Abstract
Response to environmental stimuli is critical for cell survival and function and requires high fidelity signal transduction into the nucleus to facilitate the coordinated transcriptional regulation of appropriate gene networks. The cellular response to mitogenic stimuli provides an excellent paradigm to decipher the mechanisms mediating precise gene expression control at the transcriptional level. Here we review recent advances in our understanding of this so called serum response network, which illuminate novel aspects of nuclear signaling mechanisms, combinatorial control by DNA binding proteins and regulation of RNA polymerase II (RNAPII) elongation.
Introduction
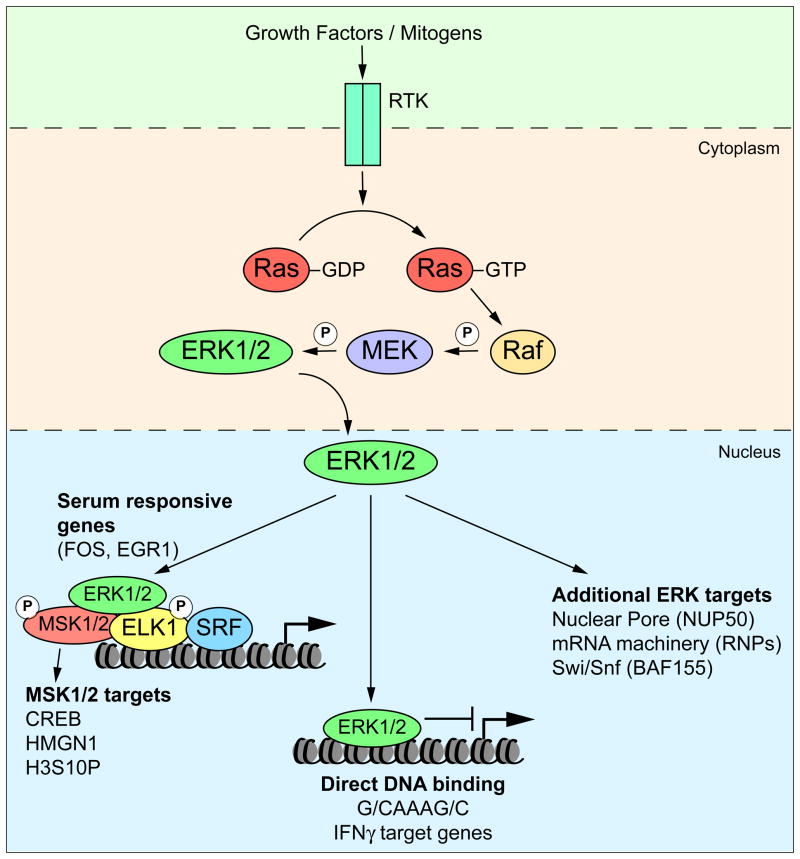
Conserved mitogen-activated protein kinase (MAPK) pathways are chief regulators of cell fate, activating specific transcriptional programs in response to extracellular growth factors [1,2]. Activation of the Ras-Raf-MEK-ERK pathway acting downstream of growth factor receptors triggers a cascade of protein phosphorylation events culminating in activation of the extracellular signal-regulated kinases ERK1/2 (Figure 1). Activated ERKs accumulate in the nucleus where they phosphorylate different types of targets, including but not restricted to DNA binding proteins, transcriptional cofactors and downstream kinases. These phosphorylation events lead to the induction of immediate early genes (IEGs) in the serum response network, which occurs within minutes of growth factor stimulation and prior to de novo protein synthesis. In turn, many IEGs encode for transcription factors of the AP-1 and EGR families, which are responsible for generating a second, larger wave of transcription that ultimately leads to the final cellular outcome. Studies of the nuclear events taking place downstream of growth factor signaling have repeatedly advanced our understanding of mechanisms of transcriptional control. Here we highlight discoveries in the past two years arising from this field that illuminate how nuclear kinases regulate transcription, how transcription factors function in a combinatorial fashion to achieve biological specificity and robustness, and how RNAPII activity is regulated at post-recruitment steps.
