Abstract
Active vision requires integrating information coming from the retina with that generated internally within the brain, especially by saccadic eye movements. Just as visual information reaches cortex via the lateral geniculate nucleus of the thalamus, this internal information reaches the cerebral cortex through other higher order nuclei of the thalamus. This review summarizes recent work on four of these thalamic nuclei. The first two pathways convey internal information about upcoming saccades (a corollary discharge) and probably contribute to the neuronal mechanisms that underlie stable visual perception. The second two pathways may contribute to the neuronal mechanisms underlying visual spatial attention in cortex and in the thalamus itself.
HIGHER-ORDER THALAMIC NUCLEI
Our vision incorporates both what falls on the retina and the consequences of moving the eye, particularly the consequences of rapid or saccadic eye movements. We refer to this vision as active vision [1]. The brain mechanisms that underlie this active vision depend on both visual inputs from the retina and information from within the brain. Both these inputs reach the cerebral cortical visual areas via nuclei in the thalamus.
Visual information from the outside world is conveyed from the retinal receptors through the sensory relay nucleus of the thalamus, the lateral geniculate nucleus (LGN), to primary visual cortex (V1 or striate cortex). The neuronal mechanisms that underlie both visual perception and the visual control of movement, however, require other information from within the brain. This internally-generated information does not reach cortex through the sensory relay nuclei, but instead through other thalamic nuclei, frequently referred to as higher-order thalamic nuclei [2].
Many pathways through the thalamus carry this internally-generated information to the cerebral cortex, including pathways from the cerebellum, the basal ganglia, and the caudal brainstem. The thalamus is the largest group of nuclei in the diencephalon and by volume is probably the least understood region of the brain. While the pathways from many brain areas have been anatomically identified (for detailed descriptions see [3]), their function has been more difficult to evaluate. A group of thalamic pathways, however, originate in the superior colliculus (SC), a structure on the roof of the midbrain that has been intensively investigated for its role in producing saccadic eye movements. The results of these investigations provide us the opportunity to use the SC-to-cortex pathways to tease apart some of the contributions that the thalamus makes to the visual functions of the cerebral cortex.
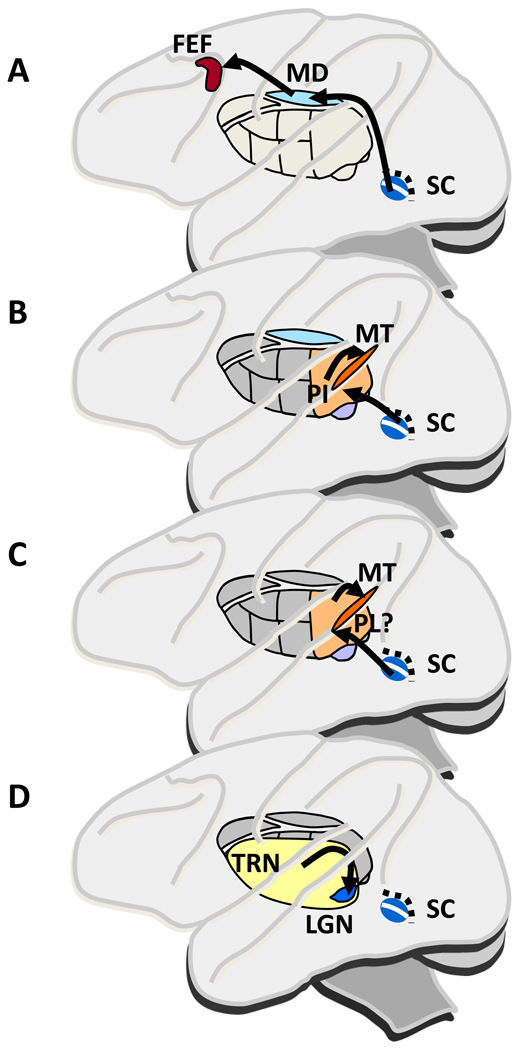
A number of pathways from the SC through the thalamus convey information to cerebral cortex. Figure 1 shows four pathways through thalamus based on anatomical evidence for which we now have enough functional information to include in this review. The first three of the pathways (Fig. 1A–C) have their origin in either the superficial layers of the SC, where neurons respond to visual stimuli in a limited part of the visual field (their receptive field, RF) or the intermediate layers, where neurons increase their activity before saccades to one part of the visual field (their movement field). Figure 1A shows a pathway from the intermediate saccade related layers to the parvocellular region of the medial dorsal thalamic nucleus (MD) and then to the frontal eye field (FEF) of the frontal cortex. Figure 1B outlines a pathway from the SC superficial visual layers to the inferior pulvinar nucleus (PI) and then to areas of occipital and parietal cortex. Figure 1C indicates a pathway from the SC intermediate layers through what is probably the lateral pulvinar and then to at least parietal and occipital cortex. Figure 1D shows a fourth pathway that includes the thalamic reticular nucleus (TRN) which is spread across much of the surface of the thalamus. The TRN does not lie on a serial path to cerebral cortex, but instead acts on the visual information passing through the LGN on its way to cortex rather than on cortex itself
Figure 1.
