Abstract
Mono- and di-substituted β-nitro derivatives have been obtained from the reaction of ttcorrFeCl with sodium nitrite in refluxing DMF. This result is unprecedented for iron corrolates and further evidences the non-innocent character of the corrole ligand.
In the last few years corrole has been a protagonist of interest in the porphyrinoid scenario.1,2 This interest is due to the striking characteristics of this macrocycle compared with the parent porphyrins, which make corrole interesting for both theoretical and application reasons.1–3
The uniqueness of corrole can be broadly defined as a result of the contracted macrocycle and of its trianionic character as a ligand: both these characteristics favour the coordination of metals in formally higher oxidation states than that observed for porphyrins.4 This aspect is of major interest when these complexes are exploited, for example, as catalysts.1
An intriguing characteristic of corrole is its so-called “non-innocent” character5 as a ligand, which makes it particularly difficult to characterize the electronic configuration of several metal complexes. A paradigmatic example is the case of iron complexes of corrole that have been described as [Fe(IV)(corr3−)]6 or as [Fe(iii)(corr•2−)]7 species by different research groups. Both theoretical and spectroscopic characterizations now favour the latter interpretation,8 but the consequences of the oxidized character of the corrole ligand in iron corrolates on the reactivity have not yet been explored in detail. We have reported9 on the nitration of free-base meso-triarylcorroles, by using AgNO2 as the nitrating system, which was consistent with the idea that the NO2− ion attacks the π-cation radical of the Ag(iii) corrolates formed by the reaction of the starting material with an excess of Ag+ ions. This hypothesis was further supported when the reaction was performed using copper corrolates as starting materials. Exploiting the temperature-dependent equilibrium between [Cu(iii)(corr3−)] and [Cu(ii)(corr•2−)],10 we successfully performed the nitration reaction in the absence of an oxidant, by simply using NaNO2 as the reagent.
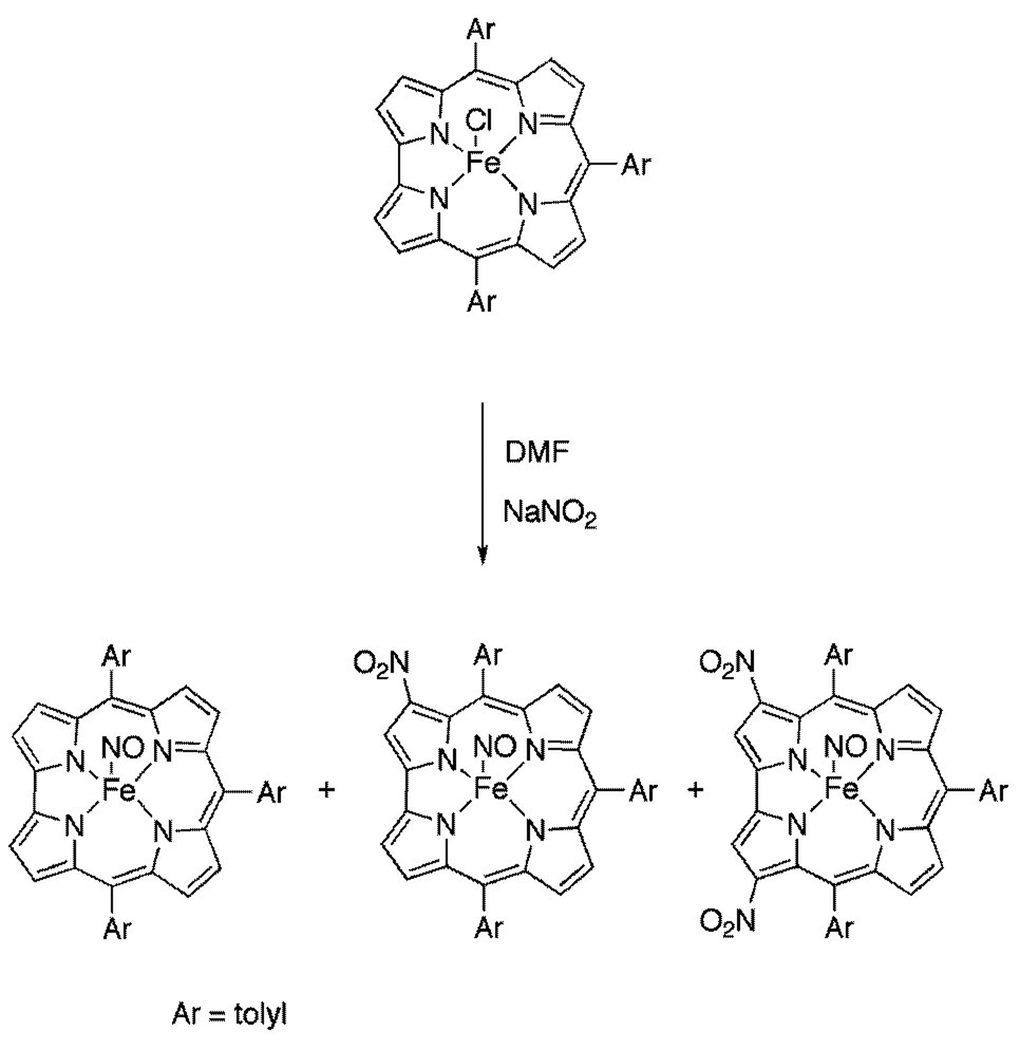
Exploitation of the same reaction in the case of iron corrolates to confirm the proposed radical character of the corrole ligand was an obvious next step, considering also that a similar approach was reported for iron(iii) tetraphenylporphyrinates.11 In this case the porphyrin π-cation radical iron complex was obtained by chemical oxidation, and subsequent reactions with different nucleophiles, including nitrite ion, led to the corresponding β-substituted derivatives. In our case the situation appears to be more complicated, because the reaction of iron corrole derivatives with NaNO2 is not unprecedented, and was used in the past for the preparation of nitrosyl complexes of iron corrolates starting from the corresponding