Abstract
Heterochromatin integrity is crucial for genome stability and regulation of gene expression, but the factors involved in mammalian heterochromatin biology are only incompletely understood. Here we identify the oncoprotein DEK, an abundant nuclear protein with a previously enigmatic in vivo function, as a Suppressor of Variegation [Su(var)] that is crucial to global heterochromatin integrity. We show that DEK interacts directly with Heterochromatin Protein 1 α (HP1α) and markedly enhances its binding to trimethylated H3K9 (H3K9me3), which is key for maintaining heterochromatic regions. Loss of Dek in Drosophila leads to a Su(var) phenotype and global reduction in heterochromatin. Thus, these findings show that DEK is a key factor in maintaining the balance between heterochromatin and euchromatin in vivo.
Keywords: heterochromatin, oncogene, HP1-α, epigenetics, Su(var)
DEK is an abundant and structurally unique constituent of metazoan chromatin (Kappes et al. 2001) that is conserved across species, and is the only member of its protein class (for review, see Sitwala et al. 2003; Waldmann et al. 2004; Riveiro-Falkenbach and Soengas 2010). Subsequent to its initial discovery as part of a fusion protein in a form of acute myeloid leukemia (AML) (von Lindern et al. 1992), multiple studies identified pleiotrophic intranuclear functions of DEK, which may be regulated by distinct post-translational modifications (see also Supplemental Fig. 1; Faulkner et al. 2001; Hollenbach et al. 2002; Waldmann et al. 2002; Kappes et al. 2004a,b, 2008; Cleary et al. 2005; Ko et al. 2006; Mor-Vaknin et al. 2006, 2011; Soares et al. 2006; Wise-Draper et al. 2006; Gamble and Fisher 2007; Sawatsubashi et al. 2010). DEK is significantly overexpressed in difficult-to-treat cancers, and was identified recently as a bona fide oncoprotein (Wise-Draper et al. 2009a,b) and a potential target for chemotherapy in malignant melanoma (Khodadoust et al. 2009; Riveiro-Falkenbach and Soengas 2010). However, the biological functions of DEK have remained only incompletely understood.
Results and Discussion
Our goal in this study was to gain a broader insight into DEK's in vivo functions. In order to address this question, we knocked down DEK expression in human cells using several nonredundant shRNAs targeting DEK mRNA that were delivered by two independent lentiviral systems (shDEK) (Fig. 1A; Supplemental Fig. 2). Strikingly, the polyclonal DEK knockdown (DEKkd) cell lines showed a reduced growth rate (Fig. 1A), and accumulation of cells in the G2/M phase of the cell cycle (Fig. 1B, no treatment). As DEK has been implicated in HDACII recruitment activities (Hollenbach et al. 2002) and exhibits H3- and H4-specific inhibitor of acetyltranferase activity (INHAT) (Ko et al. 2006), we next tested the effect of the HDAC inhibitors TSA and butyrate. Indeed, DEKkd rendered the cells more sensitive to the G2/M arrest typically seen with HDAC inhibitors (Fig. 1B, TSA and butyrate), suggesting roles for DEK in the regulation of local or global histone acetylation. Furthermore, increased aneuploidy and an overall increase in cell size were evident in DEKkd cells (Supplemental Fig. 3A,B).
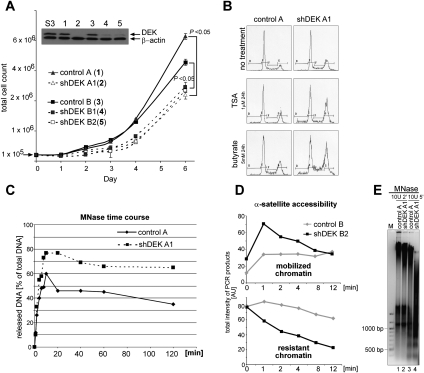
Figure 1.