Pathways through the thalamus from the superior colliculus (SC) to cerebral cortex. Side view of a monkey brain highlighting thalamic nuclei that convey visual and/or saccade related activity in SC. A. A pathway that may carry a signal related to maintaining stable visual perception despite the image displacements produced by saccades. The pathway from saccade related neurons in the intermediate layers of superior colliculus (SC) passes through the lateral parvocellular region of the thalamic medial dorsal nucleus (MD) before reaching the frontal eye field (FEF) in frontal cortex. B. A pathway that may carry a signal that contributes to the suppression of blur during a saccade. The pathway originates in the visual neurons in the superficial layers, which project to inferior pulvinar (PI) and then to regions of occipital and parietal cortex, including the middle temporal area (MT). C. A pathway that may carry the preparatory movement related activity providing the motor signal for modulating visual cortical activity with shifts of visual attention. The candidate pathway is from the saccade related intermediate SC layers to the pulvinar (probably the lateral pulvinar (PL) and area Pdm at the border of lateral and medial pulvinar) and then to parietal and occipital cortex. D. The connections between the thalamic reticular nucleus (TRN) and the lateral geniculate nucleus (LGN), which may underlie the enhanced activity in the LGN with shifts of attention. The nature of the projections to both nuclei from the SC is uncertain. Thalamus representation after Netter [70]
We consider these thalamic nuclei in two groups based on our current knowledge about the information they convey to cortex. One relates to the perception of a stable visual world despite the saccades that displace images on the retina with each saccade and that blur the images during the saccade (the pathways in Fig. 1A and B). The second relates to the shifts of attention that accompany each saccade, which might be mechanistically related to saccade generation (Figure 1C and 1D).
THALAMIC PATHWAYS CONTRIBUTING TO STABLE VISION
Each saccade displaces the image on the retina. This should produce a perceived jump of the visual scene, but it does not. With each saccade we should also perceive the blur of the image as the eye rapidly sweeps across the visual field, but we do not. There must be brain mechanisms that compensate for both image displacement and suppression of blur resulting from saccades. This compensation is a major achievement of the visual system.
Corollary discharge (CD) is a key mechanism that probably underlies much of the remarkable compensation for the disruption generated by saccades [4]. The basic concept of a CD is that the activity directed downward in the brain to produce movement is simultaneously directed upward in the brain to inform other regions that the movement is about to occur (Fig. 2A). A CD for saccades would be expected to originate in an area where neurons discharge before saccades, and the intermediate layers of the SC provide an excellent source for such a CD (Fig. 2B). The purpose of this section is to consider the upward pathways from the SC to the thalamus that might convey a CD and its effect on visual processing.
Figure 2.
The concept of a corollary discharge (CD) and its relation to thalamic function. A. The logic of a CD or efference copy (see main text). B. Applicability of CD to the SC and its pathways through thalamus to cerebral cortex. SC is part of the pathway descending to the lower midbrain and pons (downward arrow) and eventually to the motor neurons that move the eyes. A copy of this downward projection is directed upward to convey the movement information to other regions of the brain.
A Corollary Discharge to Frontal Cortex
As indicated in Figure 1A, an anatomical pathway extends from the intermediate layers of the SC, through the lateral parvocellular region of MD to the frontal cortex, specifically to FEF. While this anatomical information shows the possible connections between the SC and frontal cortex, it says nothing about which neurons in the SC are functionally connected to which neurons in the FEF. We need to link an anatomical pathway to a functional circuit, and this is done using the classical physiological techniques of orthodromic and antidromic stimulation [5, 6]. Establishing such a link begins by electrically stimulating the saccade related neurons in the SC to see if they have functional synapses onto a single neuron being recorded in MD (orthodromic activation). Then, by stimulating FEF, an MD neuron that projects to FEF can be identified by recording an action potential generated in the axons of the MD neuron (antidromic activation). A neuron in MD activated from both SC and FEF must be a relay neuron. These relay neurons lie in tiny area in the lateral region of the parvocellular region of MD [6].
These neurons must surely perform a variety of functions, and one of them is likely to be conveying a CD from SC to frontal cortex. Sommer and Wurtz [6–8] argued that the characteristics of the relay neurons in MD, and the results of their inactivation, provide strong evidence that they carry a CD.
What does such a CD circuit contribute to visual and oculomotor function? There can be little doubt that it contributes to the guidance of saccades when visual information is inadequate to guide the movement. In one such case, the double saccade task [9], the second saccade depends not on the retinotopic visual information but on updated eye position information that can be derived from a CD. The CD in the MD pathway could provide this internal information (eye proprioception probably cannot [10]). Indeed, inactivation of MD reduces the accuracy of the second saccade that depends on a CD [8].
Another function of a CD is its likely contribution to perception of a stable visual world. The basic idea is that there is internal activity (CD) that anticipates the saccade, and that anticipation affects the interpretation of the visual events resulting from the saccade [4]. Such anticipation was first observed in the parietal cortex of monkeys by Duhamel, Colby and Goldberg [11] and later in the FEF [12–14] and other cortical and subcortical areas [15–17]. Duhamel et al. found that as a monkey prepared to make a saccade, the activity of a neuron increased to a stimulus located in what would be the RF of the neuron after the saccade. This activity, in what we call the future field of the neuron, anticipates the visual consequences of the impending saccade (Fig. 3A). The example FEF neuron in Fig. 3B illustrates this anticipatory shift and shows its dependence on the MD pathway. The neuron responds to a visual stimulus flashed in the RF during fixation long before the saccade, and there is no activity in what will be the future field (Fig. 3B left, black traces). By contrast, just before the saccade, activity in the future field increases (Fig. 3B right, black traces). After the saccade the future field location obviously becomes the new RF location, and the neuron then responds to a stimulus in the RF just as it did before the saccade. The anticipatory shift requires information about the saccade before the saccade occurs, and that information is exactly what a CD conveys. Subsequent experiments showed that the CD conveyed by the MD pathway was critical for producing a shift in the FEF RFs [10, 14, 18]. As seen in the example, the future field activity observed just before the saccade is significantly and selectively reduced when MD is inactivated (Fig. 3B right, orange traces).
