Abstract
This review describes the normal biology and physiology of the mammary gland in macaques, including the typical histologic appearance across the life span (development, reproductive maturity, lactation, and senescence). The molecular events regulating breast morphogenesis are described, as well as systemic and local hormonal regulators of mammary gland proliferation, differentiation, and function. Similarities and differences to the human breast are described. Regulatory events are illuminated by discussion of genetically modified mouse models. Tissue response markers, including immunohistochemical markers of proliferation and other hormonally induced changes and studies to date, regarding the effects of exogenous hormones, are briefly summarized. In general, estrogens stimulate progesterone receptor expression and proliferation in the mammary gland, and combinations of estrogens and progestogens cause greater proliferation than estrogens alone. Evaluation of novel chemical agents in macaques requires careful evaluation of age and hormonal context to avoid the confounding effects of mammary gland development, past reproductive history, and other influences on mammary gland morphology. The expression of proliferation markers and progesterone receptors may be used as biomarkers to measure chemically induced hormonal effects.
Keywords: primate pathology, mammary gland, estrogen, progesterone, proliferation, receptors, development
INTRODUCTION
Toxicologic pathology of the mammary gland is primarily concerned with proliferative lesions, particularly neoplasms. This reflects the concern caused by the high incidence of mammary gland cancers in the human population; in the United States, breast cancer is the most common malignancy among women (Jemal et al. 2008). Major risk factors for breast cancer among women include age, nulliparity, or late first full-term pregnancy; the use of estrogen-progestogen hormone therapy after menopause; alcohol consumption; obesity after menopause; and family history (Evans and Howell 2007; Santen et al. 2007). Oral contraceptive use is associated with a slight increase in breast-cancer risk for current users that dissipates within a decade after cessation of use (Casey, Cerhan, and Pruthi 2008). Postmenopausal hormone therapy increases breast-cancer risk by approximately 50%, and that risk is clearest for combined estrogen-progestogen exposure (Collins, Blake, and Crosignani 2005). Higher circulating androgens are associated with higher breast-cancer risk in premenopausal women (Eliassen et al. 2006).
Thus, careful evaluation of the mammary glands is most important in the evaluation of drug or chemical agents that mimic sex steroids; relevant effects may be estrogenic, progestogenic, androgenic, or mediated by a novel receptor interaction or indirect mechanism. Effects of drugs and chemicals on the mammary glands change dramatically across the life span. Chemically induced mammary carcinomas in rodents are most readily induced by treatment during the period of maximal mammary gland growth, around the time of puberty (Russo and Russo 1996). There is epidemiologic evidence from studies of women treated for Hodgkin's disease during puberty that a similar period of sensitivity exists for human beings (Clemons, Loijens, and Goss 2000). The corresponding period of developmental sensitivity in macaques has been identified, and there are dramatic differences in the morphology and responsiveness of the mammary gland spanning the age of animals commonly used for toxicologic assessments (Wood, Hester, and Cline 2007). The appropriate use of nonhuman primates in studies of the mammary gland requires knowledge of the normal biology, hormonal responsiveness, and developmental context.
ANATOMY
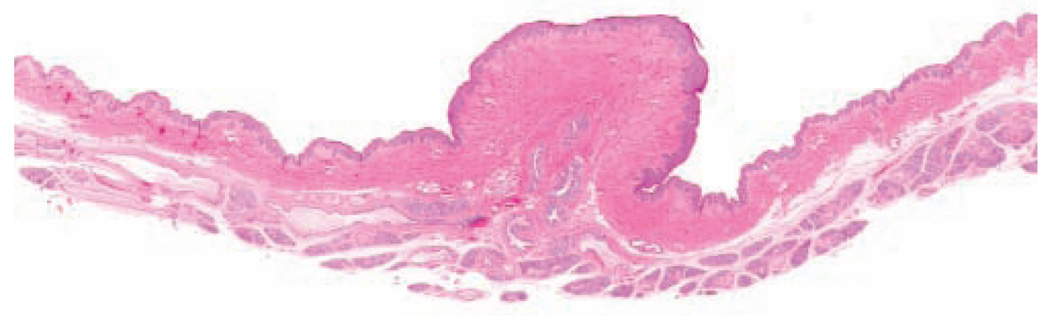
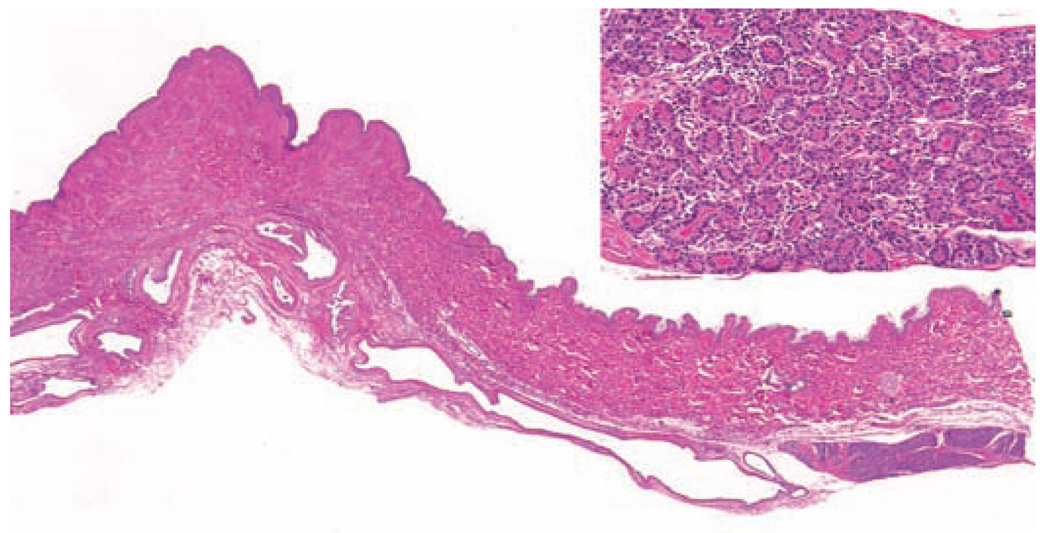
Macaques have two pectoral mammary glands. The nonlactating mammary glands are more flattened than those of nonlactating human females (Figure 1), but the histologic appearance is nearly identical. In macaques, as in women, the bulk of the glandular tissue lies above and lateral to the nipple, extending to the axilla. Primate breast tissues are easily studied at the sub-gross level by whole mount techniques (Figure 1; Cameron and Faulkin 1974; Speert 1948). The adult gland consists of a branching ductal system and terminal ductal lobular units consisting of a terminal intralobular duct and surrounding alveoli, invested with myoepithelium. There is substantial individual variation in the amount and distribution of glandular tissue among individual adult animals, due both to individual variation in development and to past reproductive history. In the nonlactating breast, approximately 5% of the organ is occupied by glandular epithelial tissue, with the remaining 95% consisting of fat, fibrous connective tissue, vascular, and nervous supply; these proportions are similar to those reported for women (Cline, Register, and Clarkson 2002). There are five to seven lactiferous ducts exiting each nipple, with varying degrees of communication between the corresponding ductal and lobular units, and occasional small clusters of glandular tissue in the nipple. The mammary gland of male cynomolgus monkeys is similar to that of females but much less developed, consisting of a small nipple and a rudimentary ductal and lobular system roughly 5 mm in diameter (Perry et al. 2007; Speert 1948).