Interference with DEK expression in cells induces a phenotype indicative of more accessible chromatin organization. (A) Comparative growth curves. Stable DEKkd in HeLa S3 cells was achieved by lentiviral delivery of either one (4,5) or two (2) distinct shRNAs targeting DEK, or no shRNA as control (control A [H1-LV vector; 1] or control B [PLKO.1 vector; 3]) (see also Supplemental Fig. S2). After the appropriate selection procedure (GFP expression [1,2] or puromycin [3–5]), total cell numbers were counted at the indicated time points. Results from three individual experiments were plotted (error bars represent ±SD) and P-values for the indicated pairwise comparisons were obtained by two-tailed Student's t-test. The inset illustrates inhibition of DEK expression as assessed by immunoblotting. (S3) Parental cell line. (B) Cell cycle analysis was performed using FACS for cell lines (as denoted in A), and representative cell cycle profiles without treatment (no treatment) or with treatment with TSA (TSA) or sodium butyrate (butyrate) for 24 h are shown. (C) MNase digestion time course assessing chromatin release. Nuclei from indicated cell lines were subjected to MNase digestion. At the denoted time points, aliquots were taken and released chromatin was separated from digestion refractory chromatin by centrifugation. DNA content of individual samples was assessed fluorimetrically (and by agarose gel electrophoresis) (see Supplemental Fig. S4A). Values displayed represent the ratio of released versus total DNA content at time point 0. Similar results were obtained in three independent experiments. (D) Assessment of α-satellite repeat accessibility. Nuclei from indicated cell lines were processed as described in C. DNA from individual samples was purified and subjected to α-satellite-specific PCR and agarose gel electrophoresis (see Supplemental Fig. S4B–D). Total product intensity in individual lanes, normalized to total intensity at time point 0, was analyzed using ImageJ software, and relative values are displayed in the graphs. (Top panel) Mobilized α-satellite repeats. (Bottom panel) Resistant, not mobilized α-satellite repeats. Similar results were obtained in three independent experiments. (E) MNase digestion of melanoma DEKkd cells. Nuclei were treated with MNase, and reactions were analyzed directly by agarose gel electrophoresis. DNA was visualized by EtBr staining, and one representative experiment out of three is shown. A DNA size marker is shown on the left (M).
The impaired genomic stability observed after DEKkd suggested that DEK might be involved in global chromatin organization in vivo. Indeed, digestion time-course experiments with micrococcal nuclease (MNase) revealed an ∼25% greater release of cellular DNA upon DEKkd (Fig. 1C), indicating a substantial global loss of digestion refractory chromatin. One example of nuclease digestion refractory sites is α-satellite repeats, which localize to trimethylated H3K9 (H3K9me3)-enriched constitutive heterochromatin flanking centromeres. Assessment of α-satellite repeat distribution revealed a striking increase in accessible α-satellite repeats in DEKkd cells (Fig. 1D, mobilized chromatin) and, in turn, a loss of nuclease refractory (silenced) repeats upon brief digestion with MNase (Fig. 1D, resistant chromatin; see Supplemental Fig. 4B–D). Furthermore, nuclease digestion experiments in a DEKkd melanoma cell line (Fig. 1E) and in Hela S3 cells (Supplemental Fig. 4E) also revealed a nuclease-sensitive chromatin structure. Thus, these experiments establish a requirement for DEK in global chromatin integrity in vivo.
We next investigated the morphological appearance of DEKkd cells in more detail by transmission electron microscopy (TEM). We found a striking reduction in size and abundance—or even loss—of constitutive heterochromatic areas in DEKkd cells (Fig. 2A).
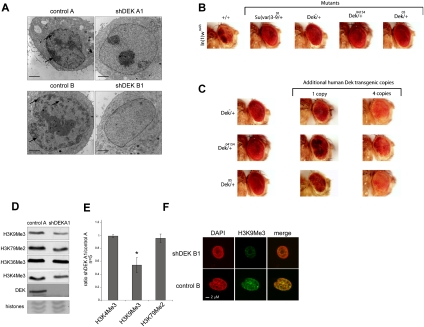
Figure 2.
DEK is a Su(var) (A) TEM reveals a global decrease in heterochromatic areas upon DEKkd. Cell lines were analyzed by standard TEM. Arrows highlight selected electron-dense heterochromatic areas. Bar, 2 μm. (B) Loss of a dose of Dek has a marked effect on white-mottled variegation in the eyes of male flies. Genomic deletion that includes the Dek coding region (Dek+/−) and P-element insertions into DEK (Dek+/04154 and Dek+/05) suppresses white-mottled variegation. The suppression was comparable with mutations in the well-characterized Su(var) KMT1 A/B [Su(var)3–9]. Representative fly heads were photographed 5–7 d after hatching. In(1)wm4h is the control fly. (C) The loss of a dose of Dek can be compensated by human DEK transgenes. The suppression of PEV in In(1)wm4h flies by Dek mutations carrying a heterozygous loss of Dek can be compensated by human DEK transgenes. (D) Assessment of epigenetic histone marks was performed by immunoblotting with modification-specific antibodies. (E) Average values from five independent experiments, as performed in D, are displayed and represent the ratio of signals obtained from total H3 amounts versus modification-specific signals. Error bars indicate ±SD. (*) P < 0.005. P-value was obtained by two-tailed Student's t-test, comparing H3K9me3 intensity in DEKkd cells with that in control cells. (F) IF staining with H3K9me3-specific antibodies was performed by confocal microscopy (see also Supplemental Fig. S5A).
