Abstract
Recent research has distinguished between anticipatory and consummatory pleasure. In the current study, we examined the psychometric properties of the Temporal Experience of Pleasure Scale (TEPS) to determine whether reliability and validity findings reported in previous research replicate in an additional sample of schizophrenia patients. Participants included 86 individuals with schizophrenia and 59 demographically matched healthy controls. Inconsistent with previous research, patients differed from controls in their reports of consummatory (TEPS-CON), but not anticipatory (TEPS-ANT) pleasure. We also failed to replicate some important correlational findings reported in previous research indicating relationships between the TEPS-ANT subscale and external validators. Analyses of the stability of the TEPS subscales were conducted in a sub-group of patients (n = 19), and indicated excellent stability for the TEPS-CON (ICC = 0.93), but somewhat lower stability for the TEPS-ANT subscale (ICC = 0.74). These findings suggest that additional studies are needed using the TEPS, as well as other measures, to determine the nature of anhedonia in individuals with schizophrenia.
Keywords: Schizophrenia, Anhedonia, Consummatory Pleasure, Anticipatory Pleasure, Negative Symptoms
1. Introduction
Anhedonia has long been considered one of the critical negative symptoms of schizophrenia (SZ). For example, more than 40 years ago Paul Meehl (1962) wrote that anhedonia is “a marked widespread, and refractory defect in pleasure capacity which, is one of the most consistent and dramatic behavioral signs of the disease”.. While anhedonia is not considered among the diagnostic criteria for SZ in the DSM-IV-TR (American Psychiatric Association, 1994), it is discussed as an important associated feature in the diagnostic nomenclature, and clinician ratings of anhedonia are central to negative symptom scales such as the Scale for the Assessment of Negative Symptoms (SANS: Andreasen, 1983) or self-report measures like the Chapman Physical (Chapman and Chapman, 1978) and Social Anhedonia (Eckblad et al., 1982) Scales. This clinical focus on anhedonia reflects the presumed motivational significance of hedonic experience. That is, if someone were unable to experience pleasure, it would be easy to understand why he or she might show the kind of broad deficits in the initiation of volitional goal-directed behavior that are characteristic of many individuals with SZ. Simply stated, why do something if there is no enjoyment associated with it?
The problem with this clinical understanding of anhedonia is that there have been a series of laboratory studies that show that individuals with SZ rate the affective value of many evocative stimuli in a nearly identical fashion as healthy control participants (Berenbaum and Oltmanns, 1992; Kring and Neale, 1996; Barch, 2005; Heerey and Gold, 2007; Kring and Moran, 2008; Cohen and Minor, 2010; Tremeau et al., 2010). While not every study finds completely “normal” levels of emotional experience, the broad scope of the literature is clear, consistent, and surprising: when presented with evocative stimuli, individuals with SZ fail to demonstrate the lack of pleasure that would be expected based on a straightforward understanding of anhedonia as an inability to experience pleasure. This leaves open the possibility that certain aspects of reward experience may be intact in SZ, whereas others may be compromised.
In an attempt to resolve this apparent paradox, investigators have recently examined the constructs of anticipatory and consummatory pleasure in hopes that they might shed light on the nature of anhedonia in SZ. Consummatory pleasure reflects the momentary pleasure that is experienced while engaged in an enjoyable activity, while anticipatory pleasure revolves around pleasure from future activities (Klein et al., 1984). Furthermore, the construct of anticipatory pleasure has been suggested to involve 2 distinct components: 1) “anticipated pleasure”- the pleasure that people anticipate from future events, and 2) “anticipatory pleasure”- the pleasure that people experience at the thought of a future event (Lowesnstein et al., 2001). Anticipatory pleasure is thought to reflect emotional processes, whereas anticipated pleasure relies more on cognitive processes. The literature examining these concepts in schizophrenia is somewhat complex at present, which is potentially due to differing methods of assessment across studies.
In a recent laboratory-based study examining “anticipated pleasure”, Tremeau et al. (2010) presented evocative stimuli and had subjects rate their immediate emotional experience, as well as their pre-test anticipated and post-test remembered pleasure. Their results indicated that anticipated pleasure was not impaired in schizophrenia. Seemingly contradictory findings were reported in experiment 1 of Gard et al. (2007), which examined daily report of pleasure using the experience sampling method, and found that patients differed from healthy controls in the amount of enjoyment they anticipated they would get out of goal-directed activities, but reported similar levels of consummatory pleasure as controls. Several additional studies have also used a new self-report questionnaire, the Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006), to assess consummatory and “anticipatory” pleasure. These studies have found that individuals with SZ report intact consummatory pleasure, but diminished anticipatory pleasure (Gard et al., 2007; Favrod et al., 2009; Chan et al., 2010; Loas et al., 2010), ostensibly signifying that patients experience less pleasure at the thought of a future event.
