Summary
The most widely held explanation for the efficacy of local radiotherapy (RT) is based on direct cytotoxicity to cancer cells through the induction of lethal DNA damage. Recent studies have demonstrated that local ablative radiation of established tumors can lead to increased T cell priming and T cell dependent tumor regression, but the underlying mechanism remains unclear. Here, we describe an essential role for type I interferon (IFN) in local tumor control mediated by local RT. We show that ablative RT increases intratumoral production of IFNβ and, more surprisingly, the anti-tumor effect of RT is abolished in type I IFN non-responsive hosts. Furthermore, the major target of RT-induced type I IFN is the hematopoietic compartment. RT drastically enhances the cross-priming capacity of tumor infiltrating DC from wild-type mice but not type I IFN receptor deficient mice. The enhanced cross-priming ability of tumor infiltrating DCs after RT was dependent on autocrine production of type I IFNs. Using adenoviral-mediated expression of IFNβ, we demonstrate that delivery of exogenous IFNβ into the tumor tissue in the absence of RT is also sufficient to selectively expand antigen-specific T cells leading to complete tumor regression. Our study reveals that local high-dose RT can trigger production of type I IFN that initiates a cascading innate and adaptive immune attack on the tumor.
Keywords: ablative radiation, interferon; CTL; immunotherapy; radiotherapy
INTRODUCTION
The current mechanistic explanation for the clinical efficacy of local RT centers on the induction of lethal DNA damage directly to tumor cells or tumor-associated stroma. Although RT has traditionally been viewed as immunosuppressive due to the inherent sensitivity of lymphocytes to radiation-induced (1), RT has been also demonstrated to enhance tumor-specific immune responses (2–4). Ionizing radiation increases production of inflammatory cytokines such as tumor necrosis factor (TNF), interleukin (IL)-1α, and IL-6 by human tumor cells in vitro (5, 6). Radiation can also modulate the peptide repertoire and enhance MHC class I expression by tumor cells(7), and alter their phenotype resulting in heightened susceptibility to T-cell killing (8). Proper localized RT given at appropriate doses and scheduling may tip the balance in favor of anti-tumor immunity both through endogenous priming mechanisms and in combination with immunotherapy (2, 3, 9). We have shown that the therapeutic effect of ablative RT depends largely on CD8+ T cells and that RT increases T cell priming (3). However, the question remains as to which immunological components link activation of innate immunity by RT with increased cross-priming and production of an anti-tumor T cell response.
Type I interferons are a family of cytokines best known for their function in the anti-viral response. In the tumor system, the role of type I IFNs is less well characterized, however, evidence suggests that type I IFNs may play some role to control tumor growth. Specifically, an early study utilizing an IFN-α/β neutralizing anti-serum showed that type I IFN may limit the growth of transplantable tumors(10). Furthermore, the complete absence of type I IFN signaling results in more rapid tumor growth and increased mortality in several tumor models (11). More recently, it has been shown that endogenous type I IFN production plays a critical role in tumor immune-editing (12, 13). Little is known about the role of type I IFNs in the therapeutic treatment of established cancers after RT for immune responses (14). Recent studies suggest that STAT1 dependent genes are upregulated following local delivery of RT, which has been associated with a radioresistant phenotype that correlates with increased tumor aggressiveness and metastasis raising (15–17). Therefore, we sought to determine the role of type I IFN in the treatment of established tumors with ablative RT and how it could potentially influence the generation of T cell responses. Here we describe an essential role for type I IFNs in tumor growth control mediated by “ablative” RT.
MATERIALS AND METHODS
Mice
B6/IFNAR1 KO mice were generously provided by Anita Chong at the University of Chicago. The source of other mice and cell lines were previously described (3). For all experiments, mice were between the ages of 6–16 weeks of age, bred under SPF conditions and used in accordance to the animal experimental guidelines set by the Institute of Animal Care and Use Committee.
Generation of bone marrow chimeras
Wild-type (WT) or IFNAR KO mice were lethally irradiated with a single dose of 1000 rads. The next day irradiated mice were adoptively transferred i.v. with 2–3×106 donor bone marrow cells. Mice were maintained on sulfamethoxazole and trimethoprim (Bactrim) antibiotics diluted in drinking water for 4 weeks after reconstitution. Mice were injected with tumor cells 5–6 weeks post reconstitution.
Adoptive transfer of T cells
A total of 2 × 106 T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) and transferred as previously described (18, 19). For reconstitution of RAG KO recipients, T cells were sorted using the Pan T Cell Isolation Kit and automated Magnetic Cell Sorting (autoMACS™ Miltenyi Biotec).
Flow cytometric analysis
Single cell suspensions of cells were isolated as before (3) and incubated with anti-CD16/32 (anti-Fc III/II receptor, clone 2.4G2) for 20 min at room temperature and then subsequently stained with conjugated antibodies: anti-CD45.2 (clone 104), anti-CD90.1 (anti-Thy-1.1, clone OX-7), anti-CD8a (clone 53-6.7), anti-CD11c (clone HL3), anti-CD11b (clone M1/70), anti-Ly6C (clone AL-21), anti-I-A/I-E (clone M5/114.15.2), anti-CD80 (B7-1, clone 16-10A1), anti-CD86 (B7-2, clone GL1), anti-CCR7 (clone 4B12), or anti-CD4 (clone GK1.5). All purified and fluorescently labeled monoclonal antibodies were purchased from BD Pharmingen. Samples were analyzed on a FACSCanto flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (TreeStar, Inc.).
