Abstract
Purpose
To describe the prevalence of and risk factors for epiretinal membrane (ERM) in a multi-ethnic population and to evaluate possible racial/ethnic differences.
Design
Cross-sectional study.
Participants
Participants of the Multi-Ethnic Study of Atherosclerosis (MESA), examined at the second visit of the MESA when retinal photography was performed.
Methods
Data on 5960 participants aged 45 to 84 years from MESA, including white, blacks, Hispanic and Chinese from six United States communities, were analysed. ERM was assessed from digital non-stereoscopic fundus photographs and defined as cellophane macular reflex (CMR) without retinal folds or pre-retinal macular fibrosis (PMF) with retinal folds. Risk factors were assessed from standardized interviews, clinical examinations, and laboratory investigations.
Main outcome measures
ERM prevalence by ethnic/racial group, and risk factors associated with ERM.
Results
The prevalence of any ERM was 28.9%, of which 25.1% were CMR and 3.8% were PMF. The prevalence of ERM was significantly higher in Chinese (39.0%), compared to Hispanics (29.3%), whites (27.5%), and blacks (26.2%), p<0.001. In multivariable models, increasing age (odds ratio [OR] 1.19, 95% confidence intervals [CI], 1.06, 1.34, per year increase in age), diabetes (OR 1.92, 95% CI, 1.39, 2.65) and hypercholesterolemia (OR 1.33, 95% CI, 1.04, 1.69) were significantly associated with CMR.
Conclusions
This study showed that ERM was significantly more common in Chinese persons compared to whites, blacks and Hispanics. Risk factors for epiretinal membrane were increasing age, presence of diabetes and hypercholesterolemia.
It has been estimated that about 30 million people in the United States have epiretinal membranes (ERM) in at least one eye.1 ERM involving the macular or peri-macular regions can cause visual impairment, metamorphopsia, micropsia and occasionally monocular diplopia. The early form of ERM, termed cellophane macular reflex (CMR), is usually asymptomatic whereas the more severe form, known as pre-retinal macular fibrosis (PMF), may be associated with vision loss.2
In recent years, studies among different ethnic groups have provided population-based prevalence data on ERM, ranging from 2.2 to 18.5%.1–9 It has been suggested that the prevalence of ERM is lower in Asians compared to that in Caucasians.5 For example, McCarty et al5 reported that the age-standardized ERM prevalence was lower in a Japanese (2.8%)6 than in whites (5.1–9.1%).1, 2, 4, 5 The Beijing Eye Study7 and Handan Eye Study10 also reported a relatively low prevalence of ERM in Chinese (2.2% and 3.0% respectively). In the Funagata Study among Japanese persons,9 and the Singapore Malay Eye Study,8 ERM was defined from photographs graded using the same protocols and graders as in the Blue Mountains Eye Study.2 After age-standardization, the prevalence of ERM was higher in Asian Malays but similar between Japanese and whites. Reasons for such variability between the different racial/ethnic groups are unclear, but could be related to different study designs, sampling methodology, different photography methods, and different rates of detection and definition of ERM, especially when the earliest stages are present. Thus, whether there is indeed a racial/ethnic difference in prevalence of ERM remains unclear.
Despite much effort in clinical and laboratory research, the pathogenesis of ERM remains incompletely understood. While ERM is known to be associated with ocular diseases (e.g., diabetic retinopathy, retinal vein occlusion, retinal detachment) and surgery (e.g., post cataract surgery), most ERM occurs in people without clinical evidence of these eye conditions and is usually classified as idiopathic. Existing studies provide little consistent evidence regarding possible risk factors, other than age, for idiopathic (primary) ERM.1–4 In this study, we report the prevalence and risk factors for ERM in a multi-ethnic population in the United States and describe racial/ethnic differences.
METHODS
Study Population
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort study of men and women aged 45 to 84 years without a history of clinical cardiovascular disease, sampled from six United States communities. Details of the methodology have been described elsewhere.11–14 In brief, a total of 6814 participants recruited underwent a baseline examination (July 2000 to August 2002). Of these, 6176 returned for a second examination and had retinal photographs taken (August 2002 to January 2004). Among those, there were 216 photographs of insufficient quality for grading of CMR or PMF, leaving 5960 for analysis.
