Abstract
Objective
Glutamate is a key excitatory neurotransmitter in the brain, and its excessive release plays a key role in the development of neuronal injury. In order to define the effect of nimodipine on glutamate release, we monitored extracellular glutamate release in real-time in a global ischemia rat model with eleven vessel occlusion.
Methods
Twelve rats were randomly divided into two groups: the ischemia group and the nimodipine treatment group. The changes of extracellular glutamate level were measured using microdialysis amperometric biosensor, in coincident with cerebral blood flow (CBF) and electroencephalogram. Nimodipine (0.025 µg/100 gm/min) was infused into lateral to the CBF probe, during the ischemic period. Also, we performed Nissl staining method to assess the neuroprotective effect of nimodipine.
Results
During the ischemic period, the mean maximum change in glutamate concentration was 133.22±2.57 µM in the ischemia group and 75.42±4.22 µM (p<0.001) in the group treated with nimodipine. The total amount of glutamate released was significantly different (p<0.001) between groups during the ischemic period. The %cell viability in hippocampus was 47.50±5.64 (p<0.005) in ischemia group, compared with sham group. But, the %cell viability in nimodipine treatment group was 95.46±6.60 in hippocampus (p<0.005).
Conclusion
From the real-time monitoring and Nissl staining results, we suggest that the nimodipine treatment is responsible for the protection of the neuronal cell death through the suppression of extracellular glutamate release in the 11-VO global ischemia model of rat.
Keywords: Nimodipine, Glutamate, Eleven vessel occlusion ischemia model, Real-time monitoring, Nissl staining
INTRODUCTION
Ischemic conditions facilitate the release of activated excitatory amino acids. The most well-known excitatory neurotransmitter, glutamate, attacks neuronal cells by postsynaptic binding to several receptors10,11,40). Various studies have demonstrated a pharmacological effect resulting from blocking the cascading excitotoxic cellular injury process1,33,34,37). Nimodipine, a dihydropyridine derivative, is a well-known neural protective drug that has been applied to some ischemic vascular diseases and may have a beneficial effect on cerebral ischemia after subarachnoid hemorrhage15). Many authors have suggested that calcium (Ca2+) channel blockers can facilitate vasodilatation of small arterioles, allowing cerebral blood flow (CBF) to increase32,38,42) and inhibiting platelet aggregation17). Bullock et al.5) reported that L-type voltage-gated Ca2+ channel blocker such as nimodipine prevented Ca2+ influx, which induced apoptosis. In contrast, Lazarewicz et al.26) proposed that nimodipine could directly protect neurons in the rabbit brain by intracellular Ca2+ antagonism rather than by inhibition of Ca2+ influx. Despite many reports that Ca2+ channel blockade induces a cascade reaction, the action of nimodipine on presynaptic glutamate release remains poorly understood14,16,18,25).
Under clinical circumstances, surgeons occasionally encounter ischemic conditions, such as multiple temporary clipping during aneurysm surgery or vascular clamping during carotid endarterectomy and bypass surgery. In order to prevent ischemic complications, several methods such as induced hypometabolism or hypothermia have been applied to various clinical conditions before and after brain surgery. We hypothesized that nimodipine would have a beneficial effect on surgical ischemic conditions if it decreases neurotoxic glutamate release as an L-type Ca2+ channel blocker at both sides of the pre- and post-synaptic membrane. Therefore, we performed real-time monitoring of glutamate release to validate the effects of nimodipine throughout induction of ischemic and reperfusion conditions in rats, using the eleven-vessel occlusion (11-VO) animal model. Changes in glutamate release were monitored with electroencephalography (EEG) and CBF.
