We studied 1,819 children with 2,592 peripherally inserted central venous catheters (PICCs). Independent predictors of CLABSI included prolonged catheter dwell time, pediatric ICU exposure, and administration of parenteral nutrition as indication for PICC insertion.
Abstract
Background. Increasingly, peripherally inserted central venous catheters (PICCs) are placed for prolonged intravenous access. Few data exist regarding risk factors for central line–associated bloodstream infection (CLABSI) complicating PICCs in hospitalized children, especially children hospitalized outside the intensive care unit (ICU).
Methods. We identified all children with a PICC inserted at The Johns Hopkins Hospital (Baltimore, MD) from 1 January 2003 through 31 December 2009 and used Poisson regression models to identify risk factors for PICC-associated CLABSIs.
Results. A total of 2592 PICCs were placed in 1819 children. One hundred sixteen CLABSIs occurred over 44,972 catheter-days (incidence rate [IR], 2.58 cases per 1000 catheter-days; 95% confidence interval [CI], 2.07–3.00 cases per 1000 catheter-days). Independent predictors of CLABSI in the entire cohort included PICC dwell time of ≥21 days (IR ratio [IRR], 1.53; 95% CI, 1.05–2.26), parenteral nutrition as indication for insertion (IRR, 2.24; 95% CI, 1.31–3.84), prior PICC-associated CLABSI (IRR, 2.48; 95% CI, 1.18–5.25), underlying metabolic condition (IRR, 2.07; 95% CI, 1.14–3.74), and pediatric ICU exposure during hospitalization (IRR, 1.80; 95% CI, 1.18–2.75). Risk factors for CLABSI in children without PICU exposure included younger age, underlying malignancy and metabolic conditions, PICCs inserted in the lower extremity, and a prior PICC-associated CLABSI.
Conclusions. Prolonged catheter dwell time, pediatric ICU exposure, and administration of parenteral nutrition as the indication for PICC insertion are important predictors of PICC-associated CLABSI in hospitalized children. A careful assessment of these risk factors may be important for future success in preventing CLABSIs in hospitalized children with PICCs.
Central line–associated bloodstream infections (CLABSIs) account for significant morbidity, mortality, and financial costs [1, 2]. The Centers for Disease Control and Prevention (CDC) estimated that, in 2002, ∼250,000 CLABSIs occurred in US hospitals, accounting for >30,000 deaths [3]. National organizations and collaborative groups have successfully reduced CLABSI rates with use of evidence-based recommendations to improve catheter insertion and maintenance practices [4, 5]. Most prevention efforts have targeted intensive care unit (ICU) patients [6], despite reports that as many as 60% of CLABSIs occur in hospitalized patients outside the ICU [3, 7].
Peripherally inserted central venous catheters (PICCs) represent a large proportion of all central venous catheters (CVCs) inserted. PICCs have become increasingly popular in pediatrics in ICU and non-ICU settings. These catheters are nontunneled and noncuffed, and prolonged use may predispose them to bacterial colonization and biofilm formation [8, 9]. PICCs have been associated with a greater risk of infection, compared with cuffed and tunneled CVCs, in hospitalized patients [9]. PICCs are frequently inserted in patients outside the ICU for administration of antibiotics, chemotherapy, and parenteral nutrition. Few data exist regarding risk factors for CLABSIs in hospitalized children with PICCs, and less is known about infectious complications of PICCs in children hospitalized outside the ICU.
The objectives of our study were to fill 2 important knowledge gaps by identifying risk factors for CLABSIs in hospitalized children with PICCs and to determine factors associated with CLABSI in children with PICCs without ICU exposure.
MATERIALS AND METHODS
Setting and Participants
We identified children admitted to the Children's Center at The Johns Hopkins Hospital (Baltimore, MD) from 1 January 2003 through 31 December 2009 who had a PICC placed by the pediatric PICC Team. The PICC Team consisted of 2 full-time nurses dedicated to inserting PICCs for all patients in the Children's Center, except those in the neonatal ICU. CLABSI determinants in the neonatal ICU have been described elsewhere [10], and this population was excluded from the current study.
The PICC Team prospectively follows up children in whom they inserted a PICC. They maintain a database that includes indications for PICC insertion. The PICC Team reviews medical records of patients from whom PICCs are removed in the hospital to document complications, including infections, line infiltrations, phlebitis, thrombosis, leakage, occlusion, dislodgement, or breakage, and they contact home care companies or health care providers to determine catheter disposition and any complications in patients whose PICCs are removed after hospital discharge.
