Abstract
Although dopamine D3 receptors have been associated with cocaine abuse, little is known about the consequences of chronic cocaine on functional activity of D3 receptor-preferring compounds. This study examined the behavioral effects of D3 receptor-selective 4-phenylpiperazines with differing in vitro functional profiles in adult male rhesus monkeys with a history of cocaine self-administration and controls. In vitro assays found that PG 619 (N-(3-hydroxy-4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl) was a potent D3 antagonist in the mitogenesis assay, but a fully efficacious agonist in the adenylyl cyclase assay, NGB 2904 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide HCl) was a selective D3 antagonist, whereas CJB 090 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl) exhibited a partial agonist profile in both in vitro assays. In behavioral studies, the D3 preferential agonist quinpirole (0.03–1.0 mg/kg, i.v.) dose-dependently elicited yawns in both groups of monkeys. PG 619 and CJB 090 elicited yawns only in monkeys with an extensive history of cocaine, whereas NGB 2904 did not elicit yawns, but did antagonize quinpirole and PG 619-elicited yawning in cocaine-history monkeys. In another experiment, doses of PG 619 that elicited yawns did not alter response rates in monkeys self-administering cocaine (0.03–0.3 mg/kg per injection). Following saline extinction, cocaine (0.1 mg/kg) and quinpirole (0.1 mg/kg), but not PG 619 (0.1 mg/kg), reinstated cocaine-seeking behavior. When given before a cocaine prime, PG 619 decreased cocaine-elicited reinstatement. These findings suggest that (1) an incongruence between in vitro and in vivo assays, and (2) a history of cocaine self-administration can affect in vivo efficacy of D3 receptor-preferring compounds PG 619 and CJB 090, which appear to be dependent on the behavioral assay.
Keywords: addiction and substance abuse, cocaine, dopamine D3 receptors, self-administration, reinstatement, nonhuman primates
INTRODUCTION
With over 2000 people initiating use every day and 1.9 million Americans reporting past-month use, cocaine abuse continues to pose a public health concern in the United States (Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies, 2008). Despite tremendous efforts to discover a pharmacotherapy to treat cocaine addiction, no approved medications are currently available (Mello and Negus, 1996; Carroll et al, 1999; Vocci et al, 2005; Karila et al, 2008).
Although many neurotransmitter systems are implicated in cocaine abuse, the dopamine (DA) system has been a primary target for treatment development owing to its extensive role in cocaine abuse (see review by Xi and Gardner, 2008). Cocaine elevates extracellular DA (Di Chiara and Imperato, 1988; Ritz et al, 1987) that subsequently stimulates DA D1- and D2-like receptors (Kebabian and Caine, 1979; Missale et al, 1998). The DA D2-like family of receptors, comprised of the D2, D3, and D4 receptor subtypes, are thought to mediate directly the reinforcing effects of cocaine (Caine et al, 2000). D3 receptors not only play a significant role in mediating the reinforcing effects of cocaine (Pilla et al, 1999; Nader and Mach, 1996, Caine and Koob, 1993; Self et al, 1996), but are also implicated in chronic cocaine use by the demonstration of higher D3 receptor densities in post-mortem cocaine-overdose victims compared with drug-naïve controls in autoradiography studies (Staley and Mash, 1996). Because D3 receptors exhibit a restricted pattern of localization to limbic brain regions (Sokoloff et al, 1990; Levant, 1997), in theory, binding at this receptor should not lead to extrapyramidal side effects or disruption of natural behaviors such as feeding. As a result, the development of novel pharmacological tools has increased interest as potential therapeutic agents (Heidbreder and Newman, 2010).
Extensive medicinal chemistry and structure–activity relationships have been described and reviewed recently (Boeckler and Gmeiner, 2006; Zhang et al, 2007), especially for the 4-phenylpiperazine series of D3-selective ligands. Despite nearly 80% sequence homology in the transmembrane regions of the D2 and D3 receptor subtypes, the discovery of compounds with >100-fold D3 selectivity over D2 and other receptor systems has been achieved. These compounds are typically evaluated for intrinsic activity in cell-based assays using cell lines transfected with hD3 or hD2 receptors and most compounds in this structural class are antagonists or partial agonists. However, to date, tractable structure–activity relationships that predict intrinsic activity have remained elusive. Moreover, a correlation between intrinsic activity as measured in vitro and behavioral actions of these compounds has not been established (Heidbreder and Newman, 2010). The compounds studied in the present experiments, the D3 compound PG 619 (N-(3-hydroxy-4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl), the D3 partial agonist CJB 090 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl), and the prototypical D3 antagonist NGB 2904 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide HCl), had previously been characterized in vitro using mitogenesis assays (Grundt et al., 2005, 2007). To further characterize these compounds, another in vitro assay was utilized, adenylyl cylase inhibition (Taylor et al., 2010), followed by examination of the behavioral effects of each compound in several in vivo functional assays. Thus, one aim of this study was to directly compare D3-selective compounds using two in vitro assays with several behavioral measures in monkeys to better determine if either in vitro assay was more predictive of D3 activity in vivo.
The in vivo evaluation of novel D3 compounds has included unconditioned and conditioned behaviors (see Newman et al, 2005; Xi and Gardner, 2008; Heidbreder and Newman, 2010). In this study, we utilize drug-elicited yawning as an unconditioned behavior that has been associated with D3 receptor activation (eg, Collins et al, 2005; Martelle et al, 2007). Of particular interest is the observation that, in vivo, full agonists such as quinpirole elicit yawning, but partial agonists do not show agonist activity (Martelle et al, 2007). In this study, we examined the ability of quinpirole, PG 619, CJB 090, and NGB 2904 to elicit yawns in cocaine-naïve and cocaine-experienced monkeys. All of the earlier work used cocaine-naïve animals or animals with a minimal cocaine history. The hypothesis was that the extensive cocaine histories of the monkeys would lead to different behavioral effects of D3 receptor-selective compounds compared with effects observed in cocaine-naïve monkeys. We also utilized the cocaine-experienced monkeys to examine these drugs on cocaine self-administration and reinstatement. An earlier study reported that CJB 090 decreased cocaine self-administration in rhesus monkeys self-administering cocaine on a second-order schedule (Martelle et al., 2007), while showing no effect on cocaine reinforcement or reinstatement in squirrel monkeys (Achat-Mendes et al, 2009). In this study, we extended this comparison to another 4-phenylpiperazine, PG 619, which binds to D3 receptors with ∼100-fold selectivity over D2 compared with the ∼50-fold D3 selectivity of CJB 090 and ∼56-fold D3 selectivity of NGB 2904 (Newman et al, 2005; Grundt et al, 2007). The use of multiple behavioral baselines allowed for the assessment of D3 receptor contribution to the behavioral effects of cocaine and show that D3 receptor mediation is dependent on the behavioral assay.