Figure 1.
Subgross appearance of the non-lactating mammary gland of a multiparous intact adult female cynomolgus macaque. Last prior lactation was > 5 years prior to necropsy. Hematoxylin and eosin.
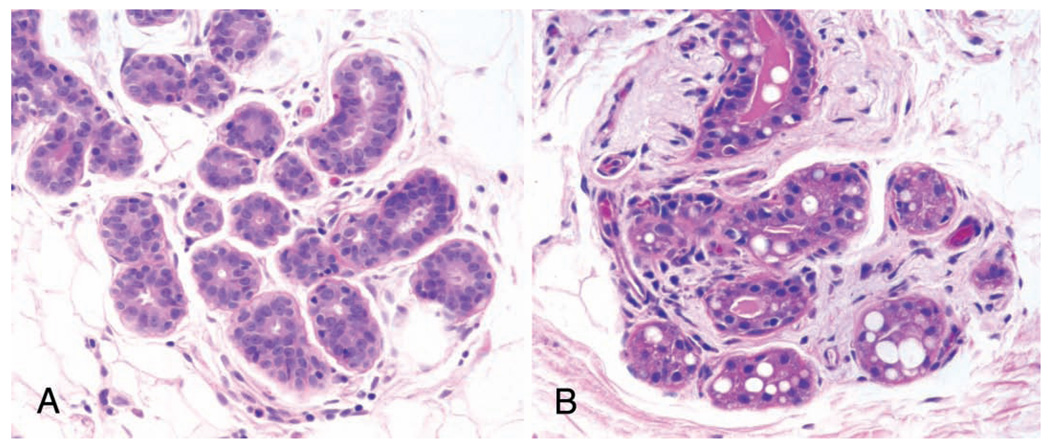
During lactation, the breast becomes enlarged and more prominent, and in older animals, the breast tissue may become pendulous. A thin but distinct mammary fat pad is present, with a slightly firmer texture and less yellow color than the adjacent subcutaneous fat; this distinction may be obscured in obese animals. Innervation of the gland is similar to that of the human, consisting of sensory nerves of the nipple, major lactiferous ducts, and some terminal ductal lobular units. Innervation is nearly absent in lactating tissue distant from the nipple (MacPherson and Montagna 1974). The cytokeratin profile of the macaque mammary gland is similar to that of the human and differs from that of rodents and other species (Tsubura et al. 1991). The nonlactating macaque mammary gland differs somewhat from the human gland in that there are more often fat droplets within secretory epithelial cells, even in the quiescent gland (Figure 3). The basis for this subtle difference in breast morphology is not yet understood; macaques and humans have nearly identical reproductive physiology and milk composition (Jenness 1979; Lonnerdal et al. 1984) and lactate for a similar developmental period of their offspring (Buss 1971).
Figure 3.
Histologic sections of non-lactating human (A) and cynomolgus monkey (B) breast. Note the presence of secretory changes (fat vacuoles, proteinaceous fluid in the lumen of the gland) in the latter. Hematoxylin and eosin stain.
GROWTH AND REGULATORY STIMULI IN THE BREAST
Growth and differentiation of the breast is dependent on ovarian and local production and activation of steroid hormones, the growth hormone (GH) or insulin-like growth factor (IGF) system, and secretory stimuli including prolactin and placental lactogen (Hennighausen and Robinson 2005). Few of these regulatory processes have been thoroughly studied in the breast of macaques; where information is lacking, comparative data from humans and other animal models have been provided.
Estrogens, Progestogens, and Androgens
The development of the breast is dependent on ovarian production of steroid hormones (Sternlicht 2006). Furthermore, among women, the life-span number of menstrual cycles and levels of endogenous estrogens and androgens are all positively correlated with risk of breast cancer (Key et al. 2002; Yager and Davidson 2006). Early first full-term pregnancy is protective against breast cancer in women, as it is in some laboratory species (e.g., rats) but not others (mice). The association between hormones and cancer risk is clearest in the setting of postmenopausal estrogen-progestogen hormone therapy (Chlebowski et al. 2003; Schairer et al. 2000), but similar associations have been shown for premenopausal ovarian production of endogenous estrogens and androgens (Eliassen et al. 2006).
Studies of mice with targeted disruption of critical receptors are instructive regarding the role of sex steroids in breast development. Estrogen receptor (ER) alpha knockout mice show only rudimentary mammary gland development (Feng et al. 2007; Mueller et al. 2002), whereas ER beta knockout mice have relatively normal mammary glands (Forster et al. 2002). Progesterone receptor knockout mice have a profound defect in ductal side-branching and lobular development (Lydon et al. 1996). Mice lacking the androgen receptor also have somewhat impaired mammary gland development (Shiina et al. 2006). Interestingly, tissue-recombinant studies have shown that in mice stromal, but not epithelial, sex steroid receptors are required for mammary growth in this species (Cunha et al. 1997). The critical role of estrogens and progestogens in breast development and growth in macaques has been demonstrated by anatomic and biomarker studies during development (Wood, Hester, and Cline 2007), during the reproductive years (Stute et al. 2004), and after menopause (Cline 2007). Major findings in macaques are described in subsequent sections of this article.
GH/IGF Axis
Growth hormone is produced both systemically and locally within the breast in macaques and human beings, and both GHs and IGFs have a critical function in breast proliferation and differentiation (Kleinberg 1997). Breast morphogenesis is markedly suppressed in IGF knockout mice (Ruan and Kleinberg 1999). In macaques, hypophysectomy suppresses mammary development (Kleinberg et al. 1985). Conversely, administration of exogenous GH to aged macaques produced mammary lobuloalveolar hyperplasia (Ng et al. 1997). There is some evidence for an association between local GH production and proliferative breast disorders in women (Raccurt et al. 2002).
Prolactin
Prolactin receptor knockout mice have a failure of mammary gland development, along with a variety of other abnormalities (Kelly et al. 2001). In contrast, prolactin is not an obligate component of mammary growth and development in macaques but is required for lactation (Kleinberg, Todd, and Niemann 1978). Administration of the prolactin antagonist pergolide to immature macaques did not slow mammary development (Kleinberg et al. 1985). Exogenous administration of prolactin to macaques caused a numerical but statistically insignificant increase in mammary lobular size and epithelial proliferation (Ng et al. 1997), indicating that prolactin is not as strong a mitogen in the primate breast as steroid hormones or GH. Galactorrhea has been reported in conjunction with prolactin-producing neoplasms of the pituitary gland in macaques (Remick et al. 2006). The role of prolactin in human mammary gland development is uncertain; prolactin receptors can be found in the pubertal and adult mammary gland, and as for other species, prolactin is necessary for milk secretion (Ben-Jonathan, LaPensee, and LaPensee 2008).