The tenets of the Declaration of Helsinki were observed, and institutional review board approval was granted at each study site. Written informed consent was obtained from each participant. All procedures followed were in accordance with institutional guidelines.
Retinal Photography and Grading
Retinal photography was performed at each site using a standardized protocol.12–15 Participants were seated in a darkened room and both eyes were photographed using a 45-degree non-mydriatic digital camera. Two photographic fields, centered at the optic disc and macula, were taken from both eyes.
All images were evaluated by research staff at the Fundus Photograph Reading Center, the University of Wisconsin, Madison, who were trained to assess retinal photographs according to standardized protocols and masked to participants’ characteristics. ERM was graded according to a standardized protocol. The first or earlier stage of ERM was defined as the presence of CMR only, a patch or patches of irregular increased reflection from the inner surface of the retina. The second or later stage of ERM was defined as the presence of PMF, characterized as thickening and contraction of the membrane, with opaque or gray colored superficial retinal folds or traction lines. Any ERM was defined as the presence of CMR or PMF in either or both eyes. Participants with both CMR and PMF were classified as having PMF. Bilateral ERM (CMR or PMF in both eyes) was determined from those with gradable photographs from both eyes. ERM was also classified as primary (idiopathic) or secondary. The latter was defined as any ERM present in eyes with other ocular conditions known to be associated with ERM, including retinal vein occlusion, diabetic retinopathy, retinal detachment or post cataract surgery state. ERM was considered to be primary if these conditions were not present in the eye assessed.
Other data was also obtained from fundus photographs, including grading of retinal vascular caliber, which was measured by a computer-based program (IVAN, University of Wisconsin, Madison), and grading of age-related macular degeneration using the modified Wisconsin Age-Related Maculopathy Grading System, as described in detail elsewhere.12–16
Refraction was determined by non-cycloplegic refraction using an Autorefractor (NIDEK ARK-760; NIDEK Co Ltd, Tokyo, Japan). For each eye, a minimum of three separate measurements were obtained and averaged. From these, the spherical equivalent of each eye was computed as the mean sphere measurement and one half of the mean cylinder measurement. Myopia was defined as spherical equivalent ≤-1.00 and hyperopia was defined as spherical equivalent ≥+1.00. Visual impairment was defined as visual acuity ≤20/40 and blindness was defined as visual acuity ≤20/200.
Assessment and Definitions of Risk Factors
All participants underwent standardized interviews, clinical examinations, and laboratory investigations for the assessment of systemic risk factors, as described in detail elsewhere.11–13, 16 Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medications. Diabetes mellitus was defined as fasting glucose ≥7.0 mmol/L (126 mg/dL) or use of insulin or oral hypoglycemic medication. Hypercholesterolemia was defined as fasting cholesterol >240mg/dL, hypertriglyceridemia as fasting triglyceride of ≥200mg/dL and homocystinemia as homocystine level of >12 μmol/L. A detailed questionnaire was used to obtain information about medical (e.g., hypertension, diabetes) and ocular (e.g., cataract surgery) history, cigarette smoking, alcohol consumption, and medication use. Fasting blood samples were drawn and blood parameters analyzed following standardized protocols.16, 17
Statistical Analysis
The age-specific prevalence of CMR, PMF, and any ERM (CMR or PMF) were determined for all participants and by ethnicity. They were compared to those from previous population-based studies after direct age-standardization using the world population aged 40 years or older by the World Health Organization as a reference population.18 ERM and its subtypes (CMR or PMF) were analyzed as binary outcome variables (present versus absent). Risk factors were analysed as present versus absent for binary traits (e.g., hypertension) and categorized based on above 80% cut off or any biologically meaningful cut off. Chi-square tests were used to compare the prevalence rates of ERM between gender and ethnic groups. We used log-likelihood test for trend to assess the linear influence of age on ERM prevalence. Logistic regression models were used to estimate odds ratios (OR) for primary CMR or PMF for each potential risk factor, adjusting for age, gender, ethnicity and study centre. In multivariable-adjusted analysis, a parsimonious model was constructed for primary ERM that includes independent risk factors with simultaneous adjustment for age, gender, ethnicity and study centre, smoking and diabetes only. All analyses were performed in SPSS version 16.0.1 (SPSS Inc, Chicago, III).