Gard et al. (2007) provided additional evidence for the construct validity of the TEPS by examining correlations with the Chapman Physical (Chapman and Chapman, 1978) and Social Anhedonia (Eckblad et al., 1982) Scales and the Behavioral Inhibition and Activation Scales (BIS/BAS: Carver and White, 1994). As predicted, both the Anticipatory (ANT) and Consummatory (CON) TEPS subscales correlated negatively with the Chapman Physical Anhedonia Scale, and positively with the BIS/BAS scales. The TEPS-ANT had a significantly higher correlation with the BAS reward responsiveness scale, which indicates that anticipatory anhedonia is specifically related to motivation. The TEPS-ANT, but not TEPS-CON, was significantly correlated with the Chapman Social Anhedonia Scale. This is likely due to the fact that the TEPS-ANT items cover physical and social activities, while the TEPS-CON examines physical and sensory, but not social pleasure. The TEPS-ANT was highly correlated with social role functioning, again more so than TEPS-CON, making the link between anticipatory pleasure and everyday functional status (Gard et al., 2007). Thus, previous data on the TEPS offer highly supportive evidence for the construct validity of the TEPS, highlight the distinction between anticipatory and consummatory pleasure, and provide a novel approach to the conceptualization of the nature of anhedonia.
In the present investigation, we sought to replicate and extend the findings of Gard et al. (2007), by examining the psychometric properties of the TEPS in a sizeable sample of stable outpatients diagnosed with SZ and healthy community controls. In order to examine the construct validity of the TEPS, we examined the correlation of TEPS-ANT and TEPS-CON with self-report on the Chapman Anhedonia Scales, functional outcome as assessed by the Level of Function Scale (LOF; Heinrichs et al., 1984), and ratings of clinical symptoms using the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1988), the Calgary Depression Scale (CDS; Addington et al., 1990), and the Schedule for the Assessment of Negative Symptoms (SANS; Andreasen, 1983). We also examined the stability of the TEPS-ANT and TEPS-CON scores measured at two time intervals.
2. Methods
2.1. Participants
Participants included 59 healthy controls and 86 individuals with DSM-IV diagnosis of SZ or Schizoaffective Disorder (n = 7) recruited from the Maryland Psychiatric Research Center, Outpatient Research Program, and Schizophrenia Related Disorders Research Program. Participant diagnoses were determined based on the Structured Clinical Interview for DSM-IV (First et al., 2002). Healthy controls were primarily recruited by random digit dialing targeting localities in the greater Baltimore area, and were free of a current Axis I diagnosis, lifetime history of psychosis, or Axis II schizophrenia spectrum disorder as determined by SCID interview. Participants were excluded from the study if they had sustained a neurological disorder, such as stroke, head injury, seizure, history of mental retardation, or diagnosis of alcohol or substance abuse/dependence in the last 6 months. To increase confidence in patient self-report and ensure a minimum literacy level for completing self-report questionnaires, a cut-off of 70 was established on the Wechsler Test of Adult Reading (WTAR). Patients and controls did not significantly differ in age, parental education, race/ethnicity, or sex. However, CN had significantly higher WTAR scaled scores and greater total years of education than patients. Basic demographic data for the study groups are presented in Table 1.
Table 1.