Placental Lactogen (Somatomammotropin)
Placentally produced mammotropic peptide hormones are found in most species but differ in evolutionary origin. Humans and macaques share an orthologous GH-related placental lactogen derived via expression of four or five GH-like genes in the placenta, whereas more distant human relatives, such as prosimians, express a single GH-like gene, and marmosets express eight. The placental lactogen in rodents and ruminants, in contrast, is more closely related to prolactin than to GH (Forsyth and Wallis 2002). The term placental lactogen may be a misnomer for primates, as deficiency of this hormone does not impair lactation in women (Forsyth and Wallis 2002).
Intratissue Hormone Production
In addition to systemic exposures, intratissue production of sex steroids and growth factors is important. In both human and nonhuman primates (macaques), the necessary hepatic and intramammary enzymatic systems are present for conversion of precursors to more bioactive estradiol (aromatase and steroid sulfatases) and for oxidation-reduction conversions (17-beta hydroxysteroid dehydrogenases), sulfation (sulfo-transferases), and glucuronidation of estrogens to move them into the large circulating reservoir of less potent estrone conjugates (Barbier and Belanger 2003; Martel et al. 1994; Stute et al. 2006). Thus, the amount of local estrogen exposure in the breast correlates only weakly with the serum concentration. In women (Pasqualini et al. 1996) and macaques (Wood, Register, and Cline 2007; unpublished data), the intrabreast concentrations of estradiol are generally higher than serum concentrations.
LIFE STAGES OF THE BREAST
Estrogen exposure of the breast tissue is high in utero during breast morphogenesis. After birth, estrogen exposure declines until early puberty, when follicular development occurs for some months prior to ovulation, thus providing an estrogen-alone phase in which longitudinal ductal growth is pronounced and, to a lesser extent, lobular development begins (Wood, Hester, and Cline 2007). With the beginning of regular ovulation the breast is exposed to cyclic patterns of estrogens and progesterone, leading to further lobular development and stromal expansion. Hormonal exposure during pregnancy brings to bear a unique pattern of placentally derived factors at high circulating concentrations including estriol, chorionic gonadotropin, placental lactogen, and progesterone, resulting in full functional differentiation of the breast. Thus, hormonal signals are not only qualitative and quantitative but also time sensitive.
Fetal/Neonatal Development
As in other species, the breast primordia arise along the “mammary line,” which runs bilaterally along the torso parallel to the midline. Initial organization of the mammary gland in most species appears to be controlled by homeobox Tbox genes; spontaneous mutation of the Tbox3 gene results in a syndrome of amastia along with other developmental disorders (ulnar agenesis) in human beings (Bamshad et al. 1997), and a similar phenotype is induced by deletion of Tbox3 in mice (Davenport, Jerome-Majewska, and Papaioannou 2003). Other critical signaling molecules, revealed by genetic modification of mice, include fibroblast growth factor 10 (fgf10); Wnt, Erbb, neuregulin-3 (Nrg3); and Lef1 (Howard and Ashworth 2006); however, the degree to which these signals are critical in primates has not