RESULTS
Participants
MESA participant characteristics have been previously presented.14, 16 In summary, from 6814 participants recruited at baseline, 6176 participated in the follow-up visit, when retinal images were obtained. Of 6176 participants, 5960 had gradable photographs for ERM. Of the 5960 participants, 39% were white, 27% black, 22% Hispanic, and 12% Chinese. The mean age of participants was 63.2 years (whites 63.8 years, Chinese 63.2 years, blacks 63.0 years, Hispanics 62.4 years; overall p from one way analysis of variance = 0.001). In pair wise comparison, only Hispanic participants were significantly younger than white (p<0.001) and black participants (p=0.03). Of the 854 non-participants, 276 (32.3%) were white, 289 (33.8%) black, 194 (22.7%) Hispanic, and 95 (11.1%) Chinese. The mean age of non-participants was 67.1 years (whites 67.1 years, blacks 65.9 years, Hispanics 64.7 years and Chinese 68.4 years).
Compared to non-participants, participants were more likely to be younger (63.2 versus 67.1 years, p<0.001), more likely to be male (47.9% versus 42.3%, p = 0.002) and white (39.4% versus 32.3%, p<0.001), but less likely to be black (27.2% versus 34.6%, p<0.001). Participants were also less likely to have a history of diabetes (14.4% versus 22.6%, p<0.001) and hypertension (43.7% versus 52.8%, p<0.001).
Prevalence of Epiretinal Membranes
In our study population, signs of any ERM were present in 28.9% (1722 of 5960) participants; 35% of these were bilateral. CMR was present in 25.1% and PMF in 3.8% of participants. Table 1 (available at http://aaojournal.org) details the prevalence of ERM by age group, gender, and ethnic group. The prevalence of all types of ERM increased significantly with age (p for trend <0.001), but did not differ between women and men after age adjustment.
Table 1.
Prevalence of Epiretinal Membranes in the Multi-Ethnic Study of Atherosclerosis population.
| Any ERM | Primary ERM | Secondary ERM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any ERM | CMR | PMF | Any ERM | CMR | PMF | Any ERM | CMR | PMF | ||||
| N | n (%) | n (%) | n (%) | N | n (%) | n (%) | n (%) | N | n (%) | n (%) | n (%) | |
| Total Cohort | 5960 | 1722 (28.9) | 1494 (25.1) | 228 (3.8) | 4761 | 1241 (26.1) | 1083 (22.7) | 158 (3.3) | 1199 | 481 (40.1) | 411 (34.3) | 70 (5.8) |
| Age, years | ||||||||||||
| 45–54 | 1429 | 191 (13.4) | 179 (12.5) | 12 (0.8) | 1281 | 169 (13.2) | 158 (12.3) | 11 (0.9) | 148 | 22 (14.9) | 21 (14.2) | 1 (0.7) |
| 55–64 | 1792 | 437 (24.4) | 384 (21.4) | 53 (3.0) | 1531 | 365 (23.8) | 319 (20.8) | 46 (3.0) | 261 | 72 (27.6) | 65 (24.9) | 7 (2.7) |
| 65–74 | 1825 | 736 (40.3) | 618 (33.9) | 118 (6.5) | 1420 | 545 (38.4) | 462 (32.5) | 83 (5.8) | 405 | 191 (47.2) | 156 (38.5) | 35 (8.6) |
| ≥75 | 914 | 358 (39.2) | 313 (34.2) | 45 (4.9) | 529 | 162 (30.6) | 144 (27.2) | 18 (3.4) | 385 | 196 (50.9) | 169 (43.9) | 27 (7.0) |
| p for trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Gender | ||||||||||||
| Men | 2852 | 850 (29.8) | 743 (26.1) | 107 (3.8) | 2263 | 604 (26.7) | 532 (23.5) | 72 (3.2) | 589 | 246 (41.8) | 211 (35.8) | 35 (5.9) |
| Women | 3108 | 872 (28.1) | 751 (24.2) | 121 (3.9) | 2498 | 637 (25.5) | 551 (22.1) | 86 (3.4) | 610 | 235 (38.5) | 200 (32.8) | 35 (5.7) |
| P | 0.14 | 0.10 | 0.93 | 0.38 | 0.25 | 0.70 | 0.44 | 0.25 | 0.72 | |||
| Race/ethnicity | ||||||||||||
| Whites | 2348 | 645 (27.5) | 557 (23.7) | 88 (3.7) | 1890 | 472 (25.0) | 413 (21.9) | 59 (3.1) | 458 | 173 (37.8) | 144 (31.4) | 29 (6.3) |
