Abstract
Purpose
Brain metastases are a common pre-terminal event in patients with metastatic melanoma and require radiation therapy. Our group has previously demonstrated that human GRM1 (hGRM1) expressing melanoma cells release excess extracellular glutamate and are growth inhibited by riluzole, an inhibitor of glutamate release. Riluzole treated cells accumulate in G2/M phase of the cell cycle at 24 hours, and then undergo apoptotic cell death. We evaluated whether riluzole enhanced radiosensitivity in melanoma cells.
Experimental Design
Clonogenic assays were performed to evaluate clonogenic survival after treatment in hGRM1 expressing and non-expressing melanoma cells. Western immunoblots were performed to confirm apoptotic cell death. A xenograft mouse model was used to validate the in vitro experiments. Tumors harvested from the xenografts were fixed and stained for apoptosis and DNA damage markers.
Results
In the hGRM1-positive cell lines C8161 and UACC903, riluzole enhanced the lethal effects of ionizing radiation; no difference was seen in the hGRM1-negative UACC930 cell line. C8161 cells treated with riluzole plus irradiation also showed the highest levels of the cleaved forms of PARP and caspase-3; excised C8161 xenografts demonstrated the greatest number of apoptotic cells by immunohistochemistry (p<0.001). On cell cycle analysis, a sequence-dependent enrichment in the G2/M phase was demonstrated with the combination of riluzole and irradiation. Xenografts treated with riluzole and weekly radiation fractions demonstrated significant growth inhibition and revealed markedly increased DNA damage.
Conclusions
We have demonstrated, in vitro and in vivo, that the combination of riluzole and ionizing radiation leads to greater cytotoxicity. These results have clinical implications for patients with brain metastases receiving whole brain radiation therapy.
Keywords: Glutamate, Melanoma, riluzole, Cell Surface Receptors, Ionizing Radiation
INTRODUCTION
Brain metastases represent a common terminal or pre-terminal event in many patients with malignancy including a large proportion of patients with metastatic melanoma. Current therapy for patients with brain metastases involves some combination of steroids, surgical resection, stereotactic radiosurgery (SRS), and whole brain radiation therapy (WBRT)1, 2. Most patients with brain metastases will receive WBRT as one component of their therapy. Available evidence suggests that the histology of brain metastases is prognostically relevant. Melanoma patients with brain metastases who receive WBRT have inferior outcomes when compared to other cancer types3. This clinical finding supports the known relative radioresistance of melanoma cell lines in vitro4. To date, efforts at combining systemic agents with WBRT to enhance the lethal effects of radiotherapy (RT) in melanoma patients have had limited success 5.
Our earlier work led to the identification and confirmation that ectopic expression of a murine neuronal receptor; metabotropic glutamate receptor 1 (mGRM1) in melanocytes was sufficient to induce spontaneous melanoma development in vivo6–8. Extension of this finding to the human system showed that normal skin and benign nevi do not express the human form of this receptor (hGRM1) while 88% of human melanoma cell lines and 68% of human melanomas biopsy samples do express this receptor, suggesting that aberrant expression of hGRM1 may be important in the onset and progression of human melanoma4. Chen and co-workers have gone on to demonstrate that inhibition of GRM1 signaling by specific GRM1 antagonists or depletion of the ligand (glutamate) by the drug riluzole in vitro and in vivo results in cell cycle arrest and subsequent apoptosis in human melanoma cells3.
Riluzole is a FDA approved drug for the treatment of amyotrophic lateral sclerosis (ALS) and has off-label uses in other psychiatric and neurologic disorders. Riluzole possess both glutamatergic modulating and neuroprotective properties, although the precise mechanisms have not been fully delineated 9–11. Investigators from our institution recently reported provocative results from a Phase 0 trial of riluzole in patients with Stage III and IV melanoma, in which about one third of the patients exhibited remarkable clinical and metabolic responses. Comparisons using biochemical markers between pre- and post-treatment samples showed suppression of components of two of the major signaling pathways important in melanoma pathogenesis, MAPK and PI3K/AKT, and an increase in the number of apoptotic cells in post-treatment tumor samples12. Therapeutic trials of riluzole in patients with advanced melanoma are ongoing at our institution.
We have already shown that treatment with riluzole results in synchronization of melanoma cells in G2/M, followed soon thereafter by a spike in the subG1 population indicating apoptosis13. This provides a strong rationale for combining ionizing radiation and riluzole; cells in G2/M are exquisitely sensitive to DNA damaging agents such as ionizing radiation. In the current communication, we examined the potential for enhanced cytotoxic effects with the addition of ionizing radiation to riluzole in human melanoma cell lines. We hypothesize that riluzole will be a radiation sensitizer for the treatment of metastatic melanoma. Because riluzole crosses the blood brain barrier, it is of particular clinical relevance since brain metastases are commonly treated with whole brain radiation therapy.