been explored. In the human breast, the primary bud is present by twelve weeks of gestation, consisting of a solid mass of epithelial cells continuous with the overlying skin and expressing cytokeratin 17 throughout and cytokeratins 14 and 19 basally (Jolicoeur 2005). Small ductal structures grow downward and outward from the primary bud during fetal development, so that in humans (Howard and Gusterson 2000) and macaques (Speert 1948), a small branching ductal system, a few hundred micrometers in diameter, is present at birth. The role of sex steroid receptors has not been explored with respect to in utero breast development in macaques, but given the high exposure of the primate fetus to estrogens, progestogens, prolactin, and placental lactogen, it is likely that the fetal mammary gland is relatively insensitive to the stimuli concurrently causing maternal breast development. Secretory activity is common in the breast of human neonates (Howard and Gusterson 2000), but this phenomenon has not been explored in macaques. Developmental disorders of the breast have not been fully described in macaques; however, single extra nipples are occasionally seen on the ventral thorax below and aligned with the ipsilateral nipple (Speert 1948).
Puberty
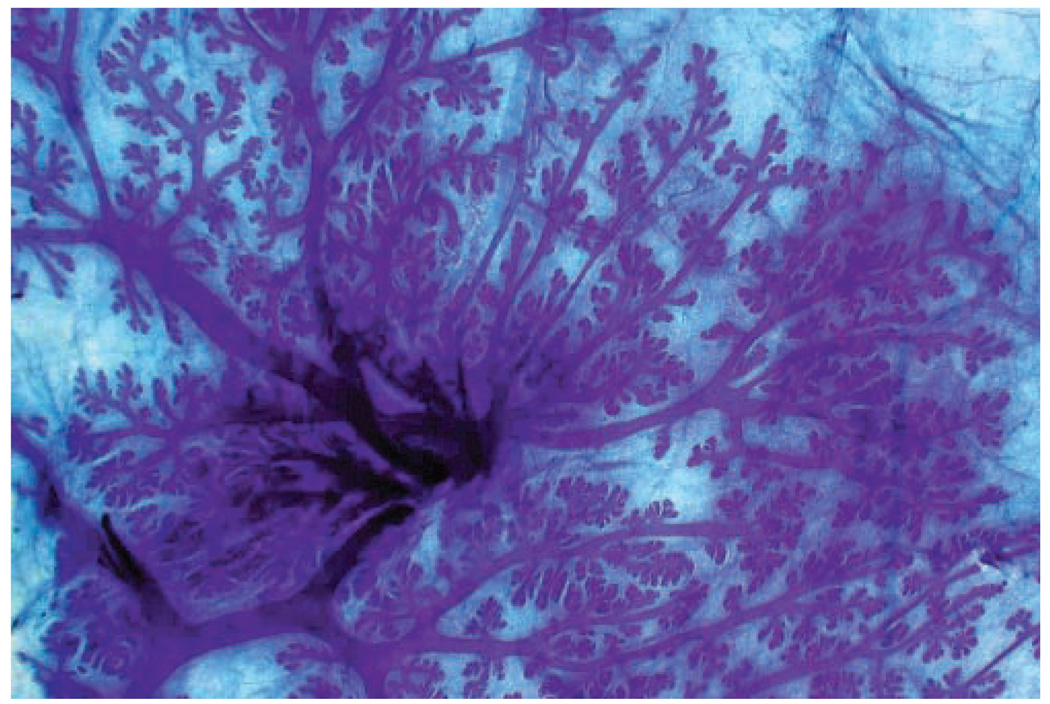
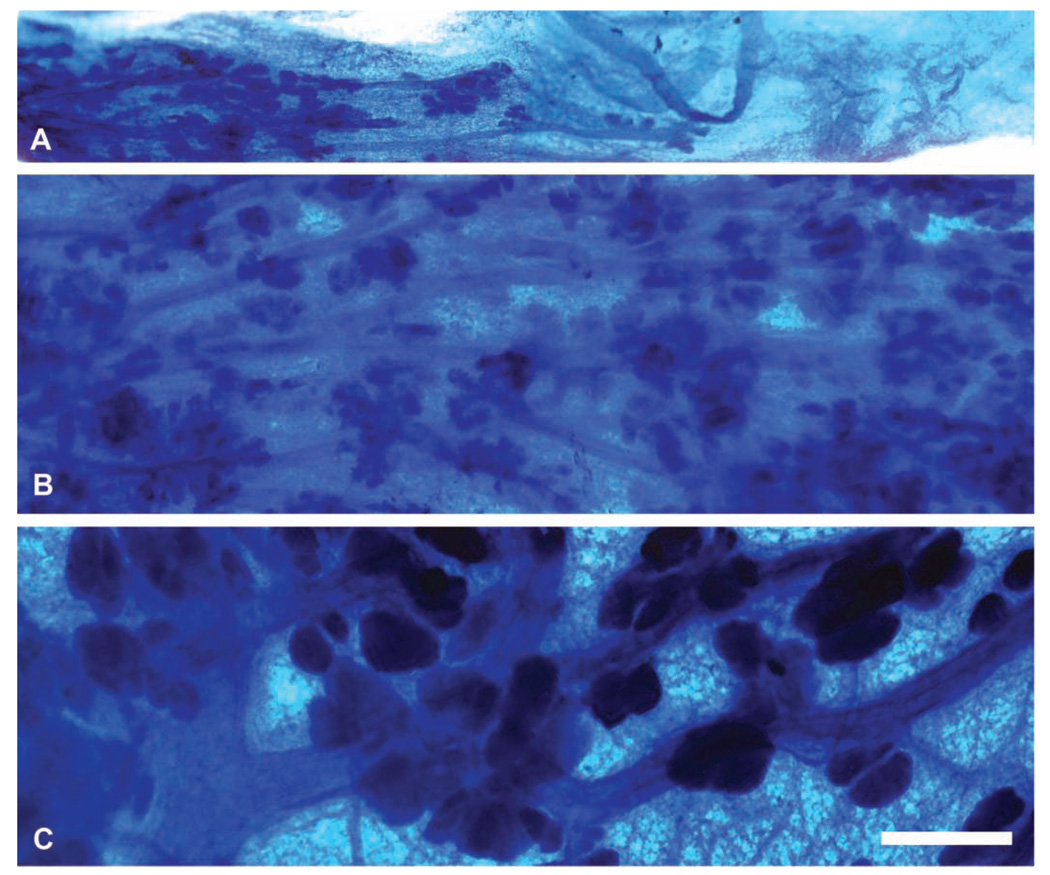
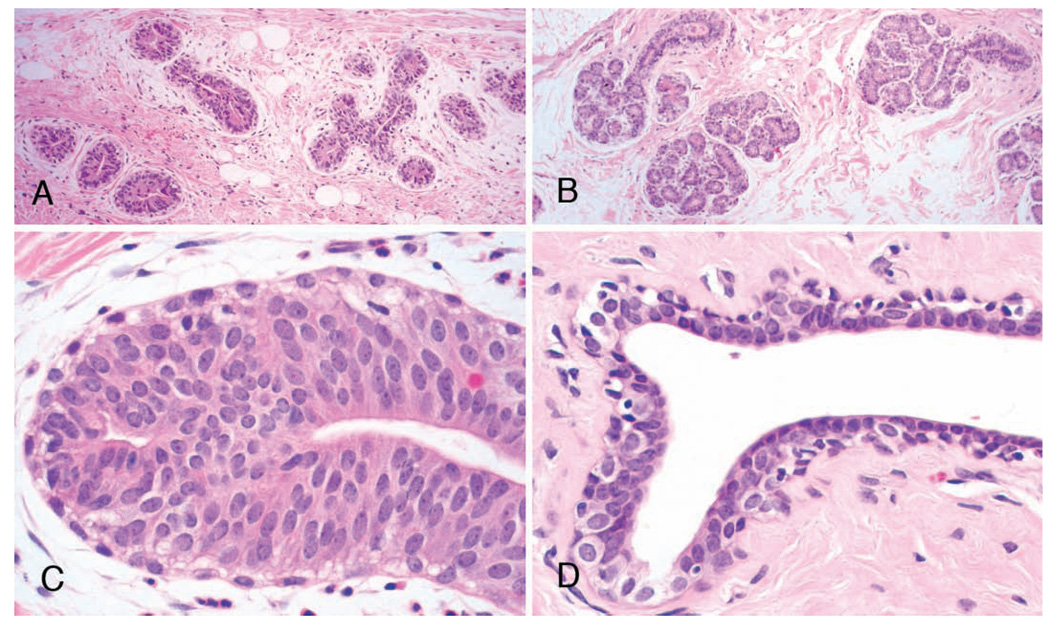
Development of the mammary glands is one of the first signs of puberty in human females, when nipple enlargement indicates entry into Tanner Stage B2 (Tanner 1952). Similarly, nipple development in macaques is distinctive and precedes regular menstruation by several months (Golub et al. 2003). Pubertal development of the breast begins with the rudimentary ductal tree established early in life and consists of rapid elongation and branching of major ducts to form a dense arborizing structure, which differentiates centrifugally with the most mature structures being near the nipple (Figure 2 and Figure 4). Distinct terminal end buds form the leading edge of mammary gland growth in human (Howard and Gusterson 2000) and macaque breasts; these structures are roughly 200 microns in diameter by 500 microns length and consist of a distinct “cap” or outer layer of myoepithelial cells, which express cytokeratin 14 and a central core of cytokeratin 18/16-positive luminal cells with a distinctive radial orientation and columnar morphology (Wood, Hester, and Cline 2007). These structures strongly express both estrogen and progesterone receptors and intermittently express the proliferation markers PCNA and Ki67, presumably during periodic episodes of higher serum-estrogen concentration. They are also highly invasive, eliciting a distinct surrounding region of loose, myxoid connective tissue (Figures 6A and 6C). Other structures prominent in the developing breast include primitive ducts with multiple layers of epithelium and nascent branching lobuloalveolar units (Figures 5B and D). Although the literature in humans is limited, a similar pattern of increased sex steroid receptor expression and rapid growth in the peripubertal breast has been documented (Bartow 1998; Howard and Gusterson 2000).
Figure 2.
Whole mount of the central portion of the mammary gland of a 2.5 year old, nulliparous, intact female cynomolgus macaque. Lactiferous ducts converging beneath the nipple are evident. Whole mount, toluidine blue.
Figure 4.