MATERIALS AND METHODS
Experiment 1: In Vitro Studies
Adenylyl cyclase
Complete details are described in Taylor et al (2010). Briefly, a whole cell-cyclic AMP accumulation assay in stably transfected HEK cells expressing human DA D2 or D3 receptors was used. The endogenous ATP pool was radioactively labeled by preincubating the cells in serum-free DMEM/25 mM HEPES medium containing 2 μCi/ml of [2,8-3H]adenine (20–Ci/mmol) in a 5% CO2 incubator at 37 °C for 75 min and the radioactive medium was removed by aspiration. Added to the flask was serum-free DMEM (5.6 ml) containing 25 mM HEPES, pH 7.4, and 0.1 mM of the phosphodiesterase inhibitor isobutylmethylxanthine. A 400-μl aliquot of cells was removed and added to 50 μl of forskolin (100 μM) and 50 μl of PG 619, CJB 090, NGB 2904, or quinpirole in DMEM-HEPES. The cells were incubated for 20 min at 37 °C (vortexing every 5 min) and the reaction was terminated by adding 0.5 ml of 10% trichloroacetic acid (w/v) containing 1.0 mM unlabeled cyclic AMP. As described by Saloman et al (1974), both the [3H]cyclic AMP and the [3H]ATP fractions were collected following separation using columns of Dowex AG-50W-X4 and alumina. The final yield of [3H]cyclic AMP was corrected for column recovery of unlabeled cyclic AMP determined spectrophotometrically (OD259). The primary dependent variable is the percent conversion of [3H]ATP into [3H]cyclic AMP.
Experiments 2–4: In Vivo Studies
Subjects
For all experiments, adult male rhesus monkeys (Macaca mulatta) served as subjects (Table 1). At the start of this study, four monkeys (R-1374, R-1375, R-1377, R-1381) had a history of cocaine self-administration (Czoty et al, 2007), with intake ranging from 635 to 1385 mg/kg, and four monkeys were drug naïve. Monkeys were individually housed in stainless-steel cages with ad libitum access to water and visual and auditory contact with other monkeys. Body weights were maintained at 95% of free-feeding weights with standard laboratory chow (Purina LabDiet 5045) and fresh produce at least three times a week. All monkeys were fitted with an aluminum collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate chair (Primate Products). All monkeys were implanted with an indwelling intravenous catheter (internal or external jugular or femoral vein) and a vascular access port (Access Technologies) under sterile surgical conditions as described previously (Czoty et al, 2007). To ensure catheter patency, the catheter was flushed with heparinized saline (100 U/ml) following each experimental session. All manipulations were performed in accordance with the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research and were approved by the Wake Forest University Institutional Animal Care and Use Committee.
Table 1. Experimental Subjects.
| Subject | Age | Weight (kg) | Lifetime cocaine intake (mg/kg) | Experiments participated in |
|---|---|---|---|---|
| R-1374 | 13 | 11.2 | 1385 | Experiments 2, 3, and 4 |
| R-1375 | 13 | 10.1 | 635 | Experiments 2 and 4 |
| R-1377 | 12 | 9.7 | 1089 | Experiments 3 and 4 |
| R-1381 | 13 | 10.4 | 922 | Experiments 2, 3, and 4 |
| R-1424 | 11 | 10.1 | 0 | Experiment 2 |
| R-1661 | 13 | 9.0 | 0 | Experiment 2 |
| R-1662 | 13 | 7.2 | 0 | Experiment 2 |
| R-1663 | 13 | 8.2 | 0 | Experiment 2 |
Apparatus
Cocaine self-administration and reinstatement studies were carried out in ventilated, sound-attenuating chambers (1.5 × 0.74 × 0.76 m3; Med Associates, East Fairfield, VT). Each chamber consisted of two response keys (5 cm wide) located on one side of the chamber with a horizontal row of three stimulus lights 14 cm above each response key, a food receptacle located between the response keys for delivery of 1-g banana-flavored food pellets and an infusion pump (Cole-Parmer Inc, Chicago, IL) for delivering drugs.
Drugs
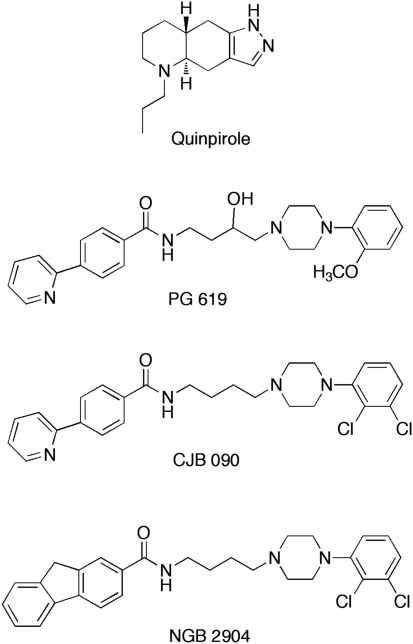
(−)Cocaine HCl (NIDA, Bethesda, MD), quinpirole (Sigma-Aldrich, St Louis, MO), and PG 619 (NIDA-Intramural Research Program, Baltimore, MD and synthesized as described in Grundt et al, 2005) were dissolved in 0.9% sodium chloride. CJB 090 and NGB 2904 were synthesized as described in Grundt et al (2005) and were dissolved in 25% β-cyclodextrin. Quinpirole, PG 619, CJB 090, and NGB 2904 (see Figure 1) were diluted to concentrations of 1.0 and 5.0 mg/ml, whereas different doses of cocaine were made by adjusting the drug concentration. Heat and sonication were used for solubility purposes and all drugs were adjusted to pH 6–7 and filtered before administration.