| Blacks | 1606 | 421 (26.2) | 393 (24.5) | 28 (1.7) | 1266 | 288 (22.7) | 268 (21.2) | 20 (1.6) | 340 | 133 (39.1) | 125 (36.8) | 8 (2.4) |
| Hispanics | 1298 | 380 (29.3) | 325 (25.0) | 55 (4.2) | 1039 | 271 (26.1) | 231 (22.2) | 40 (3.8) | 259 | 109 (42.1) | 94 (36.3) | 15 (5.8) |
| Chinese | 708 | 276 (39.0) | 219 (30.9) | 57 (8.1) | 566 | 210 (37.1) | 171 (30.2) | 39 (6.9) | 142 | 66 (46.5) | 48 (33.8) | 18 (12.7) |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.61 | 0.48 | <0.001 | |||
ERM: epiretinal membrane; CMR: cellophane macular reflex; PMF: pre-retinal macular fibrosis
Among the different ethnic groups, prevalence of any ERM and primary ERM was significantly higher in Chinese (p<0.001), compared to Hispanics, whites, and blacks. This higher prevalence rate of ERM in Chinese remains significant in pair wise comparison with each of the other ethnic categories (all p<0.001).
Prevalence of primary ERM among 4761 participants without known ocular risk factors was 26.1%, while prevalence of secondary ERM among 1199 participants with known ocular risk factors was higher at 40.1%. The prevalence of secondary PMR was significantly higher in Chinese (p<0.001) compared to other races.
The prevalence of secondary ERM, CMR and PMF by cause is listed in Table 2. Among eyes with potential causative factors, ERM were most prevalent in eyes with a history of cataract surgery (50.2% had ERM) and retinal detachment (1 of 2), followed by eyes with retinal vein occlusion (38.1%), and diabetic retinopathy (33.3%).
Table 2.
Prevalence of Secondary Epiretinal Membrane by Cause in the Multi-Ethnic Study of Atherosclerosis population.
| Secondary cause | N (eyes)* | Any ERM n (%) | CMR n (%) | PMF n (%) |
|---|---|---|---|---|
| Cataract surgery | 600 | 301 (50.2) | 255 (42.5) | 46 (7.7) |
| Diabetic retinopathy | 661 | 220 (33.3) | 188 (28.4) | 32 (4.8) |
| Retinal vein occlusion | 42 | 16 (38.1) | 12 (28.6) | 4 (9.5) |
| Retinal detachment | 2 | 1 (50.0) | 0 | 1 (50.0) |
ERM: epiretinal membrane; CMR: cellophane macular reflex; PMF: pre-retinal macular fibrosis
Reported number of eyes with secondary ERM is higher than 1199 as some eyes have more than one cause
Factors Associated with Idiopathic Epiretinal Membranes
Table 3 outlines risk factors investigated for primary ERM. Being Chinese-American was consistently associated with higher prevalence of primary CMR and PMF, while being black was associated with lower prevalence of primary PMF. Increasing age was significantly associated with increasing prevalence of primary ERM. Persons with diabetes (in the absence of diabetic retinopathy) were also more likely to have primary CMR and PMF after adjusting for age, gender, ethnicity, and study center, than persons without diabetes. After further adjusting for diabetes status and history of smoking, hypercholesterolemia was significantly associated with increased prevalence of primary CMR only; and narrower arteriolar caliber was significantly associated with increased prevalence of primary PMF only. There were no significant associations of myopia, hyperopia, hypertension, hypertriglyceridemia, homocystinemia, arteriovenous nicking, and age-related macular degeneration with primary CMR or PMF. The proportions with visual impairment (20/40 or worse) were similar between eyes with ERM (12.7%) and those without ERM (12.3%, p=0.68).
Table 3.
