Abstract
Objectives
Long-chain omega-3 polyunsaturated fatty acids (LCω3PUFAs), selenium (Se) and mercury (Hg) are three important components in fish. The cardioprotective effect of LCω3PUFA intake has been recognized; however, the hypothesis that this benefit may be greatest with high Se and low Hg levels has not been investigated.
Design
A cohort of 4,508 American adults aged 18–30, without hypertension at baseline in 1985, were enrolled. Six follow-ups were conducted at exams in 1987, 1990, 1992, 1995, 2000 and 2005. Diet was assessed by a validated interviewer-administered quantitative food frequency questionnaire at exams in 1985, 1992 and 2005. Incident hypertension was defined as first occurrence at any follow-up examination of systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or taking anti-hypertensive medication. Toenail clippings were collected in 1987, and Se and Hg levels were quantified by instrumental neutron-activation analysis.
Result
Participants in the highest LCω3PUFAintake quartile had a significantly lower incidence of hypertension (Hazard Ratio: 0.65; 95% CI: 0.53–0.79; Ptrend<0.01) compared to those in the lowest quartile after adjustment for potential confounders. Docosahexaenoic acid showed a greater inverse association than eicosapentaenoic acid. The inverse association of LCω3PUFA intake with hypertension appeared more pronounced at higher Se and lower Hg levels, although interaction tests were statistically non-significant.
Conclusions
Out findings indicated that LCω3PUFA intake was inversely associated with incidence of hypertension. The prior hypothesis that the potential anti-hypertensive effect of LCω3PUFA intake varies depending on joint levels of Se and Hg received modest support, and cannot be ruled out.
Keywords: omega-3 polyunsaturated fatty acids, selenium, mercury, hypertension, effect modification
Introduction
Fish or seafood is the major dietary source of long-chain omega-3 polyunsaturated fatty acids (LCω3PUFAs), including eicosapentaenoic acid (EPA, 20:5 ω-3), docosapentaenoic acid (DPA, 22:5 ω-3) and docosahexaenoic acid (DHA, 22:6 ω-3). Arobust body of evidence supports that fish consumption or LCω3PUFAintake may have a beneficial effect on cardiovascular disease (CVD) risk, particularly, fatal coronary heart disease (CHD).[1–5] One of the postulated mechanisms underlying this potential benefit is that LCω3PUFA intake may favorably modify blood pressure (BP),[6] a major CVD risk factor.[7–8]Although the literature is not quite consistent, the majority of experimental and epidemiological studies have suggested that LCω3PUFAs at high dose could lower BP.[9–10]A meta-analysis published in 1993 summarized 31 placebo-controlled clinical trials including a total of 1,356 individuals and found both EPA and DHA can significantly reduce BP in a dose-response fashion in hypertensive patients though not in normotensive individuals.[11] The findings were confirmed by two other meta-analyses of clinical trials.[12–13] However, data on the association of usual dietary intake of LCω3PUFA with BP and incidence of hypertension are sparse.
While fish consumption has consistently increased over the past decades, concerns have been raised regarding potential harm of exposure to mercury (Hg) found in some fish. In addition to its potential neurotoxicity,[14] Hg ingestion from fish has been linked to increased risk of CHD in men,[15–16] as well as to high BP among Nunavik Inuit adults in a recent study.[10] On the other hand, fish is rich in selenium (Se), an antioxidant that may provide cardioprotective benefits by itself at an optimal level or by interacting with LCω3PUFA intake.[17] Notably, Se may moderate the toxic effects of Hg in the body.[18] Thus, it is important to consider both Hg and Se in addition to LCω3PUFAs when investigating the potential beneficial effects of fish consumption. Recently, a hypothesis has been proposed that Se and Hg may jointly modify the association between LCω3PUFAs and CVD risk, with the greatest benefit expected in the setting of high Se and low Hg concentrations.[4] We therefore prospectively examined intakes of LCω3PUFAs and fish in relation to incidence of hypertension, and investigated the possible modifications of Se and Hg on the associations between LCω3PUFAs and hypertension using data from the Coronary Artery Risk Development in Young Adults (CARDIA) Study.
Methods
Study Population
CARDIA is an ongoing, multi-center, prospective cohort study designed to examine physiological, psychological and other lifestyle factors that might affect evolution of CVD risk among American young adults. Details of the study design have been published elsewhere.[19] Briefly, at baseline in 1985, 5,115 men and women, aged 18 to 30 years, were enrolled from four US cities including Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. By design, this cohort was roughly balanced by age (18–24 and 25–30), gender, ethnicity(AfricanAmerican and Caucasian), and education (high school or below and beyond high school). To date, six follow-up examinations have been conducted at exam years 2, 5, 7, 10, 15 and 20. Follow-up rates averaged greater than 90% and approximately 70% of the participants in the original cohort returned at year 20. We excluded participants who reported implausible total energy intake (<800 or >8000 kcal/d for men, and <600 or >6000 kcal/d for women) (n=46) and participants with missing data on exposure variables at all diet assessments (n=4). To be conservative, we also excluded pregnant women at any examination (n=237). A total of 4,828 participants remained in the analyses of LCω3PUFA or non-fried fish in relation to blood pressure. To examine the association between LCω3PUFAand non-fried fish and incidence of hypertension, we further excluded participants who had diagnosed hypertension at baseline (n=140), who had no information for defining incident hypertension during the follow-up (n=175) and had incomplete exposure information for creating cumulative diet model in the analysis (n=5).[20] After all these exclusions, 4,508 participants remained in the analyses. Of these, 3,883 participants with both toenail Se and Hg data available were included in the analyses investigating effect modification of Se and Hg on the association of LCω3PUFA intake and incident hypertension.
All participants gave informed consent. The study design, data collection, and analyses were approved by the institutional review boards of the participating institutions.