Figure 1. ERK nuclear signaling.
Stimulation of receptor tyrosine kinases (RTKs) by growth factors activates the Ras-Raf-MEK-ERK pathway and leads to the accumulation of phosphorylated active ERK1/2 in the nucleus. ERK1/2 are recruited to the promoters of target genes where they phosphorylate numerous targets in the nucleus including ETS transcription factors (e.g. ELK1), downstream kinases (e.g. MSK1) and transcriptional cofactors, which contribute to the induction of IEGs such as FOS and EGR1. In addition, ERK1/2 can also phosphorylate components of the nucleosome remodeling complex SWI/SNF, the nuclear pore, and the mRNA processing machinery. It appears that ERK2 can also act as a repressor, independently of its kinase activity, by binding to a DNA motif found at the promoters of genes regulated by IFNγ.
Lessons on nuclear signaling: ‘fold-change’ regulation and DNA binding by nuclear kinases
Many signaling pathways rely on phosphorylation events to transduce environmental stimuli into specific transcriptional programs, and the serum response network continues to spearhead our understanding of this phenomena. A recent study of ERK activation using single cell imaging techniques revealed a novel aspect of nuclear signaling. While measuring translocation of fluorescently tagged ERK2 into the nuclei of cells stimulated with EGF, Cohen-Saidon et al. [3**] observed a wide variation (up to 4-fold) in the basal levels of nuclear ERK2 across individual cells. However, the fold-increase in nuclear ERK2 upon stimulation and the overall timing of nuclear accumulation (peaking around 13 minutes) were remarkably similar among cells. Furthermore, levels of nuclear ERK2 dropped down to within 10% of their individual pre-stimulation levels by 30 minutes post-EGF addition. These results indicate that ERK signaling follows a ‘fold-change’ rather than ‘absolute change’ logic, by which the cellular outcome to ERK activation may be independent of the initial basal levels of nuclear ERK. This signaling strategy may not be exclusive to the serum response network and it has also been described for the β-catenin pathway [4].
Upon translocation into the nucleus ERK1/2 phosphorylate a number of targets, which results in the strong and rapid (yet transient) induction of IEGs. Several DNA binding transcription factors are targets of ERK kinase activity, most prominent among them are members of the ETS-domain family, such as ELK1 and ETS1/2 [5,6]. Phosphorylation of ETS factors by ERK increases their transactivation potential mostly by allowing increased interaction with cofactors such as the histone acetyl-transferases (HATs) CBP/p300 and the Mediator co-activator complex [5–8]. A recent phospho-proteomics study by Kosako et al. [9*] identified several additional nuclear targets of ERK, including the BAF155 subunit of the chromatin remodeling complex SWI/SNF, the mRNA processing factors hnRNPK and U1 snRNP70 and the nucleoporin NUP50. These observations suggest that ERK signaling may promote increased gene activity at other steps beyond mere enhancement of transactivator function, such as nucleosome remodeling, mRNA processing and export. The ERK-activated kinases MSK1/2 further contribute to gene activation, as they phosphorylate additional DNA binding proteins, histone H3 at serine 10 and the chromatin-associated protein HMGN1 (recently reviewed in [10]).
Interestingly, independent studies have shown that ERK1/2 are recruited to promoters of serum response genes upon stimulation, indicating that ERK-mediated signaling occurs in situ at target promoters where the transcriptional apparatus is assembled and activated [11,12**]. In this scenario ERK is recruited to IEGs via interaction with the DNA binding proteins it phosphorylates, as indicated by the fact that ELK1 mutants lacking ERK-docking sites fail to recruit the kinase to DNA in vitro [12**]. Surprisingly, a recent study looking for novel DNA-protein interactions found that ERK2 is able to directly bind DNA sequences matching the consensus G/CAAAG/C [13*]. Intriguingly, ERK2 binding resulted in repression of gene promoters carrying this cis element in a kinase activity-independent manner. In fact, of 82 genes upregulated upon ERK2 knockdown, 78 contained the ERK2-binding sequence. Of note, this gene set was enriched for IFNγ-inducible genes, indicating that this novel ERK2 function serves to attenuate interferon signaling [13*,14]. The DNA binding domain of ERK2 was mapped to a region called the MAPK insert, which is also found in cyclin-dependent kinases and GSK3 [14], thus opening the possibility that other kinases also bind DNA directly. In fact, recruitment of MAPKs and several other signaling kinases to the chromatin of its target genes has been extensively documented in yeast [15].