Risk Factors for Primary Epiretinal Membrane in the Multi-Ethnic Study of Atherosclerosis population.
| Risk Factors | Multivariable adjusted analysis** |
|||||||
|---|---|---|---|---|---|---|---|---|
| CMR | PMF | CMR | PMF | |||||
| OR (95% CI)* | p | OR (95% CI)* | p | OR (95% CI)** | p | OR (95% CI)** | p | |
| Age per 10 years increase | 1.57 (1.45, 1.69) | <0.001 | 1.81 (1.54, 2.14) | <0.001 | 1.19 (1.06, 1.34) | 0.004 | 1.16 (0.89, 1.52) | 0.28 |
| Race/Ethnicity: | ||||||||
| Whites (reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Blacks | 1.06 (0.91, 1.23) | 0.48 | 0.47 (0.27, 0.80) | 0.006 | 0.98 (0.81, 1.16) | 0.72 | 0.52 (0.30, 0.89) | 0.02 |
| Hispanics | 1.18 (1.00, 1.39) | 0.05 | 1.25 (0.81, 1.93) | 0.31 | 1.08 (0.89, 1.32) | 0.42 | 1.35 (0.87, 2.09) | 0.18 |
| Chinese | 1.66 (1.34, 2.06) | <0.001 | 2.46 (1.48, 4.07) | <0.001 | 1.64 (1.28, 2.10) | <0.001 | 2.58 (1.56, 4.29) | <0.001 |
| Smoking, current vs. never | 1.11 (0.90, 1.38) | 0.34 | 2.54 (1.18, 5.47) | 0.02 | 1.31 (0.93, 1.87) | 0.13 | 2.38 (0.73, 7.75) | 0.15 |
| Diabetes without retinopathy | 1.43 (1.18, 1.72) | <0.001 | 2.94 (1.61, 5.56) | 0.001 | 1.92 (1.39, 2.65) | <0.001 | 3.17 (1.26, 8.0) | 0.01 |
| Hypercholesterolemia, >240mg/dL | 1.34 (1.06, 1.68) | 0.01 | 1.23 (0.67, 2.92) | 0.50 | 1.33(1.04, 1.69) | 0.02 | 1.15 (0.56, 2.34) | 0.70 |
| +Focal retinal arteriolar narrowing | 0.91 (0.84, 0.99) | 0.03 | 0.93 (0.74, 1.17) | 0.52 | 1.03 (0.94, 1.12) | 0.52 | 1.01 (0.82, 1.24) | 0.94 |
| +Retinal arteriolar diameter per standard deviation decrease | 1.02 (0.94, 1.08) | 0.65 | 1.34 (1.11, 1.63) | 0.003 | 1.01 (0.90, 1.07) | 0.92 | 1.32 (1.09, 1.60) | 0.005 |
| +Myopia versus emmetropia | 0.83 (0.67, 1.02) | 0.08 | 1.15 (0.66, 1.99) | 0.62 | 0.83 (0.67, 1.03) | 0.09 | 1.15 (0.66, 2.00) | 0.62 |
| +Hyperopia versus emmetropia | 0.92 (0.76, 1.11) | 0.37 | 0.83 (0.49, 1.39) | 0.46 | 0.93 (0.77, 1.12) | 0.44 | 0.82 (0.49, 1.38) | 0.46 |
Odds ratio adjusted for age, gender, ethnicity and study center
Odds ratio adjusted for age, gender, ethnicity, study center, diabetes and smoking.
Analysis confined to right eyes only
CMR: cellophane macular reflex; PMF: pre-retinal macular fibrosis; OR: odds ratio; CI: confidence interval
Table 4 (available at http://aaojournal.org) summarizes the age-standardized prevalence rates of ERM in ten population-based studies to date (including the current study).
Table 4.