Ascertainment of fish consumption and LCω3PUFA intake
The CARDIA Diet History questionnaire, an interviewer-administered quantitative food frequency questionnaire (FFQ), was designed to assess habitual eating patterns. The validity and reproducibility of CARDIA FFQ has been evaluated and discussed elsewhere.[21–22] Diet assessment was conducted three times at baseline, exam years 7 and 20. Participants were asked to recall their usual dietary intakes by using the previous 30 days as the time frame. They were asked general questions about their diet, which elicited specific foods consumed in an open-ended fashion. They were then asked to report the frequency, amount, and method of food preparation for each food named. Particularly for fish consumption, participants were asked: 1) “Do you eat any fresh, frozen, or smoked fish? Include any sushi or fast food fish sandwiches eaten at places such as McDonald’s, Burger King, or Wendy’s.”; 2) “Do you eat any fresh or frozen shellfish, octopus, or squid?”; 3) “Do you eat tuna salad?”; and 4) “Do you eat any other canned fish including tuna, sardines, herring, or salmon?”. In addition, studies have suggested that the preparation method, particularly frying, may substantially alter the fatty acid content of a fish meal.[23] Consequently, the association with CVD risk may be different.[24] Therefore, we divided fish consumption into fried fish and non-fried fish. Fried fish included fried fish and fried shellfish from commercial and fast food.[25] Non-fried fish was the sum of fresh fish, smoked fish, lean fish and shellfish. Because the distribution of fried fish consumption was extremely skewed and relatively narrow, we did not treat fried fish as an exposure of interest. Instead, we adjusted for fried fish when examining non-fried fish. Similarly, we did not separate shellfish from other fish in the primary analysis. However, we excluded shellfish in a sensitivity analysis. In addition, we were not able to single fish in fast food out based on the available information from the questionnaire.
Nutrient intake was estimated using an adaptively updated nutrient database from Nutrition Coordinating Center at the University of Minnesota. This database is a food composition table containing 1609 distinct codes for exam years 0 and 7, and over 7000 codes for exam year 20, including LCω3PUFAs. In this study, LCω3PUFA intake was defined as the sum of DHA, EPAand DPA. Because of the relatively small amount and the narrow distribution, DPA was not analyzed as a separate exposure in this study.
Ascertainment of hypertension
BP was measured using the Hawksley random-zero (RZ) sphygmomanometer (W. A. Baum Co, Copiague, New York) in the first six examinations (i.e. exam years 0, 2, 5, 7, 10 and 15) and the OmROn HEM907XL at exam year 20 by trained and certified technicians.[26] Each participant had three BP measurements taken from the right arm at 1-minute intervals after a 5-minute seated rest. The second and third of the three measurements were averaged for analyses. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded as phase I and V Korotkoff sounds through year 15. Based on a study in 900 participants, at exam year 20 we estimated systolic BP (RZ) =3.74+0.96 * observed OmROn systolic BP and estimated diastolic BP =1.30+0.97 * observed OmROn diastolic BP.
The hypertension endpoint is based on BP cutoff points used in the seventh report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure.[27] Incident hypertension was defined as first occurrence at any follow-up examination of SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg or if taking anti-hypertensive medication.
Ascertainment of toenail Se and Hg
Toenail clippings were collected with a stainless steel clipper from all 10 toes by the participants themselves at exam year 2. All toenail clippings were processed with a washing procedure in a sonicator with deionized water.[28]The levels of Se and Hg were analyzed by instrumental neutron-activation analysis at the University of Missouri Research Reactor.[29] Toenail specimens were treated in random order by the laboratory personnel who were blinded to other clinical measures. The average coefficient of variation in duplicate toenail sub-samples was 2.5% for Se and 6.8% for Hg in the present study.
Toenail Se is recognized as the best available reliable biomarker reflecting relatively long-term Se intake.[30–32]Asingle sample of the toenail concentration of Se represents exposure over 9 to 12 months.[33–34] Similarly, Hg level in toenail provides the best biomarker of long-term Hg exposure.[35–36] Spearman correlation coefficients for the reproducibility of toenail levels of Se and Hg were 0.48 and 0.54, respectively, over 6 years.[37] In a pilot study conducted in 2007 among 64 randomly selected CARDIA participants from the Chicago Study Center, the Spearman correlation coefficient between two measurements of toenail concentrations collected 20 years apart by the same laboratory with the same protocol was 0.56 (Se) and 0.60 (Hg).[28]
Ascertainment of covariates
Demographic variables, including age, gender, ethnicity and education level, were collected through self-administered questionnaire and were verified during clinic examinations. Smoking status was determined based on self-report and participants were classified into three groups: non-smokers, former smokers and current smokers. Alcohol consumption was measured by validated questionnaire and classified into six groups according to total daily intake: 0 (never drink), 0.1–4.9, 5.0–9.9, 10–14.9, 15.0–19.9, and ≥ 20 g/d. Body weight and height were measured in light clothes without shoes, during the clinical visit for each examination. Physical activity was assessed using the CARDIA Physical Activity History Questionnaire (PAHQ), an interviewer-administered self-report of frequency of participation in 13 categories of recreational sports, exercise, leisure, and occupational activities over the previous 12 months. The physical activity score was calculated in exercise units (EU) reflecting the frequency and duration of activity over the previous year. A score of 100 EU is roughly equivalent to participation in a vigorous activity, 2 to 3 hours per week for 6 months of the year.[38–40]
Statistical Analysis
Analysis of variance, the Kruskal-Wallis test or the chi-squared test, when appropriate, was used to compare baseline characteristics of the study population according to quartiles of LCω3PUFA intake. Generalized estimating equations (GEEs) with identity linkage under exchangeable correlation structure assumption were used to examine intakes of LCω3PUFA and non-fried fish in relation to SBP and DBP. Cox proportional hazards regression model was used to examine intakes of total LCω3PUFA, DHA, EPA and non-fried fish in relation to incidence of hypertension.
To reduce measurement errors caused by within-person variation and to best represent the long-term dietary intakes, we used cumulative intakes of nutrients or foods in Cox models.[20] For example, we related fish consumption reported at baseline to the new cases identified at exam years 2 and 5; the average of fish consumption reported at baseline and year 7 to the new cases identified at exam years 7, 10 and 15; and the average of fish consumption reported at baseline, years 7 and 20 to the new cases identified at year 20. We categorized the exposure of interest into quartiles based on their distributions. We used a sequential covariates-adjusted strategy in both GEEs and Cox models: Model 1 adjustment for age, gender, ethnicity, and study center; Model 2 additional adjustment for body mass index (BMI), education, smoking status, alcohol consumption, physical activity, family history of hypertension, and dietary intakes of total energy, sodium, α-linolenic acid and linoleic-acid. Since information on anti-hypertensive medication was used to define incident cases of hypertension, we did not include anti-hypertensive medication in the Cox model, but it was additionally adjusted in model 2 for the GEE analyses. When studying non-fried fish, we further adjusted for fried fish consumption based on model 2. To examine interaction between gender or ethnicityand intakes of LCω3PUFAs or non-fried fish, we included corresponding interaction terms in the models followed by likelihood ratio test.
