource (Camarillo, CA). Actin was purchased from Sigma-Aldrich (St Louis, MO). Yo-Pro, annexin V conjugated to Alexa Fluor 488 and propidium iodide were purchased from Molecular Probes/Invitrogen (Carlsbad, CA). Caspase-3 fluorometric assay kit was obtained from BioVision (Palo Alto, CA, USA). Horseradish peroxidase conjugated goat anti-mouse and donkey anti-rabbit antibodies were obtained from JacksonImmuno Research Lab. Inc. (West Grove, PA). Control non-targeting siRNA and siRNA specific for S6K1, S6K2, Bid, Bax and p53 were obtained from Dharmacon (Lafayette, CO). Polyvinylidene difluoride membrane was from Millipore (Bedford, MA) and enhanced chemiluminescence detection kit was from Amersham (Arlington Heights, IL).
Cell Culture and Transfection
MCF-7 and ZR-75-1 cells were maintained in RPMI 1640 medium and MDA-MB-231 cells were maintained in DMEM supplemented with 10% fetal bovine serum and 2 mM glutamine. MCF-7 cells were obtained from Dr. Olivera J. Finn. ZR-75-1 and MDA-MB-231 cells were obtained from the UT Southwestern Medical Center. Cells were kept in a humidified incubator at 37°C with 95% air and 5% CO2. All these cells were authenticated by DNA fingerprinting at the UT Southwestern Medical Center and the Department of Forensic Genetics at the UNT Health Science Center. siRNA was transfected using Lipofectamine 2000 transfection reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA). Cells were infected with adenovirus vector containing GFP or constitutively-active (myrisotylated) Akt (MOI 10).
Immunoblot Analysis
Equivalent amounts of total cellular extracts were electrophoresed by SDS-PAGE and transferred electrophoretically to polyvinylidene difluoride membrane. Immunoblot analyses were performed as described before (40).
Cell Death Analysis
Cells were labeled with 0.5 μM YO-PRO-1 and 2 μM PI by incubating at 37°C for 15 min and visualized using a Zeiss Axiovert 40 inverted microscope with the AxioVision Rel 4.6 software (Zeiss, Göttingen, Germany).
Annexin V/Propidium Iodide Binding Assay
Cells were treated with or without TNF as indicated in the text. At the end of the incubation, both detached cells and attached cells were collected and washed with PBS. Cells were then stained with Annexin V-Alexa 488 conjugate and PI according to the manufacturer’s protocol and analyzed using a flow cytometer (Coulter Epics) (41).
Caspase assay
DEVDase activity was determined at 37°C using Ac-DEVD-AFC as the substrate and manufacturer’s protocol. The fluorescence liberated from DEVD-AFC was measured using a SpectraMax GeminiXS fluorometer and SOFTmax PRO 3.1.1 software (Molecular Devices, Sunnyvale, CA, USA) with an excitation wavelength of 400-nm and emission wavelength of 505 nm.
Statistical analysis
Data are presented as the mean ± S.E. and n = 4. Statistical significance was determined by paired Student’s t-test using PASW Statistics (SPSS, Inc., Chicago, IL). P< 0.05 was considered statistically significant.
RESULTS
S6K Homologs Exhibit Distinct Effects on TNF-Induced Apoptosis in Breast Cancer MCF-7 Cells
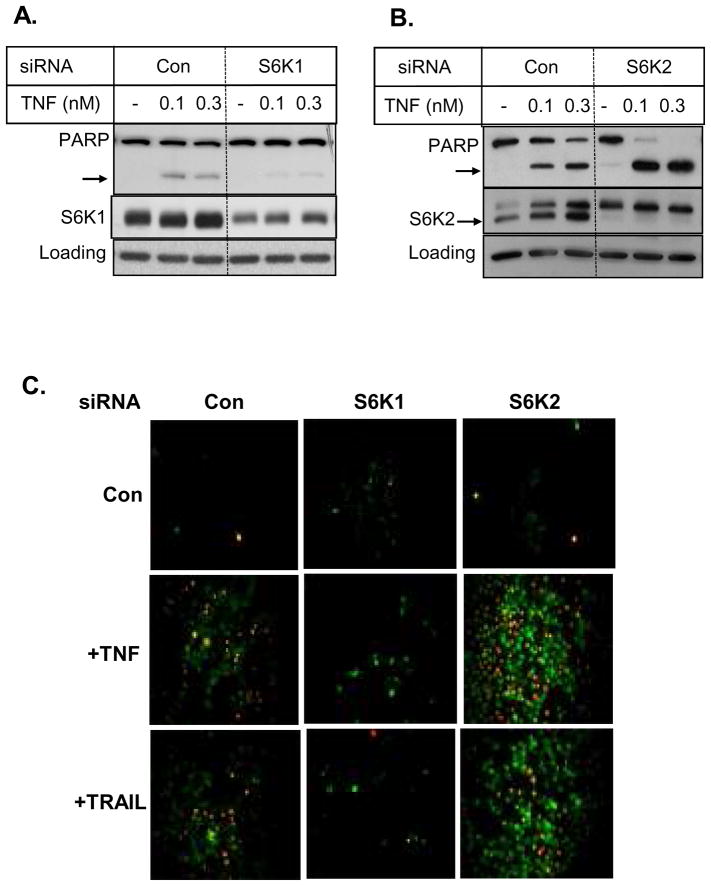
Since S6K1 is overexpressed in MCF-7 breast cancer cells (42)and has been associated with chemoresistance (43, 44), we examined if S6K1 confers resistance to TNF in MCF-7 breast cancer cells. Figure 1A shows that silencing of S6K1 by siRNA caused a modest decrease rather than an increase in the cleavage of PARP in response to TNF. Since there are two S6K homologs, we examined the effect of S6K2 knockdown on TNF-induced cell death. As shown in Figure 1B, depletion of S6K2 caused a substantial increase in TNF-induced cleavage of the 116-kDa full-length PARP to the 85-kDa form. We also monitored the effect of S6K1 and S6K2 knockdown on cell death by staining cells with YO-PRO-1 and PI (Fig. 1C). Apoptotic cells are permeable to the green fluorescent dye YO-PRO-1 whereas PI (red) is taken up only by necrotic and late-apoptotic cells. S6K2 depletion increased the number of YO-PRO-1/PI-stained cells in response to TNF and TRAIL while S6K1 depletion appears to decrease it. Thus, the two S6K homologs had distinct effects on TNF- and TRAIL-induced cell death.