Comparison of Prevalence Rates of Epiretinal Membranes in Eight Population-Based Studies
| Year | Study | Country | Race | Age | Study population | Fundus photography | Age-standardized prevalence*, % (95% CI) |
||
|---|---|---|---|---|---|---|---|---|---|
| Any ERM | CMR | PMF | |||||||
| 1987–88 | Beaver Dam Eye Study1 | USA | White | 43–84 | 4802 | 30° film stereo | - | - | - |
| 1992–93 | Blue Mountains Eye Study2 | Australia | White | 49–97 | 3490 | 30° film stereo | 3.8 (3.2–4.4) | 2.6 (2.1–3.1) | 1.2 (0.8–1.5) |
| 1992–94 | Visual Impairment Project Study5 | Australia | White | ≤40 | 4313 | 30° film stereo | 4.6 (4.1–5.2) | 3.9 (3.3–4.4) | 1.4 (1.1–1.8) |
| 1998 | Hisayama Study6 | Japan | Japanese | ≤40 | 1765 | 45° film non-stereo | 2.8 (2.0–3.5) | 2.2 (1.6 –2.9) | 0.5 (0.2–8.1) |
| 2000–02 | Funagata Study9 | Japan | Japanese | ≤35 | 1723 | 45° film non-stereo | - | - | - |
| 2001 | Beijing Eye Study7 | China | Chinese | ≤40 | 4378 | 45° digital | - | - | - |
| 2000–03 | Latino Eye Study4 | USA | Hispanic | ≤40 | 5982 | 30° film stereo | 19.0 (18.0–20.0) | 16.6 (15.7–17.6) | 2.5 (2.0–2.9) |
| 2004–06 | Singapore Malay Eye Study8 | Singapore | Malay | 40–80 | 3265 | 45° digital | 11.4 (10.3–12.5) | 4.4 (3.7–5.2) | 3.9 (3.2–4.5) |
| 2006–07 | Handan Eye Study10 | China | Chinese | 40+ | 5086 | 45° digital | 4.2 (3.4–5.0) | 2.8 (2.1–3.4) | 6.0 (4.0–8.1) |
| 2000–02 | Multi-Ethnic | USA | White | 45–84 | 2348 | 45° digital | 20.0 (18.2–21.8) | 18.2 (16.4–20.0) | 2.3 (1.7–3.0) |
| Study of Atherosclerosis | Black | 45–84 | 1606 | 17.9 (15.9–20.0) | 17.4 (15.3–19.4) | 1.1 (0.6–1.6) | |||
| Hispanic | 45–84 | 1298 | 21.6 (19.2–24.1) | 19.4 (17.0–21.8) | 2.8 (1.9–3.7%) | ||||
| Chinese | 45–84 | 708 | 31.2 (27.3–35.0) | 26.1 (22.4–29.8) | 26.1 (22.4–29.8) | ||||
| Total | 45–84 | 5960 | 22.7 (21.5–23.8) | 20.0 (18.9–21.1) | 20.0 (18.9–21.1) | ||||
CI: Confidence interval; ERM: epiretinal membrane; CMR: cellophane macular reflex; PMF: pre-retinal macular fibrosis; USA: United States of America
Age-standardized to the world population provided by the World Health Organization18
DISCUSSION
Our study found a nearly 28.9% prevalence of ERM in the MESA population (age-standardized prevalence 22.7%, confidence interval 21.5–23.8%), substantially higher than previously reported in any other study (see Table 4, available at http://aaojournal.org). Other population studies have reported prevalence rates ranging from 6.0–11.8% in whites,1, 2, 5 18.0% in Hispanics,4 2.2–3.0% in Chinese, 4.0–5.4% in Japanese,6, 9 and 7.9% in Malays.8 To our knowledge, our study is the first study to report the prevalence of ERM in blacks; and to directly compare racial/ethnic differences in ERM prevalence within the same study cohort.
The prevalence of ERM varies across studies, and comparison of these is problematic, given the variability in methods and protocols for grading and defining ERM (Table 4, available at http://aaojournal.org). The grading of ERM in the current study (MESA, from digital photographs), the Beaver Dam Eye Study1 (30 degree film stereoscopic), and the Los Angeles Latino Eye Study4 (30 degree film stereoscopic), were all performed in the same grading center at The University of Wisconsin, Madison, USA, over nearly a twenty year period. The grading of ERM in the Blue Mountains Eye Study2 (30 degree film stereoscopic), the Funagata Eye Study9 (45 degree film stereoscopic), and the Singapore Malay Eye Study8 (digital photographs), were all performed in the same grading center at the Centre for Vision Research, University of Sydney, Australia. A greater variation in the prevalence of CMR than the variation in the prevalence of PMF across these studies suggests possible systemic differences in the grading of CMR.