Figure 1.
Chemical structure of quinpirole and the 4-phenylpiperazines PG 619 (N-(3-hydroxy-4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl), CJB 090 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl), and NGB 2904 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide HCl).
Experiment 2: Effects of D3 Compounds on Drug-Elicited Yawning
Procedure
Monkeys were seated in a primate restraint chair and placed in a testing room in front of a video camera. First, single injections of saline (1.0 ml, i.v.) and quinpirole (0.03–1.0 mg/kg, i.v.) were tested in all monkeys, with yawning measured over 30 min beginning immediately after the injection. Quinpirole did not elicit yawning in R-1377 and he was removed from the study. Next, the following drugs were tested at doses of 0.03–1.0 mg/kg (i.v.) and in the following order: PG 619, CJB 090, and NGB 2904. For each drug, doses were tested in random order with a minimum of a 24-h washout period between test sessions. After completion of all dose–response curves, antagonism studies were carried out in the three cocaine-history monkeys. For these studies, NGB 2904 (0.1–0.3 mg/kg) was administered 15 min before maximally effective doses of quinpirole and PG 619 (0.1 mg/kg), followed by a 1.0 ml saline flush. Antagonism of quinpirole-elicited yawning by CJB 090 has been reported by Martelle et al (2007). Immediately following the injection, the technician left the room and the monkey was filmed for 30 min. Tapes were later scored for occurrences of yawning, which was defined as full extension of the jaws, withdrawal of the lips, and exposure of the teeth (Code and Tang, 1991). The experimenter and another observer blind to experimental conditions had inter-observer variability <5%.
Data analysis
The primary dependent variable was the number of yawns during the 30-min observation period. Data were analyzed with a two-way repeated measures ANOVA using group (cocaine history and drug naive) and drug dose, followed by post hoc Bonferroni t-tests (significance level, p<0.05).
Experiment 3: Effects of PG 619 on Cocaine Self-Administration
Procedure
Three cocaine-history monkeys (Table 1) were used in this experiment. Before the start of the session, the monkey was seated in a primate restraint chair, the animal's back containing the port was cleaned with betadine and 95% EtOH and a 22-G Huber Point Needle (Access Technologies) was inserted into the port. The Huber Point Needle was connected to an infusion pump that delivered drugs at a rate of approximately 1.5 ml/10 s. Each animal received a 3-s prime of the cocaine dose available during the self-administration session. The session began with illumination of the white stimulus light located above one of the response keys. Cocaine reinforcement (0.03 mg/kg) was contingent upon responding on a fixed-ratio (FR) 30 schedule of reinforcement. Following completion of the FR requirement, the white light extinguished, a red light above the lever was illuminated for 10 s and cocaine was delivered; a 30-s time out followed each injection. The session was terminated once the monkey received a total of 15 injections or 2 h elapsed.
A cocaine dose–response curve was generated for each animal before examining PG 619 as a pretreatment. When self-administration of the baseline dose (0.03 mg/kg) was deemed stable (±20% of the mean response rate for three consecutive sessions, with no trends), saline was substituted for cocaine for at least five consecutive sessions and until responding declined to <20% of baseline. After a return to baseline, different doses of cocaine were substituted for the baseline dose for a minimum of five sessions and until response rates were deemed stable; doses were tested in random order and there was a return to the baseline dose between substitution studies. After completion of the cocaine dose–response curve, pretreatment injections of PG 619 (0.1–1.0 mg/kg, i.v.) were administered 10 min before the self-administration session. This pretreatment time was based on the time course of drug-elicited yawning in these same animals. We also tested 3.0 mg/kg PG 619 in one monkey, but it induced catalepsy and hypothermia that persisted for nearly 3 h. Because these behaviors are attributed to D2 receptors, this dose was not tested in any other animal (Collins et al, 2007; Achat-Mendes et al, 2010). All doses of PG 619 were tested in random order and double determined; at least four cocaine self-administration doses were examined in each monkey. The cocaine dose–response curve was redetermined following PG 619 evaluation to ensure a stable baseline for each animal.
Data analysis
The primary dependent variable was response rate (total responses per session divided by session length). For the analysis of the cocaine dose–response curve, mean data from the three sessions satisfying the stability criteria for cocaine self-administration were included in the analysis using a one-way ANOVA with cocaine dose as a factor. Bonferroni post hoc testing was used to determine if PG 619 had significant effects on cocaine self-administration. For all analyses, p<0.05 was considered statistically significant.
Experiment 4: Effects of Quinpirole and PG 619 on Cocaine-Elicited Reinstatement
Procedure
All four cocaine-history monkeys were used in this study. Saline was substituted for cocaine until response rates decreased to <20% of baseline rates. Next, non-contingent injections (i.v.) of 0.1 mg/kg cocaine, PG 619, and quinpirole, administered immediately before the session, were examined. The doses of PG 619 and quinpirole were chosen because they elicited the maximum number of yawns in these monkeys, whereas the cocaine dose was the lowest dose that reliably reinstated cocaine seeking in these monkeys. There were at least two sessions of saline self-administration between each priming test session. PG 619 (0.03–1.0 mg/kg, i.v.) and quinpirole (0.1 mg/kg, i.v.) were also administered 10 min before the non-contingent cocaine prime to evaluate their effects on cocaine-elicited reinstatement.
Data analysis
Each monkey's response rate following the non-contingent 0.1 mg/kg cocaine injection was normalized to 100%. The primary dependent variable was percent cocaine-prime response rate for quinpirole and PG 619 alone and in combination with 0.1 mg/kg cocaine. Data were analyzed with a one-way ANOVA with Tukey's post hoc testing.
