Summary
The biogenesis of melanosomes is a multi-stage process that requires the function of cell-type-specific and ubiquitously expressed proteins. OCA2, the product of the gene defective in oculocutaneous albinism type 2, is a melanosomal membrane protein with restricted expression pattern and a potential role in the trafficking of other proteins to melanosomes. The ubiquitous protein complexes AP-3, BLOC-1 and BLOC-2, which contain as subunits the products of genes defective in various types of Hermanksy-Pudlak syndrome, have been likewise implicated in trafficking to melanosomes. We have tested for genetic interactions between mutant alleles causing deficiency in OCA2 (pink-eyed dilution unstable), AP-3 (pearl), BLOC-1 (pallid) and BLOC-2 (cocoa) in C57BL/6J mice. The pallid allele was epistatic to pink-eyed dilution, and the latter behaved as a semi-dominant phenotypic enhancer of cocoa and, to a lesser extent, of pearl. These observations suggest functional links between OCA2 and these three protein complexes involved in melanosome biogenesis.
Keywords: genetic interaction, Hermansky-Pudlak syndrome, melanosome, Oculocutaneous albinism, Oca2, mouse, pigmentation
Introduction
Melanosomes are membrane-bounded compartments within melanocytes and retinal-pigment-epithelium cells where eumelanins and pheomelanins are synthesized. These specialized compartments are considered lysosome-related organelles, even when melanosomes and lysosomes co-exist in melanocytes and display differential protein composition and biological functions (Raposo and Marks, 2007). The biogenesis of melanosomes involves four morphologically distinct maturation stages (I through IV), with stage II representing a compartment with characteristic intralumenal striations and, unlike stages III and IV, with no detectable melanin deposit. Extensive experimental work has led to a model whereby these maturation stages reflect the arrival, processing and activation of key melanosomal proteins in ‘waves.’ For example, stage II melanosomes would represent a point at which the Pmel17 protein (Si gene product) has been delivered and processed to form the striations, but at which the enzymes tyrosinase, tyrosinase-related protein 1 and dopachrome tautomerase have not yet been delivered or activated to begin melanin synthesis (reviewed by Hearing, 2005; Raposo and Marks, 2007). As defects in melanosome biogenesis can result in reduced pigmentation in skin, hair and eyes, several key components of the molecular machinery responsible for the formation and maturation of these organelles have been identified through the study of albinism in humans and coat color mutations in mice and other animals (Bennett and Lamoreux, 2003; Steingrímsson et al., 2006; Dessinioti et al., 2009). Among the identified components – and relevant to this work – are the adaptor protein (AP)-3 complex and biogenesis of lysosome-related organelles complex (BLOC)-1 and -2, which are expressed in a wide variety of cell types, and the oculocutaneous albinism type 2 (OCA2) protein, which is expressed mainly in melanocytes and retinal pigment epithelial cells (Brilliant, 2001; Bennett and Lamoreux, 2003; Wei, 2006).
AP-3 and BLOC-1 and -2 are stable protein complexes containing as subunits the products of genes defective in several types of Hermansky-Pudlak syndrome (HPS), which is a syndromic form of oculocutaneous albinism associated with platelet storage pool deficiency (Huizing et al., 2009). The AP-3 complex consists of δ, β3, μ3 and σ3 subunits, with the β3A isoform of the β3 subunit being defective in human HPS-2 disease and the mouse coat color mutation ‘pearl’ (Ap3b1pe) and the δ subunit being mutated in the mouse coat color allele ‘mocha’ (Ap3d1mh). BLOC-1 contains eight known subunits named pallidin, muted, cappuccino, dysbindin, snapin and BLOC subunit (BLOS)1, BLOS2 and BLOS3. Mutations in the human genes encoding dysbindin and BLOS3 are associated with HPS-7 and -8, and their mouse orthologs are mutated in the ‘sandy’ (Dtnbp1sdy) and ‘reduced pigmentation’ (Bloc1s3rp) alleles, respectively. In addition, mouse coat color mutations in the genes encoding pallidin (Pldnpa), muted (Mutedmu) and cappuccino (Cnocno) have been described. BLOC-2 is formed by the HPS3, HPS5 and HPS6 proteins that are defective in human HPS-3, -5 and -6 diseases and the mouse coat color mutations ‘cocoa’ (Hps3coa), ‘ruby-eye 2’ (Hps5ru2) and ‘ruby-eye’ (Hps6ru), respectively (reviewed by Wei, 2006; Raposo and Marks, 2007). These three complexes have been implicated in the trafficking of melanin-synthesizing enzymes, but not of Pmel17, to immature melanosomes (reviewed by Raposo and Marks, 2007). Interestingly, results from genetic, cellular and molecular studies are most consistent with the existence of more than one trafficking pathway for the delivery of melanin-synthesizing enzymes to melanosomes, with only one of them depending on the AP-3 complex (Raposo and Marks, 2007). In this context, the specific pathway(s) in which BLOC-1 and -2 act remain(s) incompletely understood. On the one hand, physical and epistatic genetic interactions imply that these two BLOCs may function in a same pathway, and most – but not all – published studies suggest that BLOC-2 and AP-3 may work independently of each other (Di Pietro et al., 2006, Gautam et al., 2006; Setty et al., 2007; Salazar et al., 2009). On the other hand, BLOC-1 also interacts physically with AP-3 (Di Pietro et al., 2006; Salazar et al., 2009; Mead et al., 2010), although genetic analyses of double-mutant mice and characterization of cellular phenotypes caused by deficiency in each complex indicate that BLOC-1 and AP-3 can function, at least in part, independently of each other (Di Pietro et al., 2006, Gautam et al., 2006; Setty et al., 2007; 2008).