chloride, without any evidence of peripheral functionalizations.12 However we decided to carry out the reaction of chloroiron 5,10,15-tris(4-methylphenyl)corrolate, ttcorrFeCl, using a large excess of sodium nitrite ([NaNO2]/[ttcorrFeCl] = 100) in refluxing DMF, as in the case of Cu corrolates. Monitoring of the reaction progress by TLC indicated, besides some unreacted starting material, the formation of three main products, which we have separated by column chromatography (Fig. 1).13
Figure 1.
The nitration reaction of ttcorrFeCl.
The first-eluted band (50% yield) afforded brilliant red crystals of the expected nitrosyl complex, ttcorrFeNO, as evidenced by spectroscopic characterization and comparison with an authentic specimen prepared by the literature method.14 Characterization of the second band (25% yield) indicated the formation of a nitrosyl complex, due to the presence of a characteristic NO stretching band at 1790 cm−1 in the IR spectrum. However the 1H NMR spectrum showed a non-symmetric substitution pattern of resonances for the β-pyrrolic protons, with a singlet at 8.57 ppm indicating a mono-substituted pyrrole subunit. The peak integration supported the characterization of the product as the nitrosyl iron 3-nitro-5,10,15-tris(4-methylphenyl)corrolate, 3-NO2ttcorrFeNO. The regioselectivity of the substitution was confirmed by preparation of the same complex starting from the corresponding 3-NO2tt-corr free base, recently obtained in our laboratory.15 Following literature methods12 we obtained a specimen identical with that obtained from the reported reaction.
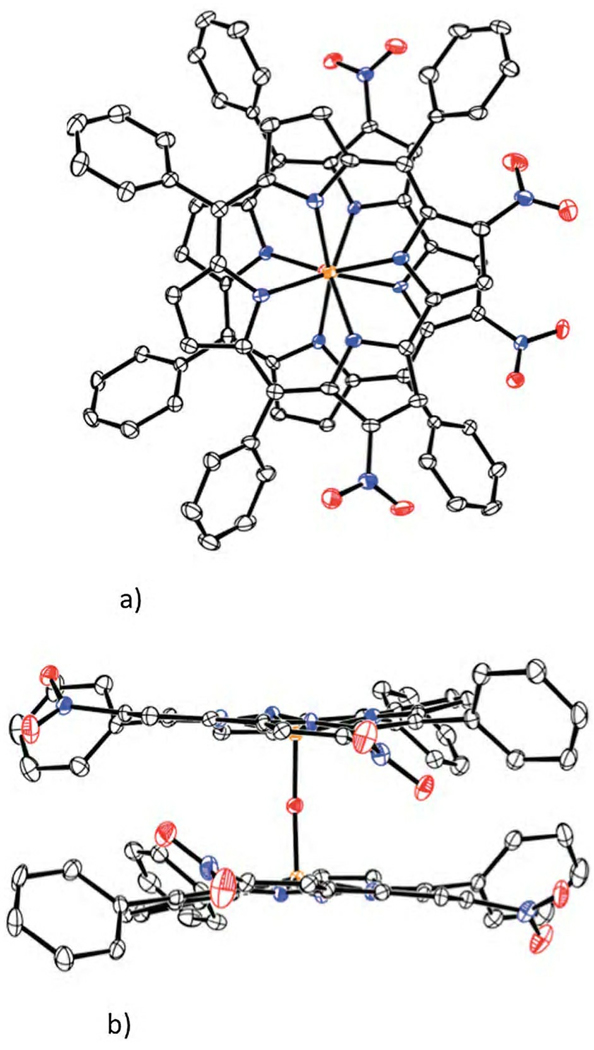
The third compound (15% yield) gave green-brown crystals. This product also featured a stretching band at 1801 cm−1 in its IR spectrum, indicating the formation of a different nitrosyl complex. The 1H NMR spectrum showed the presence of a single peak at 8.58 ppm in the β-pyrrolic region of the spectrum, consistent with its identification as the nitrosyl iron 3,17-dinitro-5,10,15-tris(4-methylphenyl)corrolate, 3,17-(NO2)2ttcorrFeNO, according to the general regioselectivity of corroles.16 Our attempts to obtain a single crystal of this complex suitable for X-ray structural characterization failed, but we were successful with the nitrosyl iron 3,17-dinitro-5,10,15-triphenylcorrolate, 3,17-(NO2)2tpcorrFeNO, obtained in similar yields from the reaction of tpcorrFeCl and sodium nitrite. In this case slow diffusion of MeOH into a CHCl3 solution of the compound afforded single crystals suitable for X-ray analysis. The molecular structure obtained is displayed in Fig. 2;17 much to our surprise the compound was found to be the corresponding μ-oxo dimmer [3,17-(NO2)2tpcorrFe]2O.
Figure 2.
Molecular structure of [3,17-(NO2)2tpcorrFe]2O with 50% ellipsoids: (a) front view; (b) side view. H atoms are not shown.