MATERIALS AND METHODS
Cell lines
hGRM1-expressing C8161 (wild-type for RAS and B-RAF) and UACC903 (wt for RAS, mutated B-RAF, V600E) human melanoma cell line and hGRM1-negative melanoma cell line UACC930 (wt for RAS, mutated B-RAF, V600E), were obtained from Dr Mary JC Hendrix (Children’s Memorial Research Center, Chicago, IL) and Dr Jeffrey M Trent (Translational Genomics Research Center, Phoenix, AZ), respectively. Cells were cultured in monolayer at 37° C in a 5% CO2 humidified incubator, in RPMI (InVitrogen) supplemented with 10% fetal bovine serum (Sigma).
Clonogenic survival assays
Cells were trypsinized for retrieval and plated on 100-mm plates and allowed to attach overnight for 20 hours. Riluzole (25 uM) was added at the 20-hour time point and allowed to incubate for 24 hours. 25 uM drug concentration was chosen based on a 96 well-plate ATP luminescence cell viability assay with increasing concentrations of drug in irradiated cells (data not shown). Cells were irradiated using a Gamma Cell 40 Exactor (MDS Nordion) irradiator and then incubated overnight (20 hours). Drug was aspirated and culture media replaced at 20 hours. Plates were then monitored for 10–21 days and stained with crystal violet for visual counting. Colonies which contained over 50 cells were scored as clonogenic survivors.
Western immunoblots
Western immunoblots of C8161 hGRM1-positive human melanoma cells either treated with riluzole for 24 hours or no treatment were conducted. Both sets of cells were irradiated at 2 or 4 Gy. Lysates were made at 24, 48 or 72 hours after irradiation. Protein lysates were prepared by washing cells with PBS, adding extraction buffer (50mM Tris, 150mM NaCl, 1mM EDTA, pH 8.0, 1% NP40, 5% glycerol, 1mM Dithiothreitol, complete protease inhibitor cocktail (Roche) and phosphatase inhibitors I and II (Sigma). The samples were resolved in 10% gels (Bio-Rad), after which they were transferred onto nitrocellulose membranes. Membranes were blocked with 5% milk and 1% bovine serum albumin and then probed with antibodies against PARP and cleaved caspase-3. The same blot was probed with α-tubulin to show equal loading.
Cell cycle analysis
C8161 cells were plated at 0.5x106 per 100-mm culture plates and treated with either 8 Gy irradiation or with 25 uM riluzole. After 24 hours, cells that had been treated with irradiation were exposed to riluzole at 25 uM, and cells treated with riluzole were treated with 8 Gy irradiation. Cells were then collected at 48 hours and washed twice with PBS. Cell pellets were fixed by drop-wise addition of ice-cold 70% ethanol and incubated overnight at 4 C. Fixed cells were washed twice with ice-cold PBS and resuspended in 500 µL PBS. Cells were treated with 50ul of RNase A solution (1mg/mL; Sigma, R-4875) and labeled with 5ul of propidium idodide (1mg/mL; Sigma P4170) overnight. Cell cycle analysis was done within 24 hours using a Beckman Coulter system (FC 500 model). The experiment was repeated three times.
Murine xenograft model
One million hGRM-positive C8161 cells were injected into the flanks of 6 weeks old nude mice (Taconic). When the tumors reached approximately 6–10mm3 the mice were randomly divided into 5 groups of 10 mice each: Group 1 was left untreated (No Treatment or NT), group 2 was treated twice with 4 Gy of irradiation only (IR), group 3 was treated with the DMSO vehicle only (Vehicle), group 4 was treated with 10mg/kg of riluzole by oral gavage only (riluzole), and group 5 was first given 10mg/kg of riluzole followed by two treatments of 4 Gy of irradiation (riluzole+IR) The irradiation was performed using the following protocol: At 24 hrs post-riluzole or vehicle administration two mice at a time were placed in individual containers that were approved by our veterinarian for use in the Gamma-irradiator (Gammator 50, cesium 137 source, Radiation Machine Corp). The mice were irradiated with 4 Gy, followed by a second dose of 4Gy at 48 hrs post-riluzole or vehicle. At 24, 48, and 72 hours after the second dose of irradiation, mice in each group were sacrificed and tumor samples were removed. Half of the samples were snap-frozen for further molecular studies and the other half was fixed in formalin for histological analysis. We used the antibody to activated caspase-3 to visualize apoptotic cells. The average number of apoptotic cells per tumor was calculated by counting the apoptotic cells in 10 random high-powered fields.
We next performed the same xenograft experiment but this time we followed the animals post-irradiation, measuring the tumors with calipers twice per week. Oral riluzole was given each day by gavage to the groups receiving riluzole and radiation was delivered in three weekly fractions of 4 Gy to the groups receiving irradiation. The experiment was terminated after 25 days of treatment due to tumor burden in the NT and vehicle treated animals.
The animals from the second xenograft experiment were sacrificed and half of the samples were snap-frozen for further molecular studies and the other half was dropped in formalin for histological analysis. We used the antibody to γH2AX (Millipore) to visualize cells with DNA damage. The level of phosphorylated histone variant H2AX is a surrogate marker for levels of DNA damage. Immediately after double strand breakage (DSB), γH2AX forms bright nuclear foci on immunoflourescent microscopy. The presence of γH2AX foci is widely regarded as a surrogate for and a sensitive marker of DSB in cells1–4 and have been shown to be specific for DSB5.