Lessons on combinatorial control of gene expression: mixing and matching for specificity and robustness
The serum response network provides a clear paradigm to understand how a limited number of transcription factors can orchestrate a myriad of biological responses in a stimulus- and cell type-specific manner. The ETS-domain family of transcription factors contains at least 27 members and it is unclear to what degree they have specialized or redundant functions [16]. Historically, ELK1 was identified as the Ternary Complex Factor (TCF) partnering with the Serum Response Factor (SRF) to activate IEGs carrying both ETS-sites and CArG-boxes, such as FOS and EGR1 [17]. However, it is now clear that both ELK1 and SRF have additional, non-overlapping functions (Figure 2). Recent genome wide analysis of ELK1 occupancy by Boros et al. [18**] revealed that a significant fraction of ELK1 target genes are not bound by SRF, and instead ELK1 binds these genes redundantly with other ETS-domain factors. Interestingly, this subset of SRF-independent ELK1 targets is enriched for several core components of the transcriptional regulation machinery [19]. Indeed, ELK1 was required for increased expression of subunits of the general transcription factors TFIIA, TFIIB and TFIID. Thus, ELK1 is not only involved in induction of IEGs (working mostly with SRF) but also in upregulation of the transcriptional apparatus (working independently of SRF), which may prepare cells for the increased transcriptional activity observed after serum stimulation. Conversely, SRF has clear separate functions not involving ELK1 or other ETS factors, but acting instead in partnership with the myocardin related transcription factors (MRTFs), which are activated by a novel signaling pathway involving Rho-family GTPases and monomeric actin (recently reviewed in [20]).
Figure 2. Combinatorial action of transcription factors within the Serum Response Network.
(A) Although ELK1 cooperates with SRF at the promoters of several IEGs, genome-wide analyses show that both of these transcription factors are able to partner with other factors to regulate additional sets of genes. SRF can partner with MRTFs to regulate genes involved in actin dynamics, cellular motility and muscle contraction. SRF-independent genes targeted by ELK1 are also bound by other ETS factors, and many of these genes are components of the basal transcription machinery. Other ETS factors are also employed in a redundant fashion with ETS1 found at the promoters of housekeeping genes in T cells, while at enhancers of genes involved in T-cell activation, ETS1 collaborates with RUNX for specific binding.
(B) Redundant binding of the TCF subfamily of ETS factors can provide robustness by allowing equivalent cofactor recruitment in different cell types. Mouse ESC cells are dependent on an interaction between ELK1 and MED23 for Mediator recruitment to the EGR1 promoter. In MEFs, SAP1 and NET (which are expressed at higher levels) are able to overcome the requirement for ELK1, and to some extent MED23, by making alternative contacts with Mediator (indicated by dashed arrows).