The Fe–O–Fe linkage is slightly nonlinear, 170.89(16)°, and the Fe–O distances are 1.709(2) and 1.713(2)Å. The coordination of the Fe atoms is square pyramidal, with Fe–N distances in the range 1.898(3)–1.922(3) Å, and the Fe atoms lying 0.3828(5) and 0.3854(5) Å out of their respective N4 planes, toward the center of the molecule. The dimeric molecule has a noncrystallographic twofold symmetry, with the two corrole ring systems rotated 60.0° from the eclipsed conformation. The two N4 planes are tilted slightly away from parallel, forming a dihedral angle of 7.38(8)°. The 23-atom corrole rings are nonplanar, one exhibiting mean and maximum deviations of 0.149 and 0.345(3) Å from coplanarity, the other 0.159 and 0.372(3) Å. Their deviation from planarity can be described as a slight saddle distortion, with pyrrole rings alternating direction of tilt around the corrole ring, and opposite pyrrole rings forming dihedral angles in the range 14.3(1)°–19.3(1)°. The nitro groups are tipped sharply out of the corrole best planes, with their N atoms having out-of-plane deviations of 0.493(3)–0.754(3) Å.
The formation of the μ-oxo dimer can be reasonably attributed to the axial liability of nitrosyl iron corrolates, reported by Joseph and Ford,18 which can lead to the replacement of the nitrosyl ligand during the slow crystallization step.
These results show that the reaction of ttcorrFeCl with NaNO2 leads to the formation of β-nitro substituted species, other than the expected nitrosyl complex. This reaction supports the hypothesis that the oxidized character of the corrole ligand is necessary for the peripheral characterization of the corrole ring. When the reaction was carried out in pyridine, the β-functionalization was not observed and the starting ttcorrFeCl was quantitatively recovered after work-up. In pyridine the formation of ttcorrFe(py)2 leads to a Fe(iii) derivative with no oxidized character for the corrole ligand,7 thus precluding the reaction with the nitrite ion. It should also be noted that the favoured β-substitution of ttcorrFeCl upon reaction with nitrite with respect to the meso-functionalization observed in the case of free base corroles19 is in accord with the a2u radical character of chloroiron corrolates,7 which in fact shows low spin density on the β-positions and large spin density on the meso-positions.
The synthetic interest of such a reaction for the preparation of β-nitro iron corrolates should also be noted, considering that the amount of ttcorrFeNO among the reaction products can be drastically reduced by increasing the excess of the NaNO2 reagent ([NaNO2]/[ttcorrFeCl] = 1/500), with 3,17-(NO2)2ttcorrFeNO becoming the major reaction product (60% yield). This result suggested that ttcorrFeNO should also react with NaNO2 to give β-functionalized species, and this hypothesis was confirmed by reaction of ttcorrFeNO and NaNO2 under the same reaction conditions, to produce both 3-NO2ttcorrFeNO and 3,17-(NO2)2ttcorrFeNO in an almost 1 : 1 ratio.