Statistics
All clonogenic assays and cell cycle analyses experiments were repeated three times and the triplicate results are shown. Results of Western blots and xenograft experiments are from single experiments. Significance was determined using unpaired Student’s two-tailed t-test. A p-value of ≤ 0.05 was considered significant.
RESULTS
Enhanced Radiosensitivity with Riluzole in hGRM1 Positive Human Melanoma Cell Lines
Clonogenic assays were repeated three times and the triplicate results are aggregated in Figure 1. In the hGRM1-positive cell line C8161, riluzole enhanced the lethal effects of ionizing radiation at the 2 and 4 Gy dose levels, with abrogation of the initial ‘shoulder’ of the cell survival curve and a dose modifying factor (DMF) of 1.48. (plating efficiency (PE): 12%). The effect in Figure 1 may appear subtle but is statistically significant (p=0.048). Similarly, the UACC903 human melanoma cell line, also hGRM1 positive, showed enhanced cell death with riluzole when compared to ionizing radiation alone (Figure 1) (DMF: 1.30, PE: 19%). Clonogenic assays in hGRM1-negative human melanoma cell line, UACC930, revealed no difference in cell survival in experiments at 25 µM and 100 µM of riluzole (PE: 25%). This demonstrates that riluzole likely has its radiation sensitizing effect on melanoma cells through inhibition of glutamergic signaling via hGRM1 receptor.
Figure 1. Clonogenic Assays.
a) hGRM1-positive C8161 human melanoma cells were treated with riluzole at 25µM for 24 hrs followed by escalating doses of ionizing radiation (IR). At 2Gy, there was a 48% reduction (0.78 ± 0.100 vs. 0.30± 0.089, p= .010) in cell survival in riluzole-treated cells versus non-treated cells. At 4 Gy, there was a 19% reduction (0.30 ± 0.121 vs. 0.11 ± 0.055, p= 0.048) in cell survival in riluzole-treated cells vs. non-treated cells. No differences were seen at 6 and 8 Gy. b) hGRM1-positive UACC903 human melanoma cells were treated with riluzole at 25 uM for 24 hours followed by escalating doses of IR. At 2 Gy, surviving fraction (SF) 0.473 ± 0.020 vs 0.233 ± 0.056 (p=0.002). At 4 Gy, SF 0.078 ± 0.010 vs 0.040 ± 0.020 (p=0.046). At 6 Gy, SF 0.013 ± 0.003 vs 0.008 ± 0.003 (p=0.110). At 8 Gy, SF 0.001 ± 0.001 vs 0.001 ± 0.001 (p=0.930). c) hGRM1-negative UACC930 human melanoma cells were treated with riluzole at 25µM for 24 hrs followed by escalating doses of IR. There was no difference between treated and untreated control UACC930 cells (p=NS).
Apoptosis of hGRM1 Expressing Cells Exposed to Riluzole and Ionizing Radiation
Cleaved forms of PARP and caspase-3 are common markers used for the assessment of apoptosis. Western Blot analysis of irradiated C8161 cells with and without riluzole showed an increase in the cleaved form of PARP at 48 and 72 hours after irradiation. Riluzole treatment appeared to further augment levels of cleaved PARP compared to irradiation alone. An example of a Western Blot from cells harvested at 72 hours post-riluzole treatment is shown in Figure 2. A second apoptosis marker, cleaved caspase-3 was also used to further confirm the increased in the number of apoptotic cells in riluzole plus ionizing radiation (Figure 2). Western blot analysis of irradiated C8161 cells with and without riluzole showed an increase in levels of cleaved caspase-3 at all time points: 0, 24, 48 and 72 hours after irradiation. Treatment with riluzole appears to sensitize the cells to ionizing radiation in comparison to irradiation alone.
Figure 2. Western Immunoblots.
a) Western immunoblots of C8161 hGRM1-positive human melanoma cells either treated with riluzole (RZ) for 24 hours or no treatment. Both sets of cells were then irradiated (IR) at 2 or 4 Gy. Lysates were made at 24, 48 or 72 hours after irradiation and probed with anti-PARP and anti-cleaved PARP antibodies to assess apoptotic cells. The same blot was probed with α-tubulin to show equal loading. b) Western immunoblots of hGRM1-positive C8161 human melanoma cells either treated with RZ for 24 hours or no treatment. Both sets of cells were then either not irradiated (0) or irradiated (IR) at 2Gy (2) or 4 Gy (4). Lysates were made immediately after IR or at 24 hours after IR and probed with anti-activated cleaved form of caspase-3 to assess apoptotic cells. The same blot was probed with α-tubulin to show equal loading.