Having found that DEK is essential to heterochromatin integrity in mammals, we next asked whether this DEK function is conserved throughout other species. We used a Drosophila melanogaster model in which an inversion of the first chromosome places the white gene into pericentromeric heterochromatin [In(1)wm4h], causing variegated expression of the white gene in the eye (Tartof et al. 1984). Spreading of heterochromatin beyond the breakpoint of the inversion will result in silencing of the white gene, which can be monitored by the eye color (see Fig. 2B). Strong, dominant suppression of this position effect variegation (PEV), comparable with the effects of KMT1 A/B {Suppressor of Variegation 3–9 [Su(var)3–9]} mutants on white expression, was observed for all Dek mutant alleles tested (Fig. 2B), demonstrating a strong genetic role for Dek in heterochromatin integrity in Drosophila, and classifying DEK as a previously unrecognized Su(var). In rescue experiments, we found that expression of human DEK can restore the variegated eye phenotype in a dosage-dependent manner in all Dek mutant alleles tested (Fig. 2C).
Changes in chromatin organization typically coincide with changes in epigenetic marks and also in the localization and composition of proteins reading the marks. Thus, we next investigated whether DEK expression affects epigenetic marks associated with heterochromatin or euchromatin (Fig. 2D). We observed an increase in global histone acetylation (Supplemental Fig. 5C) and, strikingly, an ∼50% global reduction in H3K9me3, a hallmark of repressive chromatin, in DEKkd cells (Fig. 2E). This was further confirmed by immunofluorescence (IF) staining (Fig. 2F; Supplemental Fig. 5A,D). H3K36me3, a euchromatic mark, was found to be unchanged (Fig. 2D; Supplemental Fig. 5B,D).
The best-studied protein that interacts with H3K9me3 is Heterochromatin Protein 1 α (HP1α) (Bannister et al. 2001; Lachner et al. 2001), which exhibits highly specific, although weak, binding (Eskeland et al. 2007). HP1α induces spreading of heterochromatin through self-assembly and recruitment of silencing factors such as KMT1 A/B (SUV39H1/2), the enzyme that trimethylates H3K9 to create “self-sustaining silencing loops” (Maison and Almouzni 2004; Grewal and Jia 2007). Whereas the HP1 isoforms HP1β and HP1γ also localize to euchromatic sites, HP1α is primarily found associated with pericentric heterochromatin enriched in H3K9me3 (Lomberk et al. 2006).
Prompted by the observed reduction in H3K9me3, we next investigated the subnuclear distribution of HP1 in control and DEKkd cells. In control cells, HP1α localized, as expected, exclusively to the nucleus, and was extractable from chromatin starting at 100 mM NaCl (Fig. 3A, control A). However, in DEKkd cells, we observed a striking displacement of HP1α from chromatin-bound fractions (250 or 450 mM NaCl), and HP1α accumulation in the soluble nuclear fraction (Fig. 3A, shDEK A1 and nucleosol), in agreement with reduced global H3K9me3 levels. Distribution of the linker histone H1 and distribution of HP1β and HP1γ were not affected (Fig. 3A; data not shown). IF also showed HP1α redistribution in DEKkd cells (Fig. 3A, right panels). Reciprocal experiments in HeLa cells with knocked-down HP1α expression or in KMT1 A/B knockout mouse embryonic fibroblasts (MEFs) interestingly showed considerable accumulation of DEK in the cytoplasm in both cases (Supplemental Fig. 6), pointing to a functional interdependence between DEK, HP1α, and KMT1 A/B.
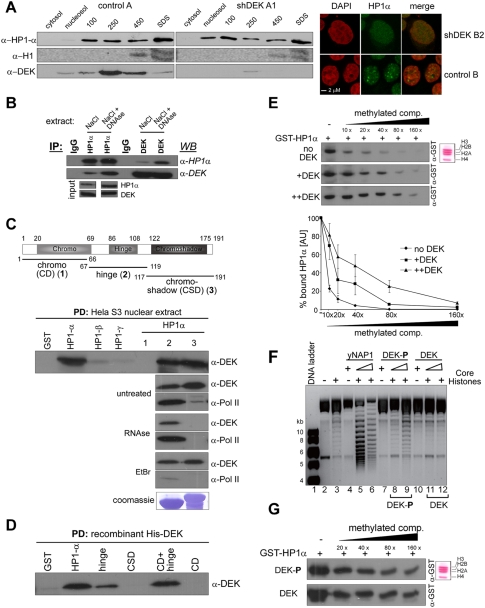
Figure 3.