This result appears to conflict with our hypothesis of a nucleophilic attack of the nitrite ion upon the iron corrolate, because it is well known that the nitrosyl complex is a diamagnetic Fe(iii) species, with no π-cation radical character of the corrole ligand, which should prevent the peripheral substitution. However one has to consider the axial liability of the nitrosyl ligand in iron corrolates,18,20 which could allow the formation of a reactive species under the experimental conditions. This hypothesis is confirmed by the complete conversion of the nitrosyl complex into the disubstituted 3,17-(NO2)2ttcorrFeNO when the reaction was carried out under irradiation, which would promote nitrosyl labilization.
While the π-cation radical nature of ttcorrFeCl is necessary for the success of the reaction, we have to consider a possible alternative reaction pathway where the β-nitro derivative is produced by reaction of the radical species with NO2•, formed by oxidation of the nitrite ion. Although this hypothesis could not be dismissed in principle, it is a fact that the corrole π-cation radical does not react with N2O4,21 and this result favours the nucleophilic attack by the nitrite ion to give the corresponding β-nitro functionalized species.
Further studies on these derivatives are currently underway in our laboratories.
Supplementary Material
Acknowledgments
This research was supported by MIUR Italy (PRIN project 2007C8RW53) and the United States National Institutes of Health (K.M.S. grant CA 132861).
Footnotes
Electronic supplementary information (ESI) available: Synthetic procedures, spectroscopic data and crystallographic data in CIF for [3,17-(NO2)2tpcorrFe]2O. CCDC 803462. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc05491g
Notes and references
- 1.Aviv-Harel I, Gross Z. Chem.–Eur. J. 2009;15:8382. doi: 10.1002/chem.200900920. [DOI] [PubMed] [Google Scholar]
- 2.(a) Paolesse R. Synlett. 2008:2215. [Google Scholar]; (b) Gryko DT, Fox JP, Goldberg DP. J. Porphyrins Phthalocyanines. 2004;8:1091. [Google Scholar]; (c) Nardis S, Monti D, Paolesse R. Mini-Rev. Org. Chem. 2005;2:546. [Google Scholar]
- 3.(a) Barbe J-M, Canard G, Brandès S, Guilard R. Chem.– Eur. J. 2007;13:2118. doi: 10.1002/chem.200601143. [DOI] [PubMed] [Google Scholar]; (b) He C-L, Ren F-L, Zhang X-B, Han Z-X. Talanta. 2006;70:364. doi: 10.1016/j.talanta.2006.02.051. [DOI] [PubMed] [Google Scholar]; (c) Gatto E, Malik MA, Di Natale C, Paolesse R, D’Amico A, Lundström I, Filippini D. Chem.–Eur. J. 2008;14:6057. doi: 10.1002/chem.200800590. [DOI] [PubMed] [Google Scholar]; (d) Paolesse R, Mandoj F, Marini A, Di Natale C. In: Encyclopedia of Nanoscience and Nanotechnology. Nalwa H, editor. Valencia, CA: American Science Publishers; 2004. [Google Scholar]; (e) Andrioletti B, Rose E. J. Chem. Soc., Perkin Trans. 1. 2002:715–716. [Google Scholar]
- 4.Erben C, Will S, Kadish KM. In: The Porphyrin Handbook. Kadish KM, Smith KM, Guilard R, editors. vol. 2. San Diego: Academic Press; 2000. p. 233. [Google Scholar]
- 5.Ghosh A, Wondimagegn T, Parusel ABJ. J. Am. Chem. Soc. 2000;122:5100. [Google Scholar]
- 6.Simkhovich L, Goldberg I, Gross Z. Inorg. Chem. 2002;41:5433. doi: 10.1021/ic020118b. [DOI] [PubMed] [Google Scholar]
- 7.Walker FA, Licoccia S, Paolesse R. J. Inorg. Biochem. 2006;100 doi: 10.1016/j.jinorgbio.2006.01.038. 810 and references therein. [DOI] [PubMed] [Google Scholar]