METHODS AND MATERIALS
Animal preparation
Male Sprague-Dawley rats (250-300 g) were used for the experiment and housed in a 22±24℃ and 12 hr light/dark cycle controlled environment. The animals were fed commercial rat chow and water ad libitum. During the entire preparation, body temperature was maintained at 37.1±0.10℃ with a Homeothermic Blanket Control Unit (Harvard Apparatus, Holliston, MA, USA). All animal use procedures were approved by the Ethical Committee of the Kyung Hee University School of Medicine and were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Eleven-vessel occlusion (11-VO) model preparation
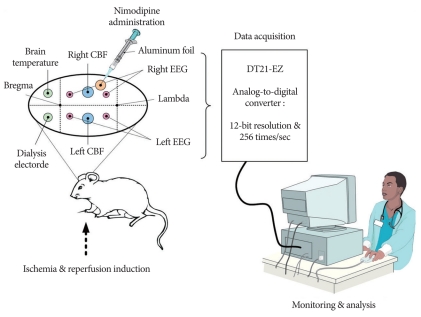
Rats were randomly divided into two groups: six ischemic rats (Group 1) and six ischemic rats treated with nimodipine (Group 2). Animals were anesthetized with an intraperitoneal injection of 300 mg/kg chloral hydrate. Additional chloral hydrate was properly administered when needed in order to maintain the same level of anesthesia during the experiment. Rectal temperature was maintained regularly at 37.1℃ using a heating pad. An 11-VO was used as the global ischemia model8). After dividing the omohyoid muscle, a pair of occipital arteries, the superior hypophyseal artery, ascending pharyngeal artery, and pterygopalatine artery were coagulated with bipolar electrocautry (Fig. 1). The ventral clivus just caudal to the basioccipital suture was then drilled and exposed to a 3-mm diameter. Next, the basilar artery was permanently coagulated.
Fig. 1.
Experimental setup for the real-time monitoring of electroencephalography (EEG), cerebral blood flow (CBF), extracellular glutamate release and brain temperature in the ischemia and reperfusion condition.
Real-time monitoring setup
Real-time monitoring setup was performed as described previously8,12,13,27,44). After placement in prone position on a stereotaxic frame, the skin over the skull of each rat was opened with a midline incision along the superior sagittal suture. Nine burr holes were made using hand drill. Two with 1.5 mm in diameter were placed 4 mm apart from the midline and 1 mm in front of the coronal suture : one for glutamate sensor insertion and the other for brain temperature measurement. Two burr holes were made for EEG electrodes at 4 mm apart from the sagittal suture and 1 mm behind the coronal suture. Two burr holes were made for the CBF probe at both the side and 2 mm behind the EEG sites. Two additional burr holes were made for EEG 2 mm behind the CBF probe on both sides. In the rats infused with nimodipine, an extra burr hole in which the dura was incised was made for the nimodipine drip. Bone wax was used to make a barrier for the nimodipine solution so as not to disturb the EEG electrode.
Four EEG electrodes were attached by screws and a reference electrode was attached to the skin of the right earlobe. EEG signals were pre-amplified using a 511 AC Amplifier (Astro-Medical Inc., USA). The signals were converted to digital information at 256 times per second using a data acquisition system designed in our laboratory. An EEG signal represented the electrical failure of a neuronal cell during an ischemic episode. The probes for the CBF were attached at both hemispheres with BLF21D laser Doppler flowmetry (Transonic Systems Inc., Ithaca, NY, USA). The microdialysis electrode, purchased from Sycopel International Ltd. (Type : General 20-10-4-4, Tyne & Wear NE32 3DT, UK), was filled with phosphate buffered saline to electropolymerize the O-phenylenediamine on the platinum electrode at 0.65 V for 20 min with Sycopel BD2000 potentiostat. Fresh phosphate buffered saline containing glutamate oxidase was then perfused in the dialysis electrode at a flow rate of 0.5 µL/min. The dialysis electrode displayed a linear response in the concentration range of 50-450 µM standard glutamate solution with a sensitivity of 0.22 nA/µM (R2, coefficient of regression of 0.998). After sensor calibration, the microdialysis electrode was inserted into the motor cortex at coordinates A 1 : L 4 : V 4 mm (from the bregma and the dura) through a small incision in the dura.
The data were collected from the analog output ports of the laser Doppler flowmetry, glutamate potentiostat, amplified EEG and temperature controller. The signal was digitized using a DT21-EZ analog-to-digital converter with a 12 bit resolution and sampling occurred 256 times per second (Data Translation Inc., Marlboro, MA, USA). The digitized signal was transferred in real-time to a personal computer and saved for further analysis (Fig. 1).
Induction of ischemia and reperfusion
Before inducing ischemia, we collected background data for 10 minutes (to ensure steady-state) from both groups. 11-VO cerebral ischemia was then initiated for 10 minutes by pulling on the tubes on the common carotid artery (CCA) and internal carotid artery (ICA) with 10-12 gm weights. Saline irrigation was applied intermittently for full restoration of the CCA at the time of reperfusion. Finally, reperfusion was allowed by releasing the pulled tubes after a 10-minute period of ischemia.
Drug administration
Nimodipine was purchased from Reyon Pharmaceutical Co. Ltd. (0.010 g/50 mL, Seoul, Korea). Nimodipine was diluted 20 times with saline and dripped (0.025 µg/100 gm/min) in the vicinity of the burr hole for the CBF probe during the 10-minute ischemic period. The nimodipine infusion syringe was protected from the light source by thin aluminum foil. Group 1 was not treated with any drug.