The OCA2 protein, also known as P protein, is considered a major determinant of pigmentation in mammals. Recessive mutations in the OCA2-encoding gene are associated with oculocutaneous albinism type 2 in humans and several ‘pink-eyed dilution’ strains in mice, including ‘pink-eyed unstable’ (Oca2p-un) (Brilliant, 2001; Suzuki and Tomita, 2008). In addition, deficient OCA2 function underlies the pigmentation defects associated with Prader-Willi and Angelman syndromes, and common variants at or near the OCA2 gene have been associated with pigmentation diversity in the normal human population (Brilliant, 2001; Sturm, 2009). OCA2 is a multi-spanning membrane protein localized to melanosomes (Rosemblat et al., 1994; Sitaram et al., 2009) for which several alternative functions have been proposed. These include, but are not limited to, the regulation of melanosomal pH (reviewed by Brilliant, 2001) and of glutathione metabolism (Staleva et al., 2002). In addition, work from several groups has pointed at a role for OCA2 in the normal trafficking of tyrosinase and related proteins, but not of Pmel17, to melanosomes (Potterf et al., 1998; Manga et al., 2001; Chen et al., 2002; Toyofuku et al., 2002).
The aim of this work was to test for genetic interactions between mutant alleles causing deficiency in OCA2 and in BLOC-1, BLOC-2 or AP-3 in mice. At least two types of functional interactions between OCA2 and these HPS-associated protein complexes could be envisioned. First, given that OCA2 is a membrane protein that following synthesis at the rough endoplasmic reticulum is targeted to melanosomes, it could very well represent a ‘cargo’ of the above-mentioned trafficking pathway(s) that depend(s) on AP-3 and the BLOCs. Along these lines, a physical interaction between the AP-3 complex and a recombinant fragment of OCA2 containing a critical dileucine motif for melanosomal targeting has been documented (Sitaram et al., 2009). Second, given the proposed role of OCA2 in the trafficking of melanin-synthesizing enzymes, which also relies on the function of AP-3 and the BLOCs, OCA2 might cooperate with these complexes in melanosomal protein targeting. It is worth noting that some of the phenotypes reported for OCA2-deficient melanocytes are reminiscent to those observed for melanocytes deficient in HPS-related protein complexes. For instance, under the electron microscope most melanosomes from OCA2-deficient melanocytes appeared smaller than normal and were classified as predominantly stages I and II, with few melanosomes classified as stage III (Manga et al., 2001); similar ultrastructural characteristics of melanosomes had been previously reported for BLOC-1-deficient melanocytes from homozygous Pldnpa mice (Theriault and Hurley, 1970). Using 3,4-dihydroxy-phenylalanine (DOPA) histochemistry to reveal sites of enzymatically active tyrosinase, few melanosomes turned positive in both OCA2- and BLOC-1-deficient melanocytes (Manga et al., 2001; Setty et al., 2008); rather, accumulation of DOPA reaction products was observed in the perinuclear region harboring the trans-Golgi network of both OCA2- and BLOC-1-deficient melanocytes (Manga et al., 2001; Setty et al., 2008) as well as in peripheral tubulovesicular structures of OCA2-deficient cells (Manga et al., 2001). Although BLOC-2-deficient melanocytes were found to contain stage III melanosomes with melanin deposits and capable of accumulating DOPA reaction products upon histochemistry, these cells also displayed DOPA-positive peripheral tubuvesicular structures (Boissy et al., 2005; Helip-Wooley, 2007) that were reminiscent of those described for OCA2-deficient melanocytes (Manga et al., 2001). Besides these similarities in cellular phenotypes, it is intriguing that the ‘pallid’ mutation (Pldnpa), which results in virtually no BLOC-1 activity in mice, had been first described in the literature as ‘pink-eyed 2’ (p2) owing to the very close similarity in eye color phenotypes of homozygous mutants for this allele and pink-eyed dilution (Roberts, 1931). Although these phenotypic similarities could have been circumstantial, the possibility that OCA2 may interact functionally with the BLOCs and/or AP-3 deserved consideration.