Whole-mount appearance of the mammary gland across the pubertal transition. (A) 1 year prior to first menstrual period; (B) 1 month after the first menstrual period; (C) 1 year after the first menstrual period. Whole mounts, toluidine blue. Bar = 1 mm.
Figure 6.
Pubertal lobular development in a juvenile male cynomolgus macaque. Inset - higher magnification of lobular proliferation. Hematoxylin and eosin.
Figure 5.
Histologic appearance of the developing breast in pubertal macaques. (A and C) Prepubertal breast with distinct terminal end buds and loose periglandular stroma; (B and D) Post-pubertal breast with lobule formation and open ductal lumens. Hematoxylin and eosin.
The extensive lobular development occurring during puberty in primates is distinct from the relatively limited lobular development occurring in nulliparous rodents. Juvenile macaques that have just begun to menstruate in some cases have mammary glands that are widely populated by well-differentiated, densely branched lobuloalveolar units of type 1 and 2 using the schema of Russo (Russo et al. 2000; Wood, Hester, and Cline 2007). Because the mammary gland of macaques grows so rapidly and invasively, care must be taken to avoid erroneously interpreting normal mammary gland development as hyperplasia or neoplasia, and the morphology of the breast must be considered in the context of the animal's stage of reproductive development. Furthermore, there is a high degree of individual variation in the pace of breast development, making animal-to-animal comparisons difficult, if not impossible, during this period. Pubertal development of mammary tissues in male macaques is not well described; however, transient glandular development (gynecomastia) has been noted (Figure 6; J. Vidal, personal communication). Transient gynecomastia occurs in more than 50% of normal adolescent boys. The incidence of this change and the degree to which it may resemble breast developmental changes seen in human males (Lazala and Saenger 2002) or normal mammary glandular development seen in male rats (Cardy 1991) remain unknown.
Adult Breast
The Adult Nonlactating Breast
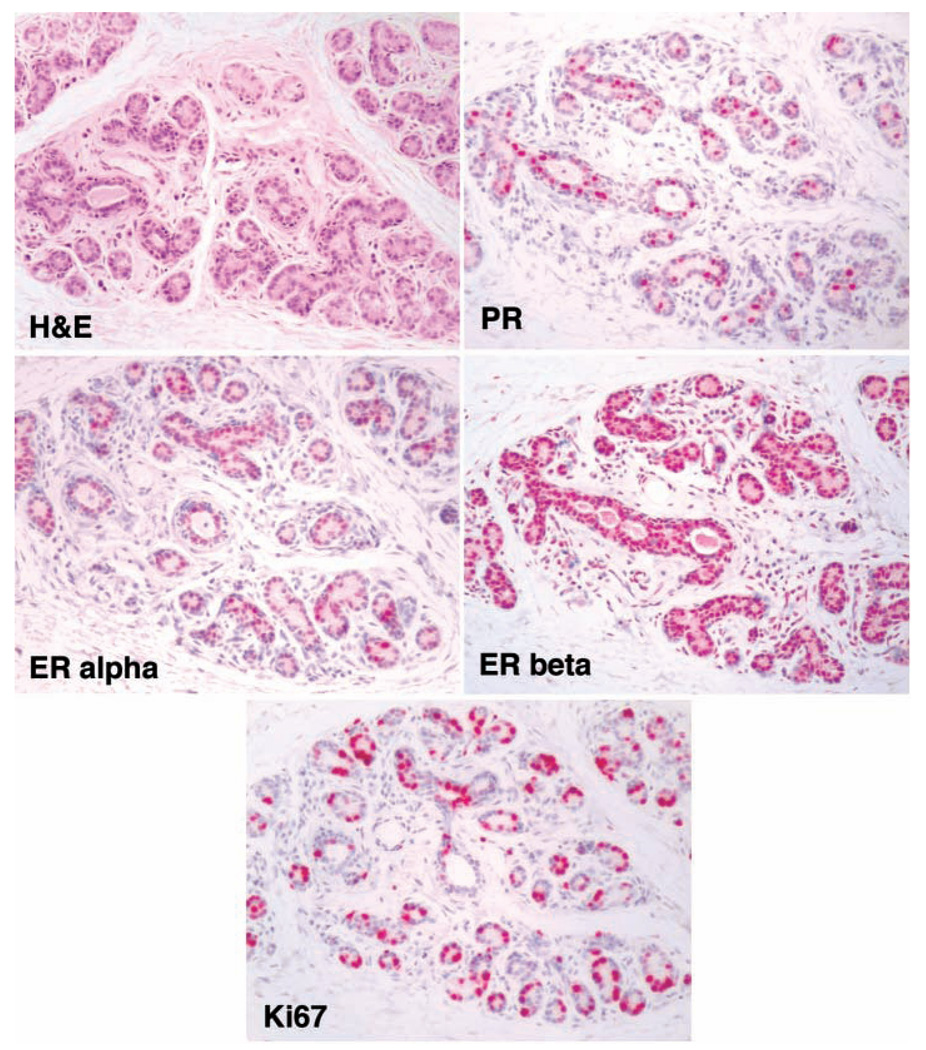
The breast of adult nonlactating animals consists primarily of a homogeneous pattern of mature type 2 lobules, and the “centrifugal” differentiation pattern seen during puberty is no longer apparent. Thus, in the adult breast, the degree of regional variation is small, and repeated breast biopsies can be taken with reasonable confidence of homogeneity (Cline et al. 1997). The sex steroid receptor expression profile in the adult macaque breast is similar to that described for the human breast (Khan, Bhandare, and Chatterton 2005). Both estrogen receptors alpha and beta are expressed, with the latter being more abundant (Figure 7; Borgerink and Cline 2004; Cline 2007; Isaksson et al. 2002). Estrogen receptor alpha is expressed multifocally in approximately 10% to 15% of breast epithelial cells, whereas expression of estrogen receptor beta is more diffuse, involving 50% or more of breast epithelial cells. Both of the known progesterone receptors (PR-A and PR-B) are expressed in the mammary gland; PR-A is the more abundant of the two, occurring in roughly 5% to 10% of epithelial cells, whereas PR-B is expressed in about 5% (Isaksson et al. 2003). Androgen receptor is also expressed in the breast tissue of macaques (Cheng et al. 2005; Zhou et al. 2000). Estrogen receptor alpha and progesterone receptors often co-express in the same cells, but steroid receptors do not co-express with the proliferation marker Ki67. Estrogen and progesterone receptors are also expressed in the skin at the tip of the nipple. For all receptors described here, expression in the periglandular stroma is occasionally but inconsistently seen.
Figure 7.
Typical patterns of sex steroid receptor expression and proliferation in the breast of a non-pregnant, intact 24-year-old female cynomolgus macaque. H&E (Hematoxylin and eosin); ER alpha (estrogen receptor alpha); (PR) (progesterone receptor); ER beta (estrogen receptor beta); and the proliferation marker Ki-67. (B–E) are immunohistochemical stains using Vector Red chromogen and a hematoxylin counterstain.