Participant Demographics
| Participant Demographics | CN (n = 59) | SZ (n = 86) |
|---|---|---|
| Ethnicity | ||
| African American | 20 | 35 |
| Caucasian | 39 | 45 |
| Asian | 0 | 3 |
| Native American | 0 | 1 |
| Mixed Race | 0 | 2 |
| Females/Males | 26/33 | 29/57 |
| Mean Age | 44(SD=10.4) | 42 (SD=10.1) |
| Mean Participant Education | 14.9 (SD = 2.3) | 12.7 (SD=2.2) |
| Mean Paternal Education | 12.9(SD=3.9) | 13.4(SD=3.9) |
| Mean Maternal Education | 13.2(SD=2.3) | 13.0(SD=2.9) |
| WTAR Scaled Score | 110.54 (SD = 11.53) | 97.57(SD = 13.35) |
All SZ participants were tested while on stable doses of antipsychotic medications (i.e., there were no changes in antipsychotic type or dose for a minimum of 4 weeks prior to study entry). The most frequently prescribed antipsychotic medication was clozapine, either alone (n=22) or in conjunction with another antipsychotic (n=19). Participants with SZ were receiving either a first generation antipsychotic (n=10), olanzapine (n=13), risperidone (n=12), aripiprazole (n=3), ziprasidone (n=3), quetiapine (n =2), or a combination of quetiapine and haloperidol (n=1) or risperidone and olanzapine (n =1). Mean (SD) BPRS subscale scores were: Positive Symptoms 2.28 (1.08); Negative Symptoms1.73 (0.70); Disogranized Symptoms 1.30 (0.38); BPRS Total 35.32 (8.31). All participants provided written informed consent after demonstrating adequate comprehension of the protocol in response to standard probe questions. Participants were paid $20 per hour as compensation for participating in the study. The study was approved by The University of Maryland’s Human Research Protection Office.
2.2. Measures and procedure
Participants completed several self-report questionnaires assessing emotion and reward sensitivity, including the TEPS, BIS/BAS, and Chapman Physical and Social Anhedonia Scales. The TEPS is composed of 18-items rated on a likert-type scale ranging from 1 (Very True for me) to 6 (Very False for me), and yields two subscales measuring Anticipatory (TEPS-ANT) and Consummatory (TEPS-CON) pleasure. Lower scores indicate greater levels of anhedonia. The BIS/BAS measures reward sensitivity in relation to self-reported behavioral inhibition and activation (fun-seeking, reward responsiveness, and drive). Controls and individuals with schizophrenia completed questionnaires on-site in the laboratory. For all patients, symptom rating assessments were performed by clinicians trained to reliably administer these measures. Reliability on symptom measures is monitored at the MPRC monthly by a “gold standard” committee, which monitors inter-rater reliability and ensures that raters are reliable at ICC = 0.80.
A subset of 19 individuals with SZ also completed these measures on a second occasion, occurring on average 88 weeks (range = 39 to 121 weeks) following the initial administration. These data formed the basis for stability estimates. These patients did not differ in their total BPRS score symptom totals from Time 1 to Time 2 (see Results).
2.3. Data Analysis
MANOVAs, one-way ANOVAs, t-tests, and chi-square analyses were calculated to determine group differences. Scheffe contrasts were performed as post hoc tests. Spearman correlations were calculated for variables that were not normally distributed (BIS-BAS, SANS, Chapman Anhedonia, Calgary Depression Scale variables). Pearson correlations were calculated for all other variables, which followed a normal distribution. Initial analyses examined between-group differences in patients and controls. However, given results of previous studies indicating differences in high and low negative symptom patients (Chan et al., 2010), we also examined the role of negative symptoms using between-group analyses (i.e., comparing high negative symptom, low negative symptom, and control groups). Negative symptom groups were determined using a median split on the average of the SANS anhedonia items (median split value = 2.8). Internal consistency was estimated by calculating Cronbach’s alpha. Effect size is reported in terms of partial eta squared. (0.01 = low; 0.06 = medium; 0.14 = large).
3. Results
3.1. Primary Analyses
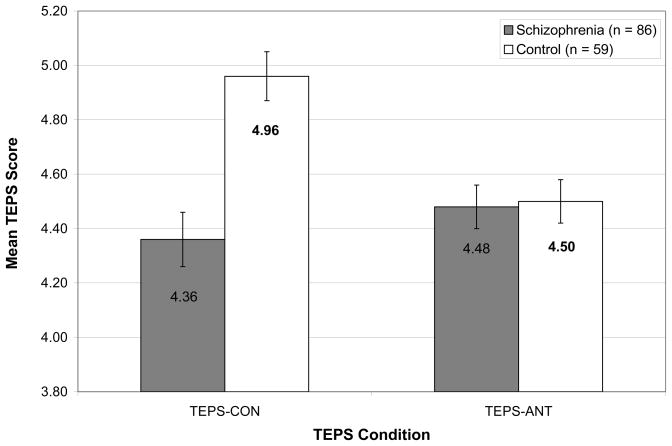
The TEPS data are shown in Figure 1. MANOVA with mean TEPS scores as the dependent variables indicated a significant effect for diagnosis, F (2, 134) = 10.21, p < 0.001 (η2=0.14). Follow-up ANOVAs computed on the TEPS subscales indicated no significant differences between groups for the TEPS-ANT scale, F (1,135) =0.03, p = 0.86 (η2 =0.00); however, significant differences were observed for the TEPS-CON scale, F (1,135) = 15.94, p < 0.001 (η2 =0.11). In order to ensure that group differences were not influenced by participant education, we re-computed the MANOVA using participant education as a covariate. There were no substantiative changes1.These results differ from those of Gard et al. (2007) who found between group differences on the TEPS-ANT, but not on the TEPS-CON scale.
