Abstract
Objective
The author measured levels of fluoroscopic radiation exposure to the surgeon's body based on the different beam directions during kyphoplasty.
Methods
This is an observational study. A series of 84 patients (96 vertebral bodies) were treated with kyphoplasty over one year. The patients were divided into four groups based on the horizontal and vertical directions of the X-Ray beams. We measured radiation exposure with the seven dosimetry badges which were worn by the surgeon in each group (total of 28 badges). Twenty-four procedures were measured in each group. Cumulative dose and dose rates were compared between groups.
Results
Fluoroscopic radiation is received by the operator in real-time for approximately 50% (half) of the operation time. Thyroid protectors and lead aprons can block radiation almost completely. The largest dose was received in the chest irrespective of beam directions. The lowest level of radiation were received when X-ray tube was away from the surgeon and beneath the bed (dose rate of head, neck, chest, abdomen and knee : 0.2986, 0.2828, 0.9711, 0.8977, 0.8168 mSv, respectively). The radiation differences between each group were approximately 2.7-10 folds.
Conclusion
When fluoroscopic guided-KP is performed, the X-Ray tube should be positioned on the opposite side of the operator and below the table, otherwise the received radiation to the surgeon's body would be 2.7-10 times higher than such condition.
Keywords: Kyphoplasty, Radiation exposure, Fluoroscopic guidance, Dosimetry, Radiation safety, Fluoroscopy
INTRODUCTION
Nowadays, percutaneous vertebroplasty or kyphoplasty (KP) is a treatment method commonly recommended for patients with stable osteoporotic compression, burst or pathologic fractures, and has such advantages as less bleeding, less pain, less risk of infection, small skin incision, early ambulation and shorter hospital stays like other minimally invasive spinal surgeries (MISS)15). The KP procedure includes level localization, cannula insertion, balloon inflation, and cement injection. Since C-arm fluoroscopy is essential in each step, exposure to ionizing radiation by surgeons is unavoidable.
X-ray beams are generated from the X-ray tube of the C-arm fluoroscopy and since images are produced and amplified by the image intensifier, the radiation exposure dose increases as the distance between the surgeon and the tube decreases. Also, when performing KP, lateral views and AP views of the C-arm fluoroscopy should be viewed alternately in succession. This means that the radiation exposure dose per unit vertebral body typically exceeds other minimally invasive spinal surgeries. Although the X-ray tube should be positioned as far away from the operator as possible during each operation, this fact is frequently forgotten during operations. Because the image intensifier is smaller than the X-ray tube, surgeons typically feel that the workspace for the operation will be reduced if the X-ray tube is located farther away. The X-ray tube and the image intensifier can be positioned in four different combinations but the degrees and risks of radiation exposure from each of these combinations have not yet been studied well.
The purpose of this study is to quantitatively analyze the doses of radiation exposure relative to the distance from the X-ray tube to major areas of the human body, and relative to the direction of the X-Ray beams generated from the C-arm fluoroscopy, and to assess the risk and degree of occupational radiation exposure.
MATERIALS AND METHODS
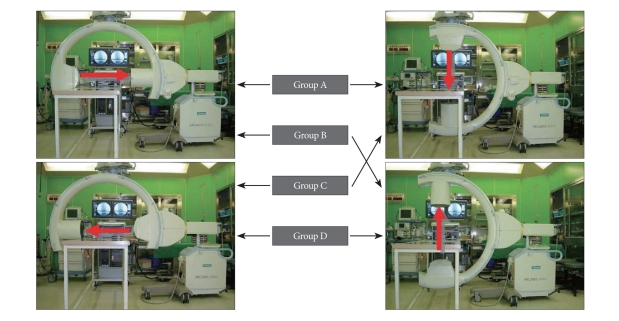
We conducted an observational study on 84 consecutive patients who underwent percutaneous kyphoplasty. All procedures were implemented by one surgeon with 7 years of experience in spinal surgeries. The patients were divided into four groups based on the positions of the X-ray tube and image intensifier of the C-arm fluoroscopy relative to the operator and the patient during surgery. In Group A, the X-Ray tube was positioned on the same side as the operator on the lateral view and above the patient on the anterioposterior (AP) view; in group B, the X-Ray tube was positioned on the same side as the operator on the lateral view and below the patient on the AP view; in group C, the X-Ray tube was positioned on the side opposite to the operator on the lateral view and above the patient on the AP view; and in group D, the X-Ray tube was positioned on the side opposite to the operator on the lateral view and below the patient on the AP view (Fig. 1). The operator always stood on the left side of the patients who were prone position when performing a surgery.