The effect of the menstrual cycle on proliferation in the breast is controversial; in the human breast, some investigators have found greater proliferation in the progesterone-dominated luteal phase of the cycle (Meyer 1977), whereas others have shown more proliferation in the estrogen-dominated follicular phase (Vogel et al. 1981). We have shown in macaques that cycle-related changes are small (less than a 6% difference in proportions of proliferating cells between luteal and follicular phases) and that there are compartmental differences in cellular proliferation in the macaque; ductal tissues proliferate more during the luteal phase, while lobuloalveolar tissues epithelium had higher proliferation during the late follicular phase (Stute et al. 2004). We also found that precise timing altered the result; when we compared the early follicular phase (day 5) to the mid-luteal phase, proliferative changes in both ducts and lobular epithelium were greater during the luteal phase (Wood, Appt, et al. 2006). Seasonal changes in the mammary gland have not been explored; thus, although some species of macaques such as the rhesus have distinctly seasonal reproductive patterns (Walker, Gordon, and Wilson 1983), no seasonal differences in hormonal responsiveness of the breast have been described. Cynomolgus macaques, in contrast to rhesus macaques, are not seasonal breeders (Dukelow, Grauwiler, and Brüggemann 1979).
Lactation
The primary biological purpose of the breast is to secrete milk, and thus, the proliferative and secretory changes induced during pregnancy and lactation represent the final state of complete differentiation and function of the mammary glands. Gestation in macaques is approximately 150 days in length, and during this time, the breast, as in other mammals, undergoes extensive growth and differentiation under the influence of high systemic concentrations of estrogens, progestogens, chorionic gonadotropin, placental lactogen, and prolactin (Neville 1983). In macaques, the change in volume of the glandular tissue is roughly tenfold to twenty fold, as a result of both epithelial proliferation and secretory distention of the ductal and alveolar system. Lobuloalveolar units are markedly increased in both number and size, and merocrine secretion of milk proteins, immunoglobulins, and fats is initiated. Macaques generally lactate for about twelve months (Buss 1971), and during this time, ovulation is suppressed (Wilson et al. 1988). The milk of macaques is similar to human milk but with a slightly higher proportion of protein (Jenness 1979; Lonnerdal et al. 1984). Macaques generally bear single offspring, and it is interesting to note that the neonate typically has a strong and generally unchanging preference for one nipple or the other (Jaffe et al. 2006). The impact of this infant behavior on secretory activity of the breast has not been fully explored, but presumably, the unused gland is less active and may undergo some degree of asymmetric involution.
Postlactational involution of the breast in macaques is not well described, but parous animals examined months to years after the last lactation have breast tissue similar to that of nulliparous animals. Although there is not sufficient data currently to state that there is a persistent breast-cancer-protective effect of lactation in macaques, such an effect is likely given the many similarities to humans.
Senescence
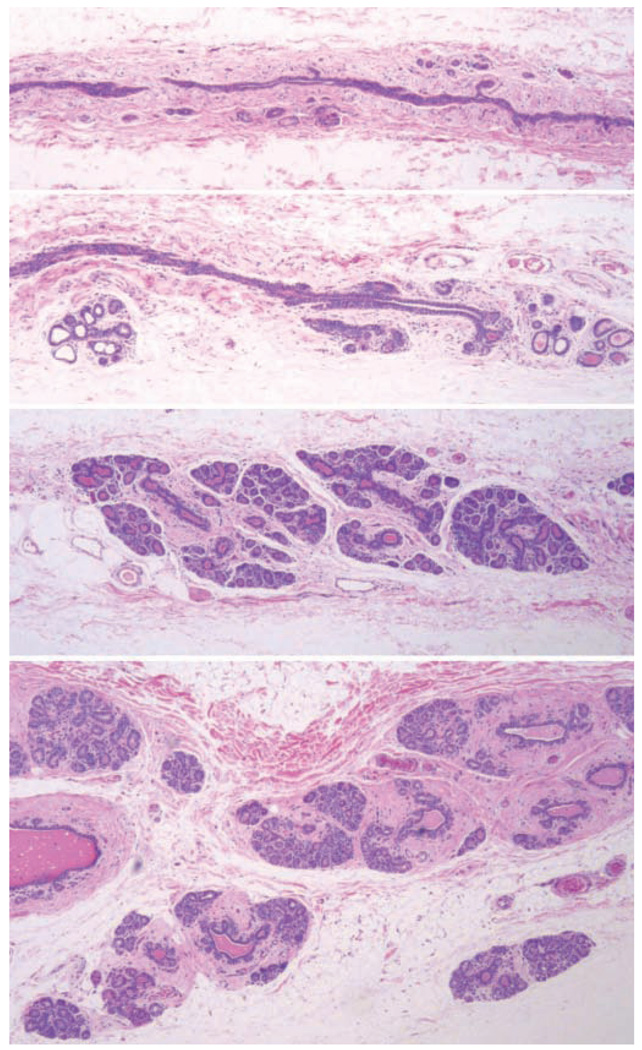
In older macaques, after natural or surgical menopause, the mammary gland regresses into a ductal network with marked lobular atrophy and little proliferative activity. However, there is substantial variation in the amount of tissue remaining (Figure 8). Estrogen and progesterone receptor expression persists in surgically postmenopausal macaques up to at least six to seven years after ovariectomy, and the breast is responsive to exogenous hormonal stimulation by estrogens and progestogens beyond twenty-five years of age (Wood, Register, et al. 2006). A variety of incidental lesions have been described in aging macaques, including cystic change (Cline 2007), columnar cell change, apocrine metaplasia, ductal hyperplasia, lobular hyperplasia, and neoplasms of the breast including ductal and lobular carcinoma in situ and invasive ductal carcinoma (Wood, Usborne, et al. 2006). Mammary gland carcinomas in macaques most commonly show a comedocarcinoma type of growth pattern, although micropapillary, solid, and cribriform patterns have also been described. Several of these findings are depicted in the “background findings” section of this issue.
Figure 8.
Range of mammary gland morphology in normal intact multiparous cycling adult female cynomolgus macaques, 20–25 years of age, at least 5 years after last giving birth. Hematoxylin and eosin.