Data Collection
Patient characteristics were extracted from medical records and administrative databases. Indication for PICC placement, PICC characteristics, and dates of PICC insertion and removal were extracted from the PICC Team database. Laboratory databases were queried to identify all positive results of blood cultures performed from the date of PICC insertion to the date of PICC removal. Medical records were reviewed to determine whether positive blood culture results met criteria outlined by the CDC's National Healthcare Safety Network for a CLABSI [11]. This study was approved by the Johns Hopkins University School of Medicine Institutional Review Board, with a waiver of informed consent.
Definitions
PICCs were defined as peripherally inserted CVCs that terminated at or close to the heart or in one of the great vessels [10, 12–14]. CLABSIs were defined as laboratory-confirmed BSIs that were not secondary to an infection at another body site [11]. A PICC-associated CLABSI was defined as a CLABSI in a patient with a PICC that could not be attributed to another CVC [15]. Seven CLABSIs were excluded because the patients had a concurrent CVC and the CLABSI could not be attributed to one catheter. The time at risk for a CLABSI was defined as the PICC dwell time, which was calculated as the number of days from the date of PICC insertion to the date of CLABSI, date of line removal, or date of death.
International Classification of Disease, Ninth Revision, codes were collected for each patient's hospitalization and were categorized into underlying complex chronic medical conditions [16, 17]. For comparisons, patients with an underlying complex chronic condition were compared with all patients without that condition. To account for severity of illness, patients were categorized as those with PICU exposure, those requiring intensive care in the pediatric ICU (PICU) during their hospitalization, and those without PICU exposure who did not require intensive care during their hospitalization.
After exploratory data analysis, certain variables were categorized for analysis. Age was not evenly distributed and was categorized into quartiles. PICC dwell time was categorized into <21 days and ≥21 days, as described elsewhere [18]. To assess changes in risk because of a history of PICC insertion, we created a variable that indicated, for each catheter, whether the patient had a prior PICC removed within the 10 days before the insertion of the current PICC or whether the current PICC dwelt simultaneously with a second PICC in the same individual.
Statistical Analysis
Descriptive analyses of the entire cohort and the subgroup with no PICU exposure were performed. Kaplan-Meier survival estimates were calculated and compared for those with and without PICU exposure with use of the log rank test. Predictors of CLABSI were assessed in univariate analysis using Poisson regression model to estimate incidence rate ratios (IRRs). Because we had previously found an association between catheter dwell time and risk of CLABSI [10], we explored this relationship with use of Poisson regression with cubic splines, but this adjustment did not improve the model's fit to the data (based on Akaike's Information Criteria; results not shown); thus, we categorized PICC dwell time, as described elsewhere [19]. A multivariable Poisson model was designed. We included covariates determined a priori to be independent predictors of CLABSI and those with P <.10 in univariate analysis. We retained variables in the final model that were significant (2-tailed P <.05) or that were observed to have a confounding effect on the association between another predictor and risk of CLABSI. A confounding effect was defined as a change in a model coefficient by >10% after removal of a single variable from the model.
Data were maintained in Microsoft Access and were analyzed using Stata, version 10.0 (Stata).
RESULTS
During the study period, 2592 PICCs were placed in 1819 children (Table 1). The median age at the time of PICC insertion was 6 years (interquartile range [IQR], 0–13 years). More than half (55.5%) of the patients were male. The median length of hospital stay was 12 days (IQR, 6–31 days), and the median PICC dwell time was 13 days (IQR, 7–21 days). Eight hundred eight children were admitted to the PICU, and their median ICU length of stay was 11 days (IQR, 4–24 days). The majority of PICCs were inserted for administration of antibiotics (52.3%). Most children in the cohort had an underlying chronic complex condition, including cardiovascular (42.4%), respiratory (14.5%), neuromuscular (14.9%), and multiple (26.3%) conditions.
Table 1.