Histological procedure
We utilized Nissl staining method for the evaluation of neuroprotective effect of nimodipine. Nissl staining method was performed as described previously28). On the third day after ischemia, the rats were anesthetized with chloral hydrate and transcardially perfused with 50 mM phosphate-buffered saline and then fixed with 4% paraformaldehyde. The brains were then removed, post-fixed in the same fixative overnight and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40 µm thickness were made with a freezing microtome (Leica, Nussloch, Germany) and mounted onto slides.
The sections were stained with cresyl violet for histological assessment of neuronal cell damage, dependent upon viable and nonviable stained cells. Viable neurons were defined with cells, with normal morphologic properties exhibiting round nuclei stained with cresyl violet. Viable neuronal cells were counted using Image Plus 2.0 (Motic, Xiamen, China) in 500×500 µm area of hippocampal CA1 regions about 300 µm from hillus of three coronal sections (about 1.4 to 1.8 mm posterior to bregma). And, surviving neurons in cortex RSGb regions were counted. Mean number of stained cells was obtained by three researchers blinded to the experimental conditions. Cell viability from each group was presented as percentages of mean number of viable cells from sham animals.
Statistical analysis
The data for CBF and glutamate changes were expressed as mean ± the standard error of the mean (SEM). A two-tailed Student's t-test was used to compare the levels of %CBF and glutamate changes in the two groups. The significant differences of neuronal cell viability were assessed by one-way analysis of variance, followed by Tukey's post hoc test using SPSS statistical software (version 17.0 for Windows, SPSS Inc., Chicage, IL). p-Values < 0.05 were considered significant.
RESULTS
Real-time changes in cerebral blood flow response
As soon as ischemia was induced, CBF declined rapidly to near zero levels, coincident with the development of a flat EEG signal in Group 1 (Fig. 2). The ischemic plateau was achieved within 10.8±4.2 sec and successfully maintained during the 10-minute ischemic period. When reperfusion was initiated, CBF significantly increased to pre-ischemic levels. The pattern of CBF response on ischemia and reperfusion in Group 2 was similar to that in Group 1 (Fig. 3). The time to reach peak %CBF levels was 659.78±89.01 sec in Group 1 and 629.44±84.17 sec in Group 2 (Table 1). The peak level of %CBF upon reperfusion was 487.89±117.47% in Group 1 and 318.55±44.25% in Group 2. The time interval of CBF restoration to control after reperfusion was 1506.58±211.62 sec in Group 1 and 1481.25±150.32 sec in Group 2. These three parameters of CBF were not significantly different between the two groups (Table 1).
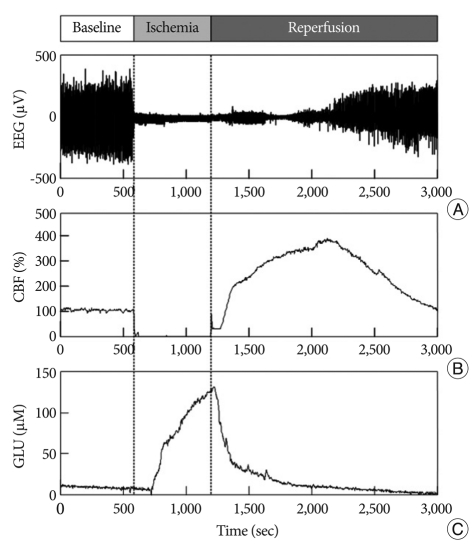
Fig. 2.
Representative real-time measurement of EEG (A), CBF (B), and glutamate (C) in Group 1 for 50 minutes. After 10 minutes pre-ischemic control, ischemia is induced for 10 minutes and then followed by reperfusion.
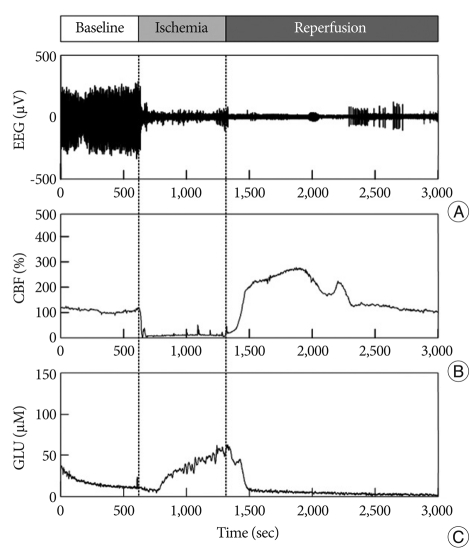
Fig. 3.