Mixing and matching for specificity has also been observed for ETS1. Despite the fact that T-cells express mRNAs for up to 17 ETS transcription factors displaying similar DNA sequence preferences, ablation of ETS1 in mouse leads to a specific defect in T cell activation [21]. Using ChIP-CHIP and ChIP-Seq experiments, the Graves lab illuminated possible mechanisms driving this specificity [22,23**]. In a first study using promoter arrays they observed two classes of ETS1-bound genes. One class showed redundant promoter occupancy of ETS1 with two other ETS factors, GABPA and ELF1, and carried an ETS consensus site (CCGGAAGT) closely matching the in vitro derived consensus for several ETS family members (Figure 2). The second class of promoters was bound only by ETS1 and carried a sequence with a core GGA motif that deviates from the ETS consensus [22]. In a second study using ChIP-Seq, they made the interesting observation that whereas ETS1 and GABPA actually co-occupy active promoters of housekeeping genes, ETS1 specifically occupies the enhancers of genes important for T cell function [23**]. Interestingly, these ETS-bound enhancers were also occupied by the transcription factor RUNX and carried a composite ETS/RUNX binding sequence. Thus, small variations in DNA sequences create different repertoires of ETS1 target genes by dictating which interacting partner is employed at different sites (Figure 2). Furthermore, CBP colocalized with ETS1 at enhancers, but not promoters, suggesting that ERK-dependent signaling, which leads to increased ETS1-CBP interaction [6], may preferentially affect histone acetylation at T cell enhancers.
A recent study by Balamotis et al. [24**] revealed another property of combinatorial control by ETS factors: robustness. They found that whereas the MED23 subunit of Mediator is fully required for ELK1-dependent activation of the EGR1 gene in embryonic stem cells (ESCs), this requirement was much decreased in murine embryonic fibroblasts (MEFs), which express lower levels of ELK1. This conundrum was solved by the observation that in MEFs Mediator was recruited by two additional ETS factors acting at the EGR1 promoter, SAP1 and NET, which interact with Mediator mostly independently of MED23 [24**] (Figure 2B). Thus, combinatorial interactions between the activation domains of related ETS factors and different Mediator subunits ensure that EGR1 is robustly activated across different cell types expressing unique ETS factor profiles.
Lessons on regulation of RNAPII at post-recruitment steps: novel roles for Mediator and histone modifications in elongation control
The serum response network has proved to be an excellent model for studying mechanisms of post-recruitment regulation of RNAPII as many IEGs have paused RNAPII at their promoters, a phenomenon which appears to be a common feature of many rapidly inducible genes [25–27]. It is well established that the Negative Elongation Factor (NELF) and DRB-Sensitivity Inducing Factor (DSIF) collaborate to mediate RNAPII pausing, which in turn is alleviated by CDK9, the catalytic subunit of the Positive Elongation Factor b (P-TEFb). CDK9 phosphorylates NELF, DSIF and the C-terminal domain (CTD) of RNAPII [28], but it is not fully understood exactly how these phosphorylation events promote elongation. A key unresolved issue in the elongation field is how P-TEFb is recruited to promoters in a stimulus- and gene-specific manner. Once again, studies of the serum response network have contributed to our understanding of this issue.
Activation of preloaded transcription complexes at promoters of many IEGs in response to serum-stimulation requires ERK-mediated phosphorylation of ELK1, which induces an interaction with MED23, thus leading to recruitment of the Mediator complex [7]. Knockout of MED23 in mice abolishes EGR1 activation in ESCs and somewhat reduces recruitment of RNAPII and GTFs but, importantly, also reduces the rate of elongation by the remaining promoter-bound RNAPII, demonstrating that Mediator affects both recruitment and post-recruitment steps [8]. In further support of a post-recruitment role for Mediator, we have recently found that the CDK8 kinase subunit of Mediator is a positive regulator of serum response genes by virtue of its ability to enhance elongation [29**,30]. In response to serum, CDK8 is recruited to the promoters of IEGs and depletion of CDK8 impairs induction of these genes. While recruitment of RNAPII to these promoters is not affected, there is a clear defect in CTD-phosphorylation and elongation rates, concomitant with reduced recruitment of CDK9. Interestingly, biochemical purification and MudPit analysis showed that both CDK8-Mediator and the free CDK8-module (formed by CDK8, Cyclin C, MED12 and MED13) interact with P-TEFb [29**], indicating that Mediator regulates elongation at least in part by enabling recruitment of this key elongation factor.