Demographic and Catheter Characteristics of 1819 Hospitalized Children with 2592 Peripherally Inserted Central Venous Catheters (PICCs)
| Demographic characteristic | Value |
| Age at PICC insertion, median (IQR), years | 6 (0–13) |
| Sex, no (%) Male Female |
1010 (55.5) 809 (44.5) |
| Race, no (%) White African American Asian Hispanic Other |
1024 (56.3) 579 (31.8) 162 (8.9) 43 (2.4) 11 (0.6) |
| Length of hospital stay, median (IQR), days | 12 (6–31) |
| PICU length of stay,a median (IQR), days | 11 (4–24) |
| Underlying conditions, b no (%) Neuromuscular Cardiovascular Respiratory Renal Gastrointestinal Hematologic and immunodeficiencies Metabolic Congenital & Genetic Malignancy None |
271 (14.9) 772 (42.4) 264 (14.5) 108 (5.9) 123 (6.8) 58 (3.2) 72 (4.0) 102 (5.6) 199 (10.9) 442 (24.3) |
| Catheter characteristic | |
| PICC dwell time, median (IQR), days | 13 (7–21) |
| Clinical indication for PICC placement, no (%) Antibiotics Total parenteral nutrition Chemotherapy Intravenous access |
1355 (52.3) 202 (7.8) 261 (10.1) 774 (29.9) |
| Site of PICC insertion, no (%) Basilic Cephalic Saphenous Facial Posterior auricular External jugular Brachial Other |
1,564 (60.4) 364 (14.1) 132 (5.1) 132 (5.1) 70 (2.7) 259 (10.0) 33 (1.3) 36 (1.4) |
| No of PICCs inserted during study period, no (%) 1 2 >2 |
1819 (70.2) 447 (17.3) 326 (12.6) |
Abbreviation: IQR, interquartile range.
For patients with PICU exposure.
Some patients had >1 underlying condition.
Of the 2592 PICCs studied, 1463 (56%) were placed in 1011 children without PICU exposure during their hospitalization. In this group, the median age was 9 years (IQR, 2–14 years). The median length of hospital stay was 7 days (IQR, 4–13 days), and the median PICC dwell time was 13 days (IQR, 7–20 days). The majority of PICCs were inserted for administration of antibiotics (71.3%), and most were placed in the upper extremity (80.7%).
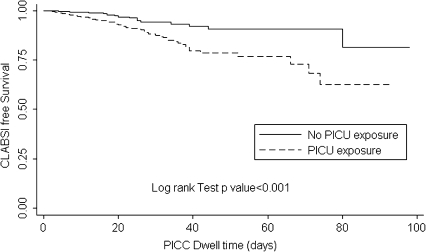
Overall, 116 CLABSIs occurred in 44,972 catheter-days. The CLABSI incidence rate (IR) in the entire cohort was 2.58 cases per 1000 catheter-days (95% confidence interval [CI], 2.07–3.00 cases per 1000 catheter-days). In patients without PICU exposure during their hospitalization, 37 CLABSIs occurred in 22,799 catheter-days (IR, 1.49 cases per 1000 catheter-days; 95% CI, 1.00–1.92 cases per 1000 catheter-days). The time to development of a CLABSI was shorter in patients with PICU exposure than in those without PICU exposure (P < .01 by log rank test) [Figure 1]. We estimate that 20.5% (95% CI, 16.6% –27.7%) of children with PICU exposure would have had a CLABSI if their PICC remained indwelling for 40 days.
Figure 1.
Time to PICC-Associated CLABSI (in days) in Hospitalized Children with and without PICU Exposure.
Among the CLABSIs, the most common organisms identified were coagulase-negative staphylococci (18 cases [13.6%]), followed by Candida parapsilosis (17 [12.9%]) [Table 2]. The majority of infections in children without PICU exposure were caused by gram-positive organisms (58%), whereas gram-negative organisms (38%) and fungi (35%) were responsible for most of the CLABSIs in children with PICU exposure.
Table 2.
Pathogens Causing Central Line–Associated Bloodstream Infection (CLABSI) in Hospitalized Children with Peripherally Inserted Central Catheters (PICCs)
| Organism | No. (%) of organisms (n = 132) |
| Gram-positive organism | 49 (37) |
| Coagulase-negative Staphylococcus species | 18 (13.6) |
| Enterococcus faecalis | 13 (9.8) |
| Staphylococcus aureus | 7 (5.3) |
| Methicillin-resistant Staphylococcus aureus | 3 (2.3) |
| Enterococcus faecium | 3 (2.3) |
| Vancomycin-resistant Enterococcus species | 3 (2.3) |
| Enterococcus raffinosus | 1 (.8) |
| Enterococcus agglomerans | 1 (.8) |
| Gram-negative organism | 50 (38) |
| Klebsiella pneumonia | 13 (9.8) |
| Enterobacter cloacae | 13 (9.8) |
| Pseudomonas aeruginosa | 4 (3.0) |
| Escherichia coli | 3 (2.3) |
| Klebsiella ozonae | 3 (2.3) |
| Klebsiella oxytoca | 3 (2.3) |
| Serratia marcescens | 4 (3.0) |
| Acinetobacter baumannii | 5 (3.8) |
| Sphingomonas paucimobilis | 1 (0.8) |
| Stenotrophomonas maltophilia | 1 (0.8) |
| Fungus | 33 (25) |
| Candida parapsilosis | 17 (12.9) |
| Candida albicans | 10 (7.6) |
| Candida glabrata | 3 (2.3) |
| Candida krusei | 1 (0.8) |
| Candida lusitaniae | 1 (0.8) |
| Candida tropicalis | 1 (0.8) |
NOTE. There were 116 events (CLABSIs); 16 events were polymicrobial, and 99 events were monomicrobial.
