Abstract
Objective
The primary objective of this study was to perform a retrospective evaluation of the radiological and pathological features influencing the formation of peritumoral brain edema (PTBE) in meningiomas.
Methods
The magnetic resonance imaging (MRI) and pathology data for 86 patients with meningiomas, who underwent surgery at our institution between September 2003 and March 2009, were examined. We evaluated predictive factors related to peritumoral edema including gender, tumor volume, shape of tumor margin, presence of arachnoid plane, the signal intensity (SI) of the tumor in T2-weighted image (T2WI), the WHO histological classification (GI, GII/GIII) and the Ki-67 antigen labeling index (LI). The edema-tumor volume ratio was calculated as the edema index (EI) and was used to evaluate peritumoral edema.
Results
Gender (p=0.809) and pathological finding (p=0.084) were not statistically significantly associated with peritumoral edema by univariate analysis. Tumor volume was not correlated with the volume of peritumoral edema. By univariate analysis, three radiological features, and one pathological finding, were associated with PTBE of statistical significance: shape of tumor margin (p=0.001), presence of arachnoid plane (p=0.001), high SI of tumor in T2WI (p=0.001), and Ki-67 antigen LI (p=0.049). These results suggest that irregular tumor margins, hyperintensity in T2WI, absence of arachnoid plane on the MRI, and high Ki-67 LI can be important predictive factors that influence the formation of peritumoral edema in meningiomas. By multivariate analysis, only SI of the tumor in T2WI was statistically significantly associated with peritumoral edema.
Conclusion
Results of this study indicate that irregular tumor margin, hyperintensity in T2WI, absence of arachnoid plane on the MRI, and high Ki-67 LI may be important predictive factors influencing the formation of peritumoral edema in meningiomas.
Keywords: Brain edema, Edema index, Intracranial meningioma, Labeling index
INTRODUCTION
Meningioma is a common brain tumor that develops in the intracranial cavity and displays a variety of radiological findings. The characteristics of meningioma include: various vascularities, benign histological traits, and slow-growing tumors. Peritumoral brain edema (PTBE) is found in more than half of all meningioma cases. Various degrees and shapes of edema have been reported, ranging from barely noticeable to up to 2-3 times the volume of the tumor1,2,5,6,13,15). PTBE increases intracranial pressure and induces neurological impairment. Also, PTBE makes surgical approach difficult and influences surgical outcome, prognosis and recurrence1,2,9,10,20).
The precise mechanisms of the development of PTBE with meningioma have not been clearly identified. Based on correlations between various factors associated with PTBE, contributors include age, gender, tumor size, location of tumor, histological findings, vascularity, sex hormone levels, the biology of the tumor and signal intensity of tumor in T2WI. However, there were no clear explanations of these correlations1,3,5,6,9,15).
This study was performed retrospectively to analyze the association between meningioma edema, radiological characteristics, histological findings, and tumor labeling index (LI) in order to evaluate the effects of these factors on brain edema. The purpose of this study was to provide a foundation for pre-operative evaluation of meningioma and prospective research on prognosis.
MATERIALS AND METHODS
We retrospectively reviewed 86 patients with brain tumors who were histologically confirmed as meningioma between September 2003 and March 2009. MRIs were checked in all patients prior to surgery, and radiological data included tumor size and peritumoral brain edema. After surgery, histological analysis and tumor LI tests were performed. We examined the correlations between PTBE and gender, tumor volume, the shape of tumor margin, the existence of arachnoid plane, the signal intensity (SI) of tumor in the T2-weighted image (T2WI), WHO histological classifications, and Ki-67 antigen LI.
The MRI findings were retrospectively analyzed by three readers. When there were variations on readings, the decision was made according to the results obtained by the majority of the readers.
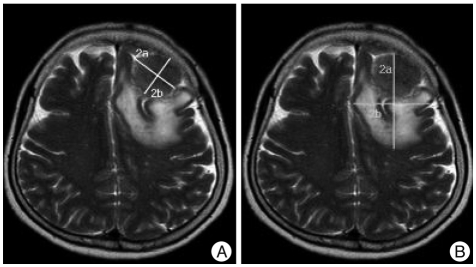
Tumor volume and edema were measured from the MRI. The longest diameter of the tumor and the vertical diameter of the tumor were measured from the axial image. The axial diameter of the tumor was measured from the coronal image. These three measurements were multiplied to calculate the volume of the elliptical sphere (Fig. 1). PTBE volume was measured in a similar manner by measuring the high SI region in T2WI that was clearly distinguished from normal tissues, including the tumor. Edema index was calculated by dividing the PTBE volume by tumor volume. The Edema index represents the degree of the PTBE, compared to tumor volume, with an index of 1.0 indicating no PTBE development. For histological diagnosis, the meningioma was divided into two groups, Grade I or Grade II/Grade III, according to WHO classification.