In this paper, we report the occurrence of genetic epistatic interactions between strong mutant alleles (null or severe hypomorphs) in the genes encoding OCA2 and representative subunits of BLOC-1 and -2 and the AP-3 complex. Although the coat color phenotypes of single mutants lacking OCA2 (OCA2p-un homozygous) or BLOC-1 (Pldnpa homozygous) in the C57BL/6J background were not identical, the Pldnpa mutation was found to be epistatic to OCA2p-un. In addition, OCA2p-un behaved as a semi-dominant enhancer of Hps3coa (null BLOC-2 subunit mutant) and, to a lesser extent, of Ap3b1pe (causing severe AP-3 deficiency). These observations support the idea of the existence of functional links between OCA2 and these three HPS-associated protein complexes.
Results and discussion
Mice homozygous for the mutant allele Oca2p-un (on chromosome 7) were crossed with those carrying Pldnpa (on chromosome 2), Hps3coa (on chromosome 3) or Ap3b1pe (on chromosome 13) to obtain homozygous double mutants as well as mice homozygous for one allele and heterozygous for the other. The yields of homozygous double mutants reaching weaning age followed the expected Mendelian frequencies in various breeding schemes (e.g., ~25% obtained through sib-crosses of mice homozygous for one allele and heterozygous for the other), implying that the combined deficiency in OCA2 and any of the HPS-related complexes under study did not compromise viability during development. For comparison, the yield of homozygous Pldnpa; Ap3b1pe double mutants obtained through similar crosses was less than half that expected from Mendelian inheritance (ECD’A, unpublished observations; see also Gautam et al., 2006).
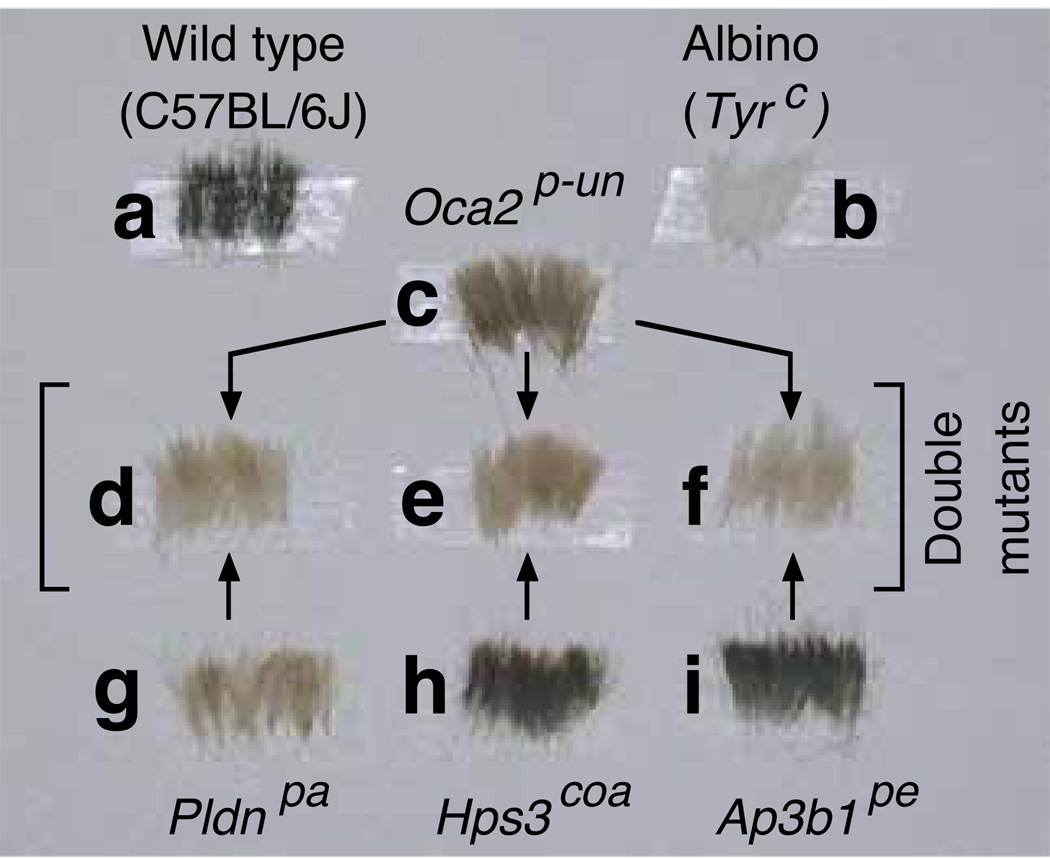
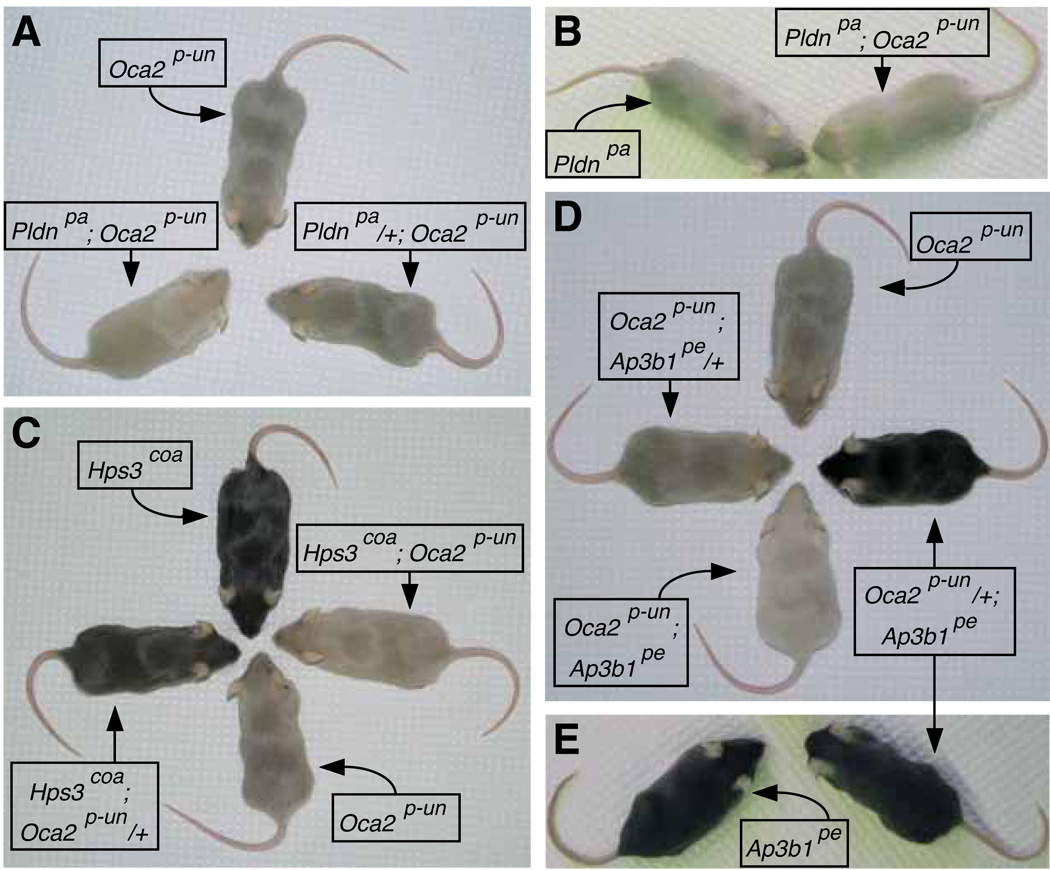