Fig. 1.
The settings of intraoperative C-arm fluoroscopy. A : X-ray tube is above the patient and near the operator (group A). B : X-ray tube is beneath the patient and near the operator (group B). C : X-ray tube is above the patient and on the opposite side of the table from the operator (group C). D : X-ray tube is beneath the patient and on the opposite side of the table from the operator (group D).
The radiation received was measured over the whole duration of the procedures using dosimetry badges. Seven dosimetry badges (Panasonic, Chiyoda Technology Corporation, Tokyo, Yushima Bunkyo-Ku, Japan) were worn : one on the head (forehead), two on the neck (one outside and one inside of the thyroid protector), two on the chest (one outside and one inside of the lead apron), one on the abdomen, and one on the knee. Seven dosimetry badges per group yields a total of 28 badges used to measure the levels of radiation exposure. All surgeries were conducted using with same C-arm fluoroscopy unit (Siemens ARCADIS Orbic, München, Germany).
All KP procedures were conducted under local anesthesia using standard approaches (transpedicular & bilateral). After aseptic draping, the operation site was level-localized using the C-arm fluoroscopy. Under AP control of C-arm fluoroscopy, an 11-gauge vertebroplasty needle was inserted into the left pedicle, a K-wire was inserted through the needle and then the vertebroplasty needle was removed, leaving the K-wire. A cannula large enough for balloons to pass through was inserted using the K-wire left in the pedicle, and a balloon was inserted through the cannula. A second balloon was inserted into the right pedicle using the same method. The two balloons were carefully inflated using steady pressure not to exceed 200psi and then deflated and removed. No intraoperative venography was conducted. Polymethylmethacrylate was mixed to the consistency of tooth-paste using the standard mixture method and then infused into the bilateral cannulas and the skin was closed to finish the operation.
The duration of each operation was measured from the point at which a vertebroplasty needle was initially inserted into the left pedicle to the point at which the last infusion of PMMA cement was finished. The fluoroscopy running time was automatically recorded in the C-arm fluoroscopy machine in real time. The energy output of the X-ray beam generation was measured by the kilovolt and milliampere values recorded in the C-arm fluoroscopy machine during each operation.
RESULTS
Demographics
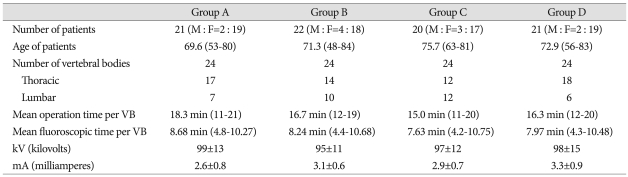
Summaries of all cases are listed in Table 1. Of the 84 subject patients; the numbers of patients in groups A, B, C and D were 21, 22, 20 and 21, respectively. The ratios between males and females for the four group were 2 : 19, 4 : 18, 3 : 17 and 2 : 19, respectively. The number of vertebral bodies (VB) involved in the operations was 24 for each group. In Group A, 17 thoracic VBs and 7 lumbar VBs were involved in KP operations; in group B, 14 thoracic VBs and 10 lumbar VBs; in group C, 12 thoracic VBs and 12 lumbar VBs; and in group D, 18 thoracic VBs and 6 lumbar VBs. Mean operation time was 18.3 minutes (11-21) for group A, 16.7 minutes (12-19) for group B, 15.0 minutes (11-20) for group C and 16.3 minutes (12-20) for group D. Mean fluoroscopic time was 8.68 minutes (4.8-10.27) for group A, 8.24 minutes (4.4-10.68) for group B, 7.63 minutes (4.2-10.75) for group C and 7.97 minutes (4.3-10.48) for group D. The results show that for approximately 50% of the operation time, the operator was exposed to fluoroscopic radiation in real-time. Energy output was 99±13 kV, 2.6±0.8 mA for group A, 95±11 kV, 3.1±0.6 mA for group B, 97±12 kV, 2.9±0.7 mA for group C and 98±15 kV, 3.3±0.9 mA for group D.