Univariate analysis of potential risk factors for CLABSI in the entire cohort and children without PICU exposure are displayed in Table 3. For the entire cohort, the incidence of CLABSI was highest among children <1 year of age. Patients with a PICC dwell time of ≥21 days had a higher risk of CLABSI, compared with those with a dwell time <21 days. Other risk factors included lower extremity as site of insertion and administration of parenteral nutrition as indication for PICC insertion. In patients without PICU exposure, risk factors for CLABSI included younger age, underlying malignancy, and metabolic conditions (IRR, 3.11 [95% CI, 1.47–6.70] and 6.20 [95% CI, 2.41–15.91], respectively), lines inserted in the lower extremity (IRR, 3.08; 95% CI, 1.09–8.76), and history of PICC complicated by a CLABSI (IRR, 9.14; 95% CI, 2.81–29.75). Longer PICC dwell time was not associated with increased risk for CLABSI in this subpopulation.
Table 3.
Univariate Analysis for Risk Factors Associated with Central Line–Associated Bloodstream Infections (CLABSIs) in Hospitalized Children with Peripherally Inserted Central venous Catheters (PICCs)
| Complete cohort |
Subcohort without PICU exposure |
|||
| IRR (95% CI) | P | IRR (95% CI) | P | |
| Age ≤1 year >1 to 7 years >7 to 13 years >13 years |
1 0.63 (.38–1.04) 0.38 (.22–.66) 0.58 (.36, .95) |
.07 <.01 .03 |
1 0.75 (.31–1.78) 0.23 (.08–.71) 0.69 (.31–1.53) |
.51 .01 .36 |
| Sex Male Female |
1 0.96 (.67–1.38) |
.83 |
1 0.65 (.34–1.27) |
.21 |
| PICC dwell timea <21 days ≥21 days |
1 1.61 (1.10–2.36) |
.01 | 1 1.50 (.75–2.98) |
.25 |
| Clinical indication for PICC insertion Intravenous access Antibiotics Total parenteral nutrition Chemotherapy |
1 0.33 (.21–.53) 1.68 (.99–2.80) 1.17 (.69– 1.99) |
<.001 .05 .55 |
1 0.26 (.11–.59) 1.66 (.68–4.10) 0.58 (.18–1.90) |
<.01 .27 .37 |
| Complex Chronic Conditionsb None Neuromuscular Cardiovascular Respiratory Renal Gastrointestinal Hematologic, immunodeficiencies Metabolic Congenital and genetic Malignancy |
1 1.17 (.72–1.89) 1.41 (.98–2.03) 0.28 (.14–.58) 1.68 (.90–3.13) 0.95 (.46–1.94) 1.33 (.59–3.02) 3.11 (1.75–5.54) 2.07 (1.16–3.69) 1.54 (.92–2.58) |
.52 .06 <.01 .10 .88 .49 <.001 .01 .10 |
1.79 (.75–4.28) 1.86 (.97–3.54) 0.13 (.03–.54) 1.76 (.54–5.72) 1.41 (.50–3.97) 1.36 (.33–5.66) 6.20 (2.41–15.91) 1.44 (.35–5.97) 3.11 (1.47–6.70) |
.19 .06 <.01 .35 .52 .67 <.001 .62 <.01 |
| Site of PICC Insertion Upper extremity Lower extremity Head |
1 2.36 (1.36–4.08) 0.73 (.42–1.27) |
<.01 .27 |
1 3.08 (1.09–8.76) 0.51 (.16–1.68) |
.03 .27 |
| Previous or concurrent PICC None Removed <10 days before insertion of new PICC Removed ≥10 days before insertion of new PICC Simultaneous PICCc |
1 1.87 (1.11–3.17) 1.03 (.64–1.66) 0.46 (.06–3.30) |
.02 .90 .44 |
1 1.91 (.67–5.50) 0.76 (.33–1.74) |
.22 .51 |
| Previous PICC complicated by CLABSI No Yes |
1 4.27 (2.23–8.17) |
<.001 |
1 9.14 (2.81–29.75) |
<.001 |
| PICU exposure No Yes |
1 2.77 (1.87–4.09) |
<.001 |
||
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; PICU, pediatric intensive care unit.
PICC Dwell time is defined as days from PICC insertion to date of CLABSI, date of line removal, or date of death.