Figure 1 shows representative dorsal hair samples obtained from 5-week-old female mice homozygous for each of the Oca2p-un, Pldnpa, Hps3coa and Ap3b1pe alleles as well as homozygous double mutants, together with hair samples from ‘wild-type’ (C57BL/6J) and ‘albino’ (Tyrc) controls. Figure 2 comprises a gallery of photographs of homozygous single- and double-mutant mice, together with selected mice that were homozygous for one mutant allele and heterozygous for the other (the shown photographs were taken on different days). Upon visual inspection, it was clear that the pigmentation defects of the three homozygous double mutants (i.e., Pldnpa; Oca2p-un, Hps3coa; Oca2p-un, and Oca2p-un; Ap3b1pe) were more severe than that of Oca2p-un single mutants (Figures 1 and 2A, C and D), such that on the basis of coat color each of the double-mutant individuals could be isolated from cages also containing Oca2p-un single mutants. Not surprisingly, the phenotypic differences between Hps3coa; Oca2p-un and Hps3coa homozygotes, as well as between Oca2p-un; Ap3b1pe and Ap3b1pe homozygotes, were even more pronounced (Figure 1). In contrast, it was not possible to unambiguously distinguish homozygous Pldnpa; Oca2p-un mice from Pldnpa single mutants of matching age (Figures 1 and 2B). Interestingly, visual inspection was sufficient to detect a phenotypic difference among homozygous Hps3coa mice depending upon the presence of a single copy of the Oca2p-un allele, with Hps3coa; Oca2p-un/+ individuals displaying a lighter coat color than Hps3coa individuals (Figure 2C).
Figure 1.
Representative dorsal hair samples from 5-week-old female mice of the C57BL/6J strain (wild type), congenic mice homozygous for the mutant alleles Tyrc (albino), Oca2p-un (pink-eyed unstable), Pldnpa (pallid), Hps3coa (cocoa) or Ap3b1pe (pearl), and homozygous double mutants generated in this study. The genotypes of hair samples (a-c) are indicated on the top, and those of samples (g-i) are indicated on the bottom. The double mutants are: Pldnpa; Oca2p-un (d), Hps3coa; Oca2p-un (e), and Oca2p-un; Ap3b1pe (f).
Figure 2.
Examples of coat color phenotypes of 5-week-old mice carrying the Oca2p-un allele and/or mutations in the Pldn gene encoding a subunit of BLOC-1 (A and B), the Hps3 gene encoding a subunit of BLOC-2 (C) or the Ap3b1 gene encoding a subunit of AP-3 (D and E).