Fig. 1.
A : Tumor volume (VTumor) is measured from MR image, that is, from maximal perpendicular diameters (radii a and b) of the tumor. Coronal diameter of the tumor is approximated by coronal section images showing tumor tissue (radius c, not shown here). B : Volume of tumor and edema (VTumor + Edema) is measured with same method. The resulting of volume is then approximated using the formula for a spheroid : Volume=4/3×π× abc. Edema Index=(VTumor + Edema)/(VTumor).
Statistical analysis was done using SPSS 11 for Windows; SPSS, Chicago, I11. The correlation between tumor volume and PTBE volume was examined using Pearson's correlation test. The correlations between the ratio of the edema index and tumor volume, gender, peritumoral arachnoid plane existence, tumor interface shape, SI in T2WI, histological classification, and Ki-67 antigen LI were examined using the χ2-test for univariate analysis as well as logistic regression for multivariate analysis. Statistical significance was p<0.05.
RESULTS
The patient population consisted of 23 men and 63 women, ranging in age from 15 to 77 years. PTBE was present in 50 cases and absent in the other 36.
Relationship between gender and peritumoral brain edema
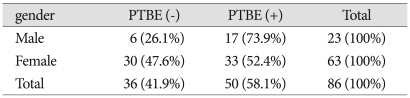
Of the 23 male cases, six had accompanying PTBE, while edema was found in 30 of the 63 female cases. No significant correlation was observed between PTBE and gender (Table 1).
Table 1.
Analysis of gender and peritumoral brain edema
p=0.809, present : +, absent : -, PTBE : peritumoral brain edema
Relationship between tumor volume and peritumoral brain edema
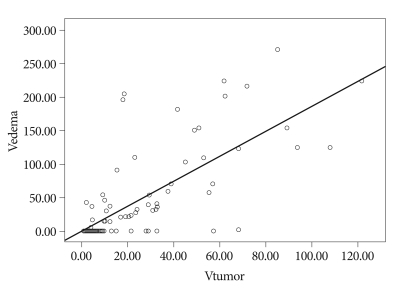
PTBE volume tended to be proportional to tumor volume. The average volume of the meningioma accompanying PTBE was 36.28 cm3, which was larger than the 8.81 cm3 average volume of meningiomas without edema. However, the relationship between tumor volume and edema size was not significant (Fig. 2).
Fig. 2.
Relationship of tumor volume and edema volume. p=0.537.
Relationship between the shape of tumor margin and peritumoral brain edema
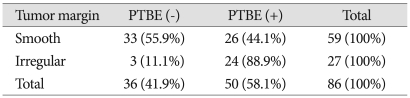
MRI was used to examine the correlation between PTBE and the shape of tumor margin. We divided the tumor margin into irregular or smooth type. We defined 'smooth margin' in contrast to marginal irregularities with nodularity, indentation or projections. Twenty-four of the 27 cases showed irregular margins and 26 of the 59 cases showed smooth surfaces, accompanied by PTBE. An irregular margin was significantly more likely to accompany PTBE (p=0.001) (Table 2).
Table 2.
Analysis of tumor margin and peritumoral brain edema
p=0.001, present : +, absent : -, PTBE : peritumoral brain edema
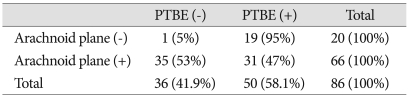
Relationship between the arachnoid plane and peritumoral brain edema
The arachnoid plane was absent in 20 cases, and 19 of these 20 cases showed PTBE. The arachnoid plane was present in 66 cases, and 31 of these 66 cases showed PTBE. These results indicate that PTBEs would occurr with a statistically higher frequency in the former cases than in the latter (p=0.001) (Table 3).
Table 3.
Analysis of arachnoid plane and peritumoral brain edema
p=0.001, present : +, absent : -, PTBE : peritumoral brain edema
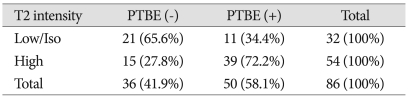
Relationship between the T2-weighted image signal intensity and peritumoral brain edema
When the SI of the tumor in T2WI was compared with brain gray matter, fifty-four cases had higher SI, and 39 of theses 54 cases showed PTBE. Thirty-two cases shoed equal or lower SI, and 11 of these 32 cases showed PTBE. These results indicate that PTBEs would occurr with a statistically higher frequency in the former cases than in the latter. Higher-signal intensity accompanied PTBE was seen significantly more often than lower signal intensity (p=0.001) (Table 4).