Each patient with an underlying complex condition is compared with those without that specific condition.
There were 13 simultaneous lines in the subcohort without PICU exposure, and there were no events in this group.
Table 4 shows the multivariable analysis with the entire cohort. After adjusting for other variables, patients with a PICC dwell time of ≥21 days had a higher risk of CLABSI, as opposed to those with a catheter dwell time of <21 days (IRR, 1.53; 95% CI, 1.05–2.26). Patients that had a PICC inserted for administration of parenteral nutrition had a higher risk of CLABSI, compared with those that had a PICC placed for intravenous access (IRR, 2.24; 95% CI, 1.31–3.84). Patients with PICU exposure had an 80% increased risk of developing a CLABSI, compared with those with no PICU exposure, after adjusting for other confounders (IRR, 1.80; 95% CI, 1.18–2.75). Patients with an underlying metabolic condition were at increased risk for CLABSI (IRR, 2.07; 95% CI, 1.14–3.74). Patients with an underlying respiratory condition were at lower risk for CLABSI, but this association may be confounded by a high antibiotic use in this population. Having a previous PICC complicated by CLABSI was also an independent risk factor for CLABSI. After adjusting for other variables, there was still a trend that age >1 year was associated with decreased risk for CLABSI; however, it was no longer statistically significant. Similarly, PICCs inserted in the lower extremity were no longer associated with increased risk for CLABSI in the adjusted analysis, nor were PICCs removed <10 days before insertion of the present PICC.
Table 4.
Multivariable Analysis for Risk Factors for Central Line–Associated Bloodstream Infections (CLABSIs) in Hospitalized Children with Peripherally Inserted Central Venous Catheters (PICCs)
| Variable | Multivariable analysis of entire cohort |
|
| IRR (95% CI) | P | |
| Age ≤1 year >1 to 7 years >7 to 13 years >13 years |
1 0.64 (.38–1.07) 0.44 (.25–.80) 0.58 (.36–1.02) |
.09 <.01 .06 |
| PICC dwell timea <21 days ≥21 days |
1 1.53 (1.05–2.26) |
.03 |
| Clinical indication for PICC insertion Intravenous access Antibiotics Total parenteral nutrition Chemotherapy |
1 0.56 (.34–.93) 2.24 (1.31–3.84) 1.33 (.78–2.28) |
.03 <.01 .30 |
| Complex chronic conditionsb None Respiratory Metabolic |
1 0.49 (.23–1.02) 2.07 (1.14–3.74) |
.06 .02 |
| Site of PICC insertion Upper extremity Lower extremity Head |
1 1.39 (.77–2.51) 0.57 (.33–1.00) |
.28 .05 |
| Previous or concurrent PICC None Removed <10 days before insertion of new PICC Removed ≥10 days before insertion of new PICC Simultaneous PICCc |
1 1.06 (.58–1.94) 1.31 (.79–2.16) 0.26 (.04–1.89) |
.84 .29 .18 |
| Previous PICC complicated by CLABSI No Yes |
1 2.48 (1.18–5.25) |
.02 |
| PICU exposure No Yes |
1 1.80 (1.18–2.75) |
.01 |
Abbreviations: CI, confidence interval; IRR, incidence rate ratio; PICU, pediatric intensive care unit.
PICC Dwell time is defined as days from PICC insertion to date of CLABSI, date of line removal, or date of death.
Each patient with an underlying complex condition is compared with those without that specific condition.
There were 13 simultaneous lines in the subcohort without PICU exposure, and there were no events in this group.
DISCUSSION
We report results from a large cohort of hospitalized children with PICCs. We found that prolonged PICC dwell time, PICU exposure during the current hospitalization, parenteral nutrition as the indication for PICC insertion, and an underlying chronic metabolic condition were among independent predictors for CLABSI. National efforts and collaborations focusing on ICU patients have successfully reduced CLABSI rates [20, 21]. In our cohort, 32% of CLABSIs occurred in patients without PICU exposure, suggesting that efforts in non-ICU patients may have similar success.
In our population, the PICC-associated CLABSI IR (2.58 cases per 1,000 catheter-days) was consistent with pooled IRs in studies of adults and neonates (1.3–2.6 cases per 1000 catheter-days) [9]. The CLABSI IR was lower among those without PICU exposure (IR, 1.49 cases per 1000 catheter days; 95% CI, 1.00–1.92 ); consistent with previous studies that found placement of CVCs in the ICU, a marker of ICU exposure, was a risk factor for pediatric CLABSI [22]. Children in the ICU have a greater severity of illness and their catheters are more frequently accessed—just 2 factors that may contribute to higher CLABSI rates among those with PICU exposure.