Figure 3.
Use of a CD to anticipate the visual consequences of a saccade. A. Experimental design for demonstrating shifting RF activity. While the monkey is looking at the fixation spot, the RF activity is determined by flashing a spot, and the activity in the future field is also determined by such a spot. These responses are determined both long before the monkey makes a saccade to the target and just before the saccade onset. B. Example of shifting RF activity in FEF in black traces, and after MD inactivation in orange traces. Just before the saccade (right column) there was activity in the future field (right column upper row), but this was eliminated by inactivation of neurons in MD by injection of muscimol. This is consistent with the hypothesis that the information for the location of the future field is derived from a CD and that the CD used by FEF is derived from the SC-MD-FEF circuit. After Sommer and Wurtz [14].
In summary, the MD thalamus pathway from SC to frontal cortex conveys information about an impending saccade that is highly likely to be a CD of that saccade. This CD provides information for guiding saccades and probably contributes to the mechanism underlying our stable visual perception in spite of incessant eye movements. A major next step is to show that the CD to frontal cortex does in fact contribute to visual stability, probably by inactivating the CD pathway and then testing for stability of perception. More generally, it would be interesting to know if the CD for saccades is just one of many inputs to frontal cortex providing internal information about eye as well as skeletal movements for multiple cognitive functions.
Consequences of a Corollary Discharge to Parietal and Occipital Cortex
Whereas the pathway through MD thalamus originates in the intermediate, saccade related layers of SC and targets the frontal cortex, another thalamic pathway originates in the superficial, visual layers of SC and targets parietal and occipital cortex. A target of considerable interest is area MT, a region specialized for visual motion processing [19, 20] and thought to be closely linked to motion perception [21]. The pulvinar is the thalamic relay in this posterior pathway, but as for the MD pathway, the first critical step was to demonstrate the presence of a functional circuit. This step was particularly challenging because existing anatomical data were equivocal. The basic pathway was postulated decades ago [22] and anatomical studies pointed to subregions of the inferior pulvinar (PI) as a probable relay to MT [23, 24], yet other anatomical data suggested the pathway was not continuous [25]. Use of the orthodromic and antidromic stimulation technique provided the identification of neurons in the pulvinar that belonged to this functional pathway. Berman and Wurtz [26] identified neurons with SC input by orthodromic activation from SC, and neurons with MT output by antidromic activation from MT. They found neurons that both received input from SC and projected to MT. These relay neurons, and other connected neurons that had either SC input or MT output, were concentrated most densely in and around a histologically verified subregion, PIm. Berman and Wurtz also found evidence for a second smaller cluster of relay neurons in the presumed lateral shell region of PI [23]. Remarkably, these physiological findings were complemented by the concurrent anatomical data of Lyon et al. [27], who used transynaptic tracers to demonstrate a disynaptic pathway from SC to MT. Their data pointed toward a pulvinar relay and highlighted the very same two clusters within PI. Together, these convergent findings represent strong evidence for a functional circuit ascending from SC to MT through PI, which we refer to here as the PI circuit.
What are the functions of this PI circuit? Unlike the MD circuit, Berman and Wurtz [28] found that it does not convey the CD itself; PI neurons rarely have the presaccadic bursts that could signal an impending saccade. Instead they are predominantly visual, like superficial SC neurons, with brisk responses to visual stimuli in their RF. This visual activity is nevertheless modulated by saccades. The modulation is a suppression of neuronal activity with each saccade, and the intriguing possibility is that it underlies the suppression of vision during saccades that has been observed behaviorally. This perceptual saccadic suppression is thought to contribute to stable vision by eliminating the blurred image that we would otherwise see with each saccade [29]. One possibility is that the PI conveys a neuronal saccadic suppression signal from SC to cortex.
The superficial SC visual neurons were the first neurons where saccadic suppression was clearly evident in the activity of single neurons [30]. This suppression is observable both as a diminution of ongoing (baseline) activity around the time of a saccade (Fig. 4) [31], and as a reduction of the visual response to stimuli presented around the time of the saccade [30]. These observations suggest that the saccade is accompanied by a wholesale suppression that affects both ongoing activity and activity in response to visual stimuli. A CD signal would be an appropriate input to produce this neuronal suppression. Indeed, a study of background suppression in SC established that it was not dependent on proprioceptive feedback but instead was attributable to a CD [32].