Table 4.
Analysis of T2 signal intensity of tumor and peritumoral brain edema
p=0.001, present : +, absent : -, PTBE : peritumoral brain edema
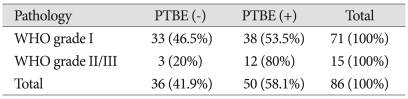
Relationship between tumor histological classification and peritumoral brain edema
Of the 86 meningioma cases, 71 were classified as WHO GI, based on histological tests, and 38 of the 71 cases had accompanying PTBE. Of the 15 cases classified as GII/GIII, 12 had accompanied PTBE. However, the relationships between the two groups were not significant (Table 5).
Table 5.
Analysis of pathology and peritumoral brain edema
p=0.084, present : + , absent : - , PTBE : peritumoral brain edema
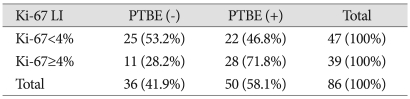
Relationship between Ki-67 antigen labeling index and peritumoral brain edema
The Ki-67 antigen LI represents the differentiation potential of the meningioma, and the average index for all cases in this study was 4.2%. For analysis of the correlation between the Ki-67 antigen LI and the edema ratio, the cases were grouped as being either above or below 4%. Of the 47 cases with a Ki-67 antigen LI below 4%, 22 had associated PTBE, while edema was found in 28 of the 39 cases with a Ki-67 antigen LI of 4% or higher. Cases with a Ki-67 antigen LI of 4% or higher showed a significant correlation with PTBE (p=0.049) (Table 6).
Table 6.
Analysis of Ki-67 antigen LI and peritumoral brain edema
p=0.049, LI : labeling index, Present : +, absent : -, PTBE : peritumoral brain edema
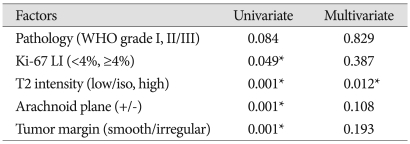
Univariate and multivariate statistical analyses of peritumoral brain edema
Based on univariate statistical analysis, factors that displayed significant correlation with PTBE were: higher than 4% Ki-67 LI, high SI of tumor, irregular tumor margin, and absence of arachnoid plane. However, as seen in Table 7, multivariate logistical analysis suggested that only a high SI in T2WI was significantly correlated with PTBE (p=0.012) (Table 7).
Table 7.
Univariate and multivariate statistical analyses of peritumoral brain edema
*p<0.05 : statistically significant, Present : +, absent : -, LI : labeling index
DISCUSSION
Meningioma is an intracranial tumor that develops in the meninges surrounding the brain. It is histologically benign, and PTBE is found in more than half of cases1,9). In addition to age and gender, radiological findings, including tumor size, vascularity, tumor location, tumor shape, and histological findings (such as macrophage infiltration), and an increase in the number of dividing cells have been reported as possible causes of PTBE5,10,11,14). However, the causes of meningioma with accompanying PTBE have not been identified. Because brain edema has been reported to influence post-operative fatality and meningioma prevalence10,19), numerous studies have investigated the causes of PTBE. One study proposed that it is caused by increased permeability through white matter fibers that become relatively loose around the limiting membrane of the tumor1,14), while another study reported that vascular brain edema is caused by a failure of the blood-brain barrier13). However, the exact pathogenesis of PTBE in meningioma has not yet explained.
From univariate analyses in this study, we concluded that cases with irregular tumor margins, as identified by MRI, absence of peritumoral arachnoid plane, high SI of the tumor in T2WI, and high Ki-67 antigen LI were significantly correlated with PTBE. From multivariate analysis, we confirmed that only cases with a high SI of the tumor in T2WI were statistically associated with PTBE.
Previous studies have reported that gender and PTBE are not correlated and, in this study, we arrived at the same conclusion5,11,20). Nevertheless, since anaplastic meningioma occurs with a higher frequency in women than in men, further pathological research is necessary to consider various factors, such as gender differences, in the release of sex hormones.