Figure 1.
Means and Standard Errors for TEPS-ANT and TEPS-CON scales in Healthy Controls and Individuals with Schizophrenia.
Note. Lower TEPS score indicate greater severity of anhedonia.
Additionally, individuals with schizophrenia were divided into high and low anhedonia groups using a median split on the average of the SANS Anhedonia items, and these anhedonia groups were then compared on TEPS-ANT and TEPS-CON scales. MANOVA indicated a significant effect of group, F (2, 142) = 8.85, p < 0.001 ( 2=0.11). Follow-up ANOVAs indicated a significant difference among the 3 groups for TEPS-CON (F = 7.12, p = 0.001; Low: 4.47 High: 4.37 CN: 4.96). Post hoc Scheffe analyses indicated that both the high (p = 0.002) and low (p = 0.041) SANS Anhedonia patient groups reported significantly less consummatory pleasure than controls; however, the high and low anhedonia groups did not differ from each other (p = 0.562). No difference was found among the 3 groups on the TEPS-ANT scale (F = 0.02, p = 0.98; Low: 4.52 High: 4.51 CN: 4.50).
Given the relatively high proportion of patients prescribed clozapine in the current sample, we also examined differences in TEPS performance in patients prescribed clozapine (CLOZ+) vs. those who were not (CLOZ−). Individual one-way ANOVAs calculated for TEPS-ANT (CLOZ+ M = 4.40, SD = 0.67; CLOZ– M = 4.69, SD = 0.75; F = 3.01, p = 0.09) and TEPS-CON (CLOZ+ M = 4.40, SD = 0.80; CLOZ– M = 4.43, SD = 0.94; F = 0.01, p = 0.97) were nonsignificant.
Cronbach’s alpha was calculated for the TEPS, BIS/BAS, and Chapman Scales to examine internal consistency between individuals with schizophrenia and controls (see Table 2). Results indicate that responses made by patients had adequate internal consistency, providing greater confidence in the validity of patient self-report in this sample. Alpha values for controls were somewhat lower in some cases (e.g., TEPS-CON, TEPS-ANT, Chapman Physical Anhedonia)
Table 2.
Internal Consistency of Self-Report Measures in individuals with Schizophrenia and Controls.
| Measure | CN (n = 59) | SZ (n = 86) |
|---|---|---|
| TEPS | ||
| Total Scale | 0.80 | 0.87 |
| TEPS-ANT | 0.64 | 0.71 |
| TEPS-CON | 0.64 | 0.78 |
| BIS/BAS | ||
| Total Scale | 0.72 | 0.72 |
| BIS Total | 0.68 | 0.62 |
| BAS Total | 0.85 | 0.87 |
| Chapman Scales | ||
| Total Scale | 0.80 | 0.82 |
| Physical Anhedonia | 0.53 | 0.76 |
| Social Anhedonia | 0.63 | 0.63 |
The TEPS-ANT and TEPS-CON subscales were significantly correlated in both patient and control groups (SZ: r = 0.54, p < 0.001; CN: r = 0.41, p < 0.001). Correlational analyses were also calculated to examine relationships between TEPS scales and measures of symptom severity, anhedonia, reward-sensitivity, and functional outcome (see Table 3). In patients, the TEPS Anticipatory subscale was not significantly correlated with any of the SANS subscales or the Level of Function scale. There were no significant correlations between the Calgary Depression Total or subscale scores and the TEPS subscales. Significant correlations were observed between the TEPS Anticipatory scale and the Chapman Physical and Social Anhedonia scales, as well as the BPRS Positive Symptom factor score. The TEPS Consummatory subscale showed a similar lack of relationship with most measures, with the exception of the Chapman Physical Anhedonia, BAS Total, and BAS Reward Responsiveness scales.