Tumor growth and treatments
Cultured cancer cells were trypsinized, washed with media, and injected subcutaneously on the back. Tumor size was determined at 3–4 day intervals. Tumor volumes were measured along three orthogonal axes (a, b, and c) and calculated as tumor volume = abc/2. The tumor nodules were inoculated with indicated amount of Ad-IFN-β or Ad-null virus intratumorally. For antibody mediated cell depletion, 200 µg/mouse anti-CD4 or anti-CD8 (YTS.169.4.2) antibody was given to mice i.p. on day 9, 11, and 13 after primary tumor inoculation. Mice received local irradiation as described previously (3). (Gy = Gray = 100 rads)
IFN determination
For RT-PCR, tumors were harvested at indicated time points after 20 Gy local RT. Real-time PCR was conducted on cDNA prepared from DNase I-treated RNA extracted from whole tumor fragments or single cell suspensions sorted on a BD FACSAria™ cell sorter into CD45.2+ and CD45.2− populations and then the CD45+ fraction was further subdivided into two sort gates based on CD11c staining. The primers and probes used are as follows. For IFN-β: Forward, 5'- ATG AGT GGT GGT TGC AGG C-3', Reverse, 5'- TGA CCT TTC AAA TGC AGT AGA TTC A-3'. For GAPDH: Forward, 5'-TTC ACC ACC ATG GAG AAG GC-3', Reverse, 5'-GGC ATG GAC TGT GGT CAT GA-3'. Reactions were run on the ABI/Prism 7300 (Applied Biosystems), in a final volume of 25 µl with 2.5 uM of the forward and reverse primers using 2x Taqman Master Mix (Applied Biosystems) containing AmpliTaq Gold polymerase. Cycling conditions were a single denaturing step at 95°C for 15 min followed by 45 cycles of 94°C for 15 s and 60°C for 1 min. Analysis of Infb1 and Gapdh gene expression were performed with a standard curve, and then normalized to sample Gapdh. The standard curves had R2 values >0.99. For ELISA, tumors were harvested and weighed at the indicated time points after local irradiation and homogenized on ice in 1X phosphate-buffered saline (PBS) plus 1X Halt Protease Inhibitor Cocktail (Thermo Fisher Scientific). Supernatants were collected and IFNβ was detected using VeriKine™ Mouse IFN-Beta ELISA Kit (PBL IFN Source) according to the manufacturer’s directions.
In vivo specific lysis assay
Mice bearing B16-SIY were transferred i.v. with equal numbers of CFSE labeled donor splenocytes pulsed with SIY peptide (1ug/ml) and OT-I peptide (1ug/ml). In vivo specific lysis assay was performed and calculated as before (18, 19).
DC cross-priming assay and cytokine detection
After tumors were established, mice received local RT (20 Gy) on the tumor and 3 days later tumors were harvested for DC purification. iIsolated cells were used for DC purification using a CD11c+ magnetic bead kit and automated Magnetic Cell Sorting (autoMACS™ Miltenyi Biotec). For in vitro culture, 1×105 DCs were plated with 2×105 naïve 2C cells with or without the addition of exogenous SIY peptide (1ug/ml), and supernatants harvested after 3 days for analysis via cytometric bead array (CBA) (BD Biosciences) according to manufacturer’s directions. T cell proliferation was assessed following similar in vitro culture conditions by pulsing with 3H-Thymidine at 72 hrs and harvesting for analysis at 96 hrs and plates were read on a liquid scintillation counter.
The generation of adenovirus-expressing IFN-β (Ad-IFN--β)
To construct recombinant Adenovirus-mIFN-β, murine IFN-β cDNA was amplified by PCR and cloned into the NotI/EcoRV sites of pAdenoVator-CMV5(CuO) under CMV5 promoter. pAdenoVator-mIFN-β was linearized by PacI digestion and electroporated into electrocompetent cells BJ5183 at 2.5kV for recombination with a backbone vector containing adenoviral genome. The hybrid cosmids were selected on Kanamycin LB agar plate. PacI digestion was used to further identify recombinant cosmid containing insert mIFN-β. The Ad-mIFN-β DNA was linearized by PacI digestion, and the mixture of PacI digestion without further purification was transfected into 293 cells for recombinant adenovirus production. The Adenovirus-mIFN-β is referred to as Ad-IFN-β.
Statistical analysis
Statistics were done using an unpaired student two-tailed t test. Error bars represent standard deviations.
RESULTS
Local radiation increases tumor infiltration by hematopoietic cells that give rise to DCs
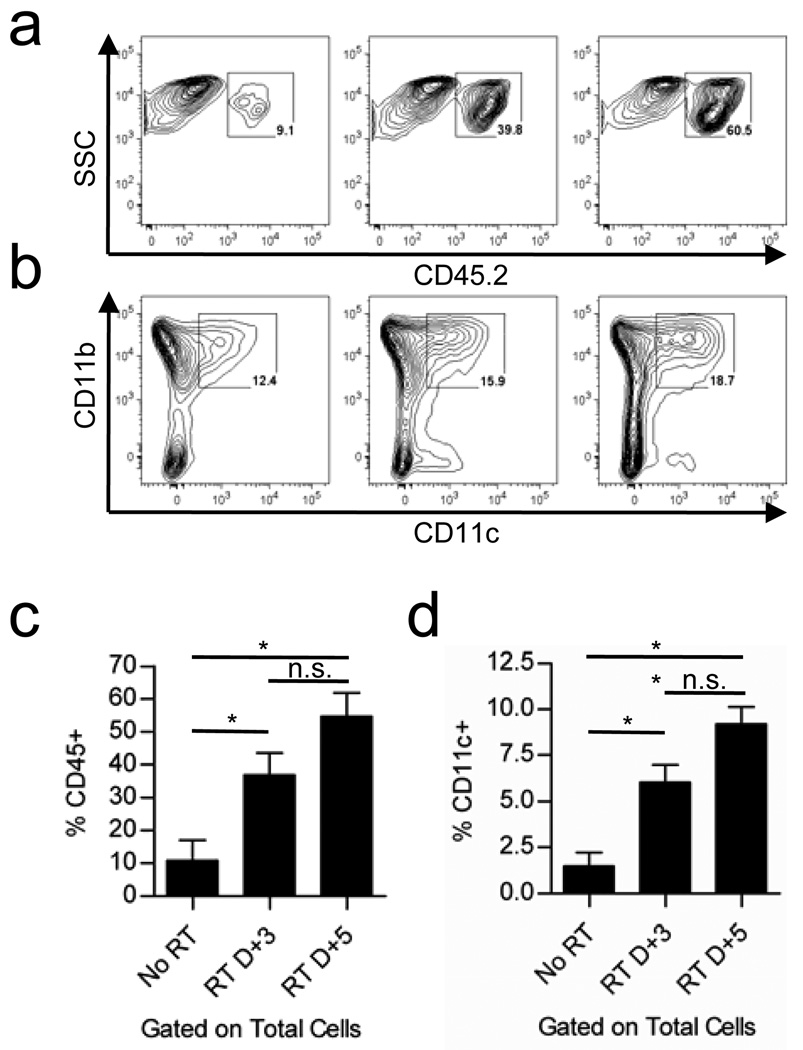
We sought to determine whether local radiation of established B16 tumors results in any quantitative differences in the populations of infiltrating immune cells. In fact, there was a time-dependent increase in the infiltration of CD45+ hematopoietic cells into the tumor in the first few days following local radiation (Figure 1a, c). CD11b+ myeloid cells of mixed phenotype including monocytes, macrophages, DC, and myeloid derived suppressor cells (MDSC) dominate the overall population of CD45+ cells. Gene expression array analysis comparing untreated tumors and those receiving local RT revealed increased production of many chemokines responsible for mediating chemoattraction of immature monocytes, macrophages, and DC suggesting that the increased frequency of CD45+/CD11b+ cells was likely due to enhanced recruitment (data not shown). Of relevance to the induction of T cell responses, the relative proportion of CD11c+ dendritic cells (DCs) was only slightly increased among the CD45+ fraction of cells isolated (Figure 1b), however, the overall representation of CD11c+ cells was consistently increased almost 3-fold and 4.5-fold among total cells isolated from the tumors on day 3 and 5 post-RT respectively (Figure 1d). Further analysis of the CD11c+ fraction revealed that these cells express markers of classical monocyte-derived DCs, also termed “inflammatory DCs”, including high expression of CD11b, Ly-6C, and MHC class II and lacking expression of B220, Langerin, and CD103 (data not shown).