Representative real-time measurement of EEG (A), CBF (B), and glutamate (C) in Group 2 for 50 minutes. After 10 minutes pre-ischemic control, ischemia is induced for 10 minutes and then followed by reperfusion.
Table 1.
Changes in cerebral blood flow (CBF) between the Group 1 and Group 2 in global ischemia induced by an eleven-vessel occlusion method
NS : not significant
Real-time changes in extracellular glutamate release
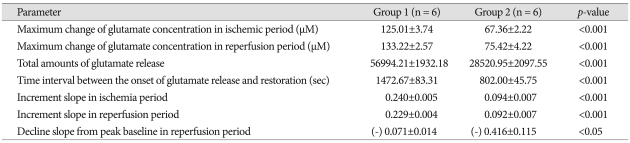
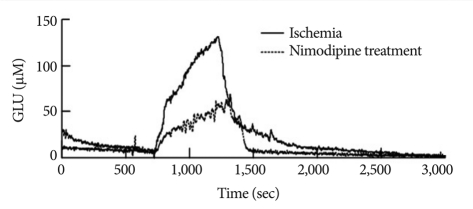
The elevation in glutamate release in Group 1 began 113.16±35.79 sec after the onset of ischemia and continued to rise throughout the entire ischemic period (Fig. 2). The glutamate level then rapidly declined to pre-ischemic levels during reperfusion. Similarly, glutamate release started changing after 99.97±50.02 sec in Group 2 (Fig. 3), but glutamate release elevation was suppressed in Group 2. The maximum change of glutamate concentration was 125.01±3.74 µM in Group 1 and 67.36±2.57 µM in Group 2 during the ischemic period (Table 2). The maximum change of glutamate concentration was 133.22±2.57 µM in Group 1 and 75.42±4.22 µM in Group 2 during the reperfusion period. The total amount of glutamate release was 56991.21±1932.18 in Group 1 and 28520.95±2098 in Group 2. After reperfusion, the mean time interval of glutamate restoration from peak levels to pre-ischemic control levels was 936.19±88.65 sec in Group 1 and 233.68±50.48 sec in Group 2. All parameters of GLU release were significantly different between the two groups (p<0.001 and p<0.05 in the decline slope). Fig. 4 shows at a glance the difference of glutamate dynamics between the Group 1 and Group 2.
Table 2.
Changes in glutamate concentration between the Group 1 and Group 2 in global ischemia induced by an eleven vessel occlusion method
Fig. 4.
Comparison of representative glutamate dynamics between the Group 1 and Group 2. Although glutamate release starts at similar time in both groups, glutamate release elevation is suppressed in Group 2.
%Cell viability in hippocampus
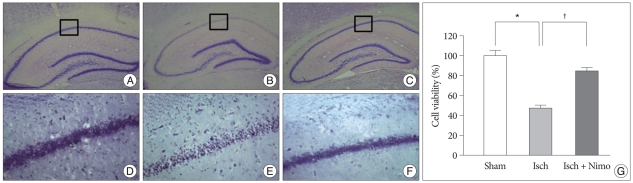
The neuroprotective effect of nimodipine on neurons in hippocampus was evaluated by measuring the neuronal cell viability in CA1 hippocampal region and cortex at three days after ischemia. Sham-operated CA1 pyramidal neurons from four hemispherical sections were counted and averaged. As shown in Fig. 5, the %cell viability in hippocampus was 47.50±5.64 in Group 1, compared with sham group. In Group 1, it was significantly lower than that in sham group (p<0.005 in hippocampal CA1 region). But, the %cell viability in Group 2 was 95.46±6.60 in hippocampus. It was significantly higher in Group 2 than Group 1 (p<0.005 in hippocampal CA1 region). And, neuronal cell viability in CA1 hippocampal region showed no significant difference between Group 2 and sham group.
Fig. 5.
Neuroprotective effect of nimodipine on CA1 pyramidal neurons after global ischemia by eleven-vessel occlusion. The hippocampus is shown in cresyl violet-stained section from sham group (A and D), Group 1 (B and E) and Group 2 (C and F). Scale bar = 100 µm. The graph (G) shows the quantitative results of CA1 neuronal cell counts. *p < 0.005, †p < 0.005.