EFFECTS OF HORMONE AND DRUG TREATMENT
The effects of estrogens, progestogens, and a variety of novel agents on the breast of macaques has been the subject of many original papers and reviews from our laboratory, and much careful and interesting work by other investigators. A detailed discussion of this entire body of work would be redundant with two recently published reviews from our group (Cline 2007; Cline and Wood 2005). However, some specific patterns of effect are noteworthy and are summarized in Table 1. In general, estrogens (estradiol, conjugated equine estrogens, ethinyl estradiol, and others) stimulate the breast to proliferate and induce expression of progesterone receptors and other “classic” estrogenic markers such as trefoil factor 1 (TFF1, formerly known as PS2; Wood, Register, et al. 2007). A standard hormone replacement dose of estradiol is a more potent agonist than the standard dose of conjugated estrogens (Wood et al. 2008). Progestogens alone in ovariectomized animals have little measurable effect on the breast, aside from down-regulation of estrogen and progesterone receptors (Cline et al. 1998). The combination of estrogens and progestogens in the breast induces a greater proliferative response than do estrogens alone (Cline, Register, and Clarkson 2002; Cline et al. 1996, 1998; Wood, Register, et al. 2007), and in this regard, the macaque model corresponds to findings in women from the Women's Health Initiative, in which combined estrogens (conjugated equine estrogens) plus progestogen (medroxyprogesterone acetate) treatment increased breast-cancer risk to a greater degree than did estrogens alone (Anderson et al. 2006). The addition of a progestogen also reduces expression of estrogen-responsive markers such as progesterone receptor and TFF1. Some synthetic progestogens, such as medroxyprogesterone acetate, may be more stimulatory than micronized progesterone (Wood, Register, et al. 2007) or alternative progestoges, such as norethindrone acetate (Suparto et al. 2003). Use of intravaginal administration of progestogens does not reduce the response of the breast to progestogen (Wood, Sitruk-Ware, et al. 2007). Dietary isoflavone phytoestrogens show little to no evidence of stimulatory effects on the macaque breast but, at supradietary doses, may antagonize the proliferative response to co-administered estradiol (Wood, Appt, et al. 2006; Wood, Kaplan, et al. 2006; Wood et al. 2006). The effects of androgens on the breast are currently unclear; Dimitrikakis et al. (2003) showed acute down-regulation of estrogen-induced proliferation by androgen co-administration, but human-observation studies indicate that higher endogenous androgens are associated with higher breast-cancer risk (Hankinson and Eliassen 2007). Selective estrogens such as raloxifene, lasofoxifene, and other novel agents have generally produced no effect on biomarkers in the breast (Sikoski et al. 2007), although, notably, the archetypal selective estrogen, tamoxifen, induces progesterone receptor (Cline et al. 1998) and, in one study, induced proliferation (Zhou et al. 2000) in the macaque breast.
Table 1.
Major findings regarding hormonal effects in the breast of macaques.
| Compound | Dosea | Duration | Major Outcome, % Changeb | Reference |
|---|---|---|---|---|
| CEE | 0.625 mg/day | 3 years | Ki67 – NSD | Cline et al. (1996, 1998); |
| PR – ↑4-fold | Cline, Register, and Clarkson (2002); | |||
| Wood et al. (2004) | ||||
| MPA | 2.5 mg/day | 3 years | Ki67, PR – NSD | Cline et al. (1998) |
| CEE+MPA | 0.625 mg/day, 2.5 mg/day | 3 years | Ki67 – ↑ 0.65-fold | Cline et al. (1996, 1998); |
| PR NSD | Cline, Register, and Clarkson (2002) | |||
| CEE+MPA | 12 months | Area – ↑ 2-fold | Suparto et al. (2003) | |
| Estradiol | 0.09 mg/day | 4 months | Ki67, PR – NSD | Wood et al. (2006) |
| Estradiol | 0.25 mg/day | Ki67, PR – NSD | Unpublished observation | |
| Estradiol | 0.5 mg/day | 4 months | Ki67, PR – 2-fold ↑ relative to 0.09 mg/day | Wood et al. (2006) |
| Estradiol | 1 mg/day | 6 months | Ki67 – 4-fold | Foth and Cline (1998) |
| EE | (0.003 mg/kg/day) | 3 months | Ki67 – ↑7-fold | Sikoski et al. (2007) |
| EE+NETA | Area – NSD | Suparto et al. (2003) | ||
| Triphasic EE+LN | ||||
| Estradiol | 2.5 mg pellet | 3 days | Ki67 – ↑ 3.8-fold | Dimitrakakis et al. (2003) |
| Estradiol | 5 mg pellet | 3 days | Ki67 – ↑ 6-fold | Zhou et al. (2000) |
| Estradiol + Progesterone | 2.5 mg pellet + 10 mg pellet | 3 days | Ki67 – ↑ 4-fold | Dimitrakakis et al. (2003) |
| Estradiol + Progesterone | 5 mg pellet +10 mg pellet | 3 days | Ki67 – ↑ 6-fold | Zhou et al. (2000) |
| Estradiol + Testosterone | 2.5 mg pellet + 35 ug/kg pellet | 3 days | Ki67 – ↑2.1-fold | Dimitrakakis et al. (2003) |
| Estradiol + Testosterone | 1 mg pellet + 42 ug/kg pellet | 3 days | Ki67 – ↑ 3.8-fold | Zhou et al. (2000) |
| Raloxifene | (3 mg/kg/day) | 2 months | Ki67, PR – NSD | Sikoski et al. (2007) |
| Lasofoxifene | (1 mg/kg/day) | 2 years | NSD | Cline et al. (2008) |
| (5 mg/kg/day) | ||||
| Tamoxifen | (1 mg/kg/day) | 3 years | Ki67 – NSD | Cline et al. (1998) |
| PR – ↑3-fold | ||||
| Tamoxifen | 50 mg pellet | 3 days | Ki-67 – ↑3-fold | Zhou et al. (2000) |
| SERM 393 | (2,4,8 mg/kg/day) | 3 months | Ki67, PR – NSD | Sikoski et al. (2007) |
| SERM 379 | (4 mg/kg/day) | 3 months | Ki67, PR – NSD | Sikoski et al. (2007) |
| Tibolone | (0.05 mg/kg/day) | 2 years | Ki67, PR – NSD | Cline, Register, and Clarkson (2002) |
| Tibolone | (0.2 mg/kg/day) | 2 years | Ki67 – NSD | Cline, Register, and Clarkson (2002) |
| PR – ↑3-fold |
Human equivalent dose; parentheses indicate mg/kg/day dose.
Relative to ovariectomized control unless otherwise stated.
In general, we have found that the number of animals required to detect statistically significant differences in qualitative histopathologic findings in the mammary gland is higher than the typically very low number of animals used in toxicology screening. Therefore, to reduce the numbers of animals used, we have adopted a biomarker approach (Cline et al. 1996, 1998; Cline, Register, and Clarkson 2002) as well as novel study designs such as crossover designs or pretreatment biopsy (Wood, Register, et al. 2006; Wood, Sitruk-Ware, et al. 2007) to reduce the effects of interindividual variability. We strongly recommend that any study involving assessment of the mammary gland should be performed in postpubertal animals.
CONCLUSIONS
Mammary gland development and hormonal responses in macaques are complex issues and show a high degree of similarity to the human breast, particularly with respect to their responses to hormonally active pharmacologic agents. Careful consideration of developmental stage, reproductive history, and hormonal context is necessary in the evaluation of breast changes in macaques. The high degree of interindividual differences in macaques may require novel strategies for evaluation of hormonal effects—for example, the use of pretreatment biopsy or the use of hormone-response biomarkers.