To examine the potential effect modification of Se or Hg levels on the association between LCω3PUFA intake and incidence of hypertension, we divided toenail Se or Hg levels into tertiles and examined the association of LCω3PUFA intake with hypertension across tertiles using Cox proportional hazards models. We also used restricted cubic splines to study the smoothed functions to detect any possible interaction. To make the graphs more stable, we deleted the observations with LCω3PUFA intake above 95th percentile, which were extremely high.[41–42]Although tests for interactions were statistically non-significant, we conducted and presented stratified analyses in relation to our strong prior hypothesis.
To further determine the joint modification of Se and Hg levels on the association between LCω3PUFA intake and incidence of hypertension, we divided participants into four sub-groups defined by joint levels of median Se (0.84 μg/g) and median Hg (0.21 μg/g). The associations were examined in each sub-group. We also used the likelihood ratio test to determine any possible significant 2- or 3-way interaction among LCω3PUFA intake, Se levels, and Hg levels.
All analyses were performed by using SAS version 9.1.3 (SAS Institute Inc, Cary, North Carolina, US) and all graphics were generated using STATA 11.0 (StataCorp LP, College Station, Texas, US). P≤0.05 was considered statistically significant.
Results
Table 1 displays baseline characteristics of 4,508 participants according to quartiles of LCω3PUFA intake. The average intakes of LCω3PUFAs were 0.03, 0.09, 0.15 and 0.37 g/day across quartiles. Comparing to participants in the lowest quartile of LCω3PUFA intake, those in the highest quartile were slightly older, more likely to be males and African-Americans, and had relatively higher education level and higher alcohol consumption. They were more likely to be active and were less likely to be current smokers.
Table 1.
Baseline characteristics of the study population according to quartile of LCω3PUFA intake, CARDIA study, 1985 to 2005*
| Quartile of LCω3PUFA intake |
||||||
|---|---|---|---|---|---|---|
| Characteristic | 1 (n=1,165) | 2 (n=1,085) | 3 (n=1,125) | 4 (n=1,133) | Total (n=4,508) | P–value† |
| LCω3PUFA intake (g/d) | 0.03±0.02 | 0.09±0.02 | 0.15±0.02 | 0.37±0.27 | 0.16±0.19 | |
| Non-fried fish (Servings/d) | 0.23±0.24 | 0.57±0.40 | 0.98±0.55 | 2.05±1.49 | 0.96±1.07 | <0.01 |
| Fried fish intake (% consumers) | 10.3 | 16.9 | 22.1 | 18.5 | 16.9 | <0.01 |
| Systolic blood pressure (mmHg) | 109.6±10.5 | 110.4±10.2 | 109.5±10.2 | 110.3±9.7 | 110.0±10.2 | 0.10 |
| Diastolic blood pressure (mmHg) | 68.1±8.9 | 68.6±8.8 | 68.2±8.7 | 68.2±8.8 | 68.3±8.8 | 0.60 |
| Selenium (μg/g) ‡ | 0.87±0.15 | 0.86±0.15 | 0.85±0.16 | 0.85±0.16 | 0.86±0.15 | <0.01 |
| Mercury (μg/g) ‡ | ||||||
| Median | 0.15 | 0.21 | 0.22 | 0.28 | 0.21 | |
| IQR§ | 0.09–0.27 | 0.13–0.34 | 0.13–0.37 | 0.16–0.50 | 0.12–0.37 | <0.01 |
| Age (year) | 24.3±3.8 | 24.9±3.6 | 25.0±3.6 | 25.2±3.5 | 24.9±3.7 | <0.01 |
| Female (%) | 59.4 | 56.2 | 51.6 | 45.0 | 53.1 | <0.01 |
| African American (%) | 48.2 | 47.6 | 50.8 | 55.7 | 50.6 | <0.01 |
| Education (year) | 13.4±2.1 | 13.9±2.2 | 14.0±2.3 | 14.1±2.3 | 13.8±2.3 | <0.01 |
| Current smokers (%) | 32.6 | 30.2 | 27.7 | 29.6 | 30.1 | 0.02 |
| Alcohol consumption (mL/d) | ||||||
| Median | 2.4 | 2.7 | 4.8 | 7.3 | 4.8 | <0.01 |
| IQR§ | 0.0–13.3 | 0.0–13.3 | 0.0–14.7 | 0.0–19.5 | 0.0–14.7 | |
| Physical activity (score) | ||||||
| Median | 306 | 349 | 364 | 435 | 364 | <0.01 |
| IQR§ | 166–514 | 192–558 | 204–567 | 250–673 | 200–579 | |
| Body mass index (kg/m2) | 24.4±4.9 | 24.5±5.1 | 24.5±5.1 | 24.3±4.6 | 24.4±4.9 | 0.78 |
| Family history of hypertension (%) | 49.4 | 50.7 | 50.2 | 47.8 | 49.5 | 0.55 |
| Dietary intake | ||||||
| Energy (kcal/day) | 2584±1325 | 2763±1439 | 2953±1550 | 3397±1742 | 2923±1551 | <0.01 |
| Sodium (mg/1000kcal/day) | 1481±308 | 1500±295 | 1506±293 | 1481±291 | 1492±297 | <0.01 |
| linoleic-acid (g/day) | 16.0±9.4 | 18.6±11.0 | 20.2±12.9 | 24.0±14.4 | 19.7±12.4 | <0.01 |
| α-linolenic acid (g/day) | 1.7±1.0 | 1.9±1.1 | 2.1±1.2 | 2.5±1.5 | 2.0±1.2 | <0.01 |
CARDIA, Coronary Artery Risk Development in Young Adults; IQR, inter–quartile range; LCω3PUFAs, long-chain omega-3 polyunsaturated fatty acids.
Mean ± SD (all such values), data are unadjusted.
P values were for any difference across the quartiles, obtained by using analysis of variance, Kruskal-Wallis test or Chi-square test as appropriate.
Based on 3883 participants with toenail data available.
25th – 75th percentiles.
Using GEE, we found that LCω3PUFAintake was inversely associated with SBP as well as DBP. The multivariable-adjusted β coefficients comparing the highest to the lowest quartiles of LCω3PUFAintake were −0.92 mmHg (95% CI: −1.49 to −0.34 mmHg; P for trend < 0.01) and −0.72 mmHg (95%CI: −1.19 to −0.25 mmHg; P for trend = 0.01) for SBP and DBP, respectively. However, non-fried fish consumption was only found to be significantly inversely related to SBP [−0.63 (95%CI: −1.20 to −0.06; P for trend = 0.04)], not DBP [−0.17 (95%CI: −0.65 to 0.31; P for trend = 0.63)], comparing the participants in the highest to those in the lowest quartile. When we examined the associations among normotensive individuals, LCω3PUFA intake, but not non-fried fish consumption, was inversely associated with SBP and DBP.