Table 1.
Summary of cases
Cumulative Dose
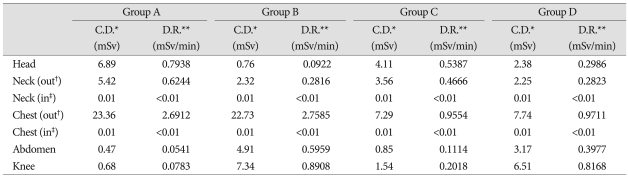
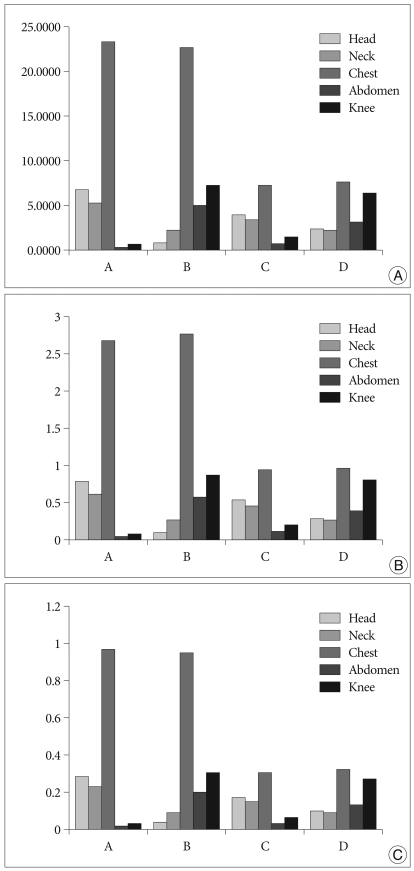
In all groups, radiation doses in lead aprons and thyroid protectors were measured at close to zero. In Group A, the area with the highest dose was the chest, with 23.36 mSv, followed by the head with 6.89 mSv, the neck with 5.42 mSv, the abdomen with 0.47 mSv and the knee with 0.68 mSv. The amount of irradiation to the head was around 10 times larger than the amount of irradiation to the knee. The amount of irradiation to the chest was around 3 times larger than the amount of irradiation received by the chest in group C or D. In Group B, the chest received the highest dose at 22.73 mSv and contrary to the results in group A, the dose of radiation delivered to the knee was 7.34 mSv, the abdomen 4.91 mSv, the neck 2.32 mSv and the head 0.76 mSv. Like in other groups, the area in Group C with the highest dose was the chest, with a dose of 7.29 mSv, followed by the head with 4.11 mSv, the neck with 3.56 mSv, the abdomen with 0.85 mSv and the knee with 1.54 mSv. The amount of irradiation to the head was around 2.7 times larger than the amount of irradiation to the knee. The amount of irradiation to the chest was approximately 1/3 than the amount of irradiation received by the chest in group A or B. Finally, in Group D, the area with the highest dose was the chest, with a dose of 7.74 mSv, followed by the knee with 6.51 mSv, the abdomen with 3.17 mSv, the neck with 2.25 mSv and the head with 2.38 mSv (Table 2, Fig. 2A).