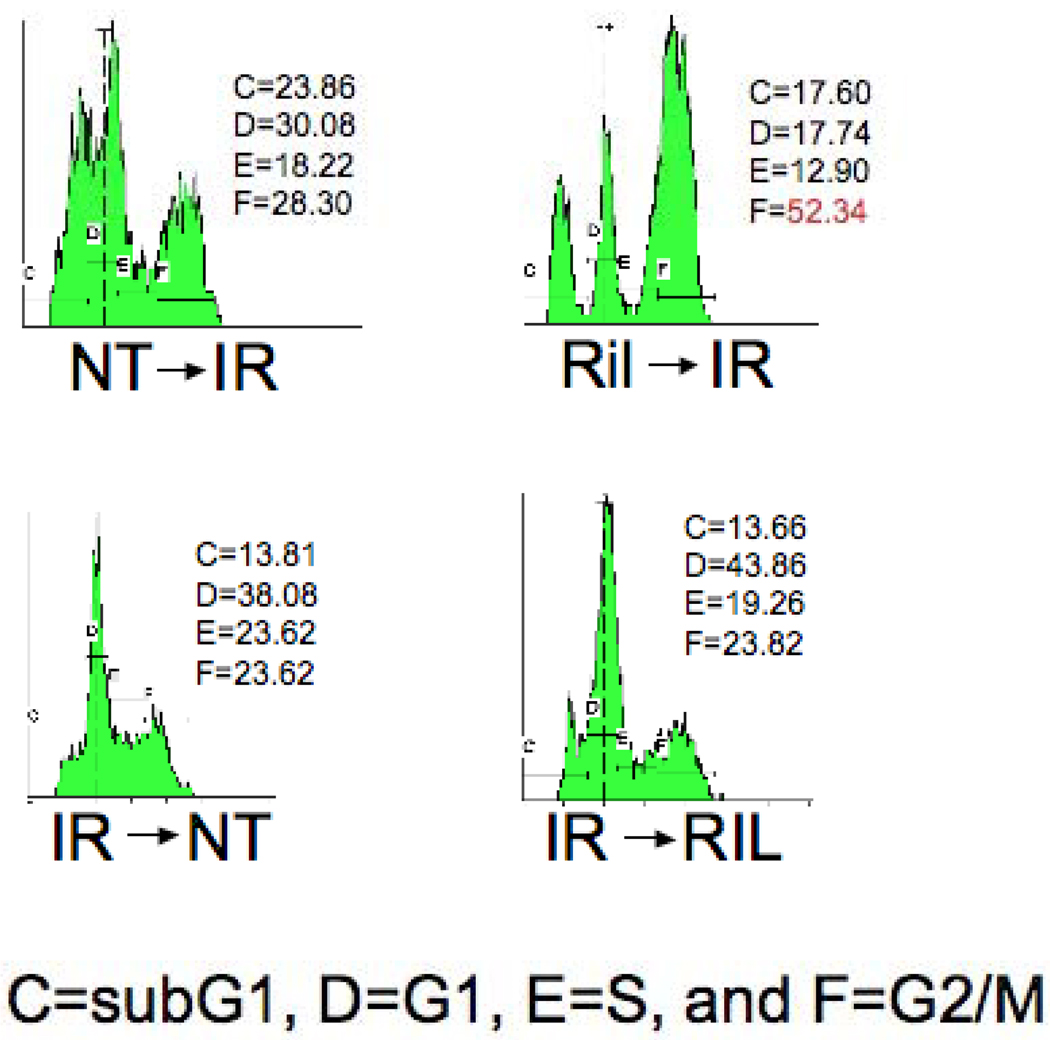
Combination therapy with Riluzole and Irradiation Leads to G2/M Synchronization that is Sequence-Dependent
We have demonstrated that C816 cells that were treated with riluzole prior to irradiation showed a clear spike in the G2/M fraction, while cells treated in the reverse sequence showed no such effect. Table 1 (Supplementary Data) displays the aggregate results from 3 experiments. Figure 3 shows the cell cycle analysis results from one experiment. This finding appears to support our hypothesis that G2/M synchronization is at least partially responsible for the enhanced radiosensitivity demonstrated in our other experiments.
Figure 3. Cell Cycle Analysis.
A representative cell cycle analysis experiment from Table 1. The G2/M fraction is increased in C8161 cells when irradiation follows treatment with 25 uM Riluzole. Each phase of the cycle is indicated: C (subG1), D (G1), E (S), and F (G2/M). NT: No Treatment; IR: ionizing irradiation; Ril: Riluzole.
In addition to these sequence experiments, we conducted cell cycle analysis after treatment of C8161 cells with the BAY 36-7620 compound, a non-competitive GRM1 antagonist, showing no G2/M synchronization (data not shown). This would suggest that the effects of riluzole are mediated by mechanisms beyond those of just receptor blockade.