Figure 1. Local Radiation Therapy increases tumor infiltration by innate immune cells.
a) Flow cytometric analysis of CD45+ hematopoietic cells infiltrating untreated B16F1 tumors or tumors treated with 25 Gy local RT at 3 and 5 days post-RT. b) Surface staining of infiltrating CD45+ cells for the myeloid marker CD11b and DC marker CD11c. Gated percentages represent the proportion of CD11b+CD11c+ cells in the parental CD45+ gate (shown in a). c) Bar graph of data in (a) (*p=0.0432 for No RT vs. RT D+3, p=0.0101 for No RT vs. RT D+5). d) Bar graph of the data in (b) (*p=0.0182, **p=0.0031). Values were calculated by the formula (%CD11c+ × %CD45+) for each sample individually and then averaged for the three samples in each group. Error bars represent the standard deviation among the three animals in each group (n.s. = not significant).
Local radiation generates infiltrating DC with enhanced functional capacity
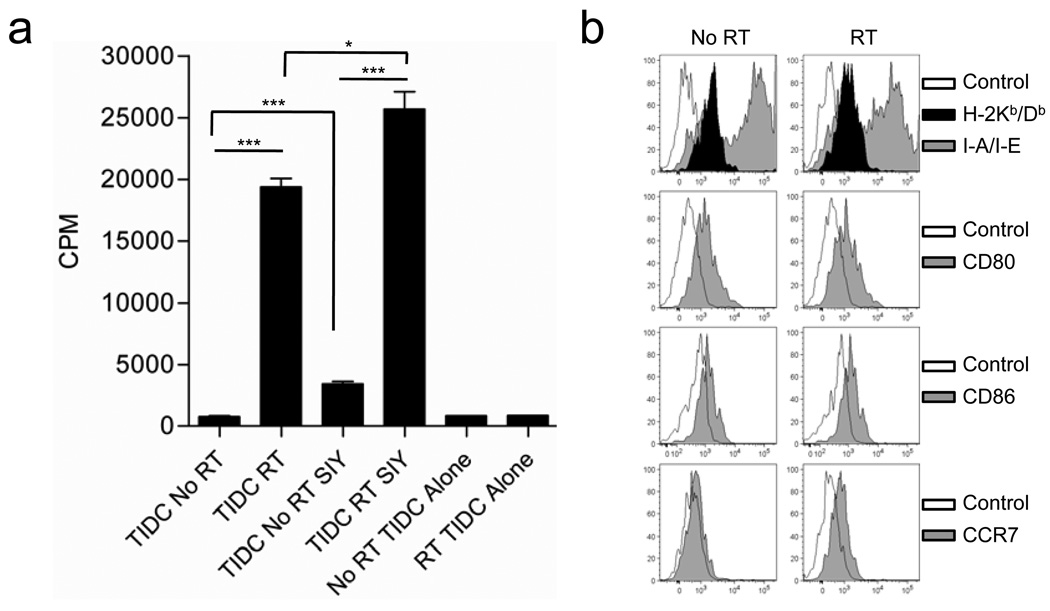
To facilitate monitoring of tumor antigen cross-presentation by Tumor Infiltrating DC (TIDCs), we introduced a Kb-binding peptide SIYRYYGL (SIY) into B16 cells (B16-SIY) (18, 19). Isolated CD11c+ cells from both untreated B16.SIY tumors and tumors receiving local RT revealed dramatic differences in T cell stimulatory potential (Figure 2a). DCs purified from tumors receiving local RT were able to induce proliferation of naïve tumor antigen-specific 2C T cells solely with endogenous tumor antigen acquired in vivo whereas, DCs from untreated tumors failed to stimulate T cell proliferation. To determine whether insufficient acquisition or reduced cross-presentation of DCs isolated from untreated tumors, we provided exogenous, SIY peptide to the in vitro culture. Despite provision of antigen, DCs from untreated tumors remained highly defective in their ability to stimulate tumor-antigen specific T cells (Figure 2a). To address whether the difference in the capacity of DCs from untreated and treated tumors to stimulate T cells was due to differences in the maturation status we analyzed the expression of several classical maturation markers. Surprisingly, we found no differences in the expression of MHC Class I, MHC Class II, B-7 (1&2), or CCR7 between DCs from untreated tumors and those receiving local RT at the same timepoint used to compare T cell stimulatory capacity (Figure 2b). Therefore, local RT of established tumors increases the T cell stimulatory potential of tumor infiltrating DCs that cannot be explained simply by differences in tumor antigen cross-presentation or DC maturation.
Figure 2. TIDC Function is altered by local RT.
a) C57Bl/6 mice were inoculated with 5×105 B16-SIY tumor cells. 15 day established tumors received 25 Gy of local RT or were left untreated. CD11c+ cells were isolated from tumors of 3 mice per group and pooled for analysis as described in the Materials and Methods. Isolated TIDCs cells were cocultured with naïve 2C TCR Tg cells under the indicated conditions and T cell proliferation assessed by tritiated-thymidine incorporation. Error bars represent the standard deviation among three triplicate wells (*p= 0.0167). b) Surface staining of maturation markers expressed by TIDCs from treated and untreated tumors at 72 hours post-RT.