In order to validate these observations with quantitative analyses, total hair melanin was extracted and quantified using a modification of the procedure originally described by Ozeki et al. (1995). Because melanin levels may vary with the age of the animals, hair samples were always collected from 5-week-old mice. We also considered the possibility of subtle, gender-specific variations in total melanin levels, even when no obvious differences in coat pigmentation between males and females could be noticed by visual inspection. Surprisingly, a small but statistically significant difference in total hair melanin was detected between Hps3coa males and females (Figure S1). Although this subtle, gender-specific variation in pigmentation was not further investigated, as a result all subsequent analyses were carried out for male and female mice separately.
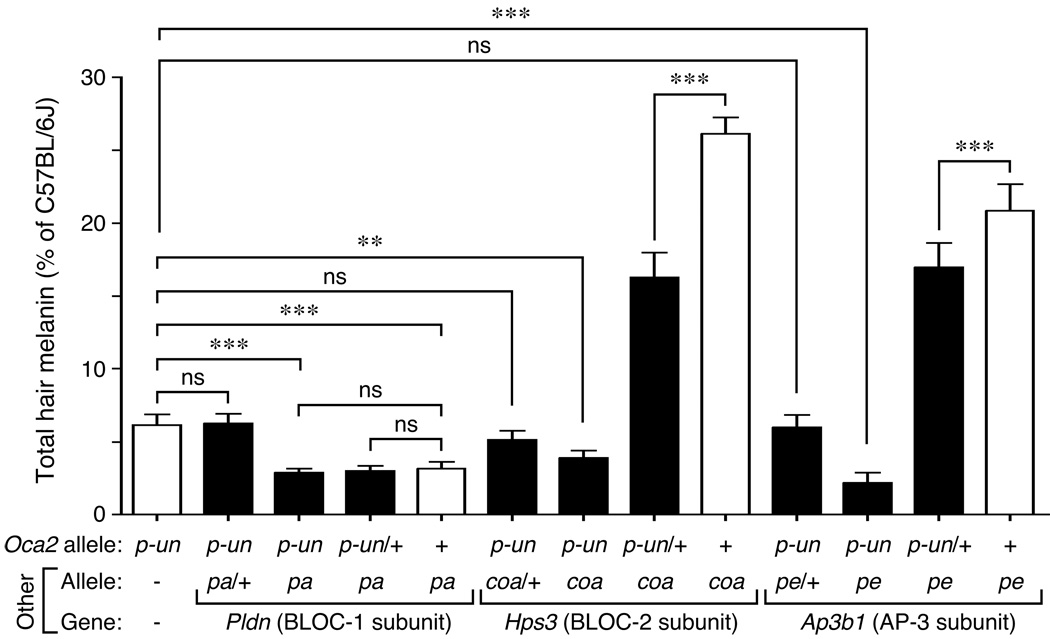
Figure 3 shows the results from quantitative analyses of hair samples from 5–8 males of each genotype, including the homozygous single mutants (open bars) and homozygous double mutants together with combinations of homozygous for one mutant allele and heterozygous for the other (closed bars). Statistical analyses (using Bonferroni’s correction for multiple testing) corroborated that the pigment dilution of the three homozygous double mutants generated in this study was greater than that of homozygous Oca2p-un single mutants, and that a single copy of Oca2p-un was sufficient to elicit a reduction in hair melanin content of Hps3coa homozygotes (from ~26% to ~16% of wild-type levels; Figure 3). The same analyses shed light on the results from crosses involving the Oca2p-un and Pldnpa alleles: while homozygous Oca2p-un; Pldnpa double mutants displayed lower melanin content than Oca2p-un single mutants (~3% and ~6% of wild-type levels, respectively), there was no statistically significant difference between the hair pigmentation of the former and that of Pldnpa single mutants (~3% of wild-type levels; Figure 3). In addition, a small but statistically significant effect of a single copy of the Oca2p-un allele on the hair melanin levels of homozygous Ap3b1pe individuals (from ~21% to ~17% of wild-type levels) was uncovered; such an effect was, however, too subtle to unambiguously distinguish Oca2p-un/+; Ap3b1pe from Ap3b1pe individuals solely by visual inspection (Figure 2E). None of the Pldnpa, Hps3coa or Ap3b1pe alleles acted semi-dominantly to enhance the phenotype of Oca2p-un homozygotes (Figure 3). Similar inferences could be made from the analyses carried out on hair samples from female mice (Figure S2), although the difference in melanin content between Oca2p-un single mutants and Hps3coa; Oca2p-un double mutants failed to reach statistical significance upon Bonferroni correction, and the relative difference between Ap3b1pe homozygotes with or without a single copy of the Oca2p-un allele was even smaller than that observed in the corresponding males.
Figure 3.
Determination of total melanin content in dorsal hair from male mice carrying the Oca2p-un allele and/or mutant alleles in genes encoding subunits of BLOC-1, BLOC-2 and AP-3. The homozygous single mutants pink-eyed unstable (Oca2p-un), pallid (Pldnpa), cocoa (Hps3coa) and pearl (Ap3b1pe) are denoted with open bars. Values are expressed as percentages of the total melanin content of control C57BL/6J male hair samples assayed in parallel. Bars represent means ± SD from 5-8 animals per genotype. One-way ANOVA followed by Bonferroni comparison of selected group pairs: ns, not significant; ** P < 0.01; *** P < 0.001.