Effects of Ionizing Radiation and Riluzole in a Murine Xenograft Model
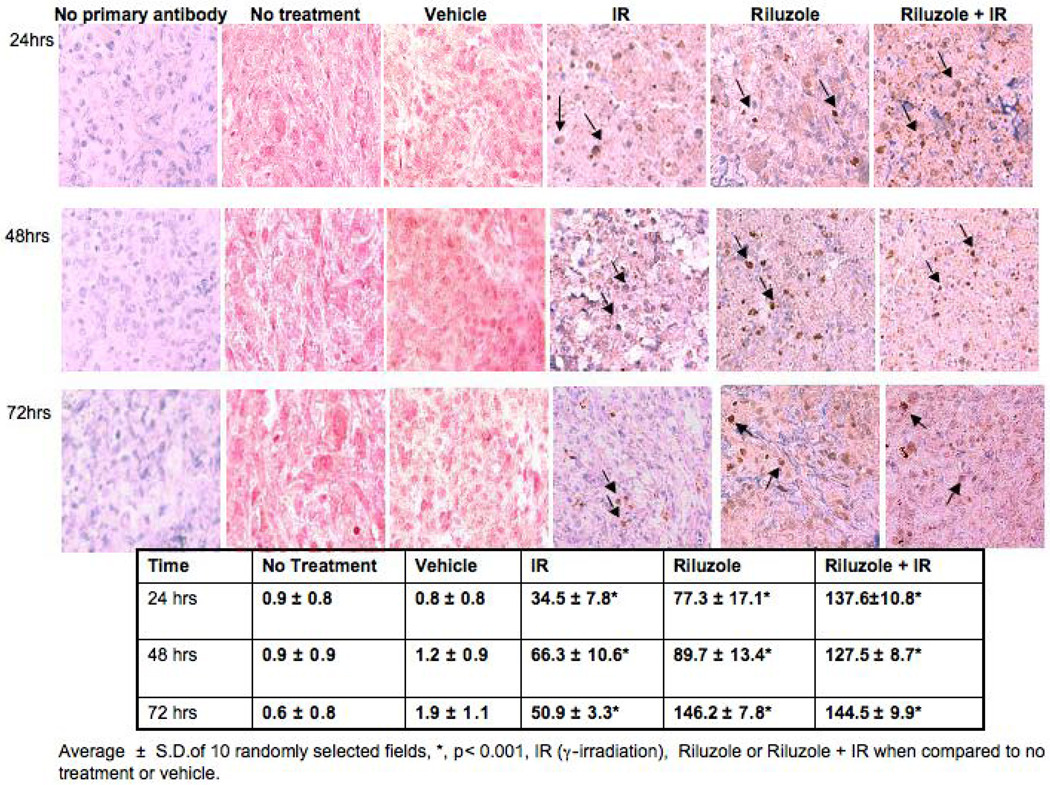
We showed in vitro cultured hGRM1-positive human melanoma cells, were more sensitive to irradiation in the presence of riluzole. We next set up xenograft experiments to determine whether this observation could be demonstrated in an in vivo animal system. We found a significant increase in the number of apoptotic tumor cells by immunohistochemistry in the riluzole plus irradiation group than in the irradiation alone group or the control groups (p<0.001). There was also a significant difference in the number of apoptotic tumor cells in the riluzole plus irradiation tumors as compared to the riluzole alone tumors at 24 hour and 48 hour time points (p<0.01). (Figure 4).
Figure 4. Murine Xenograft Model.
Immunodeficient nude mice were purchased from Taconic (Hudson, NY). C8161 cells were injected into flanks bilaterally at 106 cells per site. Tumor size was measured twice a week with a vernier caliper. When tumor volumes reach 6–10 mm3 the mice were randomly grouped into various treatments. Each treatment group consists of 10 mice. The groups were no treatment (NT), DMSO vehicle (V), V + irradiation (V+RT), riluzole (10mg/kg) (RIL), and riluzole (10mg/kg) plus irradiation (RIL+RT). Oral riluzole was given each day by oral gavage. Irradiation was delivered in three weekly fractions of 4 Gy. The experiment was terminated after 25 days of treatment due to tumor burden in the NT and vehicle treated animals. There appears to be at least an additive effect of the combination of riluzole and RT on the growth of these C8161-bearing xenografts.