Detailed analysis of the transcriptional events at the FOSL gene has revealed a different mechanism of P-TEFb recruitment involving the coordinated action of histone modifications. It has been repeatedly observed that acetylation of histones H3 and H4 as well as phosphorylation of histone H3 at serine 10 is associated with IEG activation (reviewed in [31]). Remarkably, Zippo et al. [32**] have elucidated a mechanism by which H3S10P can promote elongation. Serum-inducible phosphorylation of pre-acetylated H3 at the FOSL enhancer was found to increase RNAPII elongation and P-TEFb recruitment. Further investigation showed that H3S10P was required for binding of 14-3-3 proteins that in turn mediated recruitment of the HAT MOF to the enhancer. Subsequently, MOF-dependent acetylation of lysine 16 on histone H4 (H4K16Ac) promoted binding of the bromodomain-containing protein BRD4, which is known to interact with both acetyl-lysines and P-TEFb [33]. Thus, a relay of histone phosphorylation-acetylation leads to P-TEFb recruitment and enhanced elongation via the adaptor protein BRD4 (Figure 3).
Figure 3. Post-recruitment regulation of RNAPII.
Upon activation ERK, along with MSK1/2, is recruited to pre-loaded transcription complexes that include paused RNAPII and CBP/p300. Subsequent phosphorylation of ELK1 by ERK1/2 induces an interaction with MED23 leading to the recruitment of CDK8-Mediator, which is required for productive transcription. The HAT GCN5L and the associated factor TRRAP are known to interact with CDK8-Mediator and thus contribute to histone H3 phospho-acetylation. Importantly, release of paused RNAPII requires P-TEFb and the activity of its CDK9 subunit which phosphorylates NELF, DSIF and the RNAPII CTD to stimulate elongation. Mediator is thought to contribute to P-TEFb recruitment by via an uncharacterized interaction with the CDK module and/or by interaction with BRD4. BRD4 is also able to recruited P-TEFb by binding acetylated histones. It has been demonstrated for the FOSL enhancer that a relay from H3S10 phosphorylation to H4K16 acetylation, mediated by PIM1, 14-3-3 and MOF, leads to BRD4/P-TEFb recruitment.
A number of reports point to a connection between Mediator, BRD4 and histone phospho-acetylation. First, several independent studies showed that BRD4 interacts with Mediator suggesting that Mediator may recruit P-TEFb via BRD4 (reviewed in [33]). However, it is also clear that BRD4 interacts with Mediator independently of the presence of the CDK8-module [33]. Thus it is possible to envision at least two independent means by which Mediator may associate with P-TEFb, one via BRD4 interaction with core Mediator and a second one via P-TEFb binding to a subunit of the CDK8-module (or a CDK8-module associated factor). Importantly, a novel P-TEFb-containing complex, dubbed Super Elongation Complex (SEC) has been recently identified [34]. This complex lacks BRD4 but contains instead other potent regulators of elongation such as the ELL factors. It would be interesting to determine if SEC subunits interact with Mediator. Second, a variant of the CDK8-Mediator complex has been shown to associate with the HAT GCN5L and the associated factor TRRAP [35**]. Interestingly, this form of Mediator, named T/G Mediator, can catalyze phospho-acetylation of histone H3 in vitro, which could perhaps contribute to P-TEFb recruitment by a 14-3-3/BRD4 mechanism similar to that described by Zippo et al [32**]. Although these findings suggest a functional interplay between Mediator, P-TEFb and histone modifications, it remains unclear how these events are coordinated and whether they co-occur on specific gene loci. Of note, our studies showed that CDK8-Mediator regulates P-TEFb recruitment to the FOS and EGR1 loci without affecting histone acetylation marks recognized by BRD4 [29**].
Conclusions
Advancing our already sophisticated understanding of transcriptional regulation requires amenable and robust experimental paradigms. Because of its universality, ease of use and biomedical relevance, the serum response network has provided a powerful discovery platform, but it remains to be determined to what extent the principles established in this network apply to other transcriptional programs. How widespread is the use of the ‘fold-change’ logic described for ERK signaling? Which other signaling pathways employ direct DNA binding by their terminal kinases? What other families of related transcription factors use the mix-and-match strategy for output specificity? Is Mediator a regulator of RNAPII elongation outside of the serum response network? Future studies of other transcriptional programs will bring answer to these questions. In the meantime, we look forward to more lessons from the serum response network.