A number of papers have reported no statistical significance between meningioma volume and peritumoral edema, consistent with our findings5,9). However, a larger tumor is more likely to cause vascular, ischemic, and secondary brain edema, and some studies have claimed that venous compression of meningioma is a major factor in PTBE development1,11,19). These hypotheses suggest that additional research regarding the relationship between meningioma size and PTBE frequency is needed.
Some studies have reported that meningioma with irregular margins has a higher PTBE frequency than meningioma with smooth margins,which is in agreement with the results of our study11,12). The level of irregularity represents the relationship between tumor surface and the adjacent brain tissues, especially the vascularity of the cortex within the margin12).
The arachnoid plane around the tumor margin is also a factor representing a physiological barrier of the adjacent normal brain tissues from tumors such as meningioma. Previous studies have reported a higher frequency of PTBE in the absence of the arachnoid plane, consistent with the results of our study20). The arachnoid plane is a brain-cerebrospinal barrier that is not water-permeable. The pia mater is permeable for water and electrolytes, but is not permeable for polymers, such as plasma proteins, and the cerebral cortex is not water-permeable. Therefore, vascular edema may occur from plasma proteins and blood plasma flowing into the white matter because of increased vascular permeability. This may occur by edema fluid produced by meningioma and substances that contribute to the development of edema.
A high SI in T2WI of the tumor indicates a high water content and a high level of vascularity in the tumor10,12). Tumors with high water content can easily spread to adjacent organs because of differences in osmotic pressure12). We observed a significant association between a high SI in T2WI and PTBE. The difference in water content level in tumors appears to be the cause of this result.
Atypical and anaplastic meningiomas (GII/GIII) have been reported to show higher cell proliferation rates than benign meningiomas (GI)2). Also, PTBE in intracranial meningiomas has been reported to be associated with histological grade6). However, our results did not support these findings. The discrepancy suggests that the mechanism of PTBE development in meningioma is different from the brain edema of other intracranial tumors.
Several studies have found that the MIB-1 monoclonal antibody, which stains the Ki-67 antigen, acts as an index of growth and meningioma recurrence6,17). Meningiomas, with a high Ki-67 antigen LI, display high levels of vascularity and water permeability16). A study on Ki-67 antigen LI and recurrence of partially removed meningioma has found that the higher the Ki-67 antigen LI, the faster the tumor's doubling time and growth rate6). In our study, based on an average Ki-67 antigen LI of 4% from 86 cases, we concluded that a higher Ki-67 antigen LI leads to a higher frequency of PTBE, consistent with previous findings. PTBE must be considered when deciding on a strategy for the management of a meningioma, because it may be related to perioperative morbidity, invasion potential, and meningioma recurrence.
CONCLUSION
We suggest that irregular tumor margin, hyperintensity of tumor in T2WI, absence of arachnoid plane on the MRI, and high Ki-67 LI may be important predictive factors influencing the formation of peritumoral edema in meningiomas. According to multivariate analysis, only hyperintensity of tumors in T2WI was significantly associated with peritumoral edema.
Acknowledgements
This research was supported by Yeungnam University Research Grants in 2009.
References
- 1.Bitzer M, Wöckel L, Morgalla M, Keller C, Friese S, Heiss E, et al. Peritumoural brain oedema in intracranial meningiomas : influence of tumour size, location and histology. Acta Neurochir (Wien) 1997;139:1136–1142. doi: 10.1007/BF01410973. [DOI] [PubMed] [Google Scholar]