DISCUSSION
For real-time monitoring of the effect of nimodipine on glutamate release, we used an 11-VO global ischemia model in rats8). In contrast to four-vessel occlusion (4-VO) and seven-vessel occlusion (7-VO) models, the 11-VO model allows easy creation of an ischemic plateau after ischemia induction because the source of collateral blood flow is effectively blocked. The time interval between ischemia induction and ischemic plateau is also shorter than with other global ischemia models, facilitating a more precise estimation of glutamate12,13,44).
The release and reuptake of glutamate maintains an equilibrium status in normal neuronal cells. Excitotoxic glutamate is excessively released under specific conditions such as traumatic and hypoxic brain injury or cerebral ischemia, and many researchers have tried to demonstrate connections between extracellular glutamate levels and neurological injury2-4,6,7,9,19-21,23,43). There is no consensus for the mechanism of neurotoxic glutamate release, however. Katayama et al.22) suggested that glutamate is released by Ca2+ dependent exocytosis at the presynaptic membrane. Marcoli et al.30) proposed several mechanisms for glutamate release; primary one is a vesicular release in acute ischemia, secondary is an endogenous release of adenosine-activated A2A receptors and final one is a Ca2+-dependent control at the presynaptic receptors. Glutamate is released from dead neuronal cells15,41). Limbrick et al.29) suggested that neurotoxic depolarization by excitotoxic glutamate exposure is caused by an influx of extracellular Ca2+ ion.
There have been a few reports about the relationship between nimodipine and glutamate release in the neurological arena. Kringlstein et al.24) reported that nimodipine significantly attenuates glutamate-induced neuronal damage in a cellular media experiment. Lazarewicz et al.26) suggested that nimodipine might directly protect brain neurons by intracellular Ca2+ antagonism rather than by inhibition of Ca2+ influx. Glutamate release is reportedly not only controlled by the activation of voltage-dependent Ca2+ channels but also occurs independently without Ca2+ channel activation35).
We hypothesized that nimodipine could play an effective role in delaying glutamate response to the presynaptic membrane of presynaptic Ca2+ channels with a characteristic of L-type45). When we compared the dynamics of glutamate release between the groups treated with and without nimodipine, we found that the onset time of glutamate release was similar in the two groups (Fig. 4). The elevation of glutamate release was partially suppressed in the Group 2 during the ischemic period, however. The decline of glutamate release during the reperfusion period was also more rapid in the Group 2 than in the Group 1 (Table 2). Overall, nimodipine significantly decreased glutamate release during the ischemic period.
In contrast, Matsumoto et al.31) found that glutamate release during an ischemic period in a rabbit global ischemia model was not significantly altered by drug infusion. Nakane et al.36) reported that glutamate release with an in vivo dialysis technique in the rat brain was not affected by isradipine, an L-type voltage sensitive Ca2+ channel blocker that was infused before the occlusion. We speculate that either the injected drug did not reach the brain at all or the rat model had no route for drug injection during the ischemic period.
We successfully monitored the change of glutamate release by continuous infusion of nimodipine into rat brains during the ischemic period using an 11-VO ischemia model, confirming that nimodipine can partially regulate glutamate release during an ischemic episode and play a role in the reuptake of extracellular glutamate. Also, we evaluated the neuroprotective effect of nimodipine utilizing Nissl staining method. Partial suppression of glutamate release by nimodipine appears to influence multiple types of Ca2+ channels that mediate the exocytosis of glutamate39). Further experiments are needed to verify the precise mechanism of extracellular glutamate reuptake by nimodipine.
CONCLUSION
We have demonstrated the neuroprotective effect of nimodipine through the suppression of extracellular glutamate release using real-time monitoring of glutamate release in an 11-VO ischemia model. A reproducible 11-VO ischemia model was induced as a global ischemia. Patterns of change in CBF and EEG during ischemia and reperfusion indicate that the surgical setup and 11-VO model are suitable for monitoring the effect of nimodipine under ischemic conditions. The real-time monitoring system used in our laboratory highlights new possibilities for a detailed analysis of the in vivo dynamics of changes in the neurotransmitter glutamate during and/or after ischemia. We found that three parameters of CBF, peak level on reperfusion/baseline of CBF, time interval between the onset of reperfusion and the peak level of CBF, and time interval between the onset of reperfusion and the restoration of basal CBF level, and EEG patterns were not significantly different between the two groups. However, a significant decrease (p < 0.001) in the maximum levels of ischemic and reperfusion glutamate releases and in total amounts of glutamate release was observed in the nimodipine-treated rats. Compared with ischemia rats, a significant decrease (p < 0.001) in time interval between the onset of glutamate release and restoration was observed in Group 2. A significant decrease (p < 0.001) in the increment slopes during the ischemic and reperfusion period, and a significant increase (p < 0.05) in a decrement slope during the reperfusion period was observed in nimodipine-treated rats. Finally, from the Nissl staining, a significant increase (p < 0.005) in the %cell viability in hippocampus was observed in the nimodipine-treated rats. These results show that glutamate release can be partially regulated by nimodipine, an L-type Ca2+ channel blocker. This fact indirectly supports the hypothesis that glutamate exocytosis is mediated by multiple types of Ca2+ channels, including L-type. Therefore, we conclude that the decrease in glutamate release during ischemia is responsible for the neuroprotective effects of nimodipine treatment in a rat model. Furthermore, we propose that continuous nimodipine infusion during the ischemic period could be beneficial in neurosurgical conditions where surgeons require multiple temporary clipping or carotid occlusion.