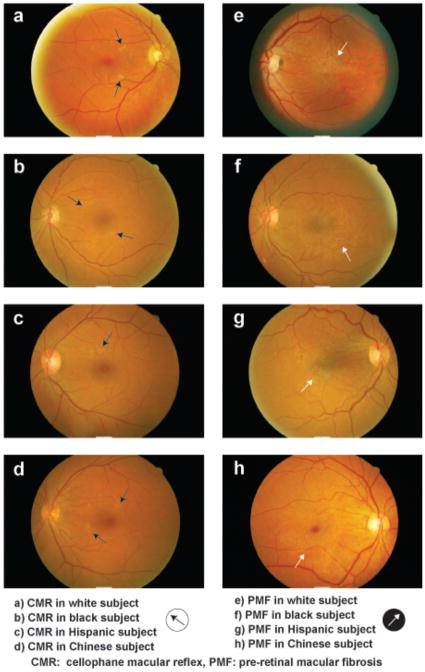
However, we are able to make valid comparisons between the four racial/ethic groups reported within our study. We found that Chinese participants had a higher prevalence of ERM across all ERM subgroups (any ERM, CMR, PMF; primary ERM, CMR PMF; and secondary PMF) compared to other races investigated in our study. Figure 1a–h (available at http://aaojournal.org) illustrates examples of CMR and PMF seen in each racial/ethnic group in our study. While it is possible that in some eyes, strong normal retinal reflex especially in Asians, might be misclassified as CMR, over-grading of PMF is highly unlikely, given that retinal folds or traction lines are essential diagnostic criterion. Interestingly, the Singapore Malay Eye Study,8 using the same grading center and identical protocol to the Blue Mountains Eye Study,2 also reported that ERM prevalence was significantly higher in Asian Malays than white participants of the Blue Mountains Eye Study. It is not clear what factors explain these racial/ethnic differences.
Figure 1.
Not surprisingly, increasing age was significantly associated with idiopathic ERM. This has been reported by numerous studies in the past.1, 2, 4–9 As shown in Table 3, diabetes, in the absence of diabetic retinopathy, was significantly associated with primary CMR (OR 1.92, CI 1.39 to 2.65) and PMF (OR 3.17, CI 1.26 to 8.00). This is consistent with findings in other studies.2, 9
Previous studies have reported inconsistent associations between primary ERM and risk factors such as hypercholesterolemia6 and retinal vascular changes including arteriovenous nicking1 and narrower arteriolar diameter.8 Our study found history of smoking, hypercholesterolemia, focal arteriolar narrowing, and narrower arteriolar diameter, to be associated with primary CMR and/or PMF when adjusted for age, gender, and ethnicity. Hypercholesterolemia and narrow arteriolar diameter remained significantly associated with primary CMR and PMF respectively, after further adjustment for other covariates. Our study and several others1, 6, 8 have described interesting but inconsistent associations between primary ERM and cardiovascular risk factors apart from diabetes. It is possible that the presence of PMF affects retinal caliber, due to traction from the membranes. The pathophysiological mechanisms for the association with hypercholesterolemia is not clear. Given the number of risk factors analyzed, the possibility of a chance finding cannot be excluded.
There are a number of limitations in our study. First, we were not able to assess some risk factors previously reported in other studies, (e.g., posterior vitreous detachment).19 ERM was diagnosed based on retinal photographic grading only. The study did not collect comprehensive data on all ocular pathology and therefore some secondary ERM could have been misclassified as primary. Secondly, our study is subject to the limitations of a cross-sectional design and smaller sample size in specific racial/ethnic groups after racial/ethnic break down.
In conclusion, this study describes a relatively high frequency of ERM and shows differences in ERM prevalence among four different racial/ethnic groups in the United States. Our findings highlight the possibility of a racial/ethnic predilection for ERM, with all subtypes of primary and secondary ERM prevalence being significantly higher in Chinese compared to whites, blacks and Hispanics. Consistent with previous studies, age and diabetes were significant risk factors for the presence of primary ERM. In addition to this, hypercholesterolemia was also found to be significantly associated with ERM.