Table 2.
Summary of the radiation exposure during kyphoplasty
*Cumulative dose, **Dose rate, †Outside of apron, ‡Inside of apron
Fig. 2.
Comparison among groups by graphs. A : Cumulative dose. B : Dose rate. C : Radiation exposure per one vertebral body (unit; mSv, vertical axis).
Dose Rate
Results indicate that dose rates behind lead aprons and thyroid protectors were close to zero. For Group A, the area with the highest DR was the chest with 2.6712 mSV/min. followed by the head with 0.7938 mSV/min., the neck with 0.6244 mSV/min., the abdomen with 0.0541 mSV/min. and the knee with 0.0783 mSV/min. For Group B, the area with the highest DR was the chest with 2.7585 mSV/min. followed by the knee with 0.8908 mSV/min., the abdomen with 0.5959 mSV/min., the neck with 0.2816 mSV/min. and the head with 0.0922 mSV/min. For Group C, the area with the highest DR was the chest with 0.9554 mSV/min. followed by the head with 0.5387 mSV/min., the neck with 0.4666 mSV/min., the abdomen with 0.1114 mSV/min. and the knee with 0.2018 mSV/min. For Group D, the area with the highest DR was the chest with 0.9711 mSV/min. followed by the knee with 0.8168 mSV/min., the abdomen with 0.3977 mSV/min, the neck with 0.2823 mSV/min. and the head with 0.2986 mSV/min.. For groups A and B, DRs to the chest were approximately three times higher than the DRs to the chest in groups C and D. In group A and B, DRs of the areas close to the X-Ray tube were approximately 10 times higher than that of the areas far from the X-ray tube. In groups C and D, DRs of the areas close to the X-Ray tube were approximately 2.5 times higher than that of the areas far from the X-ray tube (Table 2, Fig. 2B).
Radiation exposure per vertebral body
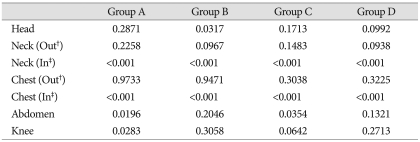
The average radiation doses per one VB during an operation are listed in Table 3. In group A, the largest dose was received by the chest with 0.9733 mSv, followed by doses received in the head, neck, abdomen and knee of 0.2871 mSv, 0.2258 mSv, 0.0196 mSv and 0.0283 mSv, respectively. In group B, the largest dose was received by the chest with 0.9471 mSv, followed by doses received by the head, neck, abdomen and knee of 0.03171 mSv, 0.0967 mSv, 0.2046 mSv and 0.3058 mSv respectively. In group C, the largest dose was received in the chest with 0.3038 mSv, followed by doses received by the head, neck, abdomen and knee of 0.17131 mSv, 0.1483 mSv, 0.0354 mSv and 0.0642 mSv, respectively. In group D, the largest dose was received by the chest with 0.3225 mSv, followed by doses received by the head, neck, abdomen and knee of 0.09921 mSv, 0.938 mSv, 0.1321 mSv and 0.2713 mSv, respectively. (Fig. 2C).
Table 3.
Radiation exposure during kyphoplasty per one vertebral body (unit = mSv)
†Outside of apron, ‡Inside of apron
Calculated KP numbers to reach the occupational annual limitation
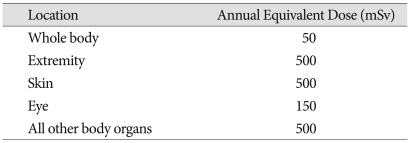
The acceptable annual limit of occupational exposure to radiation for a worker is around 50 mSv/year (Table 4). Based on this recommended dose, it was calculated how many KP surgeries would expose a spine surgeon to the limit of occupational exposure. If a spine surgeon conducts KP without wearing a lead apron, the spine surgeon will reach the acceptable annual limit of exposure after approximately 51 cases. Since thyroid protectors and lead aprons almost completely block radiation, according to the results of this study, if a spine surgeon wears these protective devices when conducting surgeries and uses the C-arm fluoroscopy as configured in group D, his or her head will reach the limit of exposure for a year after conducting approximately 504 cases and the knee will reach the limit at approximately 184 cases. However, since the radiation received by the arms and hands was excluded from this study and spine surgeons do not conduct KP operations only, it is assumed that the actual permissible number of KP operations before reaching the limit of radiation exposure should be much smaller.