Our results demonstrate non-additive (epistatic) interactions between the Oca2p-un allele causing OCA2 deficiency and representative mutant alleles causing deficiencies in BLOC-1 and -2 as well as in the AP-3 complex. First, we found that the Pldnpa allele is ‘epistatic’ to Oca2p-un allele in the most traditional meaning of the term (Cordell, 2002), i.e., that the effects of an Oca2 mutation on coat color can be masked by those caused by the null Pldnpa mutation. Although one could wonder whether visual inspection or total hair melanin quantification would allow detecting enhancement of the severe phenotype displayed by Pldnpa homozygotes, in our hands both approaches had been sensitive enough to observe such a phenotypic enhancement by Ap3b1pe in Pldnpa; Ap3b1pe double mutants (Di Pietro et al., 2006). Therefore, the simplest interpretation of these results is that OCA2 may require BLOC-1 function for expression of its biological activity. Granted, the genetic data do not allow to discriminate between a model in which OCA2 is an obligate cargo of BLOC-1-dependent trafficking and others in which OCA2 functions in a BLOC-1-dependent trafficking pathway or downstream of it. A second form of genetic interaction was observed between Oca2p-un and the Hps3coa and Ap3b1pe alleles causing deficiency in BLOC-2 and AP-3, respectively, with Oca2p-un behaving as a semi-dominant enhancer of Hps3coa and – to a lesser extent – of Ap3b1pe. This behavior of the Oca2p-un allele in the contexts of BLOC-2 and AP-3 deficiencies is noteworthy for two reasons: (i) Oca2p-un is considered a recessive allele in otherwise wild-type background (for example see Lyon et al., 1992), and (ii) the Pldnpa allele, which in homozygous form leads to a pigmentation defect slightly more severe than that caused by Oca2p-un (Figure 3), did not behave in such a semi-dominant fashion when crossed into Hps3coa or Ap3b1pe homozygotes (Di Pietro et al., 2006). Here, the simplest interpretation is that the gene dosage of Oca2 becomes rate-limiting in cells deficient in BLOC-2 or AP-3. In other words, lack of these protein complexes may partially compromise the sorting and/or activity of OCA2, such that the amount of OCA2 protein synthesized by a single copy of the gene may become inadequate to fulfill its biological role. Again, the genetic data do not allow to discriminate between OCA2 being a cargo or an active component of the trafficking pathway(s) mediated by BLOC-2 and AP-3. A potential caveat of these genetic experiments is that the hypothetical contribution of additional modifier genes, especially those in linkage disequilibrium with the mutations of interest that might have co-segregated during backcrossing onto C57BL/6J, cannot be completely ruled out. Consequently, future work will be required to understand exactly how the functions of OCA2 and these HPS-associated protein complexes may be interconnected.
Materials and methods
Generation of double-mutant mice
Animals were bred under standard conditions at a facility of the Division of Laboratory Animal Medicine of the University of California, Los Angeles (UCLA; Los Angeles, CA, USA). The C57BL/6J and C57BL/6J-Oca2p-un/J strains were from The Jackson Laboratories (JAX; Bar Harbor, ME, USA). The sources of the strains pallid (B6.Cg-Pldnpa/J), cocoa (B6.B10-Hps3coa/J) and pearl (B6.C3-Ap3b1pe/J) were described elsewhere (Di Pietro et al., 2006). The Oca2p-un allele arose spontaneously in the C57BL/6J background and was backcrossed for 49 generations at JAX and another 3 at UCLA. The Pldnpa mutation was found in a wild mouse (Roberts, 1931) and backcrossed onto C57BL/6J, for 45 generations at JAX and another at UCLA. The Hps3coa allele arose spontaneously in the C57BL/10J background and was backcrossed onto C57BL/6J, once at JAX and once at UCLA. The Ap3b1pe allele arose in the C3H/HeJ background and was backcrossed onto C57BL/6J for 25 generations. Double-mutant mice were generated using a breeding scheme analogous to that used by Meisler et al. (1984), as previously described (Di Pietro et al., 2006).