A total of 999 new cases of hypertension occurred during the follow-up. Using Cox models, we found that LCω3PUFAintake was inversely associated with incidence of hypertension. Participants in the highest quartile of LCω3PUFA intake had a significantly lower incidence of hypertension [Hazard Ratio (HR) =0.65; 95% CI: 0.53-0.79; P for trend < 0.01] compared to those in the lowest quartile after adjustment for potential dietary and non-dietary confounders (Table 2). The observed inverse associations between LCω3PUFAintake and incidence of hypertension were generally consistent in each gender or ethnicityspecific subgroup (data not shown). When examining EPA and DHAseparately, we found that DHA had a greater inverse association with incidence of hypertension than EPA. Non-fried fish consumption was marginally and inversely related to incidence of hypertension. Comparing the highest to the lowest quartile of non-fried fish consumption, HR was 0.85 (95% CI: 0.70–1.03; P for trend = 0.06] (Table 2). In addition, outcome associations with total fish with no adjustment for use of fried fish were attenuated compared to those for non-fried fish with adjustment for fried fish (data not shown).
Table 2.
Multivariable-adjusted HRs (95% CIs) of incidence of hypertension by intakes (quartiles) of LCω3PUFA, EPA, DHA and non-fried fish, CARDIA study, 1985 to 2005*
| Dietary variable | Quartiles |
P for trend† | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| LCω3PUFA intake (EPA+DPA+ DHA, g/d) | <0.060 | 0.060~0.113 | 0.114~0.200 | ≥0.201 | ||
| No. of participants | 1165 | 1085 | 1125 | 1133 | ||
| No. of events | 247 | 259 | 270 | 223 | ||
| Model 1‡ | 1.00 | 0.91 (0.76–1.08) | 0.82 (0.69–0.98) | 0.63 (0.52–0.75) | <0.01 | |
| Model 2§ | 1.00 | 0.94 (0.79–1.13) | 0.85 (0.71–1.02) | 0.65 (0.53–0.79) | <0.01 | |
| EPAintake (g/d) | <0.020 | 0.020~0.040 | 0.041~0.077 | ≥0.078 | ||
| No. of participants | 1278 | 1001 | 1059 | 1170 | ||
| No. of events | 277 | 231 | 246 | 245 | ||
| Model 1‡ | 1.00 | 0.93 (0.78–1.10) | 0.86 (0.72–1.02) | 0.76 (0.64–0.91) | <0.01 | |
| Model 2§ | 1.00 | 0.94 (0.79–1.12) | 0.87 (0.73–1.05) | 0.80 (0.66–0.96) | 0.02 | |
| DHAintake (g/d) | <0.023 | 0.023~0.050 | 0.051~0.095 | ≥0.096 | ||
| No. of participants | 1134 | 1043 | 1207 | 1124 | ||
| No. of events | 261 | 241 | 292 | 205 | ||
| Model 1‡ | 1.00 | 0.72 (0.60–0.86) | 0.68 (0.58–0.81) | 0.45 (0.37–0.54) | <0.01 | |
| Model 2§ | 1.00 | 0.72 (0.60–0.86) | 0.71 (0.59–0.84) | 0.45 (0.37–0.55) | <0.01 | |
| Non-fried fish consumption (servings/d)|| | <0.305 | 0.305~0.660 | 0.661~1.257 | ≥1.258 | ||
| No. of participants | 1127 | 1123 | 1130 | 1128 | ||
| No. of events | 245 | 259 | 257 | 238 | ||
| Model 1‡ | 1.00 | 0.95 (0.80–1.13) | 0.89 (0.74–1.06) | 0.80 (0.67–0.96) | 0.01 | |
| Model 2§ | 1.00 | 0.99 (0.83–1.19) | 0.93 (0.77–1.12) | 0.85 (0.70–1.03) | 0.06 | |
CARDIA, Coronary Artery Risk Development in Young Adults; CI, Confidence interval; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid; HR, Hazard ratio; LCω3PUFAs, long-chain omega-3 polyunsaturated fatty acids.
All models were constructed using Cox proportional hazards regression analysis.
Ordinal variables using medians in each quartile were created for the trend tests.
Model 1: adjusted for age, gender, ethnicity (African American, Caucasian) and study center.
Model 2: additionally adjusted for body mass index (continuous), physical activity (quartiles), education (<12, 12, 13–15, 16, >16 years), smoking status (non, former and current smokers), alcohol consumption (0, 0.1–4.9, 5.0–9.9, 10.0–14.9, 15.0–29.9, ≥ 30 ml/d), family history of hypertension (yes or no), and dietary intakes (quartiles) of total energy, sodium, α-linolenic acid and linolenic-acid.
Fried fish consumption (yes or no) was only adjusted in model 2 when studying non-fried fish.