Acknowledgments
Work in the Espinosa lab is supported by grants from the National Institute of Health (RO1-CA117907) and National Science Foundation (MCB-0842974). JME is a Howard Hughes Medical Institute Early Career Scientist.
Abbreviations
- BRD
bromo-domain
- CBP
CREB-binding protein
- ChIP
chromatin immunoprecipitation
- ChIP-CHIP
chromatin immunoprecipitation followed by microarray analysis
- ChIP-seq
chromatin immunoprecipitation followed by deep sequencing
- CTD
carboxy-terminal domain
- DRB
5,6-dichloro-1-b-D-ribofuranosylbenzimidazole
- DSIF
DRB sensitivity-inducing factor
- EGF
epidermal growth factor
- EGR
early growth response
- ERK
extracellular signal-regulated kinase
- ESC
embryonic stem cell
- ETS
Avian erythroblastosis virus E26 (v-ets) oncogene homolog
- GTF
general transcription factor
- H3S10P
histone H3 serine 10 phosphorylation
- H4K16Ac
histone H4 lysine 16 acetylation
- HAT
histone lysine acetyl-transferase
- IEG
immediate early gene
- MAPK
mitogen-activated protein kinase
- MEF
mouse embryonic fibroblast
- MRTF
myocardin-related transcription factor
- MSK
mitogen- and stress-activated protein kinase
- NELF
negative elongation factor
- P-TEFb
positive elongation factor b
- RNAPII
RNA polymerase II
- RUNX
runt-related transcription factor
- SEC
super elongation complex
- SRF
serum response factor
- SWI/SNF
SWItch/Sucrose NonFermentable
- TCF
ternary complex factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26:3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 2.Yang S-H, Sharrocks AD, Whitmarsh AJ. Transcriptional regulation by the MAP kinase signaling cascades. Gene. 2003;320:3–21. doi: 10.1016/s0378-1119(03)00816-3. [DOI] [PubMed] [Google Scholar]
- **3.Cohen-Saidon C, Cohen AA, Sigal A, Liron Y, Alon U. Dynamics and variability of ERK2 response to EGF in individual living cells. Mol Cell. 2009;36:885–893. doi: 10.1016/j.molcel.2009.11.025. This single-cell based study found that nuclear accumulation of fluorescently-tagged ERK2 in response to EGF stimulation exhibited a constant fold-change with precise timing despite wide variability in the basal levels of nuclear ERK, and that most cells returned to their individual pre-stimulus levels. [DOI] [PubMed] [Google Scholar]
- 4.Goentoro L, Kirschner MW. Evidence that Fold-Change, and Not Absolute Level, of β-Catenin Dictates Wnt Signaling. Mol Cell. 2009;36:872–884. doi: 10.1016/j.molcel.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulds CE, Nelson ML, Blaszczak AG, Graves BJ. Ras/mitogen-activated protein kinase signaling activates Ets-1 and Ets-2 by CBP/p300 recruitment. Mol Cell Biol. 2004;24:10954–10964. doi: 10.1128/MCB.24.24.10954-10964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson ML, Kang H-S, Lee GM, Blaszczak AG, Lau DKW, McIntosh LP, Graves BJ. Ras signaling requires dynamic properties of Ets1 for phosphorylation-enhanced binding to coactivator CBP. Proc Natl Acad Sci USA. 2010;107:10026–10031. doi: 10.1073/pnas.0915137107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens JL, Cantin GT, Wang G, Shevchenko A, Shevchenko A, Berk AJ. Transcription control by E1A and MAP kinase pathway via Sur2 mediator subunit. Science. 2002;296:755–758. doi: 10.1126/science.1068943. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Balamotis MA, Stevens JL, Yamaguchi Y, Handa H, Berk AJ. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Mol Cell. 2005;17:683–694. doi: 10.1016/j.molcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- *9.Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nature Structural & Molecular Biology. 2009 doi: 10.1038/nsmb.1656. This paper reports the results of a phosphoproteomic screen that identified 24 potentially new ERK substrates, including NUP50, BAF155, hnRNPK and U1 snRNP70. [DOI] [PubMed] [Google Scholar]