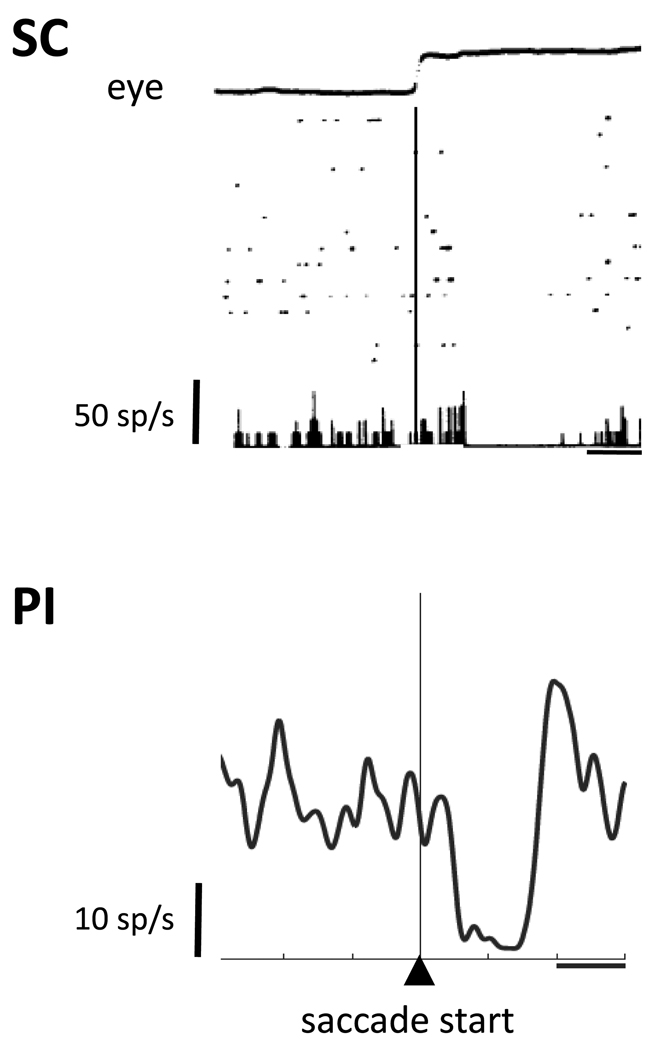
Figure 4.
Saccadic suppression in SC and PI. The top panel shows an example neuron from one of the original studies of suppression in the superficial SC. The eye trace at the top shows horizontal eye position; rasters and histograms below are aligned on saccade start (thin vertical line). Rasters show spikes in individual trials. The bottom panel shows an example neuron recorded from within the PI relay. The spike density function is aligned on saccade start. Horizontal lines indicate 100ms intervals. The SC and PI neurons each show a saccadic suppression of background activity. Reproduced with permission from Richmond and Wurtz [32] and Berman and Wurtz [28].
Few would think that perceptual suppression would result from suppression of visual responses within the SC, so the more important question is whether neuronal activity in cortical areas is associated with saccadic suppression. Such CD-derived suppression is not prominent in early visual cortical areas where a visual masking effect has been found with saccades [4], but it is present in extrastriate cortex. Notably, suppression has been observed in MT [33–35]. This suppression can even occur before the eyes begin to move [35], indicating dependence on a CD signal. Thus, the CD-driven suppression in SC appears to reach cortical regions where activity is linked to visual perception. The PI pathway is a strong candidate for conveying this suppression, and Berman and Wurtz [28] recently found that connected neurons in the identified PI pathway exhibited a perisaccadic suppression of background activity (Fig. 4). Suppression in PI, like that in SC and MT, can also begin before the saccade [28].
These findings show that saccadic suppression is present at each stage of the PI pathway from SC visual neurons to the MT visual neurons, and suggest a relay in PI that transmits this suppression to cortex. The logic here is that the visual response of the MT neurons consists of two components: one input is the directionally selective visual response that depends on cortical visual processing; the other is the input from SC. The component from SC would be suppressed before the saccade and this reduction would be seen in the response of the MT neuron before the saccade. The next critical step is to determine whether the suppression in MT is dependent upon that in PI and SC.
THALAMIC PATHWAYS FOR VISUAL ATTENTION
The Motor Theory of Attention and the Thalamic Projection of SC to Cortex
Other pathways through the thalamus (Fig. 1C) may convey an attentional modulation of visual processing to parietal and occipital cortex. Neurons in multiple areas of visual cortex have enhanced visual responses with attention [36] and more recent work has demonstrated that attention has an even greater effect on interneuronal correlations [37]. Experiments on the lateral pulvinar (particularly the dorsal medial region [38]) show attention related activity, and inactivation of this region produces deficits in visual attention [38–40]. The question is whether this attention related activity originates in SC.
The initial impetus for regarding the SC as an origin of such attentional modulation came from the observation that the responses of SC visual neurons were enhanced when a stimulus in their RFs became the target of a saccade [41]. This enhancement was inferred to be a correlate of attention because when the monkey made a saccade to the target, it must have shifted its attention to it. This inference was consistent with human psychophysical studies showing shifts of attention to saccade targets (for a summary see [42]), and was later validated by experiments on the SC that revealed neuronal correlates of the behavioral improvements (such as decreased detection thresholds) seen for stimuli that were saccade targets [43–47].
A possible source of the enhancement of SC visual responses is the activity preceding saccades in the SC intermediate layers, which might be directed upward to the superficial visual neurons to facilitate their response to visual stimuli [48]. These observations on the SC neurons along with his own observations led Rizzolatti [49] to propose a motor theory of attention, which argues that the same mechanisms that generate a saccade to a target also contribute to the shift in spatial attention to that target (for a recent review see [50]). Moore and Fallah [51] tested the theory by weakly stimulating FEF and measuring the detection threshold for stimuli in the region of the visual field to which saccades would have been directed if the electrical stimulation been stronger. The monkey’s visual detection threshold was selectively lowered at the saccade representation, providing evidence for the link between saccade planning and attention.