Quantification of hair melanin
Total melanin (eumelanins plus phenomelanins) content in mouse hair was estimated by using a modification of the spectrophotometric method of Ozeki et al. (1995). Hair was obtained by plucking from the mid-dorsal region of 5-week-old mice that had been euthanized by controlled CO2 delivery from a compressed cylinder. Care was exercised in pulling out hair by the root, rather than cutting off the tip, because hair from mice homozygous for the Ap3b1pe or Pldnpa alleles was somewhat less pigmented at the base than at the tip. Hair from albino mice (kindly provided by Guoping Fan, UCLA) was processed in parallel as a blank control. Collected hair samples were stored at −70°C until further use. To extract melanin, 3–5 mg of hair was precisely weighted and placed inside Fisherbrand™ 1.5-ml conical screw-cap microcentrifuge tubes (Fisher Scientific, Pittsburgh, PA, USA), and a solution containing Soluene®-350 (PerkinElmer, Waltham, MA, USA) and water (9:1 v/v) was added to a ratio of 0.25 ml per mg of hair. Subsequently, samples were subjected to two cycles of vigorous vortexing followed by heating at 95°C (for 30 and 15 min in first and second cycles, respectively) and cooling down to room temperature. At the end of the second cycle, 0.4 ml of each sample (or, in the case of highly pigmented hair samples from C57BL/6J mice, 0.2 ml plus an equal volume of albino hair sample prepared under identical conditions) was transferred (without prior centrifugation) to a clean screw-cap microcentrifuge tube containing 0.6 ml of the Soluene®-350/water solution, and the diluted samples were then vortexed and heated to 95°C for 15 min. Following centrifugation for 10 min at 13,000 × g to remove insoluble debris, the absorbance at 500 nm of each supernatant was measured and referred to a standard calibration curve, which was obtained using purified melanin from Sepia officinalis (Sigma-Aldrich, St. Louis, MO, USA) dissolved in Soluene®-350/water solution.
Statistical analysis
One- and two-way ANOVA and Bonferroni’s multiple comparison tests were performed using GraphPad Prism 4.0 (GraphPad Software, San Diego, CA, USA).
Significance
The biogenesis of melanosomes is expected to result from the concerted action of ubiquitous and cell-type-specific factors. This work provides genetic evidence for functional links between OCA2, which is mainly expressed in melanin-producing cells and considered a major determinant of pigmentation variation in humans, and three protein complexes, named AP-3 and BLOC-1 and -2, which are ubiquitously expressed and associated with various forms of Hermansky-Pudlak syndrome. These findings are likely to spur additional molecular and cellular studies aimed at understanding how these molecules cooperate to mediate melanosome maturation.
Supplementary Material
Acknowledgements
We thank the laboratory of Guoping Fan for kindly providing albino mouse hair samples, Donna S. Crandall for help with the preparation of Figure 2, and Michael S. Marks for critical reading of the manuscript. DJH was supported by the CARE-SEM Summer Program funded by NIH IMSD GM55052. IARF was supported by the NIH Training Grant in Genomic Analysis and Interpretation T32HG002536.
References
- Bennett DC, Lamoreux ML. The color loci of mice – a genetic century. Pigment Cell Res. 2003;16:333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Richmond B, Huizing M, Helip-Wooley A, Zhao Y, Koshoffer A, Gahl WA. Melanocyte-specific proteins are aberrantly trafficked in melanocytes of Hermansky-Pudlak syndrome-type 3. Am. J. Pathol. 2005;166:231–240. doi: 10.1016/S0002-9440(10)62247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilliant MH. The mouse p (pink-eyed dilution) and human P genes, oculocutaneous albinism type 2 (OCA2), and melanosomal pH. Pigment Cell Res. 2001;14:86–93. doi: 10.1034/j.1600-0749.2001.140203.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Manga P, Orlow SJ. Pink-eyed dilution protein controls the processing of tyrosinase. Mol. Biol. Cell. 2002;13:1953–1964. doi: 10.1091/mbc.02-02-0022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell HJ. Epistasis: what it means, what it doesn’t mean, and statistical methods to detect it in humans. Hum. Mol. Genet. 2002;11:2463–2468. doi: 10.1093/hmg/11.20.2463. [DOI] [PubMed] [Google Scholar]
- Dessinioti C, Stratigos AJ, Rigopoulos D, Katsambas AD. A review of genetic disorders of hypopigmentation: lessons learned from the biology of melanocytes. Exp. Dermatol. 2009;18:741–749. doi: 10.1111/j.1600-0625.2009.00896.x. [DOI] [PubMed] [Google Scholar]
- Di Pietro SM, Falcón-Pérez JM, Tenza D, Setty SR, Marks MS, Raposo G, Dell'Angelica EC. BLOC-1 interacts with BLOC-2 and the AP-3 complex to facilitate protein trafficking on endosomes. Mol. Biol. Cell. 2006;17:4027–4038. doi: 10.1091/mbc.E06-05-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R, Novak EK, Tan J, Wakamatsu K, Ito S, Swank RT. Interaction of Hermansky-Pudlak Syndrome genes in the regulation of lysosome-related organelles. Traffic. 2006;7:779–792. doi: 10.1111/j.1600-0854.2006.00431.x. [DOI] [PubMed] [Google Scholar]