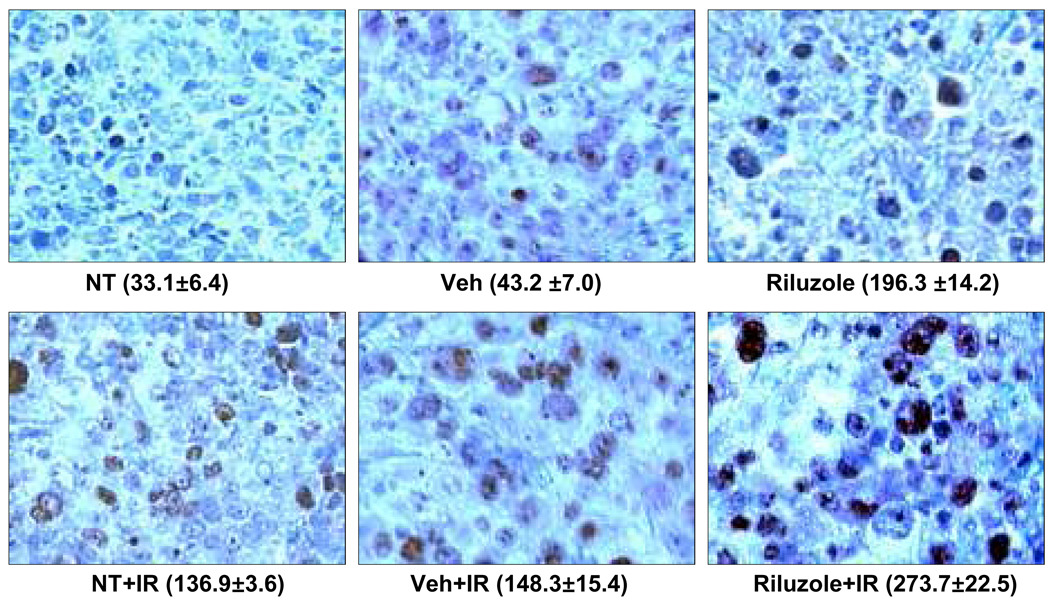
In the second xenograft experiment we measured tumor volume twice per week to determine if riluzole could sensitize xenograft tumors to the effects of ionizing radiation and arrest or delay their growth. Our results demonstrate that either riluzole or irradiation alone resulted in significantly smaller tumors at 19 and 25 days than untreated or vehicle-treated control xenografts. However, the combination of riluzole and irradiation resulted in significantly smaller tumors as compared to untreated controls or to tumors treated with riluzole or irradiation alone at both 19 and 25 days (Figure 5). Xenograft harvests from this experiment revealed markedly enhanced DNA damage as measured by foci of γH2AX on immunohistochemistry (Figure 6).
Figure 5. Cleaved Caspase-3 Immunohistochemistry.
Immunodeficient nude mice were purchased from Taconic (Hudson, NY). C8161 cells were injected into flanks bilaterally at 106 cells per site. Tumor size was measured with a vernier caliper. When tumor volumes reach 6–10 mm3 the mice were randomly grouped into various treatments. Each treatment group consists of 10 mice. Group 1 was left untreated (No Treatment), group 2 was treated twice with 4Gy of irradiation only (IR), group 3 was treated with the DMSO vehicle only (Vehicle), group 4 was treated with 10mg/kg of riluzole by oral gavage only (riluzole), and group 5 was first given 10mg/kg of riluzole followed by two treatments of 4 Gy of irradiation (riluzole+IR). The irradiation was performed using the following protocol: At 24 hrs post-riluzole or vehicle administration two mice at a time were placed in individual containers that were approved by our veterinarian for use in the Gamma-irradiator (Gammator 50, cesium 137 source, Radiation Machine Corp). The mice were irradiated with 4Gy, followed by a second dose of 4Gy at 48 hrs post-riluzole or vehicle. At 24, 48, and 72 hours after the second dose of irradiation, mice in each group were sacrificed and tumor samples were removed. Half of the samples were snap-frozen for further molecular studies and the other half was fixed in formalin for histological analysis. We used the antibody to activated caspase-3 to visualize apoptotic cells. We found a significant difference in the number of apoptotic tumor cells in the riluzole plus irradiation group than in the irradiation alone group or the control groups (p<0.001). There was also a significant difference in the number of apoptotic tumor cells in the riluzole plus irradiation tumors as compared to the riluzole alone tumors at 24hour and 48hour time points (p<0.01).
Figure 6. H2AX Immunohistochemistry.
C8161 xenografts were treated with riluzole when the tumors were 6mm3 and the mice were irradiated (4 Gy) once a week for 3 weeks as described in Figure 4. Riluzole was given for three continuous weeks. The number of H2AX positive cells was calculated from counting of 10 random fields.
DISCUSSION
In contrast to most tumor cell types, significant evidence exists that at least some melanoma cell lines have high capacity for repair of sub-lethal DNA damage caused by ionizing radiation4, 14. In an attempt to address the perceived radioresistance of melanoma, many investigators have adopted radiotherapy schedules that deliver larger daily fractions of radiation (hypofractionation) to exploit the sensitivity of these cells to larger fraction sizes15–18. However, these large daily fractions cannot be delivered to the whole brain for patients with brain metastases due to the risk of late neurotoxicity.
Melanoma is the 4th most common cause of brain metastases. It also has one of the highest rates of brain metastases; up to 50% of patients dying of melanoma have brain metastases5. The median survival of melanoma patients with brain metastases treated with whole brain radiation therapy (WBRT) ranges between 3–5 months. In a report from Fife et al., on 686 patients with metastatic melanoma to brain, 205 patients treated with resection with or without WBRT had a median survival of 9 months, 236 patients treated with WBRT had a median survival of 3.4 months, and patients treated with supportive care alone had a median survival of 2 months19.
Because of its ability to penetrate the CNS, several groups have reported results from phase II clinical trials using temozolamide in patients with brain metastases from melanoma20–25. Although the combination of WBRT and temozolamide appears to have acceptable tolerability, the response rates and survival times are modest at best. Future studies using temozolmide will likely need to be informed by the assessment of O6- methylguanine-DNA-methyltransferase methylation status to preselect patients for temozolamide therapy26. Clearly, the need for a well-tolerated agent with activity in the CNS cannot be overstated.