- 10.Vermeulen L, Vanden Berghe W, Beck IME, De Bosscher K, Haegeman G. The versatile role of MSKs in transcriptional regulation. Trends in Biochemical Sciences. 2009;34:311–318. doi: 10.1016/j.tibs.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence MC, McGlynn K, Shao C, Duan L, Naziruddin B, Levy MF, Cobb MH. Chromatin-bound mitogen-activated protein kinases transmit dynamic signals in transcription complexes in beta-cells. Proc Natl Acad Sci USA. 2008;105:13315–13320. doi: 10.1073/pnas.0806465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Zhang H-M, Li L, Papadopoulou N, Hodgson G, Evans E, Galbraith M, Dear M, Vougier S, Saxton J, Shaw PE. Mitogen-induced recruitment of ERK and MSK to SRE promoter complexes by ternary complex factor Elk-1. Nucleic Acids Res. 2008;36:2594–2607. doi: 10.1093/nar/gkn099. This study demonstrated that mitogen-stimulated recruitment of ERK1/2 and MSK1/2 to the FOS and EGR1 promoters is dependent on MAPK docking domains in ELK1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Hu S, Xie Z, Onishi A, Yu X, Jiang L, Lin J, Rho H-s, Woodard C, Wang H, Jeong J-S, et al. Profiling the human protein-DNA interactome reveals ERK2 as a transcriptional repressor of interferon signaling. Cell. 2009;139:610–622. doi: 10.1016/j.cell.2009.08.037. Using a protein-microarray to identify novel DNA binding proteins, Hu et al. found that ERK was able to directly bind a particular DNA motif. Further analysis in vivo found that ERK was able to repress a number of genes, many of which are upregulated by IFNγ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dioum EM, Wauson EM, Cobb MH. MAP-ping unconventional protein-DNA interactions. Cell. 2009;139:462–463. doi: 10.1016/j.cell.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science. 2006;313:533–536. doi: 10.1126/science.1127677. [DOI] [PubMed] [Google Scholar]
- 16.Sharrocks AD. The ETS-domain transcription factor family. Nat Rev Mol Cell Biol. 2001;2:827–837. doi: 10.1038/35099076. [DOI] [PubMed] [Google Scholar]
- 17.Buchwalter G, Gross C, Wasylyk B. Ets ternary complex transcription factors. Gene. 2004;324:1–14. doi: 10.1016/j.gene.2003.09.028. [DOI] [PubMed] [Google Scholar]
- **18.Boros J, Donaldson IJ, O’Donnell A, Odrowaz ZA, Zeef L, Lupien M, Meyer CA, Liu XS, Brown M, Sharrocks AD. Elucidation of the ELK1 target gene network reveals a role in the coordinate regulation of core components of the gene regulation machinery. Genome Res. 2009;19:1963–1973. doi: 10.1101/gr.093047.109. Genome-wide identification of ELK1 binding sites, using ChIP-CHIP technology, demonstrated that ELK1 binds many genes independently of SRF. Importantly, this set of genes is enriched for components of the basal transcription machinery, the spliceosome, and ribosomal protein genes, suggesting that ELK1 has a role in regulating the cellular gene expression apparatus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White RJ, Sharrocks AD. Coordinated control of the gene expression machinery. Trends Genet. 2010;26:214–220. doi: 10.1016/j.tig.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Posern G, Treisman R. Actin’ together: serum response factor, its cofactors and the link to signal transduction. Trends Cell Biol. 2006;16:588–596. doi: 10.1016/j.tcb.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Muthusamy N, Barton K, Leiden JM. Defective activation and survival of T cells lacking the Ets-1 transcription factor. Nature. 1995;377:639–642. doi: 10.1038/377639a0. [DOI] [PubMed] [Google Scholar]