- Hearing VJ. Biogenesis of pigment granules: a sensitive way to regulate melanocyte function. J. Dermatol. Sci. 2005;37:3–14. doi: 10.1016/j.jdermsci.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Helip-Wooley A, Westbroek W, Dorward HM, Koshoffer A, Huizing M, Boissy RE, Gahl WA. Improper trafficking of melanocyte-specific proteins in Hermansky-Pudlak syndrome type-5. J. Invest. Dermatol. 2007;127:1471–1478. doi: 10.1038/sj.jid.5700737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizing M, Helip-Wooley A, Westbroek W, Gunay-Aygun M, Gahl WA. Disorders of lysosome-related organelle biogenesis: clinical and molecular genetics. Annu. Rev. Genomics Hum. Genet. 2009;9:359–386. doi: 10.1146/annurev.genom.9.081307.164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF, King TR, Gondo Y, Gardner JM, Nakatsu Y, Eicher EM, Brilliant MH. Genetic and molecular analysis of recessive alleles at the pink-eyed dilution (p) locus of the mouse. Proc. Natl. Acad. Sci. U. S. A. 1992;89:6968–6972. doi: 10.1073/pnas.89.15.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manga P, Boissy RE, Pifko-Hirst S, Zhou BK, Orlow SJ. Mislocalization of melanosomal proteins in melanocytes from mice with oculocutaneous albinism type 2. Exp. Eye Res. 2001;72:695–710. doi: 10.1006/exer.2001.1006. [DOI] [PubMed] [Google Scholar]
- Mead CLR, Kuzyk MA, Moradian A, Wilson GM, Holt RA, Morin GB. Cytosolic protein interactions of the schizophrenia susceptibility gene dysbindin. J. Neurochem. 2010;113:1491–1503. doi: 10.1111/j.1471-4159.2010.06690.x. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Wanner L, Strahler J. Pigmentation and lysosomal phenotypes in mice doubly homozygous for both light-ear and pale-ear mutant alleles. J. Hered. 1984;75:103–106. doi: 10.1093/oxfordjournals.jhered.a109881. [DOI] [PubMed] [Google Scholar]
- Ozeki H, Ito S, Wakamatsu K, Hirobe T. Chemical characterization of hair melanins in various coat-color mutants of mice. J. Invest. Dermatol. 1995;105:361–366. doi: 10.1111/1523-1747.ep12320792. [DOI] [PubMed] [Google Scholar]
- Potterf SB, Furumura M, Sviderskaya EV, Santis C, Bennett DC, Hearing V. Normal tyrosine transport and abnormal tyrosinase routing in pink-eyed dilution melanocytes. Exp. Cell Res. 1998;244:319–326. doi: 10.1006/excr.1998.4173. [DOI] [PubMed] [Google Scholar]
- Raposo G, Marks MS. Melanosomes – dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. A new mutation in the house mouse (Mus musculus) Science. 1931;74:569. doi: 10.1126/science.74.1927.569. [DOI] [PubMed] [Google Scholar]
- Rosemblat S, Durham-Pierre D, Gardner JM, Nakatsu Y, Brilliant MH, Orlow SJ. Identification of a melanosomal membrane protein encoded by the pink-eyed dilution (type II oculocutaneous albinism) gene. Proc. Natl. Acad. Sci. U. S. A. 1994;91:12071–12075. doi: 10.1073/pnas.91.25.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar G, Zlatic S, Craige B, Peden AA, Pohl J, Faundez V. Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol 4-kinase type II α in neuronal and non-neuronal cells. J. Biol. Chem. 2009;284:1790–1802. doi: 10.1074/jbc.M805991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SR, Tenza D, Truschel ST, Chou E, Sviderskaya EV, Theos AC, Lamoreux ML, Di Pietro SM, Starcevic M, Bennett DC, Dell'Angelica EC, Raposo G, Marks MS. BLOC-1 is required for cargo-specific sorting from vacuolar early endosomes toward lysosome-related organelles. Mol. Biol. Cell. 2007;18:768–780. doi: 10.1091/mbc.E06-12-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature. 2008;454:1142–1146. doi: 10.1038/nature07163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram A, Piccirillo R, Palmisano I, Harper DC, Dell'Angelica EC, Schiaffino MV, Marks MS. Localization to mature melanosomes by virtue of cytoplasmic dileucine motifs is required for human OCA2 function. Mol. Biol. Cell. 2009;20:1464–1477. doi: 10.1091/mbc.E08-07-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staleva L, Manga P, Orlow SJ. Pink-eyed dilution protein modulates arsenic sensitivity and intracellular glutathione metabolism. Mol. Biol. Cell. 2002;13:4206–4220. doi: 10.1091/mbc.E02-05-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steingrímsson E, Copeland NG, Jenkins NA. Mouse coat color mutations: from fancy mice to functional genomics. Dev. Dyn. 2006;235:2401–2411. doi: 10.1002/dvdy.20840. [DOI] [PubMed] [Google Scholar]
- Sturm RA. Molecular genetics of human pigmentation diversity. Hum. Mol. Genet. 2009;18:R9–R17. doi: 10.1093/hmg/ddp003. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Tomita Y. Recent advances in genetic analyses of oculocutaneous albinism types 2 and 4. J. Dermatol. Sci. 2008;51:1–9. doi: 10.1016/j.jdermsci.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Theriault LL, Hurley LS. Ultrastructure of developing melanosomes in C57 black and pallid mice. Dev. Biol. 1970;23:261–275. doi: 10.1016/0012-1606(70)90098-9. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Valencia JC, Kushimoto T, Costin GE, Virador VM, Vieira WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Res. 2002;15:217–224. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 2006;19:19–42. doi: 10.1111/j.1600-0749.2005.00289.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.