- 2.Crone KR, Challa VR, Kute TE, Moody DM, Kelly DL., Jr Relationship between flow cytometric features and clinical behavior of meningiomas. Neurosurgery. 1988;23:720–724. doi: 10.1227/00006123-198812000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Elster AD, Challa VR, Gilbert TH, Richardson DN, Contento JC. Meningiomas : MR and histopathologic features. Radiology. 1989;170:857–862. doi: 10.1148/radiology.170.3.2916043. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert JJ, Paulseth JE, Coates RK, Malott D. Cerebral edema associated with meningiomas. Neurosurgery. 1983;12:599–605. doi: 10.1227/00006123-198306000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Gurkanlar D, Er U, Sanli M, Ozkan M, Sekerci Z. Peritumoral brain edema in intracranial meningiomas. J Clin Neurosci. 2005;12:750–753. doi: 10.1016/j.jocn.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Ide M, Jimbo M, Yamamoto M, Umebara Y, Hagiwara S, Kubo O. MIB-1 staining index and peritumoral brain edema of meningiomas. Cancer. 1996;78:133–143. doi: 10.1002/(SICI)1097-0142(19960701)78:1<133::AID-CNCR19>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Inamura T, Nishio S, Takeshita I, Fujiwara S, Fukui M. Peritumoral brain edema in meningiomas--influence of vascular supply on its development. Neurosurgery. 1992;31:179–185. doi: 10.1227/00006123-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan RD, Coon S, Drayer BP, Bird CR, Johnson PC. MR characteristics of meningioma subtypes at 1.5 tesla. J Comput Assist Tomogr. 1992;16:366–371. doi: 10.1097/00004728-199205000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Kim IS, Kim HD, Kim KU, Shin HC, Choin HJ, Kim KH. Factors influencing the development of peritumoral brain edema in menigiomas. J Korean Neurosurg Soc. 1997;26:940–945. [Google Scholar]
- 10.Lee KJ, Joo WI, Rha HK, Park HK, Chough JK, Hong YK, et al. Peritumoral brain edema in meningiomas : correlations between magnetic resonance imaging, angiography, and pathology. Surg Neurol. 2008;69:350–355. doi: 10.1016/j.surneu.2007.03.027. discussion 355. [DOI] [PubMed] [Google Scholar]
- 11.Lobato RD, Alday R, Gómez PA, Rivas JJ, Domínguez J, Cabrera A, et al. Brain oedema in patients with intracranial meningioma. Correlation between clinical, radiological, and histological factors and the presence and intensity of oedema. Acta Neurochir (Wien) 1996;138:485–493. doi: 10.1007/BF01411166. discussion 493-494. [DOI] [PubMed] [Google Scholar]
- 12.Nakano T, Asano K, Miura H, Itoh S, Suzuki S. Meningiomas with brain edema : radiological characteristics on MRI and review of the literature. Clin Imaging. 2002;26:243–249. doi: 10.1016/s0899-7071(02)00433-3. [DOI] [PubMed] [Google Scholar]
- 13.Nam DH, Lee SK, Whang SH, Shin HJ, Lee JI, Kim JS, et al. A study of the effects of clinicobiological factors upon the meningioma-associated peritumoral edema formation. J Korean Neurosurg Soc. 1998;27:453–459. [Google Scholar]
- 14.Otsuka S, Tamiya T, Ono Y, Michiue H, Kurozumi K, Daido S, et al. The relationship between peritumoral brain edema and the expression of vascular endothelial growth factor and its receptors in intracranial meningiomas. J Neurooncol. 2004;70:349–357. doi: 10.1007/s11060-004-9164-4. [DOI] [PubMed] [Google Scholar]
- 15.Paek SH, Kim CY, Kim YY, Park IA, Kim MS, Kim DG, et al. Correlation of clinical and biological parameters with peritumoral edema in meningioma. J Neurooncol. 2002;60:235–245. doi: 10.1023/a:1021186401522. [DOI] [PubMed] [Google Scholar]
- 16.Roser F, Samii M, Ostertag H, Bellinzona M. The Ki-67 proliferation antigen in meningiomas. Experience in 600 cases. Acta Neurochir (Wien) 2004;146:37–44. doi: 10.1007/s00701-003-0173-4. discussion 44. [DOI] [PubMed] [Google Scholar]
- 17.Salpietro FM, Alafaci C, Lucerna S, Iacopino DG, Todaro C, Tomasello F. Peritumoral edema in meningiomas : microsurgical observations of different brain tumor interfaces related to computed tomography. Neurosurgery. 1994;35:638–641. doi: 10.1227/00006123-199410000-00009. discussion 641-642. [DOI] [PubMed] [Google Scholar]
- 18.Simis A, Pires de Aguiar PH, Leite CC, Santana PA, Jr, Rosemberg S, Teixeira MJ. Peritumoral brain edema in benign meningiomas : correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg Neurol. 2008;70:471–477. doi: 10.1016/j.surneu.2008.03.006. discussion 477. [DOI] [PubMed] [Google Scholar]
- 19.Tamiya T, Ono Y, Matsumoto K, Ohmoto T. Peritumoral brain edema in intracranial meningiomas : effects of radiological and histological factors. Neurosurgery. 2001;49:1046–1051. doi: 10.1097/00006123-200111000-00003. discussion 1051-1052. [DOI] [PubMed] [Google Scholar]
- 20.Vignes JR, Sesay M, Rezajooi K, Gimbert E, Liguoro D. Peritumoral edema and prognosis in intracranial meningioma surgery. J Clin Neurosci. 2008;15:764–768. doi: 10.1016/j.jocn.2007.06.001. [DOI] [PubMed] [Google Scholar]