DEK interacts directly with HP1α and enhances binding of HP1α to H3K9me3. (A) Reduction of DEK expression alters the subnuclear distribution of HP1α. Control and DEKkd cells were subjected to cell fractionation, and the resulting individual samples, as indicated, were analyzed by immunoblotting with antibodies specific for HP1α, H1, or DEK. (Right panels) IF staining. (B) HP1α interacts with DEK. Coimmunoprecipitation was carried out in HeLa S3 nuclear extract, prepared by disruption of nuclei in either 400 mM NaCl alone or 400 mM NaCl plus DNase, using the indicated polyclonal antibodies (IP). Respective protein complexes were assessed by immunoblotting (WB). (C) DEK interacts exclusively with the α isoform of HP1 by direct or RNA-mediated interaction. GST fused to HP1α; HP1β; HP1γ; the HP1α fragments 1–66 (1), 67–119 (2), and 117–191 (3); or GST alone prebound to glutathione sepharose 4B beads was incubated with untreated, RNase A-treated (RNase), or EtBr-treated nuclear extract derived from HeLa S3 cells. Bound proteins were analyzed by immunoblotting with the indicated antibodies. (PD) Pull-down. (D) DEK interacts directly with the hinge domain of HP1α. GST fused to HP1α, the indicated HP1α fragments, or GST alone was incubated with recombinant, dephosphorylated His-DEK. Bound proteins were analyzed by immunoblotting. (PD) Pull-down. (E) Dephosphorylated DEK enhances binding of HP1α to H3K9me3. Far-Western-type overlay assay. (Inset) Core histones derived from HeLa S3 cells (or NIH 3T3) (Supplemental Fig. S7) were transferred to a nitrocellulose membrane. Individual lanes were incubated with GST-HP1α in the absence (no DEK; top panel), or presence of recombinant dephosphorylated His-DEK (+DEK: molar ratio DEK/HP1α, 5:1, middle panel; ++DEK: molar ratio 10:1, bottom panel). A trimethylated competitor peptide was added at the concentrations indicated, and samples were further processed as described in Supplemental Figure S7. (Graph at bottom) Three independent experiments were measured by densitometry, and values are expressed in arbitrary units (AU) as a percentage of bound HP1α, with reactions run without competitor being set as 100% binding (−). (F) Phosphorylation of DEK is required for its histone chaperone activity. Nucleosome reconstitution was achieved by incubating a relaxed plasmid with core histones and yeast Nap1 (yNap1; lanes 5,6), phosphorylated DEK (DEK-P; lanes 8,9), or dephosphorylated DEK (DEK; lanes 11,12), and reconstitution efficiency was analyzed by agarose gel electrophoresis. The molar ratio of Nap1 or DEK to histone octamer is 2:1 or 4:1, respectively. Controls of relaxed plasmids (lane 2) with histones (lane 3), yNap1 (lane 4), DEK-P (lane 7), or DEK (lane 10) added are shown. Lane 1 is a DNA size marker. (G) DEK augments binding of HP1α to H3K9me3 regardless of its phosphorylation status. Far-Western-type overlay assays as in E, using either phosphorylated DEK (DEK-P) or dephosphorylated DEK prepared as in F (molar ratio DEK/HP1α: 10:1), were carried out. Concentrations of the trimethylated competitor peptide used are indicated. Shown are representative results of five independent experiments.
The above findings prompted us to test whether DEK and HP1α or HP1 isoforms physically and/or functionally interact. Coimmunoprecipitation assays in nuclear extracts containing the HP1α populations extractable with up to 400 mM NaCl showed moderate interaction between DEK and HP1α (Fig. 3B, NaCl). However, in extracts additionally containing the very strongly chromatin-bound HP1α species, mutual interaction between DEK and HP1α was substantially increased (Fig. 3B, NaCl + DNase), suggesting interaction between DEK and distinct subpopulations of HP1α. Next, pull-down assays with glutathione-S-transferase (GST)-tagged HP1α, HP1β, or HP1γ showed a strong interaction between DEK and HP1α, but not HP1β or HP1γ (Fig. 3C, top panel). Structurally, HP1α contains three individual domains with known functions (Fig. 3C, 1–3; Maison and Almouzni 2004). In nuclear extracts left untreated, we identified the hinge and chromoshadow domains of HP1α as DEK-interacting domains (Fig. 3C, top panel, HP1α, 1–3). Interestingly, RNase A treatment, but not ethidium bromide (EtBr) treatment, of the extract resulted in loss of DEK binding to the chromoshadow domain of HP1α (Fig. 3C, α-DEK, RNase, and EtBr), suggesting (1) that the hinge domain is a target for direct DEK–HP1α interaction, and (2) ternary complex formation with RNA as a mediator for the DEK–HP1α interaction via the chromoshadow domain. Hinge domain-mediated binding of RNA polymerase II (Pol II) to HP1α was not affected by RNase treatment, yet was substantially reduced upon the presence of EtBr in the reactions (Fig. 3C, α-Pol II), confirming the specificity of our approach. The importance of the hinge domain of HP1α for direct interaction with DEK was further confirmed in pull-down assays using GST-tagged HP1α fusions and baculovirus-derived recombinant, dephosphorylated His-tagged DEK (Fig. 3D).