RESULTS
Experiment 1: In Vitro Comparison of PG 619, CJB 090 and NGB 2904
In vitro evaluation of the three 4-phenylpiperazines can be seen in Table 2. All three compounds were evaluated in competition binding assays in HEK 293 cells transfected with either human D2L or D3 DA receptors, as described previously (Grundt et al., 2005), and bind with high affinity to D3 receptors and moderate affinity to D2 receptors, with PG 619 having the highest D3/D2 selectivity (∼100-fold; Grundt et al, 2007). PG 619 and NGB 2904 antagonized quinpirole-induced mitogenesis in hD3-transfected CHO cells with similar potencies, whereas CJB 090 was a partial agonist in this assay, showing ∼30% agonist activity as compared with quinpirole (Grundt et al, 2005). In the second adenylyl cyclase assay, a concentration of approximately 10 times the Ki at D3 or D2 receptors was used to stimulate adenylyl cyclase in hD3- or hD2-transfected HEK 293 cells. In the D2 assay, PG 619 did not show a % maximum stimulation commensurate with an agonist profile (<20% maximal stimulation), but was fully efficacious in the D3 assay (100%) and equivalent to quinpirole as a D3 agonist. In contrast, CJB 090 was a partial agonist (by definition, between 20 and 70% maximal stimulation) at both D2 and D3 receptors and NGB 2904 was an antagonist at D3.
Table 2. In vitro Data for 4-Phenylpiperazines.
| Compound | D3 Ki (nM±SEM) | D2 Ki (nM±SEM) | D3/D2 affinity | D3 mitogenesis IC50 in nM±SEM (% maximum stimulation) | D2 mitogenesis IC50 in nM±SEM (% maximum stimulation) | D3 adenylyl cyclase % maximum stimulationd | D2 adenylyl cyclase % maximum stimulationd |
|---|---|---|---|---|---|---|---|
| PG 619 | 2.8±0.8a | 284±28a | 100 | 12.75±1.26a,b (0) | NDc | 106±23.8 | 8.5±6.6 |
| CJB 090 | 0.5±0.2e | 24.8±3.9e | 62 | 6.3±1.7e (29.7) | 92.0±4.9e (22.4) | 39.9±8.5f | 38.6±1.7f |
| NGB 2904 | 2.0±0.4e | 112±22e | 56 | 14.4±0.5b,e (0) | 1280±270b,e (0) | 5.5±1.6d | 42.7±5.1d |
Antagonist.
ND=not determined owing to low D2 affinity.
%Maximal inhibition of adenylyl cyclase was achieved using the test ligand at a concentration of ∼10 × the Ki values using methods described in Taylor et al (2010).
Experiment 2: Effects of D3 Compounds on Drug-Elicited Yawning
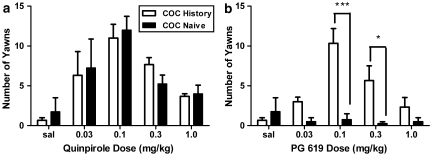
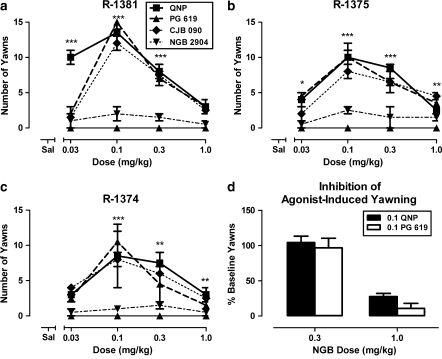
Following saline administration, monkeys yawned less than five times during the 30-min observation period (Figure 2a). Quinpirole (0.03–1.0 mg/kg) significantly (F(4,20)=9.45, p<0.001) elicited yawns in a dose-dependent manner as characterized by the inverted U-shaped function of dose (Figure 2a). There were no differences between groups, with peak yawns elicited by 0.1 mg/kg quinpirole. In contrast to the effects of quinpirole, PG 619 (0.03–1.0 mg/kg) significantly (F(4,20)=9.10, p<0.001) elicited yawns only in monkeys with an extensive history of cocaine self-administration (Figure 2b, open bars, and Figure 3, triangles). In these monkeys, the shape of the PG 619 dose–response curve was characterized as an inverted U-shaped function of dose, with peak yawns elicited following 0.1 mg/kg PG 619. In the cocaine-exposed monkeys, the frequency of yawns elicited by PG 619 was not different from the number of yawns elicited by quinpirole (Figure 3, triangles vs squares). In the cocaine-naïve monkeys, no dose of PG 619 elicited yawns (Figure 2b, closed bars). Another D3 partial agonist, CJB 090 (0.03–1.0 mg/kg), also significantly (F(4,40)=27.11, p<0.001) elicited yawns in cocaine-history monkeys (Figure 3, diamonds), whereas the D3 antagonist NGB 2904 (0.03–1.0 mg/kg) did not elicit yawns at the doses tested (Figure 3, inverted triangles). As with the quinpirole and PG 619 dose–response curves, the CJB 090 dose–response curve was characterized as an inverted U-shaped function of dose, with peak yawns elicited by 0.1 mg/kg. In drug combination studies in cocaine-history monkeys, NGB 2904 (1.0 mg/kg) significantly (F(1,8)=76.56, p<0.001) antagonized 0.1 mg/kg quinpirole- and 0.1 mg/kg PG 619-elicited yawning (Figure 3d).
Figure 2.
Dose-dependent yawning elicited by (a) the D3 agonist quinpirole (0.03–1.0 mg/kg, intravenously (i.v.)) and (b) the D3 receptor-selective compound PG 619 (N-(3-hydroxy-4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl) (0.03–1.0 mg/kg, i.v.) in cocaine-history (n=3, open bars) and cocaine-naïve monkeys (n=4, closed bars). Data are represented as mean±SEM number of yawns observed over a 30-min period. Asterisks (*p<0.05; ***p<0.001) indicate statistical differences between groups.