One possibility is that input from the SC saccade related neurons might contribute to the enhanced visual responses in cortex with attention. Two experiments have used the approach of Moore and Fallah [51] to test for attention effects in the SC [52, 53]. Both experiments used tasks in which a change in direction of motion had to be detected, and since the perception of motion is a cortical function in the primate, it seemed a reasonable assumption that the SC stimulation was acting ultimately on motion processing in cortex. In one of these experiments [52], a change blindness paradigm was used to test the monkey’s ability to detect directional changes in a set of random dot patches (Fig. 5A). The abrupt change in direction was concealed by imposing a brief blank period at the time of the direction change. When a visual cue instructed an attentional shift to one of the dot patches, the detection of the direction change improved significantly. Then the visual cue was replaced by stimulation of SC that was too weak to produce a saccade. When the SC sub-threshold stimulation targeted the region of the visual field where the change of direction occurred, the monkey was able to detect the change significantly more frequently than when the change occurred elsewhere in the visual field (Fig. 5B). Across the sample of 23 experiments, SC stimulation produced an average hit rate increase of about 9%, a highly significantly change. Essentially the same results were obtained in the study by Muller et al. [53].
Figure 5.
Modulation of visual attention in a change blindness task by stimulation of the SC neurons that are active before saccadic eye movements. A. Shift of attention by SC stimulation rather than by a visual cue. Stimulation began 300 msec before the blank period and continued for 600 ms, ending 150 ms after the motion patches reappeared. Stimulation current and pulse frequency were well below levels required to elicit saccades. In this example, the change in motion direction occurred in the upper left patch of dots (gray arrow). B. Sample results for an experiment showing stimulation overlapping a patch of moving dots (solid dots and upper drawing) and non-overlapping stimulation (open dots and lower drawing). Change in motion direction for this experiment was 40° and stimulation was always at the same SC location. When the target overlapped the site of SC stimulation, the proportion of hits increased, whereas when the target was non-overlapping, the hits did not change significantly (left plot). Reproduced with permission from Cavanaugh and Wurtz [52].
In summary, SC stimulation alters attention, and it is likely to do so by its action on cerebral cortex through a pulvinar pathway. The significance of such a pathway is that the thalamus would convey to cortex not only the signals related to a corollary discharge but also the modulatory signals related to visual attention. We do not know the exact pathway through pulvinar, although several lines of evidence implicate the lateral pulvinar, particularly the dorsal medial region Pdm [38–40, 54]. We also do not yet have any evidence that SC stimulation modulates neuronal activity in cortex, and there is some evidence that it may not [55]. The challenges for future work therefore include identification of the thalamic relay in this probable path and experiments to test its impact on cortical function.
Modulation of Thalamic Reticular and Lateral Geniculate Neurons by Attention
The final thalamic nucleus that we consider has connections that are substantially different from those described so far. The TRN is spread across the surface of a substantial proportion of the thalamus (Fig. 1D) and is polymodal, with sections devoted to visual, auditory, and somatosensory inputs [56, 57]. In its visual capacity it receives excitatory inputs from both the parvocellular and magnocellular divisions of LGN as these fibers travel to V1 (Fig. 6A). It does not receive direct retinal input. TRN sends inhibitory projections back to both divisions of the LGN [see Jones for anatomical details3]), and its high rate of discharge (frequently over 40spikes/s) is similar to that of other inhibitory areas such as the substantia nigra pars reticulata [58] and the zona incerta [59]. TRN receives descending input from layer 6 of V1 [60] and ascending input from the SC [61] as does the LGN [62].
Figure 6.
Modulation of visual activity in LGN and TRN by visual attention. A. Both the magnocellular (LGNm) and the parvocellular layers (LGNp) receive excitatory input from the retina and provide excitatory input to the TRN and V1. The TRN projection back to LGN is inhibitory. B. Increased visual response with attention into the RF of an LGNm and an LGNp neuron (increase of 12% and 24% respectively). C. Decreased TRN visual response with attention into the RF of the neuron (decrease of 13%). Horizontal lines in B, C indicate each neuron’s initial visual response. Attention changes the responses of both LGN and TRN neurons but in a reciprocal manner. Reproduced with permission from McAlonan, Cavanaugh and Wurtz [64].
Crick [63] suggested that the excitatory input from LGN to TRN and reciprocal inhibitory return was consistent with a modulatory function of TRN on LGN, specifically one related to attention. His famous searchlight hypothesis was encapsulated in his conclusion that “if the thalamus is the gateway to the cortex, the reticular complex might be described as the guardian of the gateway” [63]. This hypothesis was tested by McAlonan, Cavanaugh and Wurtz [64] who recorded from LGN and TRN neurons while the monkey attended to a stimulus either within or outside of the RF of these neurons. Figure 6B shows the increased visual responses of LGN neurons when attention was directed into their RF. Overall, the modulation was a median increase of 11% for LGNm and 9% for LGNp. Both differences were highly significant for the initial visual response. Even though the modulation is modest, i t is in the range of that observed in V1 in similar experiments [65].
By Crick’s hypothesis, attentional modulation of TRN should be in the opposite direction as that in LGN, and that is the case. Figure 6C shows a decreased visual response of a TRN neuron with attention. Across the sample of TRN neurons, the median decrease was 4% and highly significant. But if TRN gets its visual input from LGN, how can TRN modify the source of its input? One possibility is that the relatively short latency of the LGNm neurons would provide early mono-synaptic activation of TRN neurons, which in turn could act mono-synaptically on the LGN. The observed visual latency of the LGNm neurons was short enough to activate the TRN neurons, which in turn could suppress the LGNp neurons. But this timing only indicates that the modulation of LGN by TRN is feasible. The key experiment on the TRN – LGN interaction remains to be done: inactivating TRN to verify that this affects attentional modulation in LGN.