Our group has extensively studied and described the transforming ability of GRM1 in melanocytes13, 27–30. GRM1, the protein product, is a member of the seven transmembrane G-protein coupled receptors (GPCRs), which have multiple synaptic functions. In the CNS, the natural ligand of GRM1 is L-glutamate. Glutamate signaling is now known to be involved in various tissue types and various human malignancies. A phase II trial of a non-competitive glutamate inhibitor with temozolamide and irradiation has been reported in glioblastoma31. Riluzole, a glutamate release inhibitor, is an oral agent with CNS penetration that has been used chronically in patients with neurodegenerative disorders. Our previous work in melanoma has demonstrated that riluzole leads to cell death via apoptosis13.
Our earlier work has shown that treatment of human melanoma cells with riluzole resulted in an accumulation of treated cells in the G2/M phase of the cell cycle at 24 hours and progressed to the subG1 phase by 48 hours, indicative of apoptotic cells13. This finding provides a strong rationale for combining riluzole with DNA damaging agents such as ionizing radiation. In the current report, we have demonstrated, in vitro and in vivo, that the combination of riluzole and ionizing radiation leads to greater cytotoxicity than either treatment alone. It is possible that the effects we are seeing in Figures 1 and 2 are due to riluzole treatment synchronizing the hGRM1-expressing melanoma cells in the radiosensitive G2/M phase of the cell cycle. The differences in cell survival curves in a single fraction experiment are magnified considerably in a multi-fraction setting, such as whole brain radiation therapy. There was no difference in the survival curves for riluzole-treated or untreated UACC930 cells that do not express hGRM1. Earlier we reported that excess extracellular glutamate is detected in most human melanoma cell lines perhaps by creating autocrine loops to promote cell growth13. In contrast, UACC930 had the lowest levels of extracellular glutamate, had low proliferation and is nontumorigenic. It is perhaps not surprising that an inhibitor of glutamate release such as riluzole did not influence the growth of UACC930 cells.
In the xenograft models we demonstrate that the combination of riluzole and ionizing radiation results in an increase in the number of apoptotic cells in the tumors as compared to untreated controls or xenografts treated with either treatment alone. Furthermore, we have shown that the combination of riluzole and ionizing radiation leads to a significant decrease in tumor growth compared to controls or either agent alone. We also demonstrated that the combination therapy resulted in significantly enhanced levels of DNA damage. Taken together, these data suggest that the combination of riluzole and RT may have a therapeutic advantage over WBRT alone. Importantly, these findings can be rapidly translated to the clinic. Currently we are conducting a Phase I dose escalation trial of riluzole to define the maximum tolerated dose to be used in combination with conventionally fractionated whole brain irradiation.
STATEMENT OF TRANSLATIONAL RELEVANCE
Few therapeutic options exist for patients with brain metastases. A well-tolerated oral agent with CNS penetration that enhances the effects of whole brain irradiation could be of significant value. Our group has published data demonstrating the effects of riluzole on melanoma cells with aberrant glutamate signaling mediated by the hGRM1 receptor. We have previously shown that riluzole synchronizes cells in G2/M, a radiosensitive phase of the cell cycle. We hypothesize that this effect may increase radiation-induced lethality in these cells. In this report we present evidence that riluzole, a well-tolerated oral agent with known CNS penetration, enhances the effects of radiation in melanoma cells with hGRM1 upregulation.
Supplementary Material
Acknowledgement
This work is supported by New Jersey Commission for Cancer Research 09-1143-CCR-E0 (S. Chen), NIH RO1CA73077 (S. Chen), NIH RO1CA124975 (J. Goydos), UNDNJ Foundation Grant PC23-10 (A. Khan), NIEHS ES-005022 (S. Chen) and Breast Cancer Research Foundation (B. Haffty).