Acknowledgements
This study was supported by a grant from the Kyung Hee University Program for Young Researchers in Medical Science (KHU-2007-1502).
References
- 1.Alps BJ. Drugs acting on calcium channels : potential treatment for ischaemic stroke. Br J Clin Pharmacol. 1992;34:199–206. doi: 10.1111/j.1365-2125.1992.tb04125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andine P, Orwar O, Jacobson I, Sandberg M, Hagberg H. Changes in extracellular amino acids and spontaneous neuronal activity during ischemia and extended reflow in the CA1 of the rat hippocampus. J Neurochem. 1991;57:222–229. doi: 10.1111/j.1471-4159.1991.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin HA, Williams JL, Snares M, Ferreira T, Cross AJ, Green AR. Attenuation by chlormethiazole administration of the rise in extracellu lar amino acids following focal ischaemia in the cerebral cortex of the rat. Br J Pharmacol. 1994;112:188–194. doi: 10.1111/j.1476-5381.1994.tb13050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, et al. Factors affecting excitatory amino acid release following severe human head injury. J Neurosurg. 1998;89:507–518. doi: 10.3171/jns.1998.89.4.0507. [DOI] [PubMed] [Google Scholar]
- 5.Bullock R, Zauner A, Woodward J, Young HF. Massive persistent release of excitatory amino acids following human occlusive stroke. Stroke. 1995;26:2187–2189. doi: 10.1161/01.str.26.11.2187. [DOI] [PubMed] [Google Scholar]
- 6.Calo G, Sbrenna S, Bianchi C, Beani L. Immediate and delayed effects of in vitro ischemia on glutamate efflux from guinea-pig cerebral cortex slices. Brain Res. 1997;751:300–306. doi: 10.1016/s0006-8993(96)01425-4. [DOI] [PubMed] [Google Scholar]
- 7.Caron MJ, Hovda DA, Becker DP. Changes in the treatment of head injury. Neurosurg Clin N Am. 1991;2:483–491. [PubMed] [Google Scholar]
- 8.Caragine LP, Park HK, Diaz FG, Phillis JW. Real-time measurement of ischemia-evoked glutamate release in the cerebral cortex of four and eleven vessel rat occlusion models. Brain Res. 1998;793:255–264. doi: 10.1016/s0006-8993(98)00182-6. [DOI] [PubMed] [Google Scholar]
- 9.Chapman AG, Halsey MJ, Hart GP, Luff NP, Meldrum BS, Wardley-Smith B. Regional amino acid concentration in the brains of rats exposed to high pressures. J Neurochem. 1986;47:314–317. doi: 10.1111/j.1471-4159.1986.tb02864.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 11.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 12.Choi S, Kang SW, Lee GJ, Choi SK, Chae SJ, Park HK, et al. Real-time ischemic condition monitoring in normoglycemic and hyperglycemic rats. Physiol Meas. 2010;31:439–450. doi: 10.1088/0967-3334/31/3/011. [DOI] [PubMed] [Google Scholar]
- 13.Choi S, Lee GJ, Chae SJ, Kang SW, Yin CS, Lee SH, et al. Potential neuroprotective effects of acupuncture stimulation on diabetes mellitus in a global ischemic rat model. Physiol Meas. 2010;31:633–647. doi: 10.1088/0967-3334/31/5/003. [DOI] [PubMed] [Google Scholar]
- 14.Dirnagl U, Jacewicz M, Pulsinelli W. Nimodipine posttreatment does not increase blood flow in rats with focal cortical ischemia. Stroke. 1990;21:1357–1361. doi: 10.1161/01.str.21.9.1357. [DOI] [PubMed] [Google Scholar]
- 15.Drejer J, Benveniste H, Diemer NH, Schousboe A. Cellular origin of ischemia-induced glutamate release from brain tissue in vivo and in vitro. J Neurochem. 1985;45:145–151. doi: 10.1111/j.1471-4159.1985.tb05486.x. [DOI] [PubMed] [Google Scholar]
- 16.Estevez AY, O'Regan MH, Song D, Phillis JW. Effects of anion channel blockers on hyposmotically induced amino acid release from the in vivo rat cerebral cortex. Neurochem Res. 1999;24:447–452. doi: 10.1023/a:1020902104056. [DOI] [PubMed] [Google Scholar]