Having shown that DEK interacts directly with HP1α and affects its subnuclear localization, we next investigated the functional consequence of the DEK–HP1α interaction using Far-Western-type overlay assays (Muchardt et al. 2002). We found that binding of GST-HP1α to H3 was augmented in the presence of dephosphorylated recombinant His-DEK in a dose-dependent manner (Supplemental Fig. 7A), demonstrating a functional role for DEK in HP1α targeting to H3K9me3. In competition experiments using a methylated H3K9me3 peptide, we found that binding of HP1α in the absence of DEK was readily competed away with excess peptide (Fig. 3E, no DEK). However, in the presence of DEK, HP1α binding was detectable even at the highest concentrations of competitor (Fig. 3E, +DEK and ++DEK). Thus, we demonstrate that dephosphorylated DEK can markedly enhance HP1α binding to H3K9me3, suggesting a major mechanism by which DEK can regulate heterochromatin integrity.
Very recently, Sawatsubashi et al. (2010) showed histone chaperone activity for Drosophila Dek. Interestingly, this activity for DEK was found to be strictly dependent on phosphorylation by CK2 (Sawatsubashi et al. 2010). Therefore, we investigated whether human DEK, known to be targeted by CK2 phosphorylation (Kappes et al. 2004a,b), exhibits a similar activity. Indeed, phosphorylated DEK exhibited histone chaperone activity (Fig. 3F, DEK-P, lanes 8,9), although less pronounced than that seen with the well-established histone assembly factor NAP1 (Fig. 3F, lanes 5,6). Strikingly, dephosphorylation of His-DEK resulted in a complete loss of this activity (Fig. 4F, lanes 11,12). Consequently, we next studied the impact of the phosphorylation status of DEK on HP1α targeting. In contrast to what was seen in the histone chaperone assays, both phosphorylated and dephosphorylated DEK retained the ability to augment HP1α binding to H3K9me3 in Far-Western competition experiments (Fig. 4G). This observation suggests the presence of discrete, subcellular species of DEK—defined by their particular post-translational makeup—that can either modulate heterochromatin integrity or, upon phosphorylation, also participate in nucleosome assembly activity.
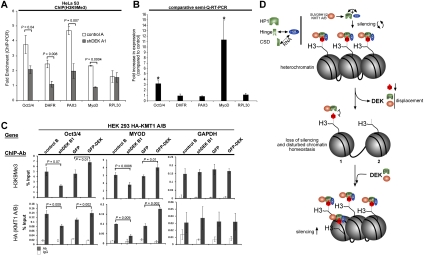
Figure 4.
DEK plays a key role in epigenetic silencing loops. (A) DEKkd in HeLa S3 cells results in reduction of H3K9me3 abundance at specific, epigenetically silenced transcriptional promoters. ChIP assays using H3K9me3 antibodies were carried out on control A and shDEK A1 cell lines, followed by promoter-specific PCR. Values represent results obtained from three independent experiments. P-values for the indicated pairwise comparisons were obtained by two-tailed Student's t-test. (B) DEKkd can induce re-expression of silenced genes in HeLa cells. (Semi-Q-RT–PCR) Total RNA from cells (parental, control A and B, shDEK A1, and shDEK B1) was subjected to semiquantitative RT–PCR. Samples from three independent experiments were assayed in triplicate. Fold change in expression was obtained by comparing averaged values from control cell lines to averaged values obtained from the DEKkd cell lines. (*) P < 0.0005. P-values were calculated using the two-tailed Student's t-test. (C) DEK participates in the recruitment of KMT1 A/B to specific genes. HEK293 cells previously constructed to express HA-tagged KMT1 A/B were engineered to express vectors that produce either stable DEKkd (shDEK B1) or stable overexpression of GFP-tagged DEK (or GST) (Supplemental Fig. S8). ChIP assays using the indicated antibodies (ChIP-Ab) were carried out in control cell lines (control B; GFP), DEKkd cells (shDEK B1), or GFP-DEK-overexpressing cells (GFP-DEK), followed by promoter-specific PCR as indicated (gene). Values shown represent results obtained from two independent experiments. P-values for the indicated pairwise comparisons were calculated using the two-tailed Student's t-test. (D) Model of DEK's function in heterochromatin. DEK can bind either directly to the hinge or through an RNA-dependent mechanism to the chromoshadow domain of HP1α. DEK then augments the binding of HP1α to histones, which brings in KMT1 A/B, which in turn adds the third methyl group to H3K9. If DEK is absent, HP1α and KMT1 A/B binding to H3K9me3 is disturbed (1), which also leads to the loss of the H3K9me3 mark (2). This causes impaired heterochromatin integrity and a loss of epigenetic silencing. In contrast, increased levels of DEK lead to increased abundance of H3K9me3, triggered by augmented recruitment of HP1α and KMT1 A/B.