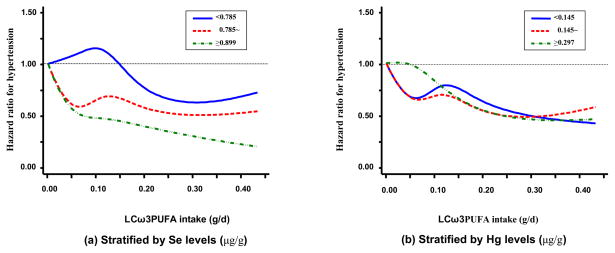
To test the prior hypothesis regarding the effect modifications of Se and Hg on LCω3PUFA, we first used restricted cubic splines to detect any possible interaction by obtaining a smooth representation of the HR as a function of LCω3PUFA intake by Se or Hg levels (tertiles) with adjustment for the potential confounders (Figure). By visual inspection, the inverse association between LCω3PUFAintake and incidence of hypertension was strengthened in the highest level of Se, though the test for interaction was non-significant (P = 0.20). However, Hg level, without reference to Se level, did not appreciably modify the inverse association of LCω3PUFA intake with hypertension. We further considered the joint modification of Se and Hg on LCω3PUFA intake, a 3-way interaction. We jointly classified data according to median levels of Se (0.84 μg/g) and Hg (0.21 μg/g). The inverse association between LCω3PUFA intake and incidence of hypertension was more pronounced at higher Se and lower Hg levels (Table 3), though the 3-way interaction was statistically non-significant (P = 0.57). Of note, the inverse associations were substantially attenuated and became statistically non-significant, when Se level was below the median and Hg level was above the median. In addition, Hg levels did not modify the association when Se level was above the median level. The incidence of hypertension was similar between participants with Hg levels above and below the median (0.21 μg/g) when toenail Se levels were greater than 0.84 μg/g. Several sensitivity analyses were conducted to test the robustness of our findings. First, when we chose not to update diet information for incident cases on anti-hypertensive medications at exam years 7 and 20, the results essentially were unchanged, and p for interaction of Se and LCω3PUFA intake was 0.17 and p for three way interaction was 0.55. Second, when we further excluded participants who had diagnosed hypertension at year 2 when toenail Se and Hg were measured, the results remained. Third, since most clinical trials found blood pressure reduction in a short time period after fish oil supplementation, we used the most-recent LCω3PUFA intake in the analysis,[20] the inverse association between LCω3PUFA intake and hypertension remained. However, the interaction of Se and LCω3PUFA intake became statistically significant (P=0.04), with a pattern similar to that shown in Table 3. Fourth, when we excluded shellfish from the fish consumption, our findings were generally consistent. Fifth, when we further adjusted for other nutrients (e.g. dietary potassium, calcium, magnesium, folic acid, saturated fat), the findings were not appreciably altered. Finally, we considered some time-dependent covariates (e.g. BMI, smoking status) in the models; the results were again not materially changed.
Figure.
Modification by toenail Se or Hg levels (tertiles) of the association between LCω3PUFA intake and incidence of hypertension (n=3883), CARDIA Study, 1985–2005. In the Cox proportional hazards models, LCω3PUFA intake was modeled as restricted cubic splines with nodes at the 25th, 50th, and 75th percentiles after deleting the observations above 95th percentile. Results were adjusted for the covariates listed for model 2 in table 2 plus Hg / Se levels (continuous in natural logarithm form) for (a) / (b).
Table 3.
Joint modification by toenail Se and Hg levels of the association between LCω3PUFA intake and incidence of hypertension, CARDIA study, 1985 to 2005*
| No. of participants | No. of Events | HRs (95%CIs) | ||
|---|---|---|---|---|
| Total cohort | 3883 | 852 | 0.81 (0.73, 0.90)† | |
| 0.82 (0.74, 0.91)‡ | ||||
| Se<0.84, | Hg ≥ 0.21 | 942 | 204 | 0.90 (0.78, 1.04) † |
| Hg <0.21 | 1003 | 278 | 0.78 (0.65, 0.95) † | |
| Se≥0.84, | Hg ≥ 0.21 | 1003 | 186 | 0.79 (0.66, 0.96) † |
| Hg <0.21 | 935 | 184 | 0.76 (0.58, 0.99) † | |
CARDIA, Coronary Artery Risk Development in Young Adults; CI, Confidence interval; HR, Hazard ratio; Hg, mercury; LCω3PUFA, long-chain omega-3 polyunsaturated fatty acid; Se, selenium.
HRs and 95% CIs were calculated based on a standard deviation (0.19g/d) increment in LCω3PUFA intake by using Cox proportional hazards regression analysis.
The model was adjusted for the covariates listed for model 2 in table 2.
Additionally adjusted for Se and Hg levels, both as binary variables (above vs. below median)
Se median: 0.84 (μg/g); Hg median: 0.21 (μg/g).
Discussion
In this unique 20-year follow-up longitudinal prospective study, we found that LCω3PUFA intake and non-fried fish consumption were inversely associated with BP and the incidence of hypertension among American young adults. DHA showed a greater inverse association with BP and incidence of hypertension than EPA. The potential favorable effect of LCω3PUFA intake on hypertension development appeared more pronounced at higher Se and lower Hg levels. An additional advantage is that both Se and Hg were measured in toenails, recognized as reliable biomarkers of long-term exposure.[15, 35, 43–44] Moreover, we have multiple in-depth dietary measurements. Further more, we had information to distinguish non-fried fish from fried fish, which contributes important additional information to the literature since the impact of different cooking methods on the effect of fish consumption has not been well investigated.[45]
Our findings are concordant with results from previous studies.[8, 46] For example, a cross-sectional study found that dietary LCω3PUFAintake was inversely related to BP among non-hypertensive individuals.[47]A meta-regression analysis published in 2002 included 36 randomized placebo-controlled trials, 22 of which had a double-blinded design, also showed an anti-hypertensive effect of fish oil[13]. Two meta-analyses of clinical trials published in 1993 reported that LCω3PUFAcan significantly reduce BP.[11–12] In short, our data are qualitatively and quantitatively similar in indicating a beneficial effect of LCω3PUFAs on BP and incidence of hypertension. Also, our study found that compared to EPA, DHA may provide a greater beneficial effect on hypertension, and the finding was supported by the literature.[48–50] In addition, few studies considered how fish was prepared before consumption. Cooking methods may alter quantities of LCω3PUFA in fish thus modifying the potential benefits of fish consumption. Our study found a potential benefit of non-fried fish consumption on incidence of hypertension, which was consistent with the results from previous studies.[51–52]
Literature regarding the interaction of Se and LCω3PUFA in relation to BP levels is limited. A randomized, double-blind study showed an interaction between LCω3PUFAand Se in affecting the plasma concentrations of lipid peroxidationproducts,[53] indicating that high Se levels may improve the benefit of LCω3PUFA, which is in agreement with our findings that the inverse association between LCω3PUFA intake and incidence of hypertension was more pronounced at high Se levels. Of note, it has been recently hypothesized that Se at very high level may not be beneficial.[54–55] However, the optimal ranges of Se level measured by different biomarkers such as levels in nails have not been established.