Fig. 1.
S6K1 and S6K2 have distinct effects on breast cancer cell survival. MCF-7 cells were transfected with control non-targeting siRNA and either S6K1 (A) or S6K2 (B) siRNA and then treated with indicated concentrations of TNF. Western blot analysis was performed with indicated antibodies. Actin was used to control for loading differences. The arrow indicates 85-kDa cleaved PARP. C, MCF-7 cells transfected with control, S6K1 or S6K2 siRNA were treated without or with TNF or TRAIL. Cells were then stained with YO-PRO-1 (Green) to detect apoptotic cells and propidium iodide (red) to detect necrotic cells using a fluorescence microscope. Merged figures are shown. Results are representative of 3 independent experiments.
S6K Homologs Exert Opposite Effects on TNF-Induced Akt Phosphorylation
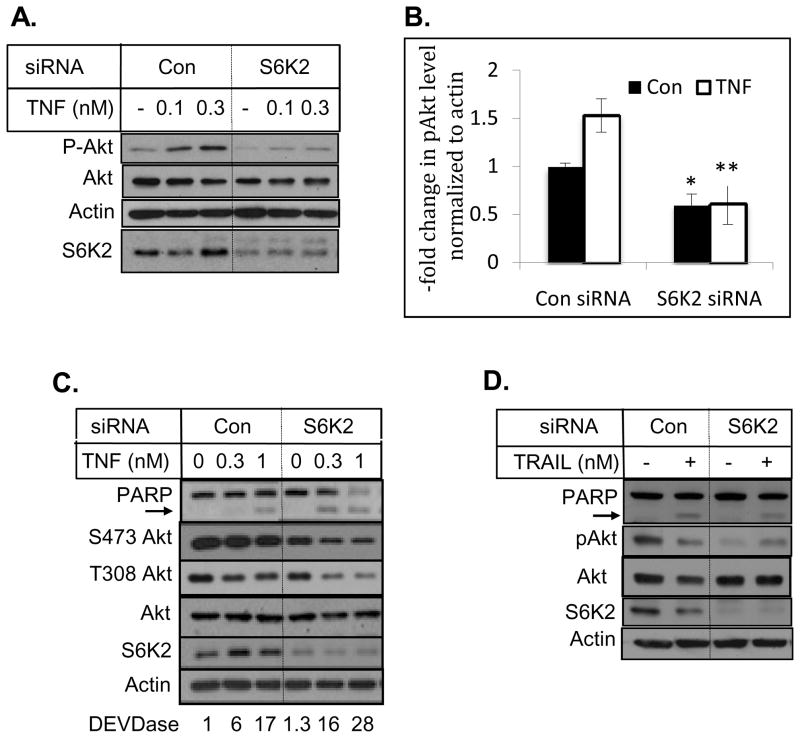
Since silencing of S6K1 caused a modest inhibition of TNF- and TRAIL-induced apoptosis (Figs. 1A and 1C), and S6K1 was shown to negatively regulate Akt via a feedback loop (26, 28–30), we examined if knockdown of S6K1 enhances TNF-induced activation of Akt in MCF-7 cells. Figure 2 shows that depletion of S6K1 in MCF-7 breast cancer cells enhanced phosphorylation of Akt. In contrast to S6K1, knockdown of S6K2 decreased both basal and TNF-induced Akt phosphorylation (Fig. 3A). Based on densitometric scanning of four independent experiments, knockdown of S6K2 decreased basal and TNF-induced Akt phosphorylation at Ser473 by 40% and 60%, respectively (Fig. 3B).
Fig. 2.