Table 4.
NCRP* recommended annual permissible equivalent dose for occupational radiation exposure
*The National Council on Radiation Protection and Measurements
DISCUSSION
Regardless of manufacturer, the image intensifiers of almost all C-arm fluoroscopy units are long and cylindrical in shape, and they are much larger and longer than the beam generator. Therefore, when surgeons conduct surgeries, they often feel that if the image intensifier is positioned over the patient and the bed, their work space would become narrow. Thus, they intentionally or unconsciously position the smaller beam generator above the bed and closer to themselves during operation. Based on the results of this study, if the beam generator is positioned close to the surgeon's head, the surgeon will receive approximately 3-10 times more radiation than when it is farther away. This shows that even if the workspace is narrow and thus inconvenient to the surgeon, the beam generator should be positioned below the bed during operation.
Ionizing radiation does occur naturally and is also generated by various kinds of machines, but the amount received by human bodies from these sources is relatively small. However, the radiation generated by the C-arm fluoroscopy is very strong and the potential dose is very high. Adverse effects of ionizing radiation on human bodies include skin diseases, thyroid cancer, brain tumors, cataracts, etc3). Such effects are largely divided into two types. The "early effects" such as acute radiation lethality, local tissue damage on skin or gonads, hematologic effects, and cytogenetic effects, and the "late effects" including radiation-induced malignancies such as leukemia and other forms of cancer, deleterious local tissue effects, chromosomal toxicity, and/or cataract formation10).
To avoid these harmful radiation effects, many surgeons should make every effort to maximally reduce radiation exposure from the C-arm fluoroscopy. The first effective method is to wear shielding devices or protectors. In addition, results suggest that wearing lead glasses and gloves is essential for blocking radiation.
Secondly, the tube in the C-arm fluoroscopy where X-ray beams are generated should be positioned on the opposite side of the operator and below the bed. However, it should be also kept in mind that this position of the beams will increase the irradiation received by an assistant positioned on the opposite side of the table 3 to 10 times. Therefore, it makes sense to have the assistant and the scrub nurse stay at the same side with the operator.
The third method is to operate the C-arm fluoroscopy in such a way as to minimize exposure. One can operate the C-arm fluoroscopy either in continuous or pulsed modes. Using the pulsed mode rather than the continuous whenever possible can reduce radiation doses6,8,11).
The forth, exposure to the radiation scattered from patients and surroundings can add to direct radiation exposure. Wearing lead gloves and using the shielding panels or screens to prevent these scattered beams when conducting cerebral or cardiac intervention therapy can be good methods for reducing exposure, but these have many technical difficulties2,4,12,13).
A fifth approach involves using spinal navigation systems. According to reports by several researchers, navigation-guided kyphoplasty can reduce the amount of irradiation during operations by almost 30%. However, several problems must be overcome, such as the high cost of the equipment and increased preparation for operations using the safer equipment5,7).
Sixth, it is important to wear personal devices to measure the amount of irradiation in order to monitor the dose to the operator. Therefore, the operator should wear a dosimetry badge at all times and periodically check the cumulative radiation exposure.
Seventh, two fluoroscopes may be used for surgery. Mroz reported quantified fluoroscopic radiation exposure to the surgeon and patient during KP. They used two fluoroscopes for their KPs. With use of two fluoroscopes, unnecessary time which were spent in repetitive viewing the AP and lateral images will be saved thereby reducing the exposure10).