- 22.Hollenhorst PC, Shah AA, Hopkins C, Graves BJ. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 2007;21:1882–1894. doi: 10.1101/gad.1561707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **23.Hollenhorst PC, Chandler KJ, Poulsen RL, Johnson WE, Speck NA, Graves BJ. DNA specificity determinants associate with distinct transcription factor functions. PLoS Genet. 2009;5:e1000778. doi: 10.1371/journal.pgen.1000778. As part of an ongoing effort to decipher determinants of transcription factor specificity, this study using ChIP-seq identified several classes of ETS1 binding sites. Of particular interest was the finding that redundant binding with other ETS factors was enriched for housekeeping genes, while ETS1-specific binding with RUNX factors was associated with T-cell enhancers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **24.Balamotis MA, Pennella MA, Stevens JL, Wasylyk B, Belmont AS, Berk AJ. Complexity in transcription control at the activation domain-mediator interface. Science signaling. 2009;2:ra20. doi: 10.1126/scisignal.1164302. This work demonstrated that MED23 was not essential for EGR1 expression of Mediator recruitment in MEFs, in direct contrast to mESCs. It was found that ELK1 is replaced in MEFs by the related TCFs, SAP1 and NET, and that these TCFs were able to recruit Mediator via alternative interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–1848. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- **29.Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nature Structural & Molecular Biology. 2010;17:194–201. doi: 10.1038/nsmb.1752. CDK8, previously thought of as a repressive Mediator component, is here demonstrated to be a positive regulator of the serum response network. Knockdown of CDK8 was found to lower RNAPII elongation and impair P-TEFb recruitment to IEGs. Furthermore, biochemical analysis showed that P-TEFb interacts with the CDK-module. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galbraith MD, Espinosa JM, Donner AJ. CDK8: a positive regulator of transcription. Transcription. 2010;1:4–12. doi: 10.4161/trns.1.1.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davie J, Drobic B, Perez-Cadahia B. Nucleosomal response, immediate-early gene expression and cell transformation. Advances in enzyme …. 2009 doi: 10.1016/j.advenzreg.2009.10.008. [DOI] [PubMed] [Google Scholar]
- **32.Zippo A, Serafini R, Rocchigiani M, Pennacchini S, Krepelova A, Oliviero S. Histone crosstalk between H3S10ph and H4K16ac generates a histone code that mediates transcription elongation. Cell. 2009;138:1122–1136. doi: 10.1016/j.cell.2009.07.031. In this detailed analysis of the role of histone H3 serine 10 phosphorylation at the intronic enhancer of FOSL, the authors demonstrate that this modification is required for binding of 14-3-3 proteins which recruit the histone acetyl-transferase MOF. The subsequent acetylation of histone H4 at lysine 16 was important for binding of BRD4 and P-TEFb, providing a link between H3S10P and elongation. [DOI] [PubMed] [Google Scholar]
- 33.Wu S-Y, Chiang C-M. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 34.Lin C, Smith ER, Takahashi H, Lai KC, Martin-Brown S, Florens L, Washburn MP, Conaway JW, Conaway RC, Shilatifard A. AFF4, a component of the ELL/P-TEFb elongation complex and a shared subunit of MLL chimeras, can link transcription elongation to leukemia. Mol Cell. 2010;37:429–437. doi: 10.1016/j.molcel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **35.Meyer KD, Donner AJ, Knuesel MT, York AG, Espinosa JM, Taatjes DJ. Cooperative activity of cdk8 and GCN5L within Mediator directs tandem phosphoacetylation of histone H3. EMBO J. 2008;27:1447–1457. doi: 10.1038/emboj.2008.78. In this work, Mediator containing the CDK8-module was found to associate with the histone acetyl-transferase GCN5L, and the TRRAP polypeptide. This ‘T/G Mediator’ was able to phosphorylate H3S10 and acetylate H3K14 in vitro suggesting a role in gene activation. [DOI] [PMC free article] [PubMed] [Google Scholar]