Knockdown of S6K1 increased Akt phosphorylation. MCF-7 cells transfected with control non-targeting or S6K1 siRNA were treated with indicated concentrations of TNF. Western blot analysis was performed with indicated antibodies.
Fig. 3.
Knockdown of S6K2 decreased Akt phosphorylation. MCF-7 (A), ZR-75-1 (C) and MDA-MB-231 (D) cells were transfected with control non-targeting or S6K2 siRNA. Cells were treated with indicated concentrations of TNF and Western blot analysis was performed with indicated antibodies. B, Intensity of phospho-Akt was determined by densitometry and standardized by loading. The data represent the -fold decrease in Akt phosphorylation in S6K2-depleted cells compared to control siRNA-transfected cells. Each bar represents the mean ± S.E. of four independent experiments. The solid bar represents untreated cells and the open bar represents TNF treatment. *, p value < 0.05; **, p value < 0.01 using paired Student’s t test.
We also examined the consequence of S6K2 knockdown on Akt phosphorylation in ZR-75-1 and MDA-MB-231 breast cancer cells (Figs 3C and 3D). Knockdown of S6K2 decreased Akt phosphorylation, and enhanced PARP cleavage and caspase activation in ZR-75-1 cells (Fig. 3C). TNF had little effect on cell death in MDA-MB-231 cells (data not shown). However, S6K2 depletion failed to enhance cell death in response to TRAIL in MDA-MB-231 cells (Fig. 3D). In contrast to MCF-7 cells, which lack caspase-3, ZR-75-1 and MDA-MB-231 cells contain functional caspase-3. Since Akt is a substrate for caspase-3, apoptotic stimuli can also induce cleavage of Akt and this may contribute to decrease in Akt level in response to TNF or TRAIL.
S6K2 Promotes MCF-7 Cell Survival via Akt
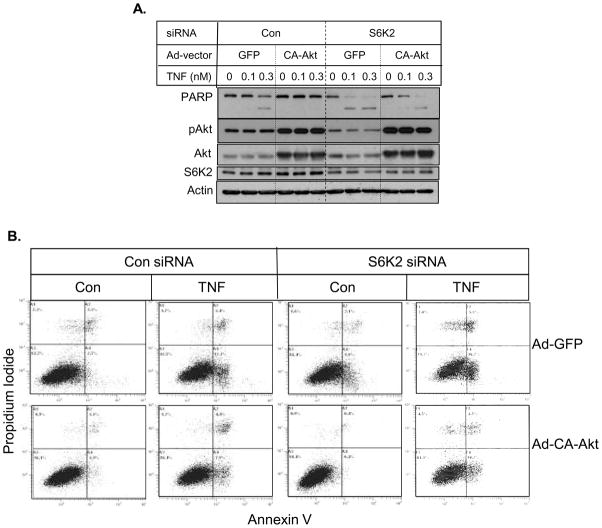
Since knockdown of S6K2 inhibits Akt phosphorylation, we examined if S6K2 promotes cell survival via Akt. We examined the ability of constitutively-active (CA) Akt to reverse the potentiation of cell death caused by S6K2 depletion. Figure 4A shows that the adenoviral vector-mediated delivery of CA-Akt in MCF-7 cells decreased TNF-induced PARP cleavage compared to cells transfected with adeno-GFP. While knockdown of S6K2 caused a substantial increase in TNF-induced PARP cleavage, overexpression of CA-Akt inhibited TNF-induced PARP cleavage in S6K2-depleted cells. Similar results were obtained when we monitored cell death by staining cells with Annexin V and PI (Fig. 4B). These results suggest that S6K2 mediates its prosurvival effect via Akt.
Fig. 4.
Overexpression of Akt reverses the effects of S6K2 knockdown on TNF-induced apoptosis. MCF-7 cells transfected with control or S6K2 siRNA were infected with adenovirus vector containing GFP or CA-Akt construct. Cells were then treated with or without indicated concentrations of TNF. A, Western blot analysis was performed with indicated antibodies. B, Cells were stained with Annexin V-Alexa 488 conjugate and PI, and analyzed using a flow cytometer. Results are representative of 3 independent experiments.