Finally, education of all spine surgeons and related workers on radiation physics is the most important.
Although radiation exposure to the torso and head of the operator is more important than other areas, the area that receives the highest doses is the hands14). Skin injury such as skin erythema occurs when the local skin exposure dose exceeds 2000 mGy. Wagner and Archer reported that the entrance skin dose (ESD) rate for fluoroscopy was typically 30 mGy/min, and longer procedures with more exposure could increase the risk of skin erythema for the patient20). This fact also applies to the hands of the operator who is in closest contact with the patient9). Direct monitoring of patient or operator skin dose during procedures is highly desirable, but current methods still have serious limitations21).
The International Commission on Radiologic Protection (ICRP) and the National Council on Radiation Protection and Measurements (NCRP) require that individuals exposed to greater than 10% of the permissible annual occupational exposure limit be regularly monitored (Table 4)13,16-19).
The surgeons are usually protected by the lead apron and thyroid shield, so the report on each protected dosimeter was less than the minimum reportable dose, as expected. However, we have to consider the unprotected areas, such as the eyes and the hands. A report by the National Council on Radiation Protection and Measurements in 1993 suggested annual occupational exposure limits of 50 mSv/year for the whole body, 150 mSv/year for the lens of eye, and 500 mSv/year for the extremities. In our study, if a surgeon conducts KP operations using the method of group D, the amount of radiation received by the torso of the surgeon will reach the annual limit after approximately 155 cases1).
CONCLUSION
During KP operations, fluoroscopic radiation is received by the operator in real-time for approximately 50% (half) of the operation time. When using the C-arm fluoroscopy, the X-ray tube should be positioned on the opposite side of the table from the operator and below the patient to reduce the amount of radiation received by the operator to the lowest level. Since thyroid protectors and lead aprons can block radiation almost completely, these must be worn without fail. Also it is recommended that personnel should additionally wear personal devices that can shield the head, eyes and hands. Finally, when assistants are standing on the opposite side of the table from the surgeon, great attention should be paid to their physical shield from radiation.
References
- 1.Bindal RK, Glaze S, Ognoskie M, Tunner V, Malone R, Ghosh S. Surgeon and patient radiation exposure in minimally invasive transforaminal lumbar interbody fusion. J Neurosurg Spine. 2008;9:570–573. doi: 10.3171/SPI.2008.4.08182. [DOI] [PubMed] [Google Scholar]
- 2.Chida K, Saito H, Otani H, Kohzuki M, Takahashi S, Yamada S, et al. Relationship between fluoroscopic time, dose-area product, body weight, and maximum radiation skin dose in cardiac interventional procedures. AJR Am J Roentgenol. 2006;186:774–778. doi: 10.2214/AJR.04.1653. [DOI] [PubMed] [Google Scholar]
- 3.Cousins C, Sharp C. Medical interventional procedures-reducing the radiation risks. Clin Radiol. 2004;59:468–473. doi: 10.1016/j.crad.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 4.D'Ercole L, Mantovani L, Thyrion FZ, Bocchiola M, Azzaretti A, Di Maria F, et al. A study on maximum skin dose in cerebral embolization procedures. AJNR Am J Neuroradiol. 2007;28:503–507. [PMC free article] [PubMed] [Google Scholar]
- 5.Izadpanah K, Konrad G, Südkamp NP, Oberst M. Computer navigation in balloon kyphoplasty reduces the intraoperative radiation exposure. Spine (Phila Pa 1976) 2009;34:1325–1329. doi: 10.1097/BRS.0b013e3181a18529. [DOI] [PubMed] [Google Scholar]