Figure 3.
(a–c) Effects of quinpirole, PG 619 (N-(3-hydroxy-4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl), CJB 090 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl), and NGB 2904 (N-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butyl)-9H-fluorene-2-carboxamide HCl) on drug-elicited yawning in cocaine-history monkeys. Data are represented as mean±SEM number of yawns observed over a 30-min period. Asterisks (**p<0.01; ***p<0.001) indicate statistical difference from saline. (d) Effects of NGB 2904 (0.3–1.0 mg/kg) on quinpirole- and PG 619-elicited yawning in cocaine-history monkeys (n=3). Asterisks (**p<0.01) indicate statistical difference when monkeys were treated with NGB 2904 before quinpirole (QNP, closed bars) or PG 619 (open bars).
Experiment 3: Effects of PG 619 on Cocaine Self-Administration
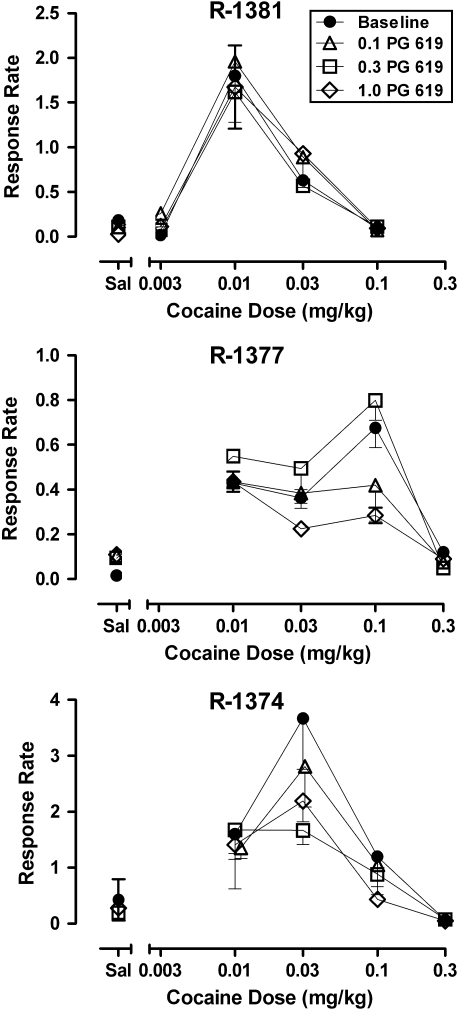
Cocaine maintained responding in all monkeys, with response rates significantly (F(4,60)=74.49, p<0.001) higher than rates of responding leading to saline injections (Figure 4, closed circles). Although the shape of the dose–response curves were generally characterized as an inverted U-shaped function of dose, peak rates of responding occurred following different doses in each monkey (0.01 mg/kg in R-1381, 0.03 mg/kg in R-1374, and 0.1 mg/kg in R-1377). When administered before sessions of cocaine availability, no dose of PG 619 (0.1–1.0 mg/kg) significantly affected cocaine-maintained responding across a range of cocaine doses (Figure 4).
Figure 4.
Effects of PG 619 (N-(3-hydroxy-4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl) (0.1–1.0 mg/kg) on response rates (responses per s) maintained by cocaine (0.003–0.3 mg/kg) in cocaine-history monkeys (n=3) responding under a fixed-ratio 30 schedule of reinforcement. Data are represented as mean±SEM.
Experiment 4: Effects of Quinpirole and PG 619 on Cocaine-Elicited Reinstatement
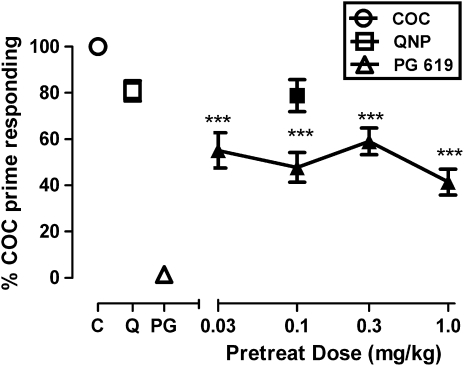
When saline was substituted for cocaine, response rates decreased by at least 80% of baseline within five sessions. A dose of PG 619 that robustly elicited yawning in these monkeys (0.1 mg/kg) did not reinstate cocaine seeking in any monkey (Figure 5, left panel, open triangle). In contrast, 0.1 mg/kg cocaine resulted in significant (t(3)=5.59, p<0.05) increases in saline-contingent response rates (2163, 1454, 2100, and 2163% in R-1374, R-1375, R-1377, and R-1381, respectively). The mean increase of 1970% served as baseline for drug combination studies (Figure 5, open circle). Quinpirole (0.1 mg/kg) also reinstated drug-seeking behavior, although the effects were less than cocaine-induced reinstatement (Figure 5, left panel, open square). Although PG 619 did not induce reinstatement, it attenuated cocaine-induced reinstatement by approximately 50% (Figure 5, filled triangles). All doses of PG 619 were equally effective. Quinpirole (0.1 mg/kg) did not alter the ability of 0.1 mg/kg cocaine to reinstate drug seeking (Figure 5, filled square).
Figure 5.
(Left panel) Effects of 0.1 mg/kg cocaine (C), quinpirole (Q), and PG 619 (N-(3-hydroxy-4-(4-(2-methoxyphenyl)piperazin-1-yl)butyl)-4-(pyridin-2-yl)benzamide HCl) (PG) administered alone (open symbols) to reinstate cocaine seeking. (Right panel) Effects of PG 619 (0.03–1.0 mg/kg) and quinpirole (0.1 mg/kg) to attenuate cocaine-induced reinstatement when administered 10 min before 0.1 mg/kg cocaine (filled symbols). Data are represented as mean±SEM percent cocaine-prime responding. Asterisks (***p<0.001) indicate statistical difference from cocaine-elicited responding.