Data on Hg and LCω3PUFA interaction are not consistent. A cross-sectional analysis of NHANES (1999–2000) found a positive association of Hg with systolic BP only among non-fish consumers, but did not find an overall significant association, which support the hypothesis that the intake of fish oils may attenuate the harmful effects of Hg on BP regulation.[56]A case-control study including participants from eight European countries and Israel reported that high Hg content might diminish the cardioprotective effect of fish intake”.[15] In the Kuopio Ischaemic Heart Disease (KIHD) risk factor study, the investigators found that high Hg content measured in hair attenuated the protective effects of LCω3PUFA intake on acute coronary events[57] and overall cardiovascular health.[16] However, a nested case-control study found no association between toenail Hg and risk of CHD, either before or after adjustment for age and other risk factors, including LCω3PUFA intake.[35] In the present study, the pattern of findings was that high Hg level tended to attenuate the anti-hypertensive effect of LCω3PUFAto some extent when Se level was low, though the three-way interaction was statistically non-significant. Of note, the anti-hypertensive effect of LCω3PUFA intake did not appear to be appreciably attenuated by high level of Hg when Se level was relatively high. If this pattern is borne out, this pattern of findings would indicate that Se could antagonize the toxicity of Hg. The first report on the protective effect of Se against Hg’s toxicity appeared in 1967.[58]Today, the ability of Se compounds to decrease the toxic action of Hg has been established in vitro and in animal studies.[59–61] Epidemiological studies indicate a possible benefit of Se intake on Hg’s cardiovascular toxicity.[62] However, Se was not found to be an effect modifier of the relations between Hg and CHD events in the prospective study that directly evaluated the potential interaction of Hg and Se.[35] Further studies are warranted.
To the best of our knowledge, no study has investigated three-way interactions of Se, Hg and LCω3PUFA in relation to incidence of hypertension. A recent cross-sectional study evaluated the influences of levels of LCω3PUFAs and Se on the association between Hg levels and BP among Nunavik Inuit adults.[10]. The investigators found that LCω3PUFAs and Se were strong confounders in the association between Hg and BP, but were not effect modifiers. In the present study, we observed a pattern in which toenail Se levels modified the inverse associations between LCω3PUFAs and hypertension. Although the tests for interactions were statistically non-significant, the possibility of joint modification of Se and Hg on the benefits of LCω3PUFA intake can not be excluded. The non-significant interaction might partly be explained by sample size limitation.
The potential anti-hypertensive effect of LCω3PUFAs may not be a mono-component action, but may involve multiple mechanisms.[63–64] LCω3PUFAs may improve vascular and endothelial functions, as well as increase arterial compliance, thus influencing BP.[65–66] Also, several studies indicate that LCω3PUFAs reduce heart rate and cardiac function, which may also be associated with the anti-hypertensive effects.[49, 67–68] Other possible mechanisms include influencing sympathetic activityand enhancing endothelial nitric oxide synthesis through hemodynamic changes.[63]
The possible mechanisms explaining the modification of Se and Hg on the anti-hypertensive effect of LCω3PUFAs may lie in that both Se and Hg are somehow involved in the process of oxidative stress and cardiac function through the same pathway by which LCω3PUFAs regulate BP. In addition, studies suggest that Se can readily bind Hg to form insoluble HgSe adducts,[69–70] prevent selenoenzyme activities from Hg-dependent inhibition, and thus protect against Hg toxicity.[70–73]
Study limitations
A few limitations of this study also need to be considered. 1) Although the study is observational, our studyis so far the only large-scale prospective data available on examining the joint modification of Se and Hg measured with biomarkers on the longitudinal association of LCω3PUFAintake with incidence of hypertension. 2) The toenail Se and Hg were measured at exam year 2 (1987), but not at baseline. However, this short timeframe difference is unlikely to substantially bias our findings since toenail Se and Hg reflect a long-term exposure and toenail Se and Hg levels are usually not materially altered in a year or so.[34] In fact, after we further excluded participants who had diagnosed hypertension at year 2, the results remained. Further, toenail Se and Hg were only used as effect modifiers in the analyses. 3) More objective measures for LCω3PUFAs such as measurement in erythrocyte cell membrane in the whole cohort were unavailable. Of note, studies suggest that the correlation of dietary LCω3PUFAs with the concentrations in erythrocyte cell membrane and adipose tissue are reasonably high (Spearman correlation = 0.42 for erythrocyte cell membrane and 0.40 for adipose tissue).[74–75]Although measurement error is inevitable, the dietary measurements of LCω3PUFA intake and fish consumption should enable us to rank participants and estimate the relative risks.Also, the diet measurement errors are likely to be random and may bias the results towards null. 4) In this cohort, Hg levels were relatively low compared to other populations where Hg levels were also measured in toenails,[15, 35, 76] which might partially explain the null findings when considering Hg as an effect modifier in the stratified analysis. Finally, the generalizability of our findings may be limited. All participants were young American adults mainly from four metropolitan areas with roughly balanced gender and ethnicity. Their characteristics including toenail Se and Hg status may be different from the general population.
Conclusions
Our findings indicate that LCω3PUFA intake or non-fried fish consumption is inversely associated with BP and the incidence of hypertension, and that the potential beneficial effect of LCω3PUFA intake on hypertension development may be more pronounced at higher Se and lower Hg levels. The results of the present longitudinal study on an apparently healthy American young adult population help us to better understand the benefits and risks of consuming fish as a package containing various nutrients and contaminants. In addition, information generated from this study may be useful for informing healthy food selection and formulating dietary recommendations on fish consumption with respect to hypertension prevention. The present study illustrates the need for fully considering of fish type and preparation method, both contributing to varying levels of LCω3PUFA, Se, Hg and other components. Additional studies are warranted to elucidate the complex interactions among Se, Hg and LCω3PUFA, three important components in fish, in terms of hypertension prevention.
Acknowledgments
We would like to thank Dr. Lyn M. Steffen for her helpful comments and Dr. Winston Hwanseok Choi for verifying SAS programming. We also thank the other investigators and the staffs of Coronary Artery Risk Development in Young Adults (CARDIA) Study for their valuable contributions.
Source of Funding
This study supported by grants from the National Institutes of Health (R01HL081572, N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095).
Footnotes
Conflicts of interest statement
None to declare.