The H3K9me3 mark also plays an important role in epigenetic silencing of genes. Therefore, we next asked whether DEKkd affects specific genes that are known to be epigenetically silenced. We studied the H3K9me3 enrichment and expression of four such genes (Oct3/4, DHFR, PAX3, and MYOD) in HeLa cells (Fig. 4A,B). Indeed, we found a significant reduction of H3K9me3 in all four promoter regions analyzed, but not in the promoter of RPL30, a constitutively expressed ribosomal gene serving as a negative control. Assessment of transcript abundance in DEKkd cells established a striking increase in MYOD and Oct3/4 transcripts, but PAX and DHFR as well as RPL30 were found to be unaffected by DEKkd (Fig. 4B). These findings suggest that DEK can modulate gene expression by interfering with epigenetic silencing events.
Our observation that knocking down DEK expression leads to a marked diminution of the H3K9me3 heterochromatin mark (Fig. 2 D,E) suggested that, as a consequence of the decrease in HP1α being brought to histones, there is less efficient recruitment of KMT1 A/B. Thus, to determine if DEK is indeed a functional member of self-sustaining silencing loops acting through coordinated recruitment of HP1α and thus KMT 1 A/B to the above-mentioned genes, we performed chromatin immunoprecipitation (ChIP) assays. As no KMT 1A/B-specific antibodies were available to us, we established stable DEKkd or stable GFP-DEK overexpression in HEK293 cell lines that had been engineered previously to stably express low levels of hemagglutinin (HA)-tagged-KMT1 A/B (Supplemental Fig. 8A,B). In the ChIP assays, we found a significantly reduced abundance of H3K9me3 at all genes examined in the HEK293 DEKkd cells (Fig. 4C, H3K9me3, control, and shDEKB1; Supplemental Fig. 9), confirming our results obtained in HeLa cells. Furthermore, reduced gene-specific H3K9me3 levels coincided with a marked reduction in the abundance of KMT1 A/B (Fig. 4C; Supplemental Fig. 9). In strong support of the knockdown data, we identified significantly increased H3K9me3 levels in GFP-DEK-overexpressing HEK293 cells at the genes investigated, accompanied by increased occupancy of KMT1 A/B at these particular genes (Fig. 4C). Thus, both knockdown and overexpression studies demonstrate that DEK coordinates the recruitment of KMT1 A/B to specific genes, thus regulating H3K9 trimethylation.
In summary, we here identify DEK as a novel Su(var) factor with a conserved role in global heterochromatin integrity that functions by augmenting the binding of HP1α (and KMT1 A/B) to the H3K9me3 heterochromatic mark (Fig. 4D). As it has been shown previously that HP1α and KMT1 A/B are crucial to self-sustaining silencing loops, disruption of this mechanism can lead to significant changes in the epigenome of a given cell (Hediger and Gasser 2006). Furthermore, to our knowledge, DEK is the only oncoprotein described that directly affects heterochromatin integrity on a global level. This effect was seen in Drosophila and cell lines derived from patients with cervical cancer (HeLa S3), human embryonic kidney cells, and melanoma cells. The observation that DEK is a key factor in heterochromatin biology suggests that disruption of the balance between euchromatin and heterochromatin could play an important role in the pathogenesis of cancers in which DEK expression is altered. Most notably, our findings indicate that DEK, a nonhistone protein with no known enzymatic activity, plays a vital role in global heterochromatin integrity across species.
Material and methods
Cell culture and lentiviral transduction procedures
Cell culture and lentiviral transduction procedures were carried out as described (Kappes et al. 2008). Melanoma cells were cultured and transduced with H1-LV lentiviral constructs as described (Khodadoust et al. 2009). Other lentiviral constructs and procedures are described in the Supplemental Material.
Cell extract preparation, assessment of cell size, FACS, TEM, Western blot of endogenous histones, IF, and antibodies
Cell extract preparation, assessment of cell size, FACS, TEM, Western blot of endogenous histones, IF, and antibodies are described in detail in the Supplemental Material.
Cell fractionations, nuclease digestions, and α-satellite-specific PCR
Cell fractionations, nuclease digestions, and α-satellite-specific PCR were carried out as described in Kappes et al. (2001, 2008), and are detailed in the Supplemental Material.
Far-Western-type overlay assays
Far-Western-type overlay assays for assessment of HP1α binding to H3K9me3 were used as described (Muchardt et al. 2002).