Knockdown of S6K2 Enhanced Cell Death via Bid
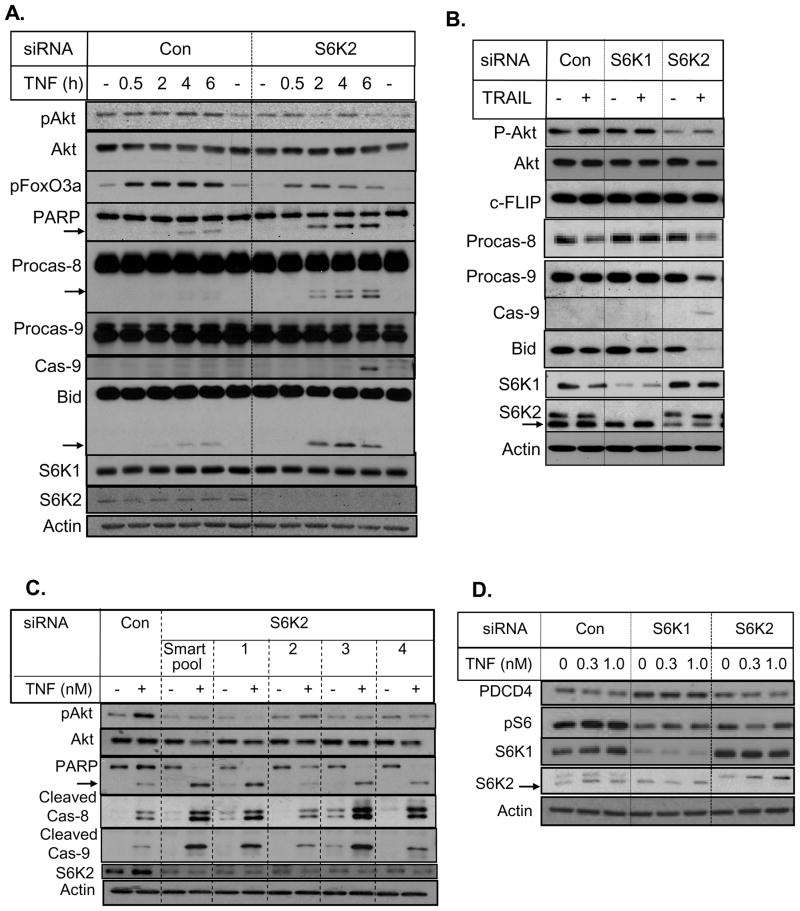
Although TNF and TRAIL trigger cell death via the receptor-initiated pathway, they can also amplify cell death via the mitochondrial pathway (10–12). To determine the mechanism(s) by which depletion of S6K2 potentiates TNF-induced cell death, we monitored TNF-induced caspase activation and processing of Bid. Figure 5A shows that TNF caused an increase in phospho-Akt which was attenuated by S6K2 knockdown. Depletion of S6K2 was associated with enhanced processing of PARP and procaspase-8 in response to TNF. This was accompanied by an increase in the cleavage of Bid, a substrate for caspase-8 (10) and increased processing of procaspase-9, the apical caspase of the mitochondrial cell death pathway. We also compared the effects of S6K1 and S6K2 knockdown on cellular responses to TRAIL (Fig. 5B). Knockdown of S6K2 had little effect on caspase-8 inhibitor c-FLIP but it enhanced processing of procaspase-8, -9 and Bid (Fig. 5B).
Fig. 5.
Knockdown of S6K2 induced apoptosis via the mitochondrial pathway. MCF-7 cells were transfected with control or S6K2 siRNA. A, Cells were treated with 1 nM TNF for indicated periods of time. B, Cells were treated with or without TRAIL. C, Cells were transfected with control, siRNA SMARTpool or four different S6K2 siRNAs and then treated with 0.3 nM TNF. D, Cells were transfected with control, S6K1 or S6K2 siRNA. Western blot analysis was performed with indicated antibodies. Western blot analyses were performed with indicated antibodies. The upper band in the S6K2 blot is likely to be S6K1. Results are representative of 3 independent experiments. The arrows indicate the processed forms of PARP, caspase-8, caspase-9 and Bid.
To further validate our observation that S6K2 depletion decreases Akt phosphorylation and increases cell death via the mitochondrial pathway, we used four different siRNA constructs against S6K2. Figure 5C shows that siRNAs 1, 3 and 4 against S6K2 decreased Akt phosphorylation, enhanced PARP cleavage and increased processing of procaspase-8 and -9 similar to S6K2 SMARTpool siRNA. In contrast, siRNA 2 was less effective in attenuating Akt phosphorylation and cleavage of PARP, caspase-8 and -9. Thus, a decrease in Akt phosphorylation by S6K2 depletion was associated with an increase in PARP cleavage.
Since PDCD4 has been implicated in TNF-induced apoptosis and acts as a tumor suppressor (45, 46), we have also examined the effects of S6K1 and -2 knockdown on the level of PDCD4. Silencing of S6K1 or S6K2 effectively depleted the homolog and attenuated phosphorylation of the substrate S6. However, while knockdown of S6K1 consistently increased PDCD4 level, depletion of S6K2 had either no effect or decreased the level of PDCD4 modestly (Fig. 5D and data not shown). Thus, it is unlikely that a decrease in PDCD4 was responsible for the potentiation of cell death caused by S6K2 knockdown.