Our data confirm previous findings that prolonged catheter dwell times are associated with an increased risk for CLABSI [10, 18, 23–28]. We found that PICCs in place for ≥21 days were 1.5 times more likely to be complicated by CLABSIs, compared with PICCs placed for a lesser duration. CLABSI occurred in 74 (3.8%) of 1918 PICCs in place for <21 days, and 42 (6.2%) of 674 PICCs in place for ≥21 days. Prolonged catheter dwell time might be predictive of CLABSI in noncuffed and nontunneled catheters, such as PICCs, which may be more susceptible to catheter colonization and biofilm formation, both of which are thought to play a central role in the pathogenesis of CLABSI [18, 29]. To further assess how the risk of CLABSI changes over catheter dwell time, we modeled time as a continuous variable to determine a linear relationship and used a more flexible model with cubic splines. Neither model fit the data as well as the simpler model using a binary variable as reported (data not shown). Even in this large cohort, assessing this relationship is challenging, because few CLABSIs occur at longer catheter dwell times; thus, any single event can have a large impact on the hazard at a given time. More research is needed in this area to build on our understanding of how the hazard of infection may change over time. Our results suggested a difference in the risk of CLABSI among patients with and without ICU exposure in the time-to-event analysis. The survival curves (Figure 1) show that CLABSI would be expected to develop in 1 of 5 children with ICU exposure who had a PICC inserted for 40 days. More frequent catheter entries in the ICU may shorten the time to PICC contamination and CLABSI. Further studies are needed to explore PICC dwell time and the risk for CLABSI, because this association may be influenced by many other variables, including the patient population, catheter type, frequency of catheter access, catheter maintenance practices, and indication for catheter insertion.
Consistent with previous studies demonstrating a strong association between receipt of parenteral nutrition and CLABSIs [30, 31], our data showed that administration of parenteral nutrition as an indication for PICC insertion was an independent risk factor for CLABSI. Potential mechanisms hypothesized to increase CLABSI risk in this group include lipid contamination, glycemic fluctuations, and alterations of gut mucosal integrity [22, 31]. Of interest, patients in our cohort with a chronic gastrointestinal condition were not at increased risk for CLABSI. This may suggest that compromised gut integrity in patients with chronic gastrointestinal conditions may not be the primary determinant of CLABSI in this population. If so, further studies and prevention strategies should focus on optimizing safe delivery of parenteral nutrition.
National CLABSI prevention guidelines recommend avoiding the femoral site for CVCs [23, 32–34]. Few data support or refute this recommendation in children. PICCs are not inserted in the femoral vein, but they are inserted in the lower extremities. In univariate analysis, lower extremity lines appeared to be associated with an increased risk for CLABSI. However, after adjusting for other important variables, insertion site was no longer a predictor for CLABSI. Younger children, especially those <1 year of age, were more likely to have CLABSI and were more likely to have lower extremity PICCs (data not shown). We did not find evidence to suggest that insertion site in the lower extremity is an independent predictor for CLABSI.
Several limitations should be considered when interpreting our findings. First, a small percentage of patients (<5%) were lost to follow-up, mostly because of transfer to other health care facilities. However, for most catheters, prospective follow-up by our PICC team reduced missing data and captured entire PICC dwell time and events, including 26 CLABSIs that occurred at home after hospital discharge. Recognizing that we still could have missed events that occurred after hospital discharge, we repeated the analysis, censoring time at risk with use of hospital discharge date, and our findings remained the same (data not shown). Second, we excluded CLABSIs that occurred in patients that had another simultaneous CVC in place at the time of the event, because we were unable to attribute the infection to the PICC. We attempted to account for simultaneous PICCs or recently removed PICC, because we recognize the impact that this may have on infection risk, especially if the previous PICC was complicated by CLABSI. Third, we were underpowered to perform a multivariable analysis of the subcohort without PICU exposure, because of fewer events. Several variables, including prolonged PICC dwell time and parenteral nutrition as the indication for PICC insertion, were significant predictors of CLABSI in the overall cohort but had non–statistically significant associations with CLABSI in the subcohort. This may have occurred because the underlying epidemiology of the cohorts was different or because the subcohort analysis was underpowered to detect a difference. Finally, despite this large cohort, because this is a single-institution study, our findings may not be generalizable to other institutions.