Radiation therapy increases IFN-β production inside tumors
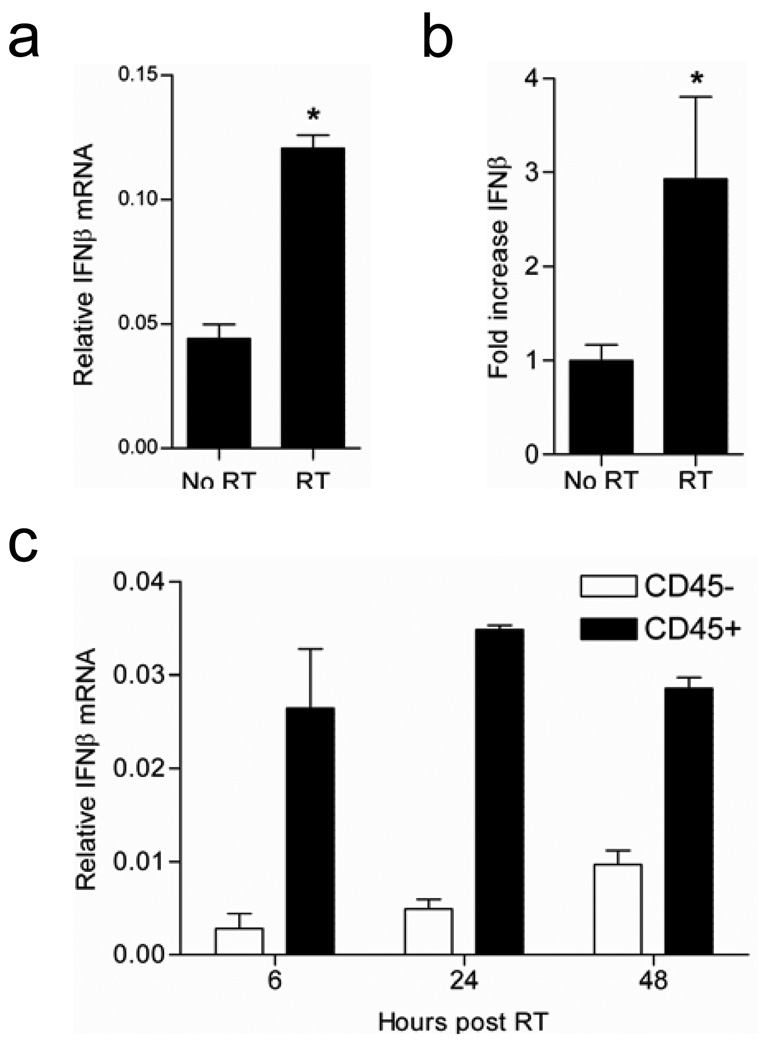
We hypothesized that local RT might augment the local tumor microenvironment and consequently DC functional capacity through changes in the local cytokine milieu. The important role of type I IFNs in enhancing cross-priming by DC has been described in the context of infection and represented a logical candidate for investigation (18, 19). RT-PCR and ELISA of tumor samples confirmed that IFN-β is indeed upregulated following local RT at both the RNA (Figure 3a) and protein level (Figure 3b). Further analysis by cell sorting demonstrated that type I IFN is produced mainly by CD45+ hematopoietic cells that infiltrate the tumor (Figure 3c). These data demonstrate that IFN-β is upregulated within the tumor following local RT and support infiltrating hematopoietic cells as the principal source of IFN-β.
Figure 3. Radiation therapy increases IFN-β within the tumor microenvironment.
a). Real-time PCR analysis of IFN-β mRNA levels from whole tumor RNA of established B16F10 tumors (16–20 days) that were untreated or received 20 Gy of local RT. Data shown are from RNA extracted at 6 hr post-RT (*p=0.0123). b). ELISA for IFNβ protein levels at 48 hrs post-RT utilizing whole tumor homogenate (*p=0.0375). c). Time course analysis of IFN-β mRNA levels by RT-PCR at the indicated time points. CD45+ and CD45− cells were sorted from established B16F10 tumors treated with 20 Gy of local RT at the indicated time points followed by RNA extraction and RT-PCR. Data shown are representative of three experiments with similar results.
IFN-α/β responsiveness is critical for the therapeutic effect of RT
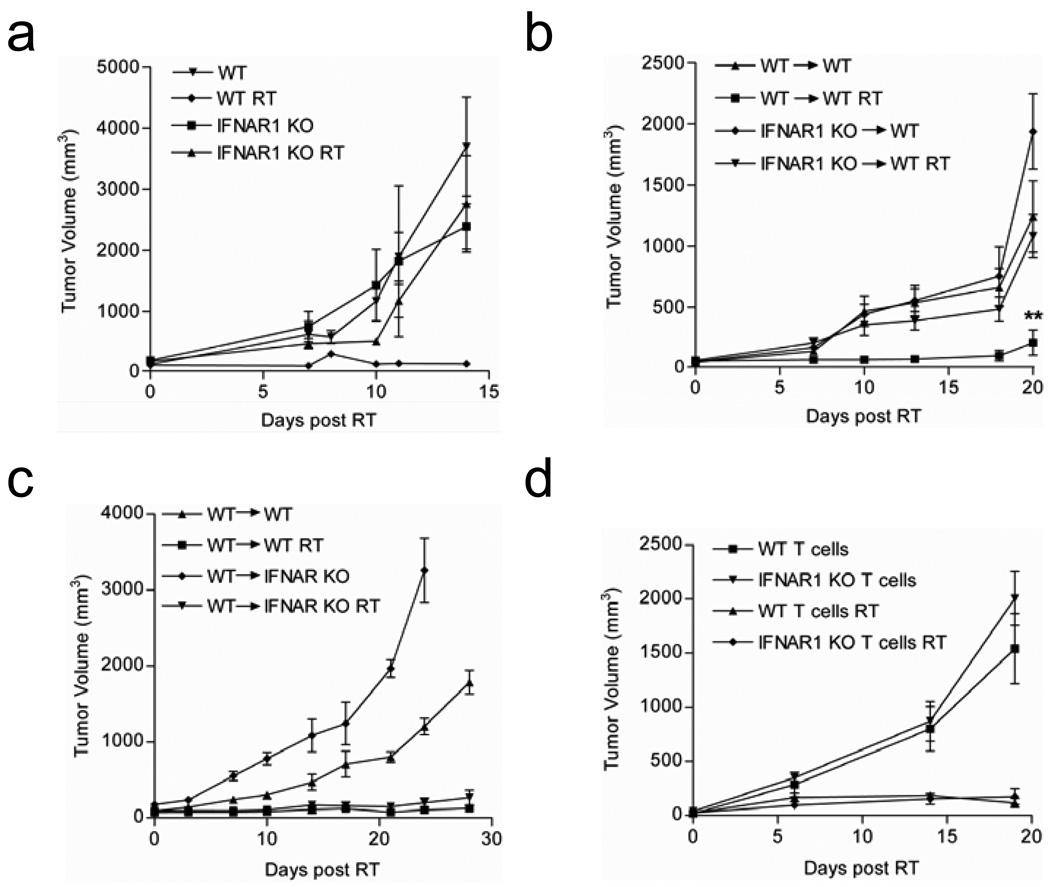
To first test whether type I IFN is essential for RT-mediated tumor reduction, we established parental B16F10 tumors in both wild-type (WT) and IFN alpha receptor 1 knockout (IFNAR1 KO) mice. These mice are unresponsive to all type I IFNs (20). Mice bearing established B16F10 tumors were treated with 15 gray (Gy) of local RT daily for three consecutive days (15 Gy x3) and tumor growth was monitored. WT mice that received local RT demonstrated strong inhibition of tumor outgrowth compared to tumors in the untreated mice. In contrast, tumors in IFNAR1 KO mice grew with similar kinetics as the untreated tumors, exhibiting almost completely resistance to even high doses of RT (Figure 4a). Therefore, the therapeutic response to RT is indeed dependent on host responsiveness to type I IFNs. IFNAR1 KO mice, unlike their WT counterparts, also failed to reject syngeneic EL4 tumors following local RT suggesting that our observations are not limited to the B16 model and that the importance of type I interferon signaling in tumor control following local RT spans tumor cell types with vastly different sensitivities to the direct effects of radiation (Figure S1).