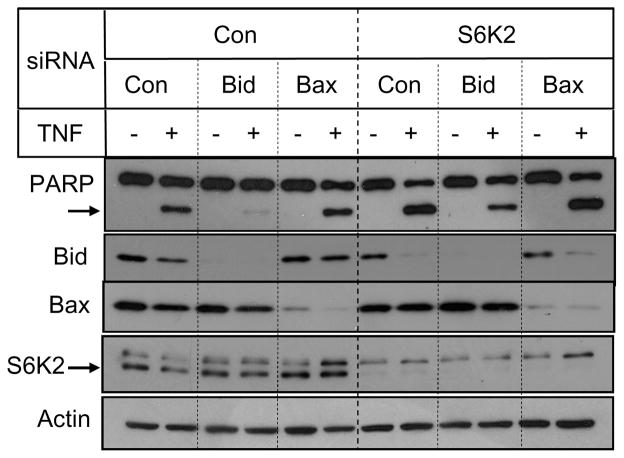
We have previously shown that activation of Akt promotes cell survival by downregulating Bid via p53 (17). We therefore examined if S6K2 knockdown affects p53 level. Figure 6 shows that knockdown of S6K2 enhanced TNF-induced p53 level, and silencing of p53 decreased Bid level, suggesting that S6K2 may regulate Bid via p53. Finally, to determine if Bid is indeed involved in the potentiation of cell death caused by S6K2 knockdown, we examined if S6K2 depletion sensitizes cells to TNF when Bid is depleted. We compared the effect of Bid with another proapoptotic Bcl-2 family member Bax. Figure 7 shows that knockdown of Bid abolished TNF-induced PARP cleavage. Additionally, knockdown of Bid but not Bax attenuated the ability of S6K2 to enhance TNF-induced PARP cleavage. These results suggest that the mechanism by which S6K2 potentiates receptor-mediated apoptosis involves the proapoptotic protein Bid.
Fig. 6.
Knockdown of p53 attenuates Bid level. MCF-7 cells were transfected with indicated siRNAs and then treated with or without TNF. Cell lysates were analyzed by Western blotting using the indicated antibodies. Actin was used to control for loading differences. Results are representative of two independent experiments.
Fig. 7.
Knockdown of Bid counteracts increase in TNF-induced apoptosis caused by S6K2 depletion. MCF-7 cells transfected with indicated siRNAs were treated with or without TNF and Western blot analysis was performed with indicated antibodies. Results are representative of two independent experiments.
DISCUSSION
The results of our present study demonstrate that the two S6K homologs, S6K1 and S6K2 exhibit distinct functions on breast cancer cell survival. While it has been reported that S6K1 can negatively regulate Akt via a negative feedback loop, we report for the first time that depletion of S6K2 inhibits Akt activity and promotes breast cancer cell death via the mitochondrial cell death pathway that involves the Bcl-2 family protein Bid.
It is generally believed that activation of PI3K/Akt stimulates the mTOR pathway by phosphorylating and inactivating the tumor suppressor protein tuberous sclerosis complex 2 (TSC2), which negatively regulates mTOR activity. mTOR is required for estrogen-induced breast tumor cell proliferation (47)and constitutive signaling through the mTOR pathway is a cause of treatment failure in breast cancer patients (48). S6K1, a downstream target of mTOR, is an important mediator of mTOR function (49). An elevation/activation of S6K has been associated with several cancers and resistance to chemotherapeutic drugs (42, 44, 50, 51). The S6K1 gene is amplified in approximately 9% of primary breast cancers (52), and S6K1 mRNA is elevated in almost 40% of the tumors (42). The status of the activated S6K1 was shown to be a predictor of patient’s survival and treatment response (42, 50, 53). Recently, it has been reported that S6K1 promotes breast cancer cell proliferation by phosphorylating ERα, leading to its transcriptional activation (54). Thus, we anticipated that knockdown of S6K1 would enhance cell death in breast cancer cells. To our surprise, depletion of S6K1 caused a modest decrease in cell death in response to TNF. Our results are, however, consistent with the recent reports that S6K1 deficiency protects against death receptor-mediated apoptosis in hepatocytes (55) and mTOR-S6K1 activates p53-dependent cell death in response to DNA damage (56). As has been reported earlier that persistent inhibition of mTOR/S6K1 can activate Akt via a negative feedback loop (26, 28–30), we also found that depletion of S6K1 resulted in an increase in TNF-induced Akt phosphorylation and this may explain why S6K1 knockdown inhibits rather than potentiates TNF-induced cell death.
Although most of the published reports have focused on S6K1, there are two homologs of S6K, S6K1 and S6K2 that act downstream of mTOR (25, 34). While the two homologs share overall similarity in structure and exhibit redundant functions, there are also important differences. S6K2 has been shown to potentiate IL3-mediated mitogenic response (57). A recent study demonstrated that S6K2 but not S6K1 interacts with heterogeneous ribonucleoproteins (hnRNPs) F/H to drive cell proliferation (58). We have consistently found that in contrast to S6K1, depletion of S6K2 caused a dramatic increase in TNF- and TRAIL-induced apoptosis, suggesting that S6K2 functions as a prosurvival protein. TNF has been shown to activate mTOR signaling (59)and we have found that TNF preferentially activates S6K1 (data not shown), presumably because the abundance of S6K1 is much greater compared to S6K2 in MCF-7 cells. We made a novel observation that in contrast to S6K1, S6K2 positively regulates Akt. Knockdown of S6K2 caused a decrease in both basal and TNF-induced Akt phosphorylation, which is indicative of its activation status, suggesting that S6K2 promotes cell survival via activation of Akt. In fact, overexpression of CA-Akt blocked increase in cell death caused by S6K2 depletion, suggesting that S6K2 acts upstream of Akt although we cannot rule out the possibility that Akt andS6K2 act in parallel pathways where Akt has a dominant role over S6K2.