GST pull-down experiments
GST fusions of HP1α, HP1β, or HP1γ, or deletion mutants of HP1α, were expressed in Escherichia coli and purified over glutathione sepharose beads (GE Healthcare). Binding assays were carried out as described in the Supplemental Material.
ChIP and expression analysis by quantitative RT–PCR (qRT–PCR)
ChIP and expression analysis by qRT–PCR were performed as described in the Supplemental Material.
Chromatin assembly assay
Chromatin assembly assay was performed as described in Lusser and Kadonaga (2004).
Drosophila cultures, stocks, and genetic analysis
Drosophila cultures, stocks, and genetic analysis are described in the Supplemental Material.
Acknowledgments
We gratefully acknowledge Dorothy Sorenson and Sasha Meshinchi for assistance with TEM procedures, and thank Rainer Dorn (University of Halle, Germany) for the human DEK-GFP transgenic flies. F.K. was supported by a William D. Robinson Fellowship from the Arthritis Foundation/Michigan Chapter and is a recipient of an Arthritis Foundation Post-doctoral Fellowship. M.S.K. was supported by NIH Training Grant T32-GM07863 and National Science Foundation and Rackham Predoctoral Fellowships. Work in D.M.M.’s laboratory was supported by NIH grants R01-AI062248 and R01-AI087128 and a Burroughs Wellcome Fund Clinical Scientist Award in Translational Research. J.Y. is supported by grants from the NIH (1K99CA129565-01A1) and the Department of Defense (PC080665). A.M.C. and K.L are investigators of the Howard Hughes Medical Institutes. Work in R.S.’s laboratory is supported by the Max Planck Society, the Deutsche Forschungsgemeinschaft (through SFB 746), Human Frontier Science Program, the EU (the Epigenome), and a European Research Council starting grant. V.M and S.E. are supported by CellNetworks-Cluster of Excellence (EXC81). V.M. is a graduate student of The Hartmut Hoffmann-Berling International Graduate School (HBIGS). R.S. and D.M.M. are joint senior authors on this paper.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.2036411.
Supplemental material is available for this article.
References
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124 [DOI] [PubMed] [Google Scholar]
- Cleary J, Sitwala KV, Khodadoust MS, Kwok RP, Mor-Vaknin N, Cebrat M, Cole PA, Markovitz DM 2005. p300/CBP-associated factor drives DEK into interchromatin granule clusters. J Biol Chem 280: 31760–31767 [DOI] [PubMed] [Google Scholar]
- Eskeland R, Eberharter A, Imhof A 2007. HP1 binding to chromatin methylated at H3K9 is enhanced by auxiliary factors. Mol Cell Biol 27: 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner NE, Hilfinger JM, Markovitz DM 2001. Protein phosphatase 2A activates the HIV-2 promoter through enhancer elements that include the pets site. J Biol Chem 276: 25804–25812 [DOI] [PubMed] [Google Scholar]
- Gamble MJ, Fisher RP 2007. SET and PARP1 remove DEK from chromatin to permit access by the transcription machinery. Nat Struct Mol Biol 14: 548–555 [DOI] [PubMed] [Google Scholar]
- Grewal SI, Jia S 2007. Heterochromatin revisited. Nat Rev Genet 8: 35–46 [DOI] [PubMed] [Google Scholar]
- Hediger F, Gasser SM 2006. Heterochromatin protein 1: don't judge the book by its cover! Curr Opin Genet Dev 16: 143–150 [DOI] [PubMed] [Google Scholar]
- Hollenbach AD, McPherson CJ, Mientjes EJ, Iyengar R, Grosveld G 2002. Daxx and histone deacetylase II associate with chromatin through an interaction with core histones and the chromatin-associated protein Dek. J Cell Sci 115: 3319–3330 [DOI] [PubMed] [Google Scholar]
- Kappes F, Burger K, Baack M, Fackelmayer FO, Gruss C 2001. Subcellular localization of the human proto-oncogene protein DEK. J Biol Chem 276: 26317–26323 [DOI] [PubMed] [Google Scholar]
- Kappes F, Damoc C, Knippers R, Przybylski M, Pinna LA, Gruss C 2004a. Phosphorylation by protein kinase CK2 changes the DNA binding properties of the human chromatin protein DEK. Mol Cell Biol 24: 6011–6020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappes F, Scholten I, Richter N, Gruss C, Waldmann T 2004b. Functional domains of the ubiquitous chromatin protein DEK. Mol Cell Biol 24: 6000–6010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappes F, Fahrer J, Khodadoust MS, Tabbert A, Strasser C, Mor-Vaknin N, Moreno-Villanueva M, Burkle A, Markovitz DM, Ferrando-May E 2008. DEK is a poly(ADP-ribose) acceptor in apoptosis and mediates resistance to genotoxic stress. Mol Cell Biol 28: 3245–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodadoust MS, Verhaegen M, Kappes F, Riveiro-Falkenbach E, Cigudosa JC, Kim DSL, Chinnaiyan AM, Markovitz DM, Soengas MS 2009. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res 69: 6405–6413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SI, Lee IS, Kim JY, Kim SM, Kim DW, Lee KS, Woo KM, Baek JH, Choo JK, Seo SB 2006. Regulation of histone acetyltransferase activity of p300 and PCAF by proto-oncogene protein DEK. FEBS Lett 580: 3217–3222 [DOI] [PubMed] [Google Scholar]