Acknowledgments
Funding: This research was supported by contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. Additional support was provided by NIH grant HL69979-03 (Klein R and Wong TY) and NEI Z01EY000403 (Cotch MF). The funding source had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: No authors have any financial/conflicting interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Klein R, Klein BE, Wang Q, Moss SE. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403–25. discussion 425–30. [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes: the Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033–40. doi: 10.1016/s0161-6420(97)30190-0. [DOI] [PubMed] [Google Scholar]
- 3.Fraser-Bell S, Guzowski M, Rochtchina E, et al. Five-year cumulative incidence and progression of epiretinal membranes: the Blue Mountains Eye Study. Ophthalmology. 2003;110:34–40. doi: 10.1016/s0161-6420(02)01443-4. [DOI] [PubMed] [Google Scholar]
- 4.Fraser-Bell S, Ying-Lai M, Klein R, Varma R Los Angeles Latino Eye Study Group. Prevalence and associations of epiretinal membranes in Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2004;45:1732–6. doi: 10.1167/iovs.03-1295. [DOI] [PubMed] [Google Scholar]
- 5.McCarty DJ, Mukesh BN, Chikani V, et al. Prevalence and associations of epiretinal membranes in the Visual Impairment Project. Am J Ophthalmol. 2005;140:288–94. doi: 10.1016/j.ajo.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki M, Nakamura H, Kubo M, et al. Prevalence and risk factors for epiretinal membranes in a Japanese population: the Hisayama study. Graefes Arch Clin Exp Ophthalmol. 2003;241:642–6. doi: 10.1007/s00417-003-0723-8. [DOI] [PubMed] [Google Scholar]
- 7.You Q, Xu L, Jonas JB. Prevalence and associations of epiretinal membranes in adult Chinese: the Beijing Eye Study. Eye (Lond) 2008;22:874–9. doi: 10.1038/sj.eye.6702786. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki R, Wang JJ, Mitchell P, et al. Singapore Malay Eye Study Group. Racial difference in the prevalence of epiretinal membrane between Caucasians and Asians. Br J Ophthalmol. 2008;92:1320–4. doi: 10.1136/bjo.2008.144626. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki R, Wang JJ, Sate H, et al. Prevalence and associations of epiretinal membranes in an adult Japanese population: the Funagata Study. Eye (Lond) 2009;23:1045–51. doi: 10.1038/eye.2008.238. [DOI] [PubMed] [Google Scholar]
- 10.Duan XR, Liang YB, Friedman DS, et al. Prevalence and associations of epiretinal membranes in a rural Chinese adult population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2009;50:2018–23. doi: 10.1167/iovs.08-2624. [DOI] [PubMed] [Google Scholar]
- 11.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Bluemke DA, Klein R, et al. Retinal arteriolar narrowing and left ventricular remodeling: left ventricular remodeling: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2007;50:48–55. doi: 10.1016/j.jacc.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung N, Sharret AR, Klein R, et al. Aortic distensibility and retinal arteriolar narrowing: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2007;50:617–22. doi: 10.1161/HYPERTENSIONAHA.107.091926. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology. 2006;113:373–80. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Cheung N, Islam FM, Jacobs DR, Jr, et al. Arterial compliance and retinal vascular caliber in cerebrovascular disease. Ann Neurol. 2007;62:618–24. doi: 10.1002/ana.21236. [DOI] [PubMed] [Google Scholar]
- 16.Wong TY, Islam FM, Klein R, et al. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the Multi-Ethnic Study of Atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–50. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson JC, Jiang XC, Tabas I, et al. Plasma sphingomyelin and subclinical atherosclerosis: findings from the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2006;163:903–12. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad OB, Boschi-Pinto C, Lopez AD, et al. Age standardization of rates: a new WHO standard. Geneva: World Health Organization; 2001. [Accessed March 27, 2010]. pp. 10–12. GPE discussion paper series: no 31. Available at: http://www.who.int/healthinfo/paper31.pdf. [Google Scholar]
- 19.Wiznia RA. Posterior vitreous detachment and idiopathic preretinal macular gliosis. Am J Ophthalmol. 1986;102:196–8. doi: 10.1016/0002-9394(86)90144-3. [DOI] [PubMed] [Google Scholar]