In summary, the transmission of visual information through the LGN is modulated by visual spatial attention, which provides another example of the LGN being far more than a passive relay [60]. Though the modulation is modest, it is in the range of that in V1, raising the question of how much V1 attentional modulation is actually passed on from LGN. The TRN is also modulated by attention in ways that make it the possible source of the LGN modulation; this possibility remains a major issue to be resolved.
CONCLUDING REMARKS
We have concentrated on the contribution of the higher-order thalamic nuclei to active vision. Several points should be emphasized in conclusion. First, in some respects these nuclei act as modulators of cortical visual processing as defined by Sherman and Guillery [66, 67]. Saccadic suppression and attentional enhancement fit nicely with their idea that thalamic input acts to modulate specific visual information. In contrast, the CD pathway to frontal cortex conveys a signal that is not simply modulatory. It combines visual information in cortex with precise saccade vector information conveyed by the CD [10, 68]. Here the interaction is closer to a combination of two inputs driving the activity of the frontal cortex neurons rather than one modulating the other. Open questions include the extent to which these ascending thalamic pathways act as modulators or drivers, and whether this distinction continues to be useful as the thalamic functions are mapped in greater detail. Second, we have used the SC as a way to investigate these thalamic visual pathways, and this obviously biases our review toward ascending information related to saccades. Other thalamic pathways might also convey movement information [69]. At this point we cannot evaluate the extent to which other types of information (non-eye-movement or non-movement) are conveyed to cortex through the thalamus. Finally, we have emphasized the differences in what is conveyed in these pathways to cortex, but it is equally important to note their similarities, particularly the parallels in the possible mechanisms underlying a CD for visual stability and those underlying visual spatial attention (see Box 1). With these considerations in mind, the continued study of higher-order thalamic pathways promises to shed new light on the neuronal mechanisms that underlie our active vision.
Box 1 Parallels between mechanisms for corollary discharge and attention.
We have treated the pathways through the thalamus that contribute to active vision individually and have emphasized what function they might perform. The differences among the pathways include the cortical targets, the signals conveyed and the timing of those signals, and the hypothesized function of each pathway. If we step back, however, and look at the circuits together, they also have substantial similarities. These include their origin in SC, a relay in the thalamus and a target in cortex, and the transmission of saccade related information, either as the activity preceding saccades or, in the case of suppression, as the consequence of saccades. In this sense, the function of all of these thalamic pathways is the result of activity preceding saccades. Perhaps instead of many circuit types, there is essentially just one.
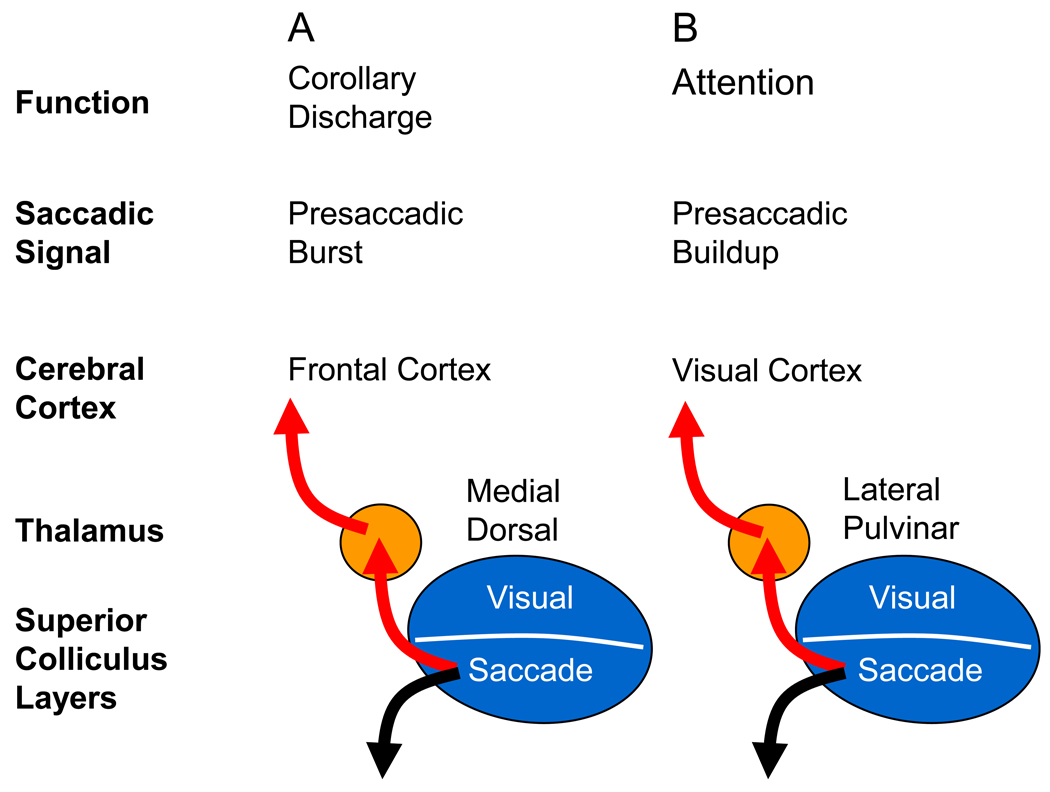
This similarity is particularly striking for the thalamic pathways contributing to visual stability and visual attention, as outlined in Fig. I. These two functions are likely supported by different pathways through thalamus and probably depend on different phases of presaccadic activity in SC. The burst preceding the saccade is critical for the CD that compensates for the displacement of the visual scene caused by the saccade (A), while the earlier buildup activity in the SC is probably most important for visual spatial attention (B) because it could alter visual processing long before the saccade is generated. The point to note, however, is that both pathways provide ascending information originating in the SC neurons related to saccadic eye movements. It will be interesting to see whether the two cognitive issues of visual stability and visual attention turn out to have very similar underlying neuronal mechanisms derived from saccade related activity.