References
- 1.He K. Fish, long-chain omega-3 polyunsaturated fatty acids and prevention of cardiovascular disease--eat fish or take fish oil supplement? Prog Cardiovasc Dis. 2009;52:95–114. doi: 10.1016/j.pcad.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 2.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–11. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 3.Konig A, Bouzan C, Cohen JT, et al. A quantitative analysis of fish consumption and coronary heart disease mortality. Am J Prev Med. 2005;29:335–46. doi: 10.1016/j.amepre.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D. Fish, mercury, selenium and cardiovascular risk: current evidence and unanswered questions. Int J Environ Res Public Health. 2009;6:1894–916. doi: 10.3390/ijerph6061894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelton SP, He J, Whelton PK, Muntner P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol. 2004;93:1119–23. doi: 10.1016/j.amjcard.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr. 2008;87:2003S–9S. doi: 10.1093/ajcn/87.6.2003S. [DOI] [PubMed] [Google Scholar]
- 7.Holub BJ. Docosahexaenoic acid (DHA) and cardiovascular disease risk factors. Prostaglandins Leukot Essent Fatty Acids. 2009;81:199–204. doi: 10.1016/j.plefa.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 9.Toft I, Bonaa KH, Ingebretsen OC, Nordoy A, Jenssen T. Effects of n-3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med. 1995;123:911–8. doi: 10.7326/0003-4819-123-12-199512150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Valera B, Dewailly E, Poirier P. Environmental mercury exposure and blood pressure among Nunavik Inuit adults. Hypertension. 2009;54:981–6. doi: 10.1161/HYPERTENSIONAHA.109.135046. [DOI] [PubMed] [Google Scholar]
- 11.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–33. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Miller ER, 3rd, Seidler AJ, Whelton PK. Does supplementation of diet with 'fish oil' reduce blood pressure? A meta-analysis of controlled clinical trials. Arch Intern Med. 1993;153:1429–38. [PubMed] [Google Scholar]
- 13.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–9. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood-brain barrier transport. Neurosci Biobehav Rev. 1990;14:169–76. doi: 10.1016/s0149-7634(05)80217-9. [DOI] [PubMed] [Google Scholar]
- 15.Guallar E, Sanz-Gallardo MI, van't Veer P, et al. Mercury, fish oils, and the risk of myocardial infarction. N Engl J Med. 2002;347:1747–54. doi: 10.1056/NEJMoa020157. [DOI] [PubMed] [Google Scholar]
- 16.Virtanen JK, Voutilainen S, Rissanen TH, et al. Mercury, fish oils, and risk of acute coronary events and cardiovascular disease, coronary heart disease, and all-cause mortality in men in eastern Finland. Arterioscler Thromb Vasc Biol. 2005;25:228–33. doi: 10.1161/01.ATV.0000150040.20950.61. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JC, Pedersen HS, Mulvad G. Fatty acids and antioxidants in the Inuit diet. Their role in ischemic heart disease (IHD) and possible interactions with other dietary factors. A review. Arctic Med Res. 1994;53:4–17. [PubMed] [Google Scholar]
- 18.Peterson SA, Ralston NV, Peck DV, Van Sickle J, Robertson JD, Spate VL, Morris JS. How might selenium moderate the toxic effects of mercury in stream fish of the western U.S.? Environ Sci Technol. 2009;43:3919–25. doi: 10.1021/es803203g. [DOI] [PubMed] [Google Scholar]
- 19.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 20.Willett W. Nutritional epidemiology. New York ; Oxford: Oxford University Press; 1998. [Google Scholar]
- 21.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–12. [PubMed] [Google Scholar]
- 22.Liu K, Slattery M, Jacobs D, Jr, et al. A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis. 1994;4:15–27. [PubMed] [Google Scholar]
- 23.Candela M, Astiasaran I, Bello J. Deep-Fat Frying Modifies High-Fat Fish Lipid Fraction. J Agric Food Chem. 1998;46:2793–6. [Google Scholar]
- 24.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–7. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 25.Archer SL, Green D, Chamberlain M, Dyer AR, Liu K. Association of dietary fish and n-3 fatty acid intake with hemostatic factors in the coronary artery risk development in young adults (CARDIA) study. Arterioscler Thromb Vasc Biol. 1998;18:1119–23. doi: 10.1161/01.atv.18.7.1119. [DOI] [PubMed] [Google Scholar]
- 26.Gunderson EP, Chiang V, Lewis CE, et al. Long-term blood pressure changes measured from before to after pregnancy relative to nonparous women. Obstet Gynecol. 2008;112:1294–302. doi: 10.1097/AOG.0b013e31818da09b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 28.Xun P, Liu K, Morris JS, Daviglus ML, Stevens J, Jacobs DR, Jr, He K. Associations of toenail selenium levels with inflammatory biomarkers of fibrinogen, high-sensitivity c-reactive protein, and interleukin-6: The CARDIA Trace Element Study. Am J Epidemiol. 2010;171:793–800. doi: 10.1093/aje/kwq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris JS, Stampfer MJ, Willett WC. Dietary selenium in humans, toenails as an indicator. Biological Trace Element Research. 1983:529–37. doi: 10.1007/BF02988944. [DOI] [PubMed] [Google Scholar]
- 30.Yoshizawa K, Ascherio A, Morris JS, et al. Prospective study of selenium levels in toenails and risk of coronary heart disease in men. Am J Epidemiol. 2003;158:852–60. doi: 10.1093/aje/kwg052. [DOI] [PubMed] [Google Scholar]
- 31.Xun P, Liu K, Morris JS, Daviglus ML, He K. Longitudinal association between toenail selenium levels and measures of subclinical atherosclerosis: The CARDIA trace element study. Atherosclerosis. 2010 doi: 10.1016/j.atherosclerosis.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter DJ, Morris JS, Chute CG, et al. Predictors of selenium concentration in human toenails. Am J Epidemiol. 1990;132:114–22. doi: 10.1093/oxfordjournals.aje.a115623. [DOI] [PubMed] [Google Scholar]
- 33.Hulka BS, Wilcosky TC, Griffith JD. Biological markers in epidemiology. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 34.Longnecker MP, Stampfer MJ, Morris JS, Spate V, Baskett C, Mason M, Willett WC. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57:408–13. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]
- 35.Yoshizawa K, Rimm EB, Morris JS, et al. Mercury and the risk of coronary heart disease in men. N Engl J Med. 2002;347:1755–60. doi: 10.1056/NEJMoa021437. [DOI] [PubMed] [Google Scholar]
- 36.Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420–3. doi: 10.1111/j.1468-3083.2009.03426.x. [DOI] [PubMed] [Google Scholar]