DISCUSSION
The main purpose of this study was to characterize the behavioral effects of the DA D3 compound PG 619 in rhesus monkeys. In addition, PG 619 and several DA D3 receptor-selective compounds with varying intrinsic efficacies as measured in vitro were directly compared using two different assays and with subsequent behavioral characterization of these compounds as agonists, partial agonists, or antagonists. An additional aim was to characterize the effects of PG 619 in cocaine-naïve and cocaine-history monkeys. We had hypothesized that if chronic cocaine exposure altered D3 receptor densities (Staley and Mash, 1996; Neisewander et al, 2004) and the effects of full D3 agonists (Nader and Mach, 1996; Collins and Woods, 2009), then it is plausible that the behavioral effects of D3 receptor-selective compounds would also be influenced by a history of cocaine self-administration. In control monkeys, PG 619 did not elicit yawning, but in cocaine-history monkeys it appeared to have unconditioned behavioral effects similar to the full agonist quinpirole. A similar behavioral profile was noted with the partial D3 agonist CJB 090, but not the D3 receptor-selective antagonist NGB 2904. Although PG 619 appeared to function as an agonist when using unconditioned behaviors, it did not reinstate cocaine seeking similar to the D3 agonist quinpirole, but did attenuate cocaine-induced reinstatement. The behavioral model of cocaine abuse influenced the characterization of PG 619, because doses that attenuated reinstatement did not block cocaine self-administration.
Initially, the in vivo effects of PG 619 were perplexing because based on the in vitro mitogenesis assay PG 619 is an antagonist at the DA D3 receptor subtype. Therefore, it was hypothesized that in vivo PG 619 would have activity similar to NGB 2904. However, the results from the adenylyl cyclase inhibition assay indicated that PG 619 can also act as a fully efficacious agonist. We have previously published the structure–activity profile of a panel of substituted 4-phenylpiperazines structurally related to PG 619, which indicated that the efficacy of this class of compound can differ in these two functional assays (Taylor et al, 2010). That finding is consistent with the theory of functional selectivity, which proposes that a ligand can have differing efficacy and/or potency for different receptor-associated effector pathways (Gay et al, 2004; Kenakin, 2002; Clarke, 2005). Therefore, a compound that is an antagonist for one receptor-linked second messenger system might also show some degree of intrinsic efficacy for a second pathway. For the 4-phenylpiperazines that we have evaluated thus far, PG 619 is unique in that it appears to be a neutral antagonist for the pertussis toxin-sensitive mitogenic assay (Pilon et al, 1994), although being a full agonist for the adenylyl cyclase inhibition assay. This finding suggests that while both pathways are mediated by G proteins, it may be the adenylyl cyclase pathway that is directly involved in the yawning response observed in the monkeys with a cocaine history, which makes in vivo responses of PG 619 appear to be more like the partial agonist CJB 090.
In this study, the ability of the full agonist quinpirole to elicit yawning was not influenced by a history of cocaine exposure, nor was the inactivity of the D3 receptor antagonist NGB 2904. However, both PG 619 and CJB 090 elicited yawns in monkeys with an extensive cocaine history, but not in cocaine-naïve monkeys. Earlier studies have consistently shown that D3 partial agonists do not display agonist-like effects in vivo (see Heidbreder and Newman, 2010). Results from this study are the first data showing in vivo agonist activity of drugs that do not show full D3 agonist profiles in the in vitro mitogenesis assay. The doses of PG 619 that were behaviorally active in yawning did not affect rates of cocaine self-administration. Interestingly, while the ability of PG 619 to elicit yawning was similar to quinpirole, only quinpirole reinstated cocaine seeking, whereas PG 619 attenuated cocaine-induced reinstatement, suggesting differential modulation by D3 receptors of the multiple stimulus effects of cocaine. Although we did not test CJB 090 and cocaine in reinstatement, Achat-Mendes et al (2009) found CJB 090 ineffective in attenuating cocaine-primed reinstatement in squirrel monkeys. Clearly, additional studies are warranted to better understand the relationship between functional selectivity at D3 receptors, as determined in vitro, with behavioral effects involving cocaine.
Woods, Collins, and co-workers have extensively characterized the role of DA D3 and D2 receptors in quinpirole-elicited yawning (Collins et al, 2005, 2007, 2008). Quinpirole, like other D3 agonists, has affinity at both D3 and D2 receptors (Gehlert et al, 1992; Levant et al, 1992), and as such, the shape of the quinpirole dose–response curve is an inverted U-shaped function of dose, with the ascending limb mediated primarily by D3 receptors and the descending limb by concomitant D2 receptor stimulation (Collins et al, 2005, 2007). We had previously reported a similar shape of the quinpirole-elicited yawning dose–response curve in cocaine-naïve rhesus monkeys (Martelle et al, 2007; Hamilton et al, 2010), which was replicated in this study. Furthermore, unpublished data from our laboratory indicates that quinpirole-elicited yawning is specific to D3 receptors as hypothermia is not elicited at the doses on the ascending limb (0.01–0.1 mg/kg). An extensive history of cocaine self-administration did not alter the shape, potency, or efficacy of quinpirole-elicited yawning. Interestingly, cocaine exposure does appear to alter the potency of low-efficacy D3 compounds as lower doses of CJB 090 and NGB 2904 elicited and antagonized, respectively, agonist-elicited yawning in cocaine-exposed monkeys compared with the doses that inhibited quinpirole-elicited yawning in cocaine-naïve rhesus monkeys (Martelle et al, 2007). Therefore, the potency of low efficacy agonists may be differentially affected as a function of cocaine exposure. Earlier work in monkeys (Nader and Mach, 1996) and rodents (Collins and Woods, 2007) has shown that a cocaine history is necessary for D3 agonists to maintain drug self-administration. Thus, it appears that the influence of cocaine self-administration on the behavioral pharmacology of D3 agonists, such as quinpirole, is dependent on the in vivo assay. These findings indicated that the consequences of cocaine exposure on the behavioral effects of the D3 partial agonist CJB 090 and the mixed efficacy compound PG 619 were also influenced by the in vivo assay, although not in a manner similar to what had been observed with full agonists. In our earlier study, CJB 090 did not elicit yawns in cocaine-naïve monkeys and did not substitute for cocaine in monkeys trained to discriminate cocaine from saline (Martelle et al, 2007). However, in monkeys with an extensive cocaine history, both CJB 090 and PG 619 elicited yawns, the magnitude of effects were comparable to quinpirole-elicited yawns. Possible reasons for these 4-phenylpiperazines showing agonist activity following a cocaine history could be due to the reported increase in D3 receptor densities (Staley and Mash, 1996; Neisewander et al, 2004) and/or the decreases in D2-like receptor densities following chronic cocaine self-administration (Moore et al, 1998; Nader et al, 2002). The fact that the quinpirole dose–response curve was not different in the cocaine-naïve and cocaine-exposed monkeys suggests that changes in receptor densities was unlikely the mechanism mediating this effect. Additional studies, perhaps combining other manipulations known to alter sensitivity to the behavioral effects of D3 compounds (eg, food restriction vs food satiation; Baladi et al, 2010) as well as assessing the role of D2 receptors by measuring body temperature (Collins et al, 2007) will be necessary to elucidate the changes in DA receptor function during chronic cocaine exposure. Alternatively, the functional selectivity of the 4-phenylpiperazines such as PG 619 might be affected by chronic cocaine use. However, further research is necessary to fully address this issue.