Table 3.
Differences in performance on self-report measures between Healthy Controls and Individuals with Schizophrenia
| Measure | CN (n = 59) | SZ (n = 86) |
|---|---|---|
| BIS Total | 18.18 (4.74) | 21.24 (3.30)*** |
| BAS Total | 38.27 (5.99) | 40.34 (6.13) |
| BAS Fun-seeking | 10.44 (3.36) | 11.79 (2.42)* |
| BAS Reward Responsiveness | 16.38 (3.56) | 17.06 (2.31) |
| BAS Drive | 10.32 (3.09) | 11.49 (2.55)* |
| Chapman Physical Anhedonia | 9.06 (6.33) | 17.90 (7.18)*** |
| Chapman Social Anhedonia | 8.13 (4.16) | 14.09 (7.38)*** |
Note.
p < 0.05;
p <0.01.;
p < 0.001
3.2. TEPS Stability Analyses
TEPS data were obtained across two time intervals for 19 individuals with SZ. Stability of scores on the two sub-scales was assessed via intraclass correlation coefficients (ICCs) (two-way mixed model) that were calculated for mean TEPS-ANT and TEPS-CON scale scores. ICCs were high for the TEPS-CON (ICC = 0.93) and moderate for the TEPS-ANT (ICC = 0.74). The test for difference between two coefficients was significant between the TEPS-ANT and TEPS-CON scales (r1-r2 = −0.19; z = −2.00, p = 0.04 two-sided). The TEPS-ANT subscale therefore appears to be less stable then the TEPS-CON in individuals with SZ. It is likely that the changes in TEPS subscale scores across time reflect psychometric properties of the subscales rather than changes in symptomatic severity given that there was no appreciable change in patient clinical presentation across the two time intervals (BPRS Total T1 = 37.75; BPRS Total Time 2 = 38.12; t = −0.20, p = 0.85; r = 0.55, p = 0.02). Note that the higher stability of the TEPS-CON subscale is likely to increase the sensitivity of this scale to individual differences relative to the TEPS-ANT scale.
4. Discussion
In this study we were unable to replicate several of the critical findings presented by Gard et al. (2006, 2007) and other studies (Favrod et al., 2009; Chan et al., 2010; Loas et al., 2010) concerning the TEPS. We found that individuals with SZ differed from controls in their ratings of consummatory but not anticipatory pleasure—the opposite of the pattern previously reported. It is important to note that our control participants did receive significantly higher scores on the TEPS-CON scale than those reported in Gard et al. (2007) (TEPS CON: Gard et al. = ~4.52 vs. 4.96 in our sample; t = 3.67, p = 0.01). However, control scores on the TEPS-ANT scale are highly consistent with those reported in Gard et al. (2007) (TEPS ANT: Gard et al. = ~4.48 vs. 4.50 in our sample; t = 0.15, p = n.s.). Although our control sample, obtained via random digit dialing, is highly representative of the individuals in our community and well-matched with the patient sample in terms of demographics, it is nonetheless possible that a selection bias in control groups could account for differences in findings on the TEPS-CON between the present investigation and previous ones.
It is also important to note that patient self-report on the TEPS-CON (M = 4.36) was nearly identical to that of Gard et al (2007) (M = 4.33); however, our patients reported greater anticipatory pleasure (M = 4.48) than Gard et al. (2007) (M = 4.04). It therefore appears that the main difference in findings across the two studies is that we failed to replicate the finding that individuals with SZ receive significantly lower anticipatory pleasure scores. We also failed to replicate group differences that have previously been reported among high and low negative symptom patients and controls on the TEPS-ANT in a Chinese sample (Chan et al., 2010) and significant correlations with the SANS in a French-speaking sample (Favrod et al., 2009). Our failure to replicate these previous results on the TEPS does not appear to be due to abnormal self-report or characteristics of the patients or controls in our sample, as individuals with SZ and controls in the current sample exhibited patterns of performance similar to other published studies on the Chapman Anhedonia and BIS/BAS scales.