- 8.Ye S, Tuttle T, Bill E, Simkhovich L, Gross Z, Thiel W, Neese F. Chem.–Eur. J. 2008;14:10839. doi: 10.1002/chem.200801265. [DOI] [PubMed] [Google Scholar]
- 9.Stefanelli M, Mastroianni M, Nardis S, Fronczek FR, Smith KM, Zhu W, Ou Z, Kadish KM, Paolesse R. Inorg. Chem. 2007;46:10791. doi: 10.1021/ic7014572. [DOI] [PubMed] [Google Scholar]
- 10.Vogel E, Will S, Tilling AS, Neumann L, Lex J, Bill E, Trautwein AX, Wieghardt K. Angew. Chem., Int. Ed. Engl. 1994;33:731. [Google Scholar]
- 11.Malek A, Latos-Grazynski L, Bartczak TJ, Zadlo A. Inorg. Chem. 1991;30:3222. [Google Scholar]
- 12.Autret M, Will S, Van Caemelbecke E, Lex J, Gisselbrecht J-P, Gross M, Vogel E, Kadish KM. J. Am. Chem. Soc. 1994;116:9141. [Google Scholar]
- 13.Spectroscopic data for 3-NO2ttcorrFeNO: 1H NMR (400 MHz, CDCl3, J [Hz]): δ = 8.57 (s, 1H, β-pyrrolic), 8.08 (d, 1H, J = 4.6, β-pyrrolic), 7.97 (d, 1H, J = 4.6, β-pyrrolic), 7.85 (d, 2H, J = 7.9, phenyl), 7.81 (dd, 2H, J=4.6, β-pyrrolic), 7.74 (m, 4H, phenyl), 7.66 (d, 1H, J=4.8, β-pyrrolic), 7.60 (d, 1H, J=4.8, β-pyrrolic), 7.52 (m, 6H, phenyl), 2.67 (s, 3H, –CH3), 2.65 (s, 3H, –CH3), 2.63 ppm (s, 3H, –CH3); UV/Vis (CHCl3): λmax (log ε)=416 (4.13), 577 nm (3.73); MS (FAB): m/z 666 (M+–NO); IR (CDCl3): νNO 1790 cm−1. Spectroscopic data for 3,17-(NO2)2ttcorrFeNO: 1H NMR (400 MHz, CDCl3, J [Hz]): δ = 8.58 (s, 2H, β-pyrrolic), 7.96 (d, 2H, J = 4.8, β-pyrrolic), 7.77 (d, 2H, J = 4.9, β-pyrrolic), 7.72 (m, 6H, phenyl), 7.53 (m, 6H, phenyl), 2.66 (s, 3H, –CH3), 2.65 (s, 6H, –CH3); UV/Vis (CHCl3): λmax (log ε) = 421 (4.35), 599 nm (3.99); MS (FAB): m/z 711 (M+–NO); IR (CDCl3): νNO 1801 cm−1.
- 14.Joseph CA, Lee MS, Iretskii AV, Wu G, Ford PC. Inorg. Chem. 2006;45:2075. doi: 10.1021/ic051956j. [DOI] [PubMed] [Google Scholar]
- 15.Stefanelli M, Shen J, Zhu W, Mastroianni M, Mandoj F, Nardis S, Ou Z, Kadish KM, Fronczek FR, Smith KM, Paolesse R. Inorg. Chem. 2009;48:6879. doi: 10.1021/ic900859a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saltsman I, Mahammed A, Goldberg I, Tkachenko E, Botoshansky M, Gross Z. J. Am. Chem. Soc. 2003;124:7420. doi: 10.1021/ja025851g. [DOI] [PubMed] [Google Scholar]
- 17.Diffraction data were collected on a Nonius Kappa CCD diffractometer equipped with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) and an Oxford Cryostream low-temperature device. Crystal data: C74H42Fe2N12O9·2CHCl3, brown lath fragment, triclinic space group P1̄, a = 15.724(2), b = 15.777(2), c = 16.522(2) Å, α = 77.023(7), β = 70.848(6), γ = 61.863(5)°, V = 3402.3(7) Å3, Z= 2, Dcalc =1.556 g cm−3, μ = 0.733 mm−1, T = 90.0(5) K, 70 569 reflections collected with θmax < 29.0°, 15 966 independent reflections (Rint=0.044) which were used in all the calculations, 10 693 data with I > 2σ(I). Final residuals (for 947 parameters) were R1 [I > 2σ(I)] = 0.063, wR2 (all data) = 0.178, CCDC 803462.
- 18.Joseph CA, Ford PC. J. Am. Chem. Soc. 2005;127:6737. doi: 10.1021/ja044090+. [DOI] [PubMed] [Google Scholar]
- 19.Nardis S, Pomarico G, Fronczek FR, Vicente MGH, Paolesse R. Tetrahedron Lett. 2007;48:8643. [Google Scholar]
- 20.Hocking RK, DeBeer George S, Gross Z, Walker FA, Hodgson KO, Hedman B, Solomon EI. Inorg. Chem. 2009;48:1678. doi: 10.1021/ic802248t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaquinod L, Paolesse R, Smith KM. unpublished results. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.