There are several potential mechanisms by which S6K2 affects phosphorylation/activity of Akt. Since mTORC2 activates Akt by phosphorylating at the hydrophobic site, it is conceivable that knockdown of S6K2 decreases Akt phosphorylation by inhibiting mTORC2. Others and we have also shown that Ser473 phosphorylation of Akt is also regulated by DNA-dependent protein kinase (41). Since PTEN inhibits PI3K/Akt, another possibility is that S6K2 knockdown increases PTEN level resulting in inhibition of Akt. It has been reported that a major kinase downstream of mTORC2 is SGK1 (60). Thus, it is also important to determine if S6K2 regulates cell survival via SGK1. Moreover, since activation of Akt would lead to the activation of mTORC1, there may be a positive feedback loop between S6K2 and Akt. Thus, mTORC1 and its downstream targets may mediate some of the effects of the potential functional interaction between S6K2 and Akt. Future studies should discern the mechanisms by which S6K2 regulate Akt and the functional interaction between S6K2 and Akt.
Our results suggest that the mechanism by which S6K2 promotes cell survival via Akt involves the proapoptotic Bcl-2 family protein Bid. We have previously shown that activation of Akt can cause a decrease in p53 levels in MCF-7 cells by phosphorylating and stabilizing Hdm2, which degrades p53 via the ubiquitin proteasome-mediated pathway (17). We have also shown that Bid is a transcriptional target of p53 and Akt can decrease Bid expression by inducing downregulation of p53 (17). The results of our present study demonstrate that knockdown of S6K2 increased p53 and silencing of p53 was associated with a decrease in Bid. However, depletion of S6K2 was not associated with upregulation of Bid. We have previously shown that overexpression of Bid is sufficient to cause cell death (19). Since Bid is a proapoptotic protein, an increase in Bid may also lead to its cleavage. Therefore, it may be difficult to demonstrate an increase in Bid level. Nevertheless, knockdown of S6K2 had little effect on enhancing TNF-induced cell death when Bid was depleted by siRNA silencing. Moreover, knockdown of S6K2 failed to enhance cell death in MDA-MB-231 cells, which express mutant p53. Thus, the mechanism by which S6K2 promotes cell survival via Akt may involve downregulation of Bid.
S6K2 has also been implicated in fibroblast growth factor-mediated chemoresistance of small cell lung cancer H69 cells (38). It has been reported that PKCε interacts with S6K2 and mediates the prosurvival effects of S6K2 via Raf/MAPK signaling pathway by increasing the levels of antiapoptotic proteins XIAP and Bcl-xL (38). We were unable to detect a decrease in XIAP and Bcl-xL in S6K-2-depleted MCF-7 cells (data not shown) although we cannot rule out the possibility of other Bcl-2 family members. Interestingly, we have previously shown that PKCε also acts upstream of Akt during TNF-induced apoptosis in MCF-7 breast cancer cells (41), and inhibits TNF- and TRAIL-mediated apoptosis by increasing antiapoptotic Bcl-2 and decreasing proapoptotic Bid levels (19). Moreover PKCε caused a decrease in Bid via Akt (17). Thus, depending on the cellular context and apoptotic stimulus, PKCε may promote cell survival either via the Raf/MEK/ERK pathway or via the Akt signaling pathway.
Aberrations in Akt/mTOR/S6K pathway have been associated with many cancers. Consequently, this pathway is an important target for cancer therapy. Rapamycin and its analogues that inhibit mTOR, however, were of limited success (26–30). Since S6K1 and S6K2 appear to have opposite effects on cell death, targeting mTOR which acts upstream of both S6K1 and S6K2 may not be effective. Our observation that S6K2 rather than S6K1 is needed for the survival of breast cancer cells has significant implications in the treatment of the disease. Inhibition of S6K2 rather than of S6K1 should sensitize cancer cells to chemotherapeutic agents, providing a basis for rational combination chemotherapy. Since Akt signaling pathway is often deregulated in cancer, the observation that knockdown of S6K2 results in inhibition of Akt demonstrates positive feedback regulation of Akt by S6K2, and has significant impact in cancer therapy.
Acknowledgments
Grant Support: Supported by the grant CA071727 (A. Basu) from the NIH/NCI
We thank Mr. Timothy Break and Dr. Eswar Shankar for help with flow cytometry and YO-PRO-1/PI assay, respectively. We also gratefully acknowledge Ms. Soumya Krishnamurthy and Deepanwita Pal for help with several experiments, and Deepanwita Pal for critical reading of the manuscript. Adenovirus containing constitutively active Akt was a kind gift from Dr. Santosh DeMello (University of Texas, Dallas).