Interestingly, our pattern of correlations observed between the TEPS and the BIS/BAS and Chapman Anhedonia scales was largely consistent with findings from Gard et al. (2007)2. Similar to Gard et al. (2007), we also found that: 1) the TEPS scales were not correlated with the BIS, but were positively correlated with the total BAS score; 2) the TEPS-ANT scale was robustly correlated with the BAS Reward Responsiveness subscale, 3) the TEPS scales were negatively correlated with Chapman Scale Physical Anhedonia; 4) Chapman Social Anhedonia was negatively correlated with TEPS-ANT, but not TEPS-CON. However, we also failed to replicate several correlational findings reported in Gard et al. (2007), as we found that the TEPS-CON was significantly correlated with BAS Reward Responsiveness and that the TEPS-ANT scale correlated unexpectedly with the severity of BPRS positive symptoms. This pattern of findings strengthens the interpretation of the current findings on null group differences. That is, our TEPS findings are not simply a byproduct of some peculiar responses on the TEPS that are inconsistent with prior reports. Rather, we show substantial replication of the pattern of correlations observed in Gard et al. (2007), and find meaningful associations across multiple measures. The validity of patient self-report in the current study is also strengthened by the fact that the scales were internally consistent in both patients and controls and showed reasonable stability over time. Thus, the validity of the patient self-report in the current study does not seem in question- we simply did not replicate the finding of intact consummatory and impaired anticipatory pleasure that has been found in other studies using the TEPS.
We do not have a ready explanation for the differences in findings across studies. Both studies involved groups of stably treated outpatients with long established illness. While there are some demographic differences across the studies3 it would be hard to explain why any such difference would selectively impact performance on ratings of anticipatory versus consummatory pleasure. One of the main differences across samples appears to be the percentage of patients prescribed conventional vs. atypical antipsychotics. In the present study, only 12% of patients were prescribed typical antipsychotics, compared to 31% in Gard et al. (2007), and 29% in Chan et al. Given previous evidence indicating that typical antipsychotics result in more reward prediction dysfunction (Juckel et al., 2006), it is possible that conventional antipsychotics play an important role in producing anticipatory pleasure deficits. It is also important to note that our sample of schizophrenia patients, while large, had a greater proportion of individuals prescribed clozapine than what is common in many samples. This likely reflects the long history of clozapine clinical trials conducted at MPRC, resulting in clinicians having more experience using the drug than is common in community. However, the lack of significant differences between individuals with SZ prescribed clozapine vs. those not prescribed clozapine in the current sample argues against clozapine being responsible for the different pattern of results across studies. In the Gard et al (2007) study, only 1 patient was prescribed clozapine, yet their sample had higher mean BPRS total scores than patients in the current study. The patients in Gard et al. (2007) therefore appear to be more globally symptomatic, yet less likely to be on clozapine, potentially suggesting that anticipatory pleasure is more related to symptom severity than treatment resistance. In line with this possibility, our data suggest that greater severity of psychosis is associated with lower self-reported anticipatory pleasure. It therefore appears that additional research is needed to determine the roles of conventional antipsychotics and psychotic symptoms in anticipatory pleasure.
It is also noteworthy that we were able to observe differences on the TEPS-CON scale, which proved to be the more stable of the two subscales in our sample of 19 individuals with SZ who completed the measure across two time points. Differences in the consistency of measurement across the two time points did not appear to be due to changes in clinical symptoms, therefore suggesting that the lower consistency noted for the TEPS-ANT might be due to relatively lower stability of this scale (which is still high by most standards). However, it is also possible that anticipatory pleasure is not as “trait-like” as consummatory pleasure, and that it may be influenced by a number of unique outside factors. If anticipatory pleasure is in fact more susceptible to being influenced by such factors, this could certainly explain the lower stability of the TEPS-ANT scale. Given that the TEPS primarily examines physical pleasure, it may be beneficial for future studies to examine the possibility that anticipatory pleasure is more affected in other domains of pleasure, such as social, recreational, or intellectual domains.
While our empirical results are disappointing, we continue to believe that the theoretical approach embedded in the TEPS, distinguishing between different aspects of reward experience and pleasure, may provide a way forward in understanding the nature of anhedonia and broader motivational deficits in individuals with SZ. Indeed, we were motivated to do this study because we considered the preliminary evidence for the construct validity of the TEPS, gathered in both healthy controls and in SZ, to be extremely promising, and therefore, our results are surprising and disappointing. It will only be possible to determine if the different findings across studies is a result of sampling error with the study of additional SZ cohorts. Future studies examining these aspects of anhedonia will benefit from using additional behavioral, experience sampling, and psychophysiological measures in conjunction with the TEPS.