This study also investigated the ability of PG 619 to attenuate cocaine self-administration. As we had reported earlier with CJB 090 and the D3 antagonist NGB 2904, PG 619 had no effect on cocaine self-administration. These findings in monkeys may be at odds with the view that D3 compounds may be effective treatments for drug addiction (Le Foll et al, 2005). One possible reason for the lack of effect in this study was the schedule of reinforcement used in the cocaine self-administration paradigm. It has been hypothesized that ratio-based schedules are not as sensitive to the effects of D3 partial agonists or antagonists as other reinforcement schedules, such as second-order schedules or progressive-ratio schedules (Pilla et al, 1999; Xi et al, 2006). Our earlier study (Martelle et al, 2007) used a second-order schedule and CJB 090 decreased cocaine self-administration. However, in that study, CJB 090 also decreased food-maintained responding under an identical second-order schedule of reinforcement. Thus, while the schedule of reinforcement may increase or decrease sensitivity of D3 compounds to alter cocaine self-administration, the concurrent study of behavior maintained by a non-drug reinforcer suggests possible non-selectivity in decreasing cocaine-maintained behavior. Future studies should utilize reinforcement schedules that are less sensitive to rate-decreasing effects of the pretreatment drug, such as using cocaine-food choice paradigms (eg, Negus, 2003).
Although the unconditioned behavioral effects of PG 619 were altered by chronic cocaine exposure, the D3 agonist-like effects of PG 619 were not apparent in reinstatement studies, in contrast to quinpirole, which reinstated cocaine seeking. Thus, it appears that not all behavioral assays deemed sensitive to D3 agonists will be sensitive to D3 partial agonists, nor are they equally sensitive to the effects of chronic cocaine exposure. From a cocaine addiction treatment perspective, the fact that PG 619 did not reinstate cocaine-seeking behavior, but did attenuate these effects of cocaine is intriguing and potentially promising from a drug discovery point of view (see also Cervo et al, 2003; Achat-Mendes et al, 2009).
Acknowledgments
We thank Tonya Calhoun and Susan Nader for excellent technical assistance. This research was supported by the National Institute on Drug Abuse Grant R01 DA12460 (to MAN) and by the NIDA Intramural Research Program (to AHN).
The authors declare no conflict of interest.
References
- Achat-Mendes C, Grundt P, Cao J, Platt DM, Newman AH, Spealman RD. Dopamine D3 and D2 receptor mechanisms in the abuse-related behavioral effects of cocaine: studies with preferential antagonists in squirrel monkeys. J Pharmacol Exp Ther. 2010;334:556–565. doi: 10.1124/jpet.110.167619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achat-Mendes C, Platt DM, Newman AH, Spealman RD. The dopamine D3 receptor partial agonist CJB 090 inhibits the discriminative stimulus but not the reinforcing or priming effects of cocaine in squirrel monkeys. Psychopharmacology. 2009;206:73–84. doi: 10.1007/s00213-009-1581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. J Pharmacol Exp Ther. 2010;332:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckler F, Gmeiner P. The structural evolution of dopamine D3 receptor ligands: structure–activity relationships and selected neuropharmacological aspects. Pharmacol Ther. 2006;112:281–333. doi: 10.1016/j.pharmthera.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Effects of dopamine D1-like and D2-like agonists on cocaine self-administration in rhesus monkeys: rapid assessment of cocaine dose–effect curves. Psychopharmacology. 2000;148:41–51. doi: 10.1007/s002130050023. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- Cervo L, Carnovali F, Stark JA, Mennini T. Cocaine-seeking behavior in response to drug-associated stimuli in rats: involvement of D3 and D2 dopamine receptors. Neuropsychopharmacology. 2003;28:1150–1159. doi: 10.1038/sj.npp.1300169. [DOI] [PubMed] [Google Scholar]
- Clarke WP. What's for lunch at the conformational cafeteria. Mol Pharmacol. 2005;67:1819–1821. doi: 10.1124/mol.105.013060. [DOI] [PubMed] [Google Scholar]
- Code RA, Tang AH. Yawning produced by dopamine agonists in rhesus monkeys. Eur J Pharmacol. 1991;201:235–238. doi: 10.1016/0014-2999(91)90351-p. [DOI] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, et al. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology. 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, et al. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther. 2007;323:599–605. doi: 10.1124/jpet.107.123042. [DOI] [PubMed] [Google Scholar]
- Collins GT, Woods JH. Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behav Pharmacol. 2009;20:492–504. doi: 10.1097/FBP.0b013e328330ad9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader SH, Reboussin BA, Bounds M, Nader MA. PET imaging of dopamine D2 receptor and transporter availability during acquisition of cocaine self-administration in rhesus monkeys. J Addict Med. 2007;1:33–39. doi: 10.1097/ADM.0b013e318045c038. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay EA, Urban JD, Nichols DE, Oxford GS, Mailman RB. Functional selectivity of D2 receptor ligands in a Chinese hamster ovary hD2L cell line: evidence for induction of ligand-specific receptor states. Mol Pharmacol. 2004;66:97–105. doi: 10.1124/mol.66.1.97. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Gackenheimer SL, Seeman P, Schaus J. Autoradiographic localization of [3H]quinpirole binding to dopamine D2 and D3 receptors in rat brain. Eur J Pharmacol. 1992;211:189–194. doi: 10.1016/0014-2999(92)90528-c. [DOI] [PubMed] [Google Scholar]