The abbreviations used are
- 4E-BP
eIF4E-binding protein
- CA
constitutively-active
- FLIP
Flice-like inhibitory protein
- GFP
green fluorescent protein
- Mdm2
murine double minute 2
- Hdm2
Human homolog of Mdm2
- MAPK
mitogen-activated protein kinase
- mTOR
mammalian target of rapamycin
- PARP
poly(ADP-ribose) polymerase
- PDCD4
programmed cell death 4
- PKB
protein kinase B
- PI
propidium iodide
- PKC
protein kinase C
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- S6K
40S ribosomal protein S6 kinase
- SGK1
serum- and glucocorticoid-inducible kinase
- TNF
tumor necrosis factor-α
- TRAIL
TNF-related apoptosis-inducing ligand
References
- 1.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, et al. Role of translocation in the activation and function of protein kinase B. The Journal of biological chemistry. 1997;272:31515–24. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 2.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. Journal of cellular and molecular medicine. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–92. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 4.Bozulic L, Hemmings BA. PIKKing on PKB: regulation of PKB activity by phosphorylation. Current opinion in cell biology. 2009;21:256–61. doi: 10.1016/j.ceb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Carswell EA, Old LJ, Kassel RL, Greem S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugarman BJ, Aggarwal BB, Hass PE, Figari IS, Palladino MA, Jr, Shepard HM. Recombinant tumor necrosis factor-α: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230:943–5. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- 7.O’Toole A, Moule SK, Lockyer PJ, Halestrap AP. Tumour necrosis factor-alpha activation of protein kinase B in WEHI-164 cells is accompanied by increased phosphorylation of Ser473, but not Thr308. The Biochemical journal. 2001;359:119–27. doi: 10.1042/0264-6021:3590119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen GM. Caspases: the executioners of apoptosis. The Biochemical journal. 1997;326 ( Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunez G, Benedict MA, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–45. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Zhu H, Xu C, Yuan J. Cleavage of BID by caspase-8 mediates the mitochondrial damage to the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 11.Kuwana T, Smith JJ, Muzio M, Dixit V, Newmeyer DD, Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273:16589–94. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 12.Gross A, Yin X-M, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while Bcl-xL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–63. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 13.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends in cell biology. 2008;18:157–64. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nature reviews. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 15.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 16.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. The Journal of biological chemistry. 2006;281:813–23. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 17.Shankar E, Sivaprasad U, Basu A. Protein kinase C epsilon confers resistance of MCF-7 cells to TRAIL by Akt-dependent activation of Hdm2 and downregulation of p53. Oncogene. 2008;27:3957–66. doi: 10.1038/onc.2008.39. [DOI] [PubMed] [Google Scholar]
- 18.She QB, Solit DB, Ye Q, O’Reilly KE, Lobo J, Rosen N. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer cell. 2005;8:287–97. doi: 10.1016/j.ccr.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivaprasad U, Shankar E, Basu A. Downregulation of Bid is associated with PKCepsilon-mediated TRAIL resistance. Cell death and differentiation. 2007;14:851–60. doi: 10.1038/sj.cdd.4402077. [DOI] [PubMed] [Google Scholar]
- 20.Zhu S, Evans S, Yan B, Povsic TJ, Tapson V, Goldschmidt-Clermont PJ, et al. Transcriptional regulation of Bim by FOXO3a and Akt mediates scleroderma serum-induced apoptosis in endothelial progenitor cells. Circulation. 2008;118:2156–65. doi: 10.1161/CIRCULATIONAHA.108.787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 23.Baldo P, Cecco S, Giacomin E, Lazzarini R, Ros B, Marastoni S. mTOR pathway and mTOR inhibitors as agents for cancer therapy. Current cancer drug targets. 2008;8:647–65. doi: 10.2174/156800908786733513. [DOI] [PubMed] [Google Scholar]
- 24.Jacinto E, Lorberg A. TOR regulation of AGC kinases in yeast and mammals. The Biochemical journal. 2008;410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- 25.Lee-Fruman KK, Kuo CJ, Lippincott J, Terada N, Blenis J. Characterization of S6K2, a novel kinase homologous to S6K1. Oncogene. 1999;18:5108–14. doi: 10.1038/sj.onc.1202894. [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer research. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer research. 2005;65:7052–8. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 28.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–40. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Aktactivation. Cancer research. 2008;68:7409–18. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Gao Z, Yin J, Quon MJ, Ye J. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. The Journal of biological chemistry. 2008;283:35375–82. doi: 10.1074/jbc.M806480200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–6. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. The Journal of biological chemistry. 2009;284:8023–32. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koh H, Jee K, Lee B, Kim J, Kim D, Yun YH, et al. Cloning and characterization of a nuclear S6 kinase, S6 kinase-related kinase (SRK); a novel nuclear target of Akt. Oncogene. 1999;18:5115–9. doi: 10.1038/sj.onc.1202895. [DOI] [PubMed] [Google Scholar]