The TEPS presents an unusual task demand that might explain the variable findings to date. In order to rate TEPS items, a participant must be able to call into mind some kind of representation of the “value” of the experiences that are being probed. The distinctions that are made between how excited one is before an experience versus during or after an experience, are very subtle and may often be represented in one overall schema. It is clearly plausible that some people may be better at searching through these memories and making subtle value distinctions. Additionally, prospective emotion reports that are based upon hypothetical situations, such as those used in the TEPS, are often made by accessing situation-specific schemas about how one should respond in a given-situation (Robinson & Clore, 2001). It is likely that such schemas differ greatly among patients, and that variable beliefs regarding how one should respond in a given situation could cause substantial variability in performance on prospective self-report measures. In contrast, we suspect that experimental paradigms that present a standard set of evocative stimuli that are then rated using a very concrete rating scale may be less susceptible to individual differences in cognitive processes that are not directly related to anhedonia and reward processing.
There is reason to suspect that ratings of emotional experience based upon verbal prompts may present challenges for many individuals with SZ. For example, we have previously reported that individuals with SZ abnormally discount the value of larger future rewards relative to smaller immediate rewards, and thought that this behavioral finding might reflect a compromised ability to represent the relative values of differing rewards (Heerey et al., 2007). We also found that SZ patients made more inconsistent value-based preference judgments in a simple two-choice preference task, and made less fine grained distinctions in their preferences for emotional stimuli within the same valence category in comparison to CN (Strauss et al., in press). Thus, the ability to distinguish the relative value of different rewards, or perhaps the ability to distinguish the enjoyment associated with different aspects of a reward experience, may draw upon cognitive and affective processes that are compromised in many individuals with SZ. It will remain for future work to examine the contribution of different cognitive abilities to TEPS performance.
In summary, while the conceptual distinction between reward anticipation and consumption is innovative and theoretically well-motivated, we were unable to replicate the major findings of previous studies using the TEPS (i.e., impaired anticipatory and intact consummatory pleasure) (Gard et al., 2007; Favrod et al., 2009; Chan et al., 2010; Loas et al., 2010).. The advantages and limitations of online evocative testing, retrospective self-report, and prospective self-report measures of emotional experience are well-documented (Robinson & Clore, 2002). Each holds its own distinctive pros and cons, and provides unique insight into the affective lives of individuals with SZ. Developing a more precise understanding of the nature of anhedonia and motivational impairment remains an important issue, and the apparent contradictions between the clinical and experimental laboratory literatures addressing this topic suggest that there is still important work to do in this area.
Table 4.
Correlations between TEPS subscales and measures of anhedonia and reward-sensitivity.
| Measure | TEPS-ANT | TEPS-CON | ||
|---|---|---|---|---|
| CN | SZ | CN | SZ | |
| BIS | −0.03 | −0.05 | 0.03 | 0.06 |
| BAS Total | 0.69*** | 0.28* | 0.52*** | 0.29* |
| BAS Fun-seeking | 0.21 | 0.19 | 0.28* | 0.20 |
| BAS Reward Responsiveness | 0.06 | 0.32** | 0.10 | 0.41*** |
| BAS Drive | 0.18 | 0.18 | 0.17 | 0.14 |
| Chapman Physical Anhedonia | −0.28* | −0.36** | −0.65*** | −0.41*** |
| Chapman Social Anhedonia | −0.10 | −0.26* | −0.22 | −0.05 |
Note.
p < 0.05;
p <0.01.;
p < 0.001
Table 5.
Correlations between TEPS subscales and measures of symptom severity and functional outcome in individuals with schizophrenia.
| Measure | TEPS-ANT | TEPS-CON |
|---|---|---|
| LOF Social Total | −0.07 | 0.15 |
| LOF Work Total | 0.08 | 0.13 |
| SANS AFB Global | 0.02 | −0.08 |
| SANS Alogia Global | 0.12 | 0.17 |
| SANS Avol Global | 0.04 | −0.19 |
| SANS Anhedonia | 0.05 | −0.10 |
| BPRS Positive Symptoms | −0.39*** | −0.18 |
| BPRS Total | −0.50*** | −0.31** |
| Calgary Total | 0.09 | 0.02 |
Note.
p < 0.05;
p <0.01.;
p < 0.001
Footnotes
It should be noted that Gard et al. (2007) used the Role Functioning Scales by Goodman et al. (1993) to measure functional outcome, whereas we used the LOF; however both scales assessed similar variables.