Figure 4. The therapeutic response to RT is dependent on host responsiveness to type I IFNs.
a) Tumor growth curves for WT and IFNAR1 KO mice bearing established B16F10 tumors that were either untreated or received local RT. Tumor growth was plotted starting from the initial dose of RT (n=6–9 mice pooled). b) WT mice were lethally irradiated and reconstituted with either WT or IFNAR1 KO bone marrow (BM). Established tumors received ablative local RT (15 Gy) on day 14, day 15, and day 16. (n= 7–8/group, **p=0.0011 for RT groups WT >WT vs. IFNAR KO > WT at day 20 post treatment). c) Same as (b) except, IFNAR1 KO mice were also used as recipients of WT BM. d) B6/RAG1−/− mice were reconstituted with purified, polyclonal T cells from WT or IFNAR1 KO mice according to procedures detailed in the Materials and Methods. Tumors were treated with 25 Gy of local RT (n=4 mice/group). Data shown are representative of at least two experiments with similar results.
The common heterodimeric IFNα/β receptor is ubiquitously expressed but the effects of IFN receptor engagement can vary depending on the cell type (21, 22). The lack of response to RT in the IFNAR1 KO hosts could be due to receptor deficiency in non-hematopoietic tumor-associated stromal cells or immune cells. In order to examine the requirement for type I IFN responsiveness among these populations, we generated reciprocal bone marrow (BM) chimeras. Treated tumors in WT and IFNAR1 KO mice reconstituted with WT BM responded similarly as WT mice. However, in WT and IFNAR1 KO mice receiving IFNAR1 KO BM, the tumors no longer responded to RT (Figure 4b,c). Therefore among non-tumor cells, IFNα/β responsiveness in the hematopoietic compartment is necessary for the therapeutic effect of RT.
The direct stimulatory effect of type I interferon on T cell expansion, differentiation, and effector function have been described previously in both human and mouse cells and in the context of melanoma specific CD8+ T cell vaccines (23–27). To specifically study whether T cell responsiveness to type I IFN is critical for RT-mediated tumor control, recombinase-activating gene knock-out (RAG−/−) mice were reconstituted with total T cells purified from either WT or IFNAR1 KO mice. These T cell chimeric mice were inoculated with tumor cells one week after T cell transfer, at which time the homeostatic proliferation of transferred cells had ceased (data not shown). Tumors established in T cell chimeric mice were treated with local ablative RT and the tumor growth was monitored. As expected, treated mice that received WT T cells were able to control tumor outgrowth (Figure 4d). To our surprise, mice receiving IFNAR1 KO T cells were still able to mediate equivalent tumor control following local RT. Therefore, direct T cell responsiveness to type I IFNs is not required for RT-mediated tumor control.
Restoration of TIDC function by local RT is type I IFN dependent
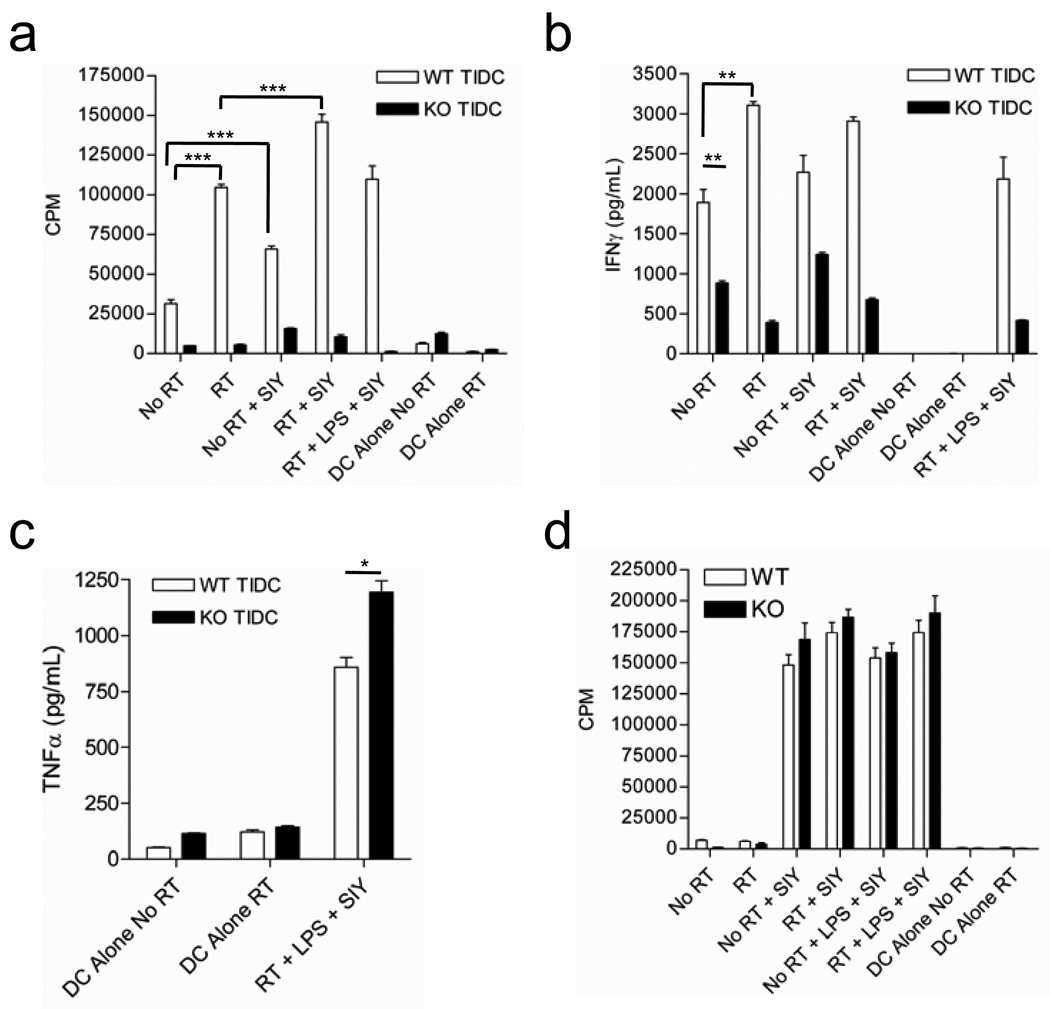
We sought to determine whether responsiveness to type I interferon could explain the functional differences we observed between DCs from untreated and locally irradiated tumors. When we analyzed the function of tumor infiltrating DC from untreated B16-SIY tumors in IFNAR1 KO mice, we found that they had severely diminished capacity to stimulate naïve 2C T cell proliferation (Figure 5a), a defect that was similarly observed when WT DCs from untreated tumors were used (Figure 2a and 5a). Interestingly, local radiation of tumors in IFNAR1 KO mice failed to restore the ability of TIDC to