- 37.Garland M, Morris JS, Rosner BA, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. 1993;2:493–7. [PubMed] [Google Scholar]
- 38.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29:S1–205. [PubMed] [Google Scholar]
- 39.Schmitz KH, Jacobs DR, Jr, Leon AS, Schreiner PJ, Sternfeld B. Physical activity and body weight: associations over ten years in the CARDIA study. Coronary Artery Risk Development in Young Adults. Int J Obes Relat Metab Disord. 2000;24:1475–87. doi: 10.1038/sj.ijo.0801415. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201–8. doi: 10.1016/s0169-2607(97)00043-6. [DOI] [PubMed] [Google Scholar]
- 43.Rajpathak S, Rimm E, Morris JS, Hu F. Toenail selenium and cardiovascular disease in men with diabetes. J Am Coll Nutr. 2005;24:250–6. doi: 10.1080/07315724.2005.10719472. [DOI] [PubMed] [Google Scholar]
- 44.Hunter DJ, Morris JS, Stampfer MJ, Colditz GA, Speizer FE, Willett WC. A prospective study of selenium status and breast cancer risk. JAMA. 1990;264:1128–31. [PubMed] [Google Scholar]
- 45.He K, Daviglus ML. A few more thoughts about fish and fish oil. J Am Diet Assoc. 2005;105:350–1. doi: 10.1016/j.jada.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 46.Howe PR. Dietary fats and hypertension. Focus on fish oil. Ann N Y Acad Sci. 1997;827:339–52. doi: 10.1111/j.1749-6632.1997.tb51846.x. [DOI] [PubMed] [Google Scholar]
- 47.Ueshima H, Stamler J, Elliott P, et al. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension. 2007;50:313–9. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 49.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–60. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 50.McLennan P, Howe P, Abeywardena M, et al. The cardiovascular protective role of docosahexaenoic acid. Eur J Pharmacol. 1996;300:83–9. doi: 10.1016/0014-2999(95)00861-6. [DOI] [PubMed] [Google Scholar]
- 51.Chung H, Nettleton JA, Lemaitre RN, Barr RG, Tsai MY, Tracy RP, Siscovick DS. Frequency and type of seafood consumed influence plasma (n-3) fatty acid concentrations. J Nutr. 2008;138:2422–7. doi: 10.3945/jn.108.089631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–21. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 53.Meltzer HM, Folmer M, Wang S, Lie O, Maage A, Mundal HH, Ydersbond TA. Supplementary selenium influences the response to fatty acid-induced oxidative stress in humans. Biol Trace Elem Res. 1997;60:51–68. doi: 10.1007/BF02783309. [DOI] [PubMed] [Google Scholar]
- 54.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bleys J, Navas-Acien A, Guallar E. Selenium and diabetes: more bad news for supplements. Ann Intern Med. 2007;147:271–2. doi: 10.7326/0003-4819-147-4-200708210-00177. [DOI] [PubMed] [Google Scholar]
- 56.Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP. Blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999–2000. Environ Res. 2005;97:195–200. doi: 10.1016/j.envres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, Salonen JT. Fish oil-derived fatty acids, docosahexaenoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation. 2000;102:2677–9. doi: 10.1161/01.cir.102.22.2677. [DOI] [PubMed] [Google Scholar]
- 58.Parizek J, Ostadalova I. The protective effect of small amounts of selenite in sublimate intoxication. Experientia. 1967;23:142–3. doi: 10.1007/BF02135970. [DOI] [PubMed] [Google Scholar]
- 59.Cuvin-Aralar ML, Furness RW. Mercury and selenium interaction: a review. Ecotoxicol Environ Saf. 1991;21:348–64. doi: 10.1016/0147-6513(91)90074-y. [DOI] [PubMed] [Google Scholar]
- 60.Frisk P, Wester K, Yaqob A, Lindh U. Selenium protection against mercury-induced apoptosis and growth inhibition in cultured K-562 cells. Biol Trace Elem Res. 2003;92:105–14. doi: 10.1385/BTER:92:2:105. [DOI] [PubMed] [Google Scholar]
- 61.Stoewsand GS, Bache CA, Lisk DJ. Dietary selenium protection of methylmercury intoxication of Japanese quail. Bull Environ Contam Toxicol. 1974;11:152–6. doi: 10.1007/BF01684595. [DOI] [PubMed] [Google Scholar]
- 62.Buettner C. Mercury and the risk of myocardial infarction. N Engl J Med. 2003;348:2151–4. author reply -4. [PubMed] [Google Scholar]
- 63.Beilin LJ. Dietary fats, fish, and blood pressure. Ann N Y Acad Sci. 1993;683:35–45. doi: 10.1111/j.1749-6632.1993.tb35690.x. [DOI] [PubMed] [Google Scholar]
- 64.Mori TA. Omega-3 fatty acids and hypertension in humans. Clin Exp Pharmacol Physiol. 2006;33:842–6. doi: 10.1111/j.1440-1681.2006.04451.x. [DOI] [PubMed] [Google Scholar]
- 65.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102:1264–9. doi: 10.1161/01.cir.102.11.1264. [DOI] [PubMed] [Google Scholar]
- 66.Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76:326–30. doi: 10.1093/ajcn/76.2.326. [DOI] [PubMed] [Google Scholar]
- 67.Dallongeville J, Yarnell J, Ducimetiere P, et al. Fish Consumption Is Associated With Lower Heart Rates. Circulation. 2003;108:820–5. doi: 10.1161/01.CIR.0000084542.64687.97. [DOI] [PubMed] [Google Scholar]
- 68.Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci U S A. 1994;91:4427–30. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ralston NV, Ralston CR, Blackwell JL, 3rd, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–11. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 70.Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;175:1122–4. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- 71.Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol. 1999;21:83–8. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe C, Yoshida K, Kasanuma Y, Kun Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environ Res. 1999;80:208–14. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- 73.Prohaska JR, Ganther HE. Interactions between selenium and methylmercury in rat brain. Chem Biol Interact. 1977;16:155–67. doi: 10.1016/0009-2797(77)90125-9. [DOI] [PubMed] [Google Scholar]
- 74.Lucas M, Asselin G, Merette C, Poulin MJ, Dodin S. Validation of an FFQ for evaluation of EPA and DHA intake. Public Health Nutr. 2009;12:1783–90. doi: 10.1017/S1368980008004333. [DOI] [PubMed] [Google Scholar]
- 75.Tjonneland A, Overvad K, Thorling E, Ewertz M. Adipose tissue fatty acids as biomarkers of dietary exposure in Danish men and women. Am J Clin Nutr. 1993;57:629–33. doi: 10.1093/ajcn/57.5.629. [DOI] [PubMed] [Google Scholar]
- 76.Ohno T, Sakamoto M, Kurosawa T, Dakeishi M, Iwata T, Murata K. Total mercury levels in hair, toenail, and urine among women free from occupational exposure and their relations to renal tubular function. Environ Res. 2007;103:191–7. doi: 10.1016/j.envres.2006.06.009. [DOI] [PubMed] [Google Scholar]