- 17.Feinberg WM, Bruck DC. Effect of oral nimodipine on platelet function. Stroke. 1993;24:10–13. doi: 10.1161/01.str.24.1.10. [DOI] [PubMed] [Google Scholar]
- 18.Fujisawa A, Matsumoto M, Matsuyama T, Ueda H, Wanaka A, Yoneda S, et al. The effect of the calcium antagonist nimodipine on the gerbil model of experimental cerebral ischemia. Stroke. 1986;17:748–752. doi: 10.1161/01.str.17.4.748. [DOI] [PubMed] [Google Scholar]
- 19.Guyot LL, Diaz FG, O'Regan MH, Song D, Phillis JW. The effect of streptozotocin-induced diabetes on the release of excitotoxic and other amino acids from the ischemic rat cerebral cortex. Neurosurgery. 2001;48:385–390. doi: 10.1097/00006123-200102000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Huang SJ, Chang L, Han YY, Lee YC, Tu YK. Efficacy and safety of hypertonic saline solutions in the treatment of severe head injury. Surg Neurol. 2006;65:539–546. doi: 10.1016/j.surneu.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Katayama Y, Becker DP, Tamura T, Hovda DA. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J Neurosurg. 1990;73:889–900. doi: 10.3171/jns.1990.73.6.0889. [DOI] [PubMed] [Google Scholar]
- 22.Katayama Y, Kawamata T, Tamura T, Hovda DA, Becker DP, Tsubokawa T. Calcium-dependent glutamate release concomitant with massive potassium flux during cerebral ischemia in vivo. Brain Res. 1991;558:136–140. doi: 10.1016/0006-8993(91)90730-j. [DOI] [PubMed] [Google Scholar]
- 23.Koura SS, Doppenberg EM, Marmarou A, Choi S, Young HF, Bullock R. Relationship between excitatory amino acid release and outcome after severe human head injury. Acta Neurochir Suppl. 1998;71:244–246. doi: 10.1007/978-3-7091-6475-4_70. [DOI] [PubMed] [Google Scholar]
- 24.Krieglstein J, Lippert K, Pöch G. Apparent independent action of nimodipine and glutamate antagonists to protect cultured neurons against glutamate-induced damage. Neuropharmacology. 1996;35:1737–1742. doi: 10.1016/s0028-3908(96)00104-9. [DOI] [PubMed] [Google Scholar]
- 25.Kulik A, Trapp S, Ballanyi K. Ischemia but not anoxia evokes vesicular and Ca(2+)independent glutamate release in the dorsal vagal complex in vitro. J Neurophysiol. 2000;83:2905–2915. doi: 10.1152/jn.2000.83.5.2905. [DOI] [PubMed] [Google Scholar]
- 26.Lazarewicz JW, Pluta R, Puka M, Salinska E. Diverse mechanisms of neuronal protection by nimodipine in experimental rabbit brain ischemia. Stroke. 1990;21:IV108–IV110. [PubMed] [Google Scholar]
- 27.Lee GJ, Choi SK, Eo YH, Kang SW, Choi S, Park JH, et al. The effect of extracellular glutamate release on repetitive transient ischemic injury in global ischemia model. Korean J Physiol Pharmacol. 2009;13:23–26. doi: 10.4196/kjpp.2009.13.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SR, Lok J, Rosell A, Kim HY, Murata Y, Atochin D, et al. Reduction of hippocampal cell death and proteolytic responses in tissue plasminogen activator knockout mice after transient global cerebral ischemia. Neuroscience. 2007;150:50–57. doi: 10.1016/j.neuroscience.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limbrick DD, Jr, Sombati S, DeLorenzo RJ. Calcium influx constitutes the ionic basis for the maintenance of glutamate-induced extended neuronal depolarization associated with hippocampal neuronal death. Cell Calcium. 2003;33:69–81. doi: 10.1016/s0143-4160(02)00054-4. [DOI] [PubMed] [Google Scholar]