Figure I, Box 1.
Similarity of modulation of cortical activity by CD and by visual attention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Merriam EP, Colby CL. Active vision in parietal and extrastriate cortex. Neuroscientist. 2005;11:484–493. doi: 10.1177/1073858405276871. [DOI] [PubMed] [Google Scholar]
- 2.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones EG. The Thalamus Second Edition. Cambridge University Press; 2007. pp. 1270–1271. [Google Scholar]
- 4.Wurtz RH. Neuronal mechanisms of visual stability. Vision Res. 2008;48:2070–2089. doi: 10.1016/j.visres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemon R. IBRO Handbook Series: Methods in the Neurosciences. Vol. 4. J. Wiley & Sons; 1984. Methods for neuronal recording in conscious animals; pp. 95–102. [Google Scholar]
- 6.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004;91:1381–1402. doi: 10.1152/jn.00738.2003. [DOI] [PubMed] [Google Scholar]
- 7.Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- 8.Sommer MA, Wurtz RH. What the Brain Stem Tells the Frontal Cortex. II. Role of the SC-MD-FEF Pathway in Corollary Discharge. J Neurophysiol. 2004;91:1403–1423. doi: 10.1152/jn.00740.2003. [DOI] [PubMed] [Google Scholar]
- 9.Hallett PE, Lightstone AD. Saccadic eye movements to flashed targets. Vision Res. 1976;16:107–114. doi: 10.1016/0042-6989(76)90084-5. [DOI] [PubMed] [Google Scholar]
- 10.Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movments. Annu Rev Neuroscience. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duhamel JR, et al. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- 12.Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- 13.Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. II. Memory responses. J Neurophysiol. 2001;86:2344–2352. doi: 10.1152/jn.2001.86.5.2344. [DOI] [PubMed] [Google Scholar]
- 14.Sommer MA, Wurtz RH. Influence of the thalamus on spatial visual processing in frontal cortex. Nature. 2006;444:374–377. doi: 10.1038/nature05279. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura K, Colby CL. Updating of the visual representation in monkey striate and extrastriate cortex during saccades. Proc Natl Acad Sci U S A. 2002;99:4026–4031. doi: 10.1073/pnas.052379899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker MF, et al. Neurons in the monkey superior colliculus predict the visual result of impending saccadic eye movements. J Neurophysiol. 1995;73:1988–2003. doi: 10.1152/jn.1995.73.5.1988. [DOI] [PubMed] [Google Scholar]
- 17.Dunn CA, et al. Spatial updating in monkey superior colliculus in the absence of the forebrain commissures: dissociation between superficial and intermediate layers. J Neurophysiol. 2010;104:1267–1285. doi: 10.1152/jn.00675.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crapse TB, Sommer MA. Corollary discharge circuits in the primate brain. Curr Opin Neurobiol. 2008;18:552–557. doi: 10.1016/j.conb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubner R, Zeki SM. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 1971;35:528–532. doi: 10.1016/0006-8993(71)90494-x. [DOI] [PubMed] [Google Scholar]
- 20.Maunsell JH, Van Essen DC. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J Neurophysiol. 1983;49:1127–1147. doi: 10.1152/jn.1983.49.5.1127. [DOI] [PubMed] [Google Scholar]
- 21.Britten KH, et al. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diamond IT, Hall WC. Evolution of neocortex. Science. 1969;164:251–262. doi: 10.1126/science.164.3877.251. [DOI] [PubMed] [Google Scholar]
- 23.Cusick CG, et al. Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr. J Comp Neurol. 1993;336:1–30. doi: 10.1002/cne.903360102. [DOI] [PubMed] [Google Scholar]
- 24.Harting JK, et al. Ascending pathways from the monkey superior colliculus: An autoradiographic analysis. J. Comp. Neurol. 1980;192:853–882. doi: 10.1002/cne.901920414. [DOI] [PubMed] [Google Scholar]
- 25.Stepniewska I, et al. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis Neurosci. 2000;17:529–549. doi: 10.1017/s0952523800174048. [DOI] [PubMed] [Google Scholar]
- 26.Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci. 2010;30:6342–6354. doi: 10.1523/JNEUROSCI.6176-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyon DC, et al. A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron. 2010;65:270–279. doi: 10.1016/j.neuron.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berman RA, Wurtz RH. Signals Conveyed in the Pulvinar Pathway from Superior Colliculus to Cortical Area MT. J Neurosci. 2011:373–384. doi: 10.1523/JNEUROSCI.4738-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross J, et al. Changes in visual perception at the time of saccades. Trends Neurosci. 2001;24:113–121. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey: I. Visual receptive fields of single neurons. J. Neurophysiol. 1972;35:542–559. doi: 10.1152/jn.1972.35.4.542. [DOI] [PubMed] [Google Scholar]
- 31.Robinson DL, Wurtz RH. Use of an extraretinal signal by monkey superior colliculus neurons to distinguish real from self-induced stimulus movement. J Neurophysiol. 1976;39:852–870. doi: 10.1152/jn.1976.39.4.852. [DOI] [PubMed] [Google Scholar]
- 32.Richmond BJ, Wurtz RH. Vision during saccadic eye movements. II. A corollary discharge to monkey superior colliculus. J. Neurophysiol. 1980;43:1156–1167. doi: 10.1152/jn.1980.43.4.1156. [DOI] [PubMed] [Google Scholar]