stimulate T cell proliferation (Figure 5a) and effector cytokine production (Figure 5b). Similar to our earlier results with WT TIDC, the functional capacity of IFNAR1 KO TIDCs could not be restored by addition of exogenous SIY peptide or following stimulation with bacterial lipopolysaccharide (LPS) (Figure 5a,b). Furthermore, maturation of IFNAR1 KO DCs revealed no differences compared to WT TIDC in both untreated and treated tumors (data not shown). It is unlikely that IFNAR1 KO TIDCs are globally functionally impaired because TNFα production in response to LPS was equivalent, if not increased, in comparison with WT TIDC from irradiated tumors (Figure 5c). To confirm that there is not a global defect in DC function in IFNAR1 KO mice, we also analyzed lymph node DCs isolated from WT and IFNAR1 KO mice. Lymph node DCs were functionally indistinguishable between WT and IFNAR1 KO mice in their ability to drive T cell proliferation and effector cytokine production (Figure 5d and data not shown). Therefore, responsiveness to type I IFN is required for the acquisition DC cross-priming capacity within the tumor microenvironment.
Figure 5. Local ablative RT endows TIDC with T-cell stimulatory capacity in WT mice but fails to generate functional TIDC in IFNAR KO mice.
a) CD11c+ cells were isolated from tumors (a) and draining lymph nodes (d) of 3 mice/group and pooled for analysis. Isolated CD11c+ cells were cocultured with naïve 2C T cells under various conditions and T cell proliferation assessed. Error bars represent the standard deviation among three triplicate wells. b,c) Cytokine concentrations in supernatents from in vitro culture with TIDCs. Levels of IFNγ (b) and TNFα (c) are shown (*p= 0.0192). Data shown are representative of two experiments with similar results. (Unless otherwise indicated ***p<0.001, **p<0.01; individual p values are available where noted above)
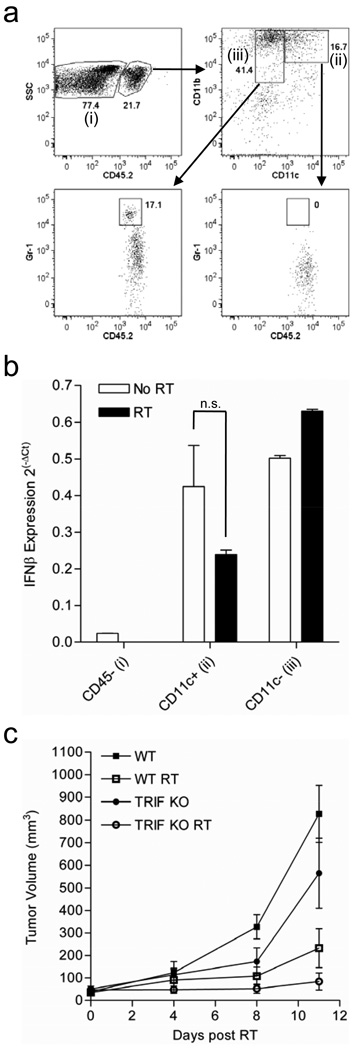
Infiltrating myeloid cells produce constitutive IFN-β that is independent of cononical TRIF dependent TLR signaling
To determine which hematopoietic cells in the tumor microenvironment are responsible for the increased IFN-β production we further sorted CD45+ myeloid cells from untreated tumors and tumors receiving local RT based on expression of CD11c and used the CD45− fraction of cells as a negative control (Figure 6a). TIMo and MDSCs both fall into the CD11c- gate and were analyzed together (referred to collectively as MDSC). RT-PCR analysis of IFN-β expression by these three subsets of cells revealed that both TIDCs and MDSCs produce IFN-β at near equivalent levels (Figure 6b). When we tested the tumor response to radiation in TRIF knock-out mice, tumor control was indistinguishable from that in WT B6 mice, and perhaps slightly enhanced, suggesting that IFN-β production downstream of canonical TLR signaling is not essential and distinguishing our model from published data (Figure 6c).
Figure 6. Tumor infiltrating myeloid cells produce autocrine IFN-β that is independent of TRIF signaling.
a) Gating strategy used to purify infiltrating myeloid cells from tumors for Real-Time PCR analysis of IFNβ expression. Cells were sorted into three populations, (i)CD45−, (ii)CD45+/CD11b+/CD11c+, and (iii)CD45+/CD11b+/CD11c−. Expression of Gr-1 is shown for both the CD11c+ and CD11c− fractions. b) RT-PCR analysis of IFN-β expression in the three sorted populations depicted in (a) (n.s. p=0.1741). c) Established B16.SIY tumors in WT and TRIF−/− mice were treated with 20Gy local RT and tumor growth was monitored (n=5).