- 30.Marcoli M, Bonfanti A, Roccatagliata P, Chiaramonte G, Ongini E, Raiteri M, et al. Glutamate efflux from human cerebrocortical slices during ischemia : vesicular-like mode of glutamate release and sensitivity to A(2A) adenosine receptor blockade. Neuropharmacology. 2004;47:884–891. doi: 10.1016/j.neuropharm.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto M, Scheller MS, Zornow MH, Strnat MA. Effect of S-emopamil, nimodipine, and mild hypothermia on hippocampal glutamate concentrations after repeated cerebral ischemia in rabbits. Stroke. 1993;24:1228–1234. doi: 10.1161/01.str.24.8.1228. [DOI] [PubMed] [Google Scholar]
- 32.Milde LN, Milde JH, Michenfelder JD. Delayed treatment with nimodipine improves cerebral blood flow after complete cerebral ischemia in the dog. J Cereb Blood Flow Metab. 1986;6:332–337. doi: 10.1038/jcbfm.1986.56. [DOI] [PubMed] [Google Scholar]
- 33.Miljanich GP, Ramachandran J. Antagonists of neuronal calcium channels : structure, function, and therapeutic implications. Annu Rev Pharmacol Toxicol. 1995;35:707–734. doi: 10.1146/annurev.pa.35.040195.003423. [DOI] [PubMed] [Google Scholar]
- 34.Morley P, Hogan MJ, Hakim AM. Calcium-mediated mechanisms of ischemic injury and protection. Brain Pathol. 1994;4:37–47. doi: 10.1111/j.1750-3639.1994.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 35.Muir KW, Lees KR. Excitatory amino acid antagonists for acute stroke. Cochrane Database Syst Rev. 2003:CD001244. doi: 10.1002/14651858.CD001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakane H, Ooboshi H, Ibayashi S, Yao H, Sadoshima S, Fujishima M. Isradipine, a calcium channel blocker, attenuates the ischemia-induced release of dopamine but not glutamate in rats. Neurosci Lett. 1995;188:151–154. doi: 10.1016/0304-3940(95)11417-u. [DOI] [PubMed] [Google Scholar]
- 37.Siesjö BK, Bengtsson F. Calcium fluxes, calcium antagonists, and calcium-related pathology in brain ischemia, hypoglycemia, and spreading depression : a unifying hypothesis. J Cereb Blood Flow Metab. 1989;9:127–140. doi: 10.1038/jcbfm.1989.20. [DOI] [PubMed] [Google Scholar]
- 38.Steen PA, Newberg LA, Milde JH, Michenfelder JD. Nimodipine improves cerebral blood flow and neurologic recovery after complete cerebral ischemia in the dog. J Cereb Blood Flow Metab. 1983;3:38–43. doi: 10.1038/jcbfm.1983.4. [DOI] [PubMed] [Google Scholar]
- 39.Terrian DM, Dorman RV, Gannon RL. Characterization of the presynaptic calcium channels involved in glutamate exocytosis from rat hippocampal mossy fiber synaptosomes. Neurosci Lett. 1990;119:211–214. doi: 10.1016/0304-3940(90)90836-x. [DOI] [PubMed] [Google Scholar]
- 40.Van Harreveld A, Fifková E. Mechanisms involved in spreading depression. J Neurobiol. 1973;4:375–387. doi: 10.1002/neu.480040406. [DOI] [PubMed] [Google Scholar]
- 41.Wahl F, Obrenovitch TP, Hardy AM, Plotkine M, Boulu R, Symon L. Extracellular glutamate during focal cerebral ischaemia in rats : time course and calcium dependency. J Neurochem. 1994;63:1003–1011. doi: 10.1046/j.1471-4159.1994.63031003.x. [DOI] [PubMed] [Google Scholar]
- 42.Welsch M, Nuglisch J, Krieglstein J. Neuroprotective effect of nimodipine is not mediated by increased cerebral blood flow after transient forebrain ischemia in rats. Stroke. 1990;21:IV105–IV107. [PubMed] [Google Scholar]
- 43.Yi JH, Hazell AS. Excitotoxic mechanisms and the role of astrocytic glutamate transporters in traumatic brain injury. Neurochem Int. 2006;48:394–403. doi: 10.1016/j.neuint.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Yin CS, Choi SK, Lee GJ, Eo YH, Kim BS, Oh BS, et al. A real-time glutamate release in rat striatum of 11-vessel-occlusion ischemia model treated with acupuncture. Korean J Orient Physiol Pathol. 2008;22:835–840. [Google Scholar]
- 45.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]