In exploratory analyses we found that within the healthy control group only, Caucasian subjects had significantly higher ratings of TEPS-CON than African American subjects. We therefore ran an ANCOVA, controlling for the effects of race, and re-examined between group effects: the difference between SZ and controls on TEPS consummatory remained significant F(1, 136) = 11.86, p < 0.001 (η2 =0.08). We also examined the possibility of a Group X Ethnicity interaction among Caucasian and African-American subjects (since these are the two largest ethnic groups), and found a nonsignificant diagnosis X race interaction (F = 0.14, p = 0.87).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addington D, Addington J, Schissel BA. A depression rating scale for schizophrenics. Schizophrenia Research. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms. University of Iowa Press; Iowa City: 1983. [Google Scholar]
- Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: How much and how little we know. Schizophrenia Bulletin. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality & Social Psychology. 1994;67:319–333. [Google Scholar]
- Chan RCK, Wang Y, Huang J, Shi Y, Wang Y, Hong X, Ma Z, Li Z, Lai MK, Kring AM. Anticipatory and consummatory components of the experience of pleasure in schizophrenia: Cross-cultural validation and extension. Psychiatry Research. 2010;175:181–183. doi: 10.1016/j.psychres.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP. The Revised Physical Anhedonia Scale. University of Wisconsin; Madison: 1978. Unpublished test. [Google Scholar]
- Cohen A, Minor K. Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophrenia Bulletin. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP, Mishlove M. The Revised Social Anhedonia Scale. University of Wisconsin; Madison: 1982. Unpublished test. [Google Scholar]
- Favrod J, Ernst F, Giuliani F, Bonsack C. Validation of the Temporal Experience of Pleasure Scale (TEPS) in a French-speaking environment. Encephale. 2009;35:241–248. doi: 10.1016/j.encep.2008.02.013. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York, NY: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen. [Google Scholar]
- Gard DE, Gard-Germans M, Kring AM, Oliver JP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. Journal of Research in Personality. 2006;40:1086–1102. [Google Scholar]
- Gard DE, Kring AM, Gard-Germans M, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Sewell DR, Cooley EL, Leavitt N. Assessing levels of adaptive functioning: The role of functioning scale. Community Mental Health Journal. 1993;29:119–131. doi: 10.1007/BF00756338. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. Journal of Abnormal Psychology. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RR, Gold JM. Delay discounting in schizophrenia. Cognitive Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs DW, Hanlon TE, Carpenter WT. The quality of life scale: An instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29:409–16. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Klein D. Depression and anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and Affect Deficit States. PMA Publishing; New York: 1984. pp. 1–34. [Google Scholar]
- Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophrenia Bulletin. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? Journal of Abnormal Psychology. 1996;105:249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- Loas G, Monestes JL, Ameller A, Bubrovszky M, Yon V, Wallier J, Berthoz S, Corcos M, Thomas P, Gard DE. Traduction et étude de validation de la version française de l’échelle d’expérience temporelle du plaisir. Annales Médico-Psychologiques. 2009;167:641–648. [Google Scholar]
- Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychological Bulletin. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Schizotaxia, schizotypy, schizophrenia. American Psychologist. 1962;17:827–838. [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale (BPRS): Recent developments in ascertainment and scaling. Psychopharmacology Bulletin. 1988;24:97–99. [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychology Bulletin. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Robinson BM, Waltz JA, Frank MJ, Kasanova Z, Herbener ES, Gold JM. Schizophrenia Bulletin. Patients With Schizophrenia Demonstrate Inconsistent Preference Judgments for Affective and Nonaffective Stimuli. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F, Antonius D, Cacioppo JT, Ziwich R, Jalbrzikowski M, Saccente E, et al. In support of Bleuler: objective evidence for increased affective ambivalence in schizophrenia based upon evocative testing. Schizophrenia Research. 2009;107:223–231. doi: 10.1016/j.schres.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Tremeau F, Antonius D, Cacioppo JT, Ziwich R, Butler P, Malaspina D, Javitt DC. Anticipated, on-line and remembered positive experience in schizophrenia. Schizophrenia Research. 2010;122:199–205. doi: 10.1016/j.schres.2009.10.019. [DOI] [PubMed] [Google Scholar]