Tumor reduction by exogenous local delivery of type I IFN is dependent on CD8+ T cells
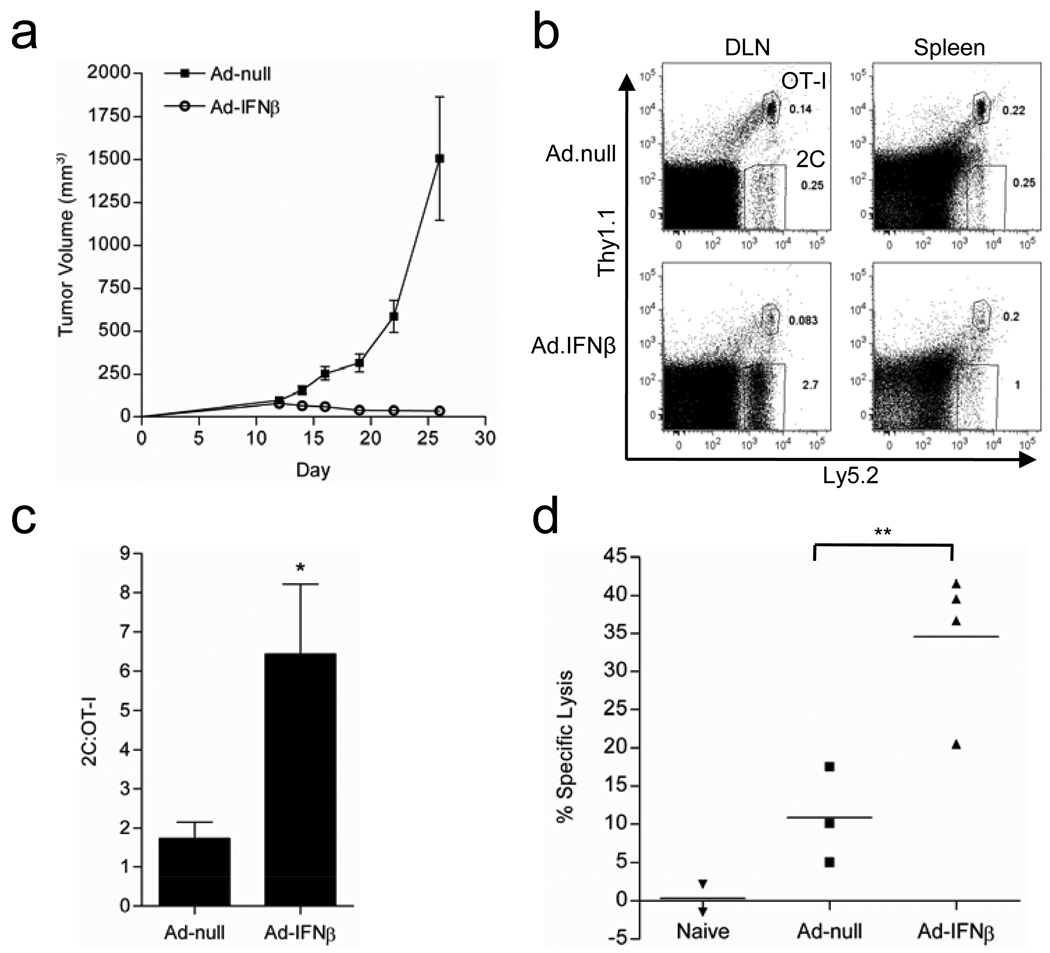
To study the sufficient role of type I IFN in the absence of RT, we tested whether local delivery of type I IFN into tumors through a recombinant adenoviral (Ad-IFN-β) vector could cause tumor rejection. We treated established B16-SIY tumors with either Ad-null, an empty vector control, or Ad-IFN-β and monitored tumor growth. Surprisingly, even for such an aggressive tumor, Ad-IFN-β demonstrated very potent anti-tumor effects (Figure 7a). To test whether T cells are required for the inhibition, we used B6/RAG KO mice, which are deficient in lymphocytes, and compared non-transferred hosts to those reconstituted with T cells collected from WT donors. Interestingly, tumor responsiveness to Ad-IFN-β was not detectable in Rag-1−/− mice but was restored by transfer of peripheral T cells (Figure S2a). Thus, as with RT, treatment with Ad-IFN-β is immune-mediated and dependent on T cells.
Figure 7. Ad-IFN-β promotes preferential expansion of tumor Ag-specific cells.
a) Established B16-SIY tumors were treated with 2×1010vp ad-null or ad-IFN-β on day 13 and 15 and tumor growth was monitored (n=4). b,c) WT mice bearing day 12 established B16-SIY tumors were adoptively transferred with a mixture of CFSE labeled 2C and OT-I/Thy1+ T cells. b) Representative FACS plot showing the frequency of Ag-specific 2C cells vs. non-specific OT-I T cells. c) Pooled data showing the ratio of 2C to OT-I T cells (n=5–7), (*p=0.0128). d) Specific lysis of SIY-loaded target cells (**p=0.0015). Data shown are representative of two experiments with similar results.
In order to further distinguish the role of CD4+ and CD8+ T cell subsets, we used WT mice and antibody-mediated depletion of CD8+ T cells or CD4+ T cells. Surprisingly, depletion of CD4+ T cells had almost no effect on Ad-IFN-β treatment as tumors responded equivalently with or without CD4 depletion (Figure 4c). However, depletion of CD8+ T cells drastically reduced treatment efficacy and tumors rapidly progressed (Figure S2b). Therefore CD8+ T cells are essential for the anti-tumor effect of Ad-IFN-β.
IFN-β leads to preferential expansion of Ag-specific T cells
To test whether Ad-IFN-β could induce Ag-specific T cell responses, we transferred naïve 2C transgenic T cells, which recognize the SIY antigen, into B16-SIY tumor bearing mice to quantify antigen-specific CD8+ T cell expansion. In order to test whether this expansion was limited to antigen-specific cells, we labeled both 2C (antigen-specific) and OT-I (non-specific) cells with CFSE and then adoptively transferred them into B16-SIY tumor bearing mice Ad-IFN-β induced a preferential expansion of the Ag-specific 2C cells compared to the non-specific OT-I cells (Figures 7b and S3a). With the Ad-null control group, the ratio of 2C to OT-I cells was approximately equivalent, confirming that similar numbers of transgenic T cells were transferred into recipients, yet significantly increased with Ad-IFN-β treatment (Figure 7c). In addition, 2C cells showed robust proliferation as evidenced by almost complete CFSE dilution (Figure S3b). In contrast, the non-specific cells failed to proliferate (data not shown). Finally, we investigated whether Ad-IFN-β therapy leads to increased lytic activity of tumor antigen-specific T cells in vivo. Ad-IFN-β treated mice showed significantly higher Ag-specific lysis than Ad-null treated mice (Figure 7d). These data indicate that the expansion of antigen specific cells observed in Ad-IFN-β treated mice results in robust effector CTL activity.