- 33.Thiele A, et al. Neural mechanisms of saccadic suppression. Science. 2002;295:2460–2462. doi: 10.1126/science.1068788. [DOI] [PubMed] [Google Scholar]
- 34.Ibbotson MR, et al. Enhanced motion sensitivity follows saccadic suppression in the superior temporal sulcus of the macaque cortex. Cereb Cortex. 2007;17:1129–1138. doi: 10.1093/cercor/bhl022. [DOI] [PubMed] [Google Scholar]
- 35.Bremmer F, et al. Neural dynamics of saccadic suppression. J Neurosci. 2009;29:12374–12383. doi: 10.1523/JNEUROSCI.2908-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MR, Maunsell JH. Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci. 2009;12:1594–1600. doi: 10.1038/nn.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson DL, Petersen SE. The pulvinar and visual salience. Trends Neurosci. 1992;15:127–132. doi: 10.1016/0166-2236(92)90354-b. [DOI] [PubMed] [Google Scholar]
- 39.Wilke M, et al. Pulvinar inactivation disrupts selection of movement plans. J. Neurosci. 2010;30:8650–8659. doi: 10.1523/JNEUROSCI.0953-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Desimone R, et al. Attentional control of visual perception: Cortical and subcortical mechanisms. Cold Spring Harbor Symp. Quant. Biol. 1990;60:963–971. doi: 10.1101/sqb.1990.055.01.090. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972;35:560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- 42.Moore T, et al. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 43.Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- 44.Ignashchenkova A, et al. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- 45.Fecteau JH, et al. Neural correlates of the automatic and goal-driven biases in orienting spatial attention. J Neurophysiol. 2004;92:1728–1737. doi: 10.1152/jn.00184.2004. [DOI] [PubMed] [Google Scholar]
- 46.Li X, Basso MA. Preparing to move increases the sensitivity of superior colliculus neurons. J Neurosci. 2008;28:4561–4577. doi: 10.1523/JNEUROSCI.5683-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2009;13:261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wurtz RH, Mohler CW. Organization of monkey superior colliculus: enhanced visual response of superficial layer cells. J Neurophysiol. 1976;39:745–765. doi: 10.1152/jn.1976.39.4.745. [DOI] [PubMed] [Google Scholar]
- 49.Rizzolatti G. Mechanisms of selective attention in mammals. In: Ewert J-P, et al., editors. Advances in Vertebrate Neuroethology. Plenum Publishing Corp.; 1983. [Google Scholar]
- 50.Noudoost B, et al. Top-down control of visual attention. Curr Opin Neurobiol. 2010;20:183–190. doi: 10.1016/j.conb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci U S A. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muller JR, et al. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci U S A. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clower DM, et al. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krauzlis RJ, Zenon A. Effects of SC inactivation on covert selective attention and neuronal activity in MST. Program No. 304.13. 2010 Neuroscience Meeting Planner; San Diego, CA. Society for Neuroscience; 2010. 2010. Online. [Google Scholar]
- 56.Jones EG. Some aspects of the organization of the thalamic reticular complex. J. Comp. Neurol. 1975;162:285–308. doi: 10.1002/cne.901620302. [DOI] [PubMed] [Google Scholar]
- 57.Guillery RW, et al. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 1998;21:28–32. doi: 10.1016/s0166-2236(97)01157-0. [DOI] [PubMed] [Google Scholar]
- 58.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J. Neurophysiol. 1983;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- 59.Trageser JC, Keller A. Reducing the uncertainty: gating of peripheral inputs by zona incerta. J Neurosci. 2004;24:8911–8915. doi: 10.1523/JNEUROSCI.3218-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sillito AM, et al. Always returning: feedback and sensory processing in visual cortex and thalamus. Trends Neurosci. 2006;29:307–316. doi: 10.1016/j.tins.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Kolmac CI, Mitrofanis J. Patterns of brainstem projection to the thalamic reticular nucleus. J Comp Neurol. 1998;396:531–543. [PubMed] [Google Scholar]
- 62.Harting JK, et al. Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J Comp Neurol. 1991;304:275–306. doi: 10.1002/cne.903040210. [DOI] [PubMed] [Google Scholar]
- 63.Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci U S A. 1984;81:4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McAlonan K, et al. Guarding the gateway to cortex with attention in visual thalamus. Nature. 2008;456:391–394. doi: 10.1038/nature07382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman SM, Guillery RW. Functional organization of thalamocortical relays. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 67.Sherman SM, Guillery RW. Exploring the Thalamus. Academic Press; 2006. [Google Scholar]
- 68.Wurtz RH, et al. Drivers from the deep: the contribution of collicular input to thalamocortical processing. Prog Brain Res. 2005;149:207–225. doi: 10.1016/S0079-6123(05)49015-9. [DOI] [PubMed] [Google Scholar]
- 69.Sommer MA. The role of the thalamus in motor control. Curr Opin Neurobiol. 2003;13:663–670. doi: 10.1016/j.conb.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 70.Netter FH. The Ciba Collection of Medical Illustrations. Ciba Pharmaceutical Company; 1957. [Google Scholar]