- Lachner M, O'Carroll D, Rea S, Mechtler K, Jenuwein T 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116–120 [DOI] [PubMed] [Google Scholar]
- Lomberk G, Wallrath L, Urrutia R 2006. The heterochromatin protein 1 family. Genome Biol 7: 228 doi: 10.1186/gb-2006-7-7-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusser A, Kadonaga JT 2004. Strategies for the reconstitution of chromatin. Nat Methods 1: 19–26 [DOI] [PubMed] [Google Scholar]
- Maison C, Almouzni G 2004. HP1 and the dynamics of heterochromatin maintenance. Nat Rev Mol Cell Biol 5: 296–304 [DOI] [PubMed] [Google Scholar]
- Mor-Vaknin N, Punturieri A, Sitwala K, Faulkner N, Legendre M, Khodadoust MS, Kappes F, Ruth JH, Koch A, Glass D, et al. 2006. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol Cell Biol 26: 9484–9496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor-Vaknin N, Kappes F, Dick AE, Legendre M, Damoc C, Teitz-Tennenbaum S, Kwok R, Ferrando-May E, Adams BS, Markovitz DM 2011. DEK in the synovium of JIA patients: characterization of DEK antibodies and posttranslational modification of the DEK autoantigen. Arthritis Rheum 63: 556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchardt C, Guilleme M, Seeler JS, Trouche D, Dejean A, Yaniv M 2002. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1α. EMBO Rep 3: 975–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveiro-Falkenbach E, Soengas MS 2010. Control of tumorigenesis and chemoresistance by the DEK oncogene. Clin Cancer Res 16: 2932–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawatsubashi S, Murata T, Lim J, Fujiki R, Ito S, Suzuki E, Tanabe M, Zhao Y, Kimura S, Fujiyama S, et al. 2010. A histone chaperone, DEK, transcriptionally coactivates a nuclear receptor. Genes Dev 24: 159–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitwala KV, Mor-Vaknin N, Markovitz DM 2003. Minireview: DEK and gene regulation, oncogenesis and AIDS. Anticancer Res 23: 2155–2158 [PubMed] [Google Scholar]
- Soares LM, Zanier K, Mackereth C, Sattler M, Valcarcel J 2006. Intron removal requires proofreading of U2AF/3′ splice site recognition by DEK. Science 312: 1961–1965 [DOI] [PubMed] [Google Scholar]
- Tartof KD, Hobbs C, Jones M 1984. A structural basis for variegating position effects. Cell 37: 869–878 [DOI] [PubMed] [Google Scholar]
- von Lindern M, Fornerod M, van Baal S, Jaegle M, de Wit T, Buijs A, Grosveld G 1992. The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA. Mol Cell Biol 12: 1687–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann T, Eckerich C, Baack M, Gruss C 2002. The ubiquitous chromatin protein DEK alters the structure of DNA by introducing positive supercoils. J Biol Chem 277: 24988–24994 [DOI] [PubMed] [Google Scholar]
- Waldmann T, Scholten I, Kappes F, Hu HG, Knippers R 2004. The DEK protein—an abundant and ubiquitous constituent of mammalian chromatin. Gene 343: 1–9 [DOI] [PubMed] [Google Scholar]
- Wise-Draper TM, Allen HV, Jones EE, Habash KB, Matsuo H, Wells SI 2006. Apoptosis inhibition by the human DEK oncoprotein involves interference with p53 functions. Mol Cell Biol 26: 7506–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Draper TM, Mintz-Cole RA, Morris TA, Simpson DS, Wikenheiser-Brokamp KA, Currier MA, Cripe TP, Grosveld GC, Wells SI 2009a. Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo. Cancer Res 69: 1792–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Draper TM, Morreale RJ, Morris TA, Mintz-Cole RA, Hoskins EE, Balsitis SJ, Husseinzadeh N, Witte DP, Wikenheiser-Brokamp KA, Lambert PF, et al. 2009b. DEK proto-oncogene expression interferes with the normal epithelial differentiation program. Am J Pathol 174: 71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]