DISCUSSION
Despite these well-known functions of type I IFN during infection, the role of type I IFN in tumor immunity is not well defined. Here, we highlight several important features regarding the essential role of type I IFNs in RT-induced immunity. 1) IFN-α/β is essential for tumor reduction or eradication following local RT. 2) IFN-α/β is not produced by tumor cells in our model, but instead, is produced in an autocrine fashion by tumor infiltrating myeloid cells. 3) IFN-α/β signaling is required to endow TIDCs with T cell cross-priming capacity following local RT, however, T cells do not need to bear the type I IFN receptor to mediate tumor rejection. 4) Inducing a local increase in type I IFN within the tumor microenvironment through a therapeutically relevant adenoviral delivery system can mimic the therapeutic effect of RT for tumor regression. Together, these results suggest that a local increase in type I IFN signaling with the tumor microenvironment is essential and sufficient to reverse the suppressive tumor microenvironment and induce anti-tumor immunity that mediates tumor regression.
Infiltration of DC into tumors has been shown to augment the suppression through both direct suppression of CD8+ T cell effector function and aberrant skewing of CD4+ T cell cytokine production (28, 29). Furthermore, depletion of tumor infiltrating DCs can sensitize the tumor to immune mediated killing by removing their suppressive influence in the tumor microenvironment (30). The ability of local RT to functionally reinstate TIDCs may represent a more multifaceted way to reduce suppression in the tumor microenvironment but the source and nature of TIDC after RT remains to be determined.
This study highlights the importance of identifying and fostering the immune-dependency of RT in order to maximize therapeutic outcomes. Our study reveals that ablative RT can initiate a cascading innate and adaptive immune attack against tumor cells that is dependent on type I interferon. This study has also elucidated the mechanisms by which ad-IFN-β induces tumor specific T cell responses leading to the tumor reduction. It would be of interest to study whether increased type I IFN production is correlated with better immune responses in patients following RT and whether such responses can be used to predict the therapeutic efficacy of RT and survival of cancer patients.
Acknowledgments
This research was in part supported by US National Institutes of Health grants CA115540 and CA97296 to Y.X.F, CA111423 to R.R.W. and a grant from the Ludwig Foundation to R.R.W. and Y.X.F. S.L.A. is supported by a Medical Scientist National Research Service Award (5 T32 GM07281). and B.B. is supported by the Foglia Fellowship.
Footnotes
Disclosure of Potential conflicts of Interest
The authors declare that they have no competing financial interests.
References
- 1.Wasserman J, Blomgren H, Rotstein S, Petrini B, Hammarstrom S. Immunosuppression in irradiated breast cancer patients: in vitro effect of cyclooxygenase inhibitors. Bull N Y Acad Med. 1989;65:36–44. [PMC free article] [PubMed] [Google Scholar]
- 2.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 3.Lee Y, Sogyong L Auh, Yugang Wang, Byron Burnette, Yang Wang, Yuru Meng, Michael Beckett, Rohit Sharma, Robert Chin, Tony Tu, Ralph R Weichselbaum, Yang-Xin Fu. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009 doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 5.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang JS, Nakatsugawa S, Niwa O, Ju GZ, Liu SZ. Ionizing radiation-induced IL-1 alpha, IL-6 and GM-CSF production by human lung cancer cells. Chin Med J (Engl) 1994;107:653–657. [PubMed] [Google Scholar]
- 7.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–4337. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 9.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 10.Gresser I, Belardelli F, Maury C, Maunoury MT, Tovey MG. Injection of mice with antibody to interferon enhances the growth of transplantable murine tumors. J Exp Med. 1983;158:2095–2107. doi: 10.1084/jem.158.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Picaud S, Bardot B, De Maeyer E, Seif I. Enhanced tumor development in mice lacking a functional type I interferon receptor. J Interferon Cytokine Res. 2002;22:457–462. doi: 10.1089/10799900252952244. [DOI] [PubMed] [Google Scholar]
- 12.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 13.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nat Immunol. 2005;6:722–729. doi: 10.1038/ni1213. [DOI] [PubMed] [Google Scholar]
- 14.Ma Y, Kepp O, Ghiringhelli F, Apetoh L, Aymeric L, Locher C, et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin Immunol. 2010;22:113–124. doi: 10.1016/j.smim.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Khodarev NN, Beckett M, Labay E, Darga T, Roizman B, Weichselbaum RR. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci U S A. 2004;101:1714–1719. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weichselbaum RR, Ishwaran H, Yoon T, Nuyten DS, Baker SW, Khodarev N, et al. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105:18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khodarev NN, Roach P, Pitroda SP, Golden DW, Bhayani M, Shao MY, et al. STAT1 pathway mediates amplification of metastatic potential and resistance to therapy. PLoS One. 2009;4:e5821. doi: 10.1371/journal.pone.0005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, et al. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- 19.Yu P, Lee Y, Wang Y, Liu X, Auh S, Gajewski TF, et al. Targeting the primary tumor to generate CTL for the effective eradication of spontaneous metastases. J Immunol. 2007;179:1960–1968. doi: 10.4049/jimmunol.179.3.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 21.Uddin S, Platanias LC. Mechanisms of type-I interferon signal transduction. J Biochem Mol Biol. 2004;37:635–641. doi: 10.5483/bmbrep.2004.37.6.635. [DOI] [PubMed] [Google Scholar]
- 22.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher KM, Lauder S, Rees IW, Gallimore AM, Godkin AJ. Type I interferon (IFN alpha) acts directly on human memory CD4+ T cells altering their response to antigen. J Immunol. 2009;183:2915–2920. doi: 10.4049/jimmunol.0801607. [DOI] [PubMed] [Google Scholar]
- 24.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 25.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikora AG, Jaffarzad N, Hailemichael Y, Gelbard A, Stonier SW, Schluns KS, et al. IFN-alpha enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J Immunol. 2009;182:7398–7407. doi: 10.4049/jimmunol.0802982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, et al. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 28.Norian LA, Rodriguez PC, O'Mara LA, Zabaleta J, Ochoa AC, Cella M, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, et al. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huarte E, Cubillos-Ruiz JR, Nesbeth YC, Scarlett UK, Martinez DG, Buckanovich RJ, et al. Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Res. 2008;68:7684–7691. doi: 10.1158/0008-5472.CAN-08-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]