We have identified several important predictors for PICC-associated CLABSI in hospitalized children. Future studies should validate these predictors and determine how prevention strategies can target groups at high risk (eg, children in the PICU with PICCs in place for >21 days). Reducing CLABSIs is a Joint Commission 2010 National Patient Safety Goal [35], and many institutions, including ours, are standardizing catheter insertion and maintenance practices in all pediatric units. In addition to standardizing insertion and maintenance practices, clinicians need to customize how they think about the individual patient's risk of CLABSI. As CLABSI rates in ICUs decrease nationally and the burden of CLABSIs outside ICUs become apparent, there is an urgent need for future studies to identify specific patient characteristics and practices on which to focus prevention strategies.
Acknowledgments
We thank Drs Trish Perl and Pranita Tamma, for their support and comments on the manuscript; John Shepard, for assistance with data management; Alicia Budd, for consultation on applying CDC definitions; and members of The JHH Department of Hospital Epidemiology and Infection Control and the Pediatric PICC Team, for their support of this study.
Financial support. This work was supported by National Institute of Allergy and Infectious Disease, National Institutes of Health (1 K23 AI081752-01 to AM).
Potential conflicts of interest. A.M. receives grant support from Sage Products and BioMerieux. All other authors: no conflicts.
References
- 1.Blot SI, Depuydt P, Annemans L, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41:1591–8. doi: 10.1086/497833. [DOI] [PubMed] [Google Scholar]
- 2.Saint S, Veenstra DL, Lipsky BA. The clinical and economic consequences of nosocomial central venous catheter-related infection: are antimicrobial catheters useful? Infect Control Hosp Epidemiol. 2000;21:375–80. doi: 10.1086/501776. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Edwards JR, Richards CL, Jr, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKee C, Berkowitz I, Cosgrove SE, et al. Reduction of catheter-associated bloodstream infections in pediatric patients: experimentation and reality. Pediatr Crit Care Med. 2008;9:40–6. doi: 10.1097/01.PCC.0000299821.46193.A3. [DOI] [PubMed] [Google Scholar]
- 5.Marschall J, Mermel LA, Classen D, et al. Strategies to prevent central line-associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S22–30. doi: 10.1086/591059. [DOI] [PubMed] [Google Scholar]
- 6.Trick WE, Miranda J, Evans AT, Charles-Damte M, Reilly BM, Clarke P. Prospective cohort study of central venous catheters among internal medicine ward patients. Am J Infect Control. 2006;34:636–41. doi: 10.1016/j.ajic.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Kallen AJ, Patel PR, O'Grady NP. Preventing catheter-related bloodstream infections outside the intensive care unit: expanding prevention to new settings. Clin Infect Dis. 2010;51:335–41. doi: 10.1086/653942. [DOI] [PubMed] [Google Scholar]
- 8.Abedin S, Kapoor G. Peripherally inserted central venous catheters are a good option for prolonged venous access in children with cancer. Pediatr Blood Cancer. 2008;51:251–5. doi: 10.1002/pbc.21344. [DOI] [PubMed] [Google Scholar]
- 9.Safdar N, Maki DG. Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest. 2005;128:489–95. doi: 10.1378/chest.128.2.489. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta A, Lehmann C, Diener-West M, Perl TM, Milstone AM. Catheter duration and risk of CLA-BSI in neonates with PICCs. Pediatrics. 2010;125:648–53. doi: 10.1542/peds.2009-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Central Line-Associated Bloodstream Infection (CLABSI) Event. Available at: http://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf. Accessed 3 December 2010. [Google Scholar]
- 12.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linck DA, Donze A, Hamvas A. Neonatal peripherally inserted central catheter team. Evolution and outcomes of a bedside-nurse-designed program. Adv Neonatal Care. 2007;7:22–9. doi: 10.1097/00149525-200702000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Mermel LA. Prevention of intravascular catheter-related infections. Ann Intern Med. 2000;132:391–402. doi: 10.7326/0003-4819-132-5-200003070-00009. [DOI] [PubMed] [Google Scholar]
- 15.de Jonge RC, Polderman KH, Gemke RJ. Central venous catheter use in the pediatric patient: mechanical and infectious complications. Pediatr Crit Care Med. 2005;6:329–39. doi: 10.1097/01.PCC.0000161074.94315.0A. [DOI] [PubMed] [Google Scholar]
- 16.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106:205–9. [PubMed] [Google Scholar]