- Grundt P, Carlsson EE, Cao J, Bennett CJ, McElveen E, Taylor M, et al. Novel heterocyclic trans olefin analogues of N-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butyl} arylcaroxamides as selective probes with high affinity for the dopamine D3 receptor. J Med Chem. 2005;48:839–884. doi: 10.1021/jm049465g. [DOI] [PubMed] [Google Scholar]
- Grundt P, Prevatt KM, Cao J, Taylor M, Floresca CZ, Choi JK, et al. Heterocyclic analogues of N-(4-(4-(2,3 dichlorophenyl)piperazin-1-yl)butyl)arylcarboxamides with functionalized linking chains as novel dopamine D3 receptor ligands: potential substance abuse therapeutic agents. J Med Chem. 2007;50:4135–4146. doi: 10.1021/jm0704200. [DOI] [PubMed] [Google Scholar]
- Hamilton LR, Czoty PW, Gage HD, Nader MA. Characterization of the dopamine receptor system in adult rhesus monkeys exposed to cocaine throughout gestation. Psychopharmacology. 2010;210:481–488. doi: 10.1007/s00213-010-1847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Newman AH. Current perspectives on selective dopamine D3 receptor antagonists as pharmacotherapeutics for addictions and related disorders. Ann NY Acad Sci. 2010;1187:4–34. doi: 10.1111/j.1749-6632.2009.05149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karila L, Gorelick D, Weinstein A, Noble F, Benyamina A, Coscas S, et al. New treatments for cocaine dependence: a focused review. Int J Neuropsychopharmacol. 2008;11:425–438. doi: 10.1017/S1461145707008097. [DOI] [PubMed] [Google Scholar]
- Kebabian JW, Caine DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Ann Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR, Sokoloff P. The dopamine D3 receptor and drug dependence: effects on reward or beyond. Neuropharmacology. 2005;49:525–541. doi: 10.1016/j.neuropharm.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Levant B, Grigoriadis DE, DeSouza EB. Characterization of [3H]quinpirole binding to D2-like dopamine receptors in rat brain. J Pharmacol Exp Ther. 1992;262:929–935. [PubMed] [Google Scholar]
- Levant B. The D3 dopamine receptor: neurobiology and potential clinical relevance. Pharmacol Rev. 1997;49:231–252. [PubMed] [Google Scholar]
- Martelle JL, Claytor R, Ross JT, Reboussin BA, Newman AH, Nader MA. Effects of two novel D3-selective compounds, NGB 2904 and CJB 090, on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:573–582. doi: 10.1124/jpet.106.113571. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opiod abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. The effect of cocaine self-administration on dopamine D2 receptors in rhesus monkeys. Synapse. 1998;30:88–96. doi: 10.1002/(SICI)1098-2396(199809)30:1<88::AID-SYN11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, et al. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology. 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Fuchs RS, Tran-Nguyen LT, Weber SM, Coffey GP, Joyce JN. Increases in dopamine D3 receptor binding in rats receiving a cocaine challenge at various time points after cocaine self-administration: implications for cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1479–1487. doi: 10.1038/sj.npp.1300456. [DOI] [PubMed] [Google Scholar]
- Newman AH, Grundt P, Nader MA. Dopamine D3 receptor partial agonists and antagonists as potential drug abuse therapeutic agents. J Med Chem. 2005;48:3664–3679. doi: 10.1021/jm040190e. [DOI] [PubMed] [Google Scholar]
- Pilla M, Perachon S, Sautel F, Garrido F, Mann A, Wermuth CG, et al. Selective inhibition of cocaine-seeking behavior by a partial dopamine D3 receptor agonist. Nature. 1999;400:371–375. doi: 10.1038/22560. [DOI] [PubMed] [Google Scholar]
- Pilon C, Levesque D, Dimitriadou V, Griffon N, Martres MP, Schwartz JC, et al. Functional coupling of the human dopamine D3 receptor in a transfected NG 108-15 neuroblastoma–glioma hybrid cell line. Eur J Pharmacol. 1994;268:129–139. doi: 10.1016/0922-4106(94)90182-1. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Saloman Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271:1586–1589. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres M, Bouthenet M, Schwartz J. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration SAMHSA Office of Applied Studies Results from National Survey on Drug Use and Health 2008.
- Taylor M, Grundt P, Griffin SA, Newman AH, Luedtke RR. Dopamine D3 receptor selective ligands with varying intrinsic efficacies at adenylyl cyclase inhibition and mitogenic signaling pathways. Synapse. 2010;64:251–266. doi: 10.1002/syn.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocci JF, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. Am J Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gardner EL. Hypothesis-driven medication discovery for the treatment of psychostimulant addiction. Curr Drug Abuse Rev. 2008;1:303–327. doi: 10.2174/1874473710801030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR, Jr, et al. The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology. 2006;7:1393–1405. doi: 10.1038/sj.npp.1300912. [DOI] [PubMed] [Google Scholar]
- Zhang A, Neumeyer JL, Baldessarini RJ. Recent progress in development of dopamine receptor subtype-selective agents: potential therapeutics for neurological and psychiatric disorders. Chem Rev. 2007;107:274–302. doi: 10.1021/cr050263h. [DOI] [PubMed] [Google Scholar]