- 35.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. The EMBO journal. 1998;17:6649–59. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, et al. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Molecular and cellular biology. 2004;24:3112–24. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, et al. Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat Cell Biol. 2005;7:286–94. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- 38.Pardo OE, Wellbrock C, Khanzada UK, Aubert M, Arozarena I, Davidson S, et al. FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCepsilon, B-Raf and S6K2. The EMBO journal. 2006;25:3078–88. doi: 10.1038/sj.emboj.7601198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goh ET, Pardo OE, Michael N, Niewiarowski A, Totty N, Volkova D, et al. Involvement of heterogeneous ribonucleoprotein F in the regulation of cell proliferation via the mammalian target of rapamycin/S6 kinase 2 pathway. The Journal of biological chemistry. 2010;285:17065–76. doi: 10.1074/jbc.M109.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Basu A, Akkaraju GR. Regulation of caspase activation and cis-diamminedichloroplatinum(II)-induced cell death by protein kinase C. Biochemistry. 1999;38:4245–51. doi: 10.1021/bi982854q. [DOI] [PubMed] [Google Scholar]
- 41.Lu D, Huang J, Basu A. Protein kinase Cepsilon activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-alpha-induced cell death. The Journal of biological chemistry. 2006;281:22799–807. doi: 10.1074/jbc.M603390200. [DOI] [PubMed] [Google Scholar]
- 42.Barlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. Journal of the National Cancer Institute. 2000;92:1252–9. doi: 10.1093/jnci/92.15.1252. [DOI] [PubMed] [Google Scholar]
- 43.Dhar R, Basu A. Constitutive activation of p70 S6 kinase is associated with intrinsic resistance to cisplatin. International journal of oncology. 2008;32:1133–7. [PubMed] [Google Scholar]
- 44.Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer research. 2007;67:6325–32. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- 45.Wang WQ, Zhang H, Wang HB, Sun YG, Peng ZH, Zhou G, et al. Programmed cell death 4 (PDCD4) enhances the sensitivity of gastric cancer cells to TRAIL-induced apoptosis by inhibiting the PI3K/Akt signaling pathway. Mol Diagn Ther. 2010;14:155–61. doi: 10.1007/BF03256368. [DOI] [PubMed] [Google Scholar]
- 46.Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, et al. A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene. 2001;20:669–76. doi: 10.1038/sj.onc.1204137. [DOI] [PubMed] [Google Scholar]
- 47.Boulay A, Rudloff J, Ye J, Zumstein-Mecker S, O’Reilly T, Evans DB, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–28. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 48.Crowder RJ, Ellis MJ. Treating breast cancer through novel inhibitors of the phosphatidylinositol 3′-kinase pathway. Breast Cancer Res. 2005;7:212–4. doi: 10.1186/bcr1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. The EMBO journal. 1997;16:3693–704. doi: 10.1093/emboj/16.12.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klos KS, Wyszomierski SL, Sun M, Tan M, Zhou X, Li P, et al. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer research. 2006;66:2028–37. doi: 10.1158/0008-5472.CAN-04-4559. [DOI] [PubMed] [Google Scholar]
- 51.Seufferlein T, Rozengurt E. Rapamycin inhibits constitutive p70s6k phosphorylation, cell proliferation, and colony formation in small cell lung cancer cells. Cancer research. 1996;56:3895–7. [PubMed] [Google Scholar]
- 52.Wu GJ, Sinclair CS, Paape J, Ingle JN, Roche PC, James CD, et al. 17q23 amplifications in breast cancer involve the PAT1, RAD51C, PS6K, and SIGma1B genes. Cancer research. 2000;60:5371–5. [PubMed] [Google Scholar]
- 53.Guo L, Abraham J, Flynn DC, Castranova V, Shi X, Qian Y. Individualized survival and treatment response predictions for breast cancers using phospho-EGFR, phospho-ER, phospho-HER2/neu, phospho-IGF-IR/In, phospho-MAPK, and phospho-p70S6K proteins. The International journal of biological markers. 2007;22:1–11. doi: 10.1177/172460080702200101. [DOI] [PubMed] [Google Scholar]
- 54.Yamnik RL, Digilova A, Davis DC, Brodt ZN, Murphy CJ, Holz MK. S6 Kinase 1 Regulates Estrogen Receptor {alpha} in Control of Breast Cancer Cell Proliferation. The Journal of biological chemistry. 2009;284:6361–9. doi: 10.1074/jbc.M807532200. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez-Rodriguez A, Alba J, Zimmerman V, Kozma SC, Valverde AM. S6K1 deficiency protects against apoptosis in hepatocytes. Hepatology (Baltimore, Md. 2009;50:216–29. doi: 10.1002/hep.22915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lai KP, Leong WF, Chau JF, Jia D, Zeng L, Liu H, et al. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. The EMBO journal. 2010;29:2994–3006. doi: 10.1038/emboj.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cruz R, Hedden L, Boyer D, Kharas MG, Fruman DA, Lee-Fruman KK. S6 kinase 2 potentiates interleukin-3-driven cell proliferation. Journal of leukocyte biology. 2005;78:1378–85. doi: 10.1189/jlb.0405225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goh ET, Pardo OE, Michael N, Niewiarowski A, Totty N, Volkova D, et al. Involvement of heterogeneous ribonucleoprotein F in the regulation of cell proliferation via the mammalian target of rapamycin/S6 kinase 2 pathway. The Journal of biological chemistry. 285:17065–76. doi: 10.1074/jbc.M109.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, et al. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci U S A. 2001;98:4640–5. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–86. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]