- 17.Zaoutis T, Localio AR, Leckerman K, Saddlemire S, Bertoch D, Keren R. Prolonged intravenous therapy versus early transition to oral antimicrobial therapy for acute osteomyelitis in children. Pediatrics. 2009;123:636–42. doi: 10.1542/peds.2008-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chathas MK, Paton JB, Fisher DE. Percutaneous central venous catheterization. Three years' experience in a neonatal intensive care unit. Am J Dis Child. 1990;144:1246–50. doi: 10.1001/archpedi.1990.02150350078030. [DOI] [PubMed] [Google Scholar]
- 19.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–23. 10.1109/TAC.1974.1100705. [Google Scholar]
- 20.Pronovost P. Interventions to decrease catheter-related bloodstream infections in the ICU: the Keystone Intensive Care Unit Project. Am J Infect Control. 2008;36(Suppl 171):e1–5. doi: 10.1016/j.ajic.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Miller MR, Griswold M, Harris JM, 2nd, et al. Decreasing PICU catheter-associated bloodstream infections: NACHRI's quality transformation efforts. Pediatrics. 2010;125:206–13. doi: 10.1542/peds.2009-1382. [DOI] [PubMed] [Google Scholar]
- 22.Wylie MC, Graham DA, Potter-Bynoe G, et al. Risk factors for central line-associated bloodstream infection in pediatric intensive care units. Infect Control Hosp Epidemiol. 2010;31:1049–56. doi: 10.1086/656246. [DOI] [PubMed] [Google Scholar]
- 23.Mermel LA, McCormick RD, Springman SR, Maki DG. The pathogenesis and epidemiology of catheter-related infection with pulmonary artery Swan-Ganz catheters: a prospective study utilizing molecular subtyping. Am J Med. 1991;91:197S–205S. doi: 10.1016/0002-9343(91)90369-9. [DOI] [PubMed] [Google Scholar]
- 24.McLaws ML, Berry G. Nonuniform risk of bloodstream infection with increasing central venous catheter-days. Infect Control Hosp Epidemiol. 2005;26:715–9. doi: 10.1086/502608. [DOI] [PubMed] [Google Scholar]
- 25.Odetola FO, Moler FW, Dechert RE, VanDerElzen K, Chenoweth C. Nosocomial catheter-related bloodstream infections in a pediatric intensive care unit: risk and rates associated with various intravascular technologies. Pediatr Crit Care Med. 2003;4:432–6. doi: 10.1097/01.PCC.0000090286.24613.40. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Teresa MA, Casado-Flores J, Delgado Dominguez MA, et al. Infectious complications of percutaneous central venous catheterization in pediatric patients: a Spanish multicenter study. Intensive Care Med. 2007;33:466–76. doi: 10.1007/s00134-006-0508-8. [DOI] [PubMed] [Google Scholar]
- 27.Goes-Silva E, Abreu TF, Frota AC, Pessoa-Silva CL, Cunha AJ, Hofer CB. Use of peripherally inserted central catheters to prevent catheter-associated bloodstream infection in children. Infect Control Hosp Epidemiol. 2009;30:1024–6. doi: 10.1086/606040. [DOI] [PubMed] [Google Scholar]
- 28.Milstone AM, Sengupta A. Do prolonged peripherally inserted central venous catheter dwell times increase the risk of bloodstream infection? Infect Control Hosp Epidemiol. 2010;31:1184–7. doi: 10.1086/656589. [DOI] [PubMed] [Google Scholar]
- 29.Ullman RF, Gurevich I, Schoch PE, Cunha BA. Colonization and bacteremia related to duration of triple-lumen intravascular catheter placement. Am J Infect Control. 1990;18:201–7. doi: 10.1016/0196-6553(90)90185-u. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Sommerfeld E, Snyder G, Rossi TM, Lebenthal E. Catheter-related complications in 35 children and adolescents with gastrointestinal disease on home parenteral nutrition. JPEN J Parenter Enteral Nutr. 1990;14:148–51. doi: 10.1177/0148607190014002148. [DOI] [PubMed] [Google Scholar]
- 31.Christensen ML, Hancock ML, Gattuso J, et al. Parenteral nutrition associated with increased infection rate in children with cancer. Cancer. 1993;72:2732–8. doi: 10.1002/1097-0142(19931101)72:9<2732::aid-cncr2820720934>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.O'Grady NP, Alexander M, Dellinger EP, et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U.S. Pediatrics. 2002;110:e51. doi: 10.1542/peds.110.5.e51. [DOI] [PubMed] [Google Scholar]
- 33.Goetz AM, Wagener MM, Miller JM, Muder RR. Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19:842–5. doi: 10.1086/647742. [DOI] [PubMed] [Google Scholar]
- 34.Merrer J, De Jonghe B, Golliot F, et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700–7. doi: 10.1001/jama.286.6.700. [DOI] [PubMed] [Google Scholar]
- 35.Hospitals: 2010 National patient safety goals. Available at: http://www.jointcommission.org/assets/1/18/NPSG_Chapter_Outline_FINAL_HAP_2010.pdf. Accessed 3 December 2010. [Google Scholar]