- 6.Kallmes DF, O E, Roy SS, Piccolo RG, Marx WF, Lee JK, et al. Radiation dose to the operator during vertebroplasty : prospective comparison of the use of 1-cc syringes versus an injection device. AJNR Am J Neuroradiol. 2003;24:1257–1260. [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CW, Lee YP, Taylor W, Oygar A, Kim WK. Use of navigation-assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J. 2008;8:584–590. doi: 10.1016/j.spinee.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Kruger R, Faciszewski T. Radiation dose reduction to medical staff during vertebroplasty : a review of techniques and methods to mitigate occupational dose. Spine (Phila Pa 1976) 2003;28:1608–1613. [PubMed] [Google Scholar]
- 9.Mehdizade A, Lovblad KO, Wilhelm KE, Somon T, Wetzel SG, Kelekis AD, et al. Radiation dose in vertebroplasty. Neuroradiology. 2004;46:243–245. doi: 10.1007/s00234-003-1156-0. [DOI] [PubMed] [Google Scholar]
- 10.Mroz TE, Yamashita T, Davros WJ, Lieberman IH. Radiation exposure to the surgeon and the patient during kyphoplasty. J Spinal Disord Tech. 2008;21:96–100. doi: 10.1097/BSD.0b013e31805fe9e1. [DOI] [PubMed] [Google Scholar]
- 11.Müller LP, Suffner J, Wenda K, Mohr W, Rommens PM. Radiation exposure to the hands and the thyroid of the surgeon during intramedullary nailing. Injury. 1998;29:461–468. doi: 10.1016/s0020-1383(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 12.Perisinakis K, Damilakis J, Theocharopoulos N, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. Patient exposure and associated radiation risks from fluoroscopically guided vertebroplasty or kyphoplasty. Radiology. 2004;232:701–707. doi: 10.1148/radiol.2323031412. [DOI] [PubMed] [Google Scholar]
- 13.Rampersaud YR, Foley KT, Shen AC, Williams S, Solomito M. Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine (Phila Pa 1976) 2000;25:2637–2645. doi: 10.1097/00007632-200010150-00016. [DOI] [PubMed] [Google Scholar]
- 14.Seibert JA. Vertebroplasty and kyphoplasty : do fluoroscopy operators know about radiation dose, and should they want to know? Radiology. 2004;232:633–634. doi: 10.1148/radiol.2323040968. [DOI] [PubMed] [Google Scholar]
- 15.Synowitz M, Kiwit J. Surgeon's radiation exposure during percutaneous vertebroplasty. J Neurosurg Spine. 2006;4:106–109. doi: 10.3171/spi.2006.4.2.106. [DOI] [PubMed] [Google Scholar]
- 16.Radiation protection in in educational institutions (National Council on Radiation Protection and Measurements Web site) The National Council on Radiation Protection and Measurements. 2006. Jul, [Accessed 01/05, 2007]. Available at: http://www.ncrponline.org/Docs_in_Review/NCRP0640.pdf.
- 17.Recommendations of the International Commission on Radiologic Protection. ICRP Publication 26. Bethesda, MD: International Commission on Radiological Protection; 1977. p. 1. [Google Scholar]
- 18.Recommendations on limits for exposure to ionizing radiation. Bethesda, MD: National Council on Radiation Protection and Measurements; 1987. p. 91. [Google Scholar]
- 19.Recommendations of the International Commission on Radiologic Protection. ICRP Publication 60. Bethesda, MD: International Commission on Radiological Protection; 1991. p. 21. [Google Scholar]
- 20.Wagner LK, Archer BR. Minimizing Risks From Fluoroscopic X Rays; Bioeffects, Instrumentation, and Examination. 3rd ed. The Woodlands, TX: Partners in radiation management; 2000. [Google Scholar]
- 21.Mahesh M. Fluoroscopy : patient radiation exposure issues. Radiographics. 2001;21:1033–1045. doi: 10.1148/radiographics.21.4.g01jl271033. [DOI] [PubMed] [Google Scholar]