REFERENCES
- 1.Patchell RA, Regine WF, Loeffler JS, Sawaya R, Andrews DW, Chin LS. Radiosurgery plus whole-brain radiation therapy for brain metastases. JAMA. 2006;296:2089–2090. doi: 10.1001/jama.296.17.2089. author reply 90-1. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 3.Broadbent AM, Hruby G, Tin MM, Jackson M, Firth I. Survival following whole brain radiation treatment for cerebral metastases: an audit of 474 patients. Radiother Oncol. 2004;71:259–265. doi: 10.1016/j.radonc.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 4.McKay M, Kefford R. The spectrum of in vitro radiosensitivity in four human melanoma cell lines is not accounted for by differential induction of rejoining of DNA double strand breaks. Int J Radiat Oncol Biol Phys. 1995;31:345–352. doi: 10.1016/0360-3016(94)e0147-c. [DOI] [PubMed] [Google Scholar]
- 5.Amer MH, Al-Sarraf M, Baker LH, Vaitkevicius VK. Malignant melanoma and central nervous system metastases: incidence, diagnosis, treatment and survival. Cancer. 1978;42:660–668. doi: 10.1002/1097-0142(197808)42:2<660::aid-cncr2820420237>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 6.Marin YE, Namkoong J, Cohen-Solal K, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–1286. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Namkoong J, Shin SS, Lee HJ, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 8.Pollock PM, Cohen-Solal K, Sood R, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 9.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 10.McGeer EG, McGeer PL. Pharmacologic approaches to the treatment of amyotrophic lateral sclerosis. BioDrugs. 2005;19:31–37. doi: 10.2165/00063030-200519010-00004. [DOI] [PubMed] [Google Scholar]
- 11.Miller R. Riluzole for ALS: what is the evidence? Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:135. [PubMed] [Google Scholar]
- 12.Yip D, Le MN, Chan JL, et al. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–3902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Namkoong J, Shin SS, Lee HJ, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Research. 2007;67:2298–2305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 14.Habermalz HJ, Fischer JJ. Radiation therapy of malignant melanoma: experience with high individual treatment doses. Cancer. 1976;38:2258–2262. doi: 10.1002/1097-0142(197612)38:6<2258::aid-cncr2820380611>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 15.Ballo MT, Ang KK. Radiation therapy for malignant melanoma. Surg Clin North Am. 2003;83:323–342. doi: 10.1016/S0039-6109(02)00096-8. [DOI] [PubMed] [Google Scholar]
- 16.Ballo MT, Ang KK. Radiotherapy for cutaneous malignant melanoma: rationale and indications. Oncology (Williston Park) 2004;18:99–107. discussion -10, 13-4. [PubMed] [Google Scholar]
- 17.Ballo MT, Garden AS, Myers JN, et al. Melanoma metastatic to cervical lymph nodes: Can radiotherapy replace formal dissection after local excision of nodal disease? Head Neck. 2005;27:718–721. doi: 10.1002/hed.20233. [DOI] [PubMed] [Google Scholar]
- 18.Bonnen MD, Ballo MT, Myers JN, et al. Elective radiotherapy provides regional control for patients with cutaneous melanoma of the head and neck. Cancer. 2004;100:383–389. doi: 10.1002/cncr.11921. [DOI] [PubMed] [Google Scholar]
- 19.Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol. 2004;22:1293–1300. doi: 10.1200/JCO.2004.08.140. [DOI] [PubMed] [Google Scholar]
- 20.Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: a phase II study. J Clin Oncol. 2004;22:2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Danson S, Lorigan P, Arance A, et al. Randomized phase II study of temozolomide given every 8 hours or daily with either interferon alfa-2b or thalidomide in metastatic malignant melanoma. J Clin Oncol. 2003;21:2551–2557. doi: 10.1200/JCO.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann M, Kiecker F, Wurm R, et al. Temozolomide with or without radiotherapy in melanoma with unresectable brain metastases. J Neurooncol. 2006;76:59–64. doi: 10.1007/s11060-005-2914-0. [DOI] [PubMed] [Google Scholar]
- 23.Hwu WJ, Lis E, Menell JH, et al. Temozolomide plus thalidomide in patients with brain metastases from melanoma: a phase II study. Cancer. 2005;103:2590–2597. doi: 10.1002/cncr.21081. [DOI] [PubMed] [Google Scholar]
- 24.Krown SE, Niedzwiecki D, Hwu WJ, Hodgson L, Houghton AN, Haluska FG. Phase II study of temozolomide and thalidomide in patients with metastatic melanoma in the brain: high rate of thromboembolic events (CALGB 500102) Cancer. 2006;107:1883–1890. doi: 10.1002/cncr.22239. [DOI] [PubMed] [Google Scholar]
- 25.Margolin K, Atkins B, Thompson A, et al. Temozolomide and whole brain irradiation in melanoma metastatic to the brain: a phase II trial of the Cytokine Working Group. J Cancer Res Clin Oncol. 2002;128:214–218. doi: 10.1007/s00432-002-0323-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mutter N, Stupp R. Temozolomide: a milestone in neuro-oncology and beyond? Expert Rev Anticancer Ther. 2006;6:1187–1204. doi: 10.1586/14737140.6.8.1187. [DOI] [PubMed] [Google Scholar]
- 27.Pollock PM, Cohen-Solal K, Sood R, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–112. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 28.Shin SS, Namkoong J, Wall BA, Gleason R, Lee HJ, Chen S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res. 2008;21:368–378. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu H, Reuhl K, Zhang X, et al. Development of heritable melanoma in transgenic mice. J Invest Dermatol. 1998;110:247–252. doi: 10.1046/j.1523-1747.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Ryan K, Chen S. Cloning of novel splice variants of mouse mGluR1. Brain Res Mol Brain Res. 1999;73:93–103. doi: 10.1016/s0169-328x(99)00239-9. [DOI] [PubMed] [Google Scholar]
- 31.Grossman SA, Ye X, Chamberlain M, et al. Talampanel with standard radiation and temozolomide in patients with newly diagnosed glioblastoma: a multicenter phase II trial. J Clin Oncol. 2009;27:4155–4161. doi: 10.1200/JCO.2008.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.