Abbreviations
- CEE
conjugated equine estrogens
- GH
growth hormone
- IGF
insulin-like growth factor
- MPA
medroxyprogesterone acetate
- NMGA
nomegestrol acetate
- NETA
norethindrone acetate
- Laso
Lasofoxifene
- Tam
Tamoxifen
- Ral
Raloxifene
- SERM 393 and 379
investigative selective estrogen receptor modulators.
References
- Anderson GL, Chlebowski RT, Rossouw JE, Rodabough RJ, McTiernan A, Margolis KL, Aggerwal A, David Curb J, Hendrix SL, Allan Hubbell F, Khandekar J, Lane DS, Lasser N, Lopez AM, Potter J, Ritenbaugh C. Prior hormone therapy and breast cancer risk in the Women's Health Initiative randomized trial of estrogen plus progestin. Maturitas. 2006;55:103–115. doi: 10.1016/j.maturitas.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, Franceschini P, Lala R, Holmes LB, Gebuhr TC, Bruneau BG, Schinzel A, Seidman JG, Seidman CE, Jorde LB. Mutations in human TBX3 alter limb, apocrine, and genital development in ulnar-mammary syndrome. Nat Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- Barbier O, Belanger A. The cynomolgus monkey (Macaca fascicularis) is the best animal model for the study of steroid glucuronidation. J Steroid Biochem Mol Biol. 2003;85:235–245. doi: 10.1016/s0960-0760(03)00235-8. [DOI] [PubMed] [Google Scholar]
- Bartow SA. Use of the autopsy to study ontogeny and expression of the estrogen receptor gene in human breast. J Mammary Gland Biol Neoplasia. 1998;3:37–48. doi: 10.1023/a:1026641401184. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, LaPensee CR, LaPensee EW. What can we learn from rodents about prolactin in humans? Endocr Rev. 2008;29:1–41. doi: 10.1210/er.2007-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgerink H, Cline JM. Triple immunolableing in a single section of mammary gland using Ki67-MIB1, ER alpha and ER beta antibodies. J Histotechnol. 2004;27:38–41. [Google Scholar]
- Buss DH. Mammary glands and lactation. In: Hafez ESE, editor. Comparative reproduction of nonhuman primates. Springfield, IL: Charles C. Thomas; 1971. pp. 315–333. [Google Scholar]
- Cameron AM, Faulkin LT., Jr Subgross evaluation of the non-human primate mammary gland: Method and initial observations. J Med Primatol. 1974;3:298–310. doi: 10.1159/000460031. [DOI] [PubMed] [Google Scholar]
- Cardy RH. Sexual dimorphism of the normal rat mammary gland. Vet Pathol. 1991;28:139–145. doi: 10.1177/030098589102800206. [DOI] [PubMed] [Google Scholar]
- Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and risk of breast cancer. Mayo Clin Proc. 2008;83:86–90. doi: 10.4065/83.1.86. [DOI] [PubMed] [Google Scholar]
- Cheng G, Li Y, Omoto Y, Wang Y, Berg T, Nord M, Vihko P, Warner M, Piao YS, Gustafsson JA. Differential regulation of estrogen receptor (ER)alpha and ERbeta in primate mammary gland. J Clin Endocrinol Metab. 2005;90:435–444. doi: 10.1210/jc.2004-0861. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, Rodabough RJ, Gilligan MA, Cyr MG, Thomson CA, Khandekar J, Petrovitch H, McTiernan A. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: The Women's Health Initiative randomized trial. Jama. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- Clemons M, Loijens L, Goss P. Breast cancer risk following irradiation for Hodgkin's disease. Cancer Treat Rev. 2000;26:291–302. doi: 10.1053/ctrv.2000.0174. [DOI] [PubMed] [Google Scholar]
- Cline JM. Assessing the mammary gland of nonhuman primates: Effects of endogenous hormones and exogenous hormonal agents and growth factors. Birth Defects Res B Dev Reprod Toxicol. 2007;80:126–146. doi: 10.1002/bdrb.20112. [DOI] [PubMed] [Google Scholar]
- Cline JM, Botts S, Lees CJ, Brommage R. Effects of lasofoxifene on the uterus, vagina, and breast in ovariectomized cynomolgus monkeys (Macaca fascicularis) Am J Obstet Gynecol. 2008;199:158.el–8. doi: 10.1016/j.ajog.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Cline JM, Register TC, Clarkson TB. Effects of tibolone and hormone replacement therapy on the breast of cynomolgus monkeys. Menopause. 2002;9:422–429. doi: 10.1097/00042192-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Cline JM, Soderqvist G, von Schoultz B, Skoog L. Regional distribution of proliferating cells and hormone receptors in the mammary gland of surgically postmenopausal macaques. Gynecol Obstet Invest. 1997;44:41–46. doi: 10.1159/000291407. [DOI] [PubMed] [Google Scholar]
- Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of hormone replacement therapy on the mammary gland of surgically postmenopausal cynomolgus macaques. Am J Obstet Gynecol. 1996;174:93–100. doi: 10.1016/s0002-9378(96)70379-4. [DOI] [PubMed] [Google Scholar]
- Cline JM, Soderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of conjugated estrogens, medroxyprogesterone acetate, and tamoxifen on the mammary glands of macaques. Breast Cancer Res Treat. 1998;48:221–229. doi: 10.1023/a:1005984932268. [DOI] [PubMed] [Google Scholar]
- Cline JM, Wood CE. Hormonal effects on the mammary gland of postmenopausal nonhuman primates. Breast Dis. 2005;24:59–70. doi: 10.3233/bd-2006-24105. [DOI] [PubMed] [Google Scholar]
- Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545–560. doi: 10.1093/humupd/dmi028. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Horn YK, Cooke PS, Taylor JA, Lubahn DB. Elucidation of a role for stromal steroid hormone receptors in mammary gland growth and development using tissue recombinants. J Mammary Gland Biol Neoplasia. 1997;2:393–402. doi: 10.1023/a:1026303630843. [DOI] [PubMed] [Google Scholar]
- Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- Dimitrakakis C, Zhou J, Wang J, Belanger A, LaBrie F, Cheng C, Powell D, Bondy C. A physiologic role for testosterone in limiting estrogenic stimulation of the breast. Menopause. 2003;10:292–298. doi: 10.1097/01.GME.0000055522.67459.89. [DOI] [PubMed] [Google Scholar]
- Dukelow WR, Grauwiler J, Brüggemann S. Characteristics of the menstrual cycle in nonhuman primates. I. Similarities and dissimilarities between Macaca fascicularis and Macaca arctoides. J Med Primato. 1979;8:39–47. doi: 10.1159/000460174. [DOI] [PubMed] [Google Scholar]
- Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006;98:1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- Evans DG, Howell A. Breast cancer risk-assessment models. Breast Cancer Res. 2007;9:213–221. doi: 10.1186/bcr1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA. 2007;104:14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, Warner M, Gustafsson JA. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci USA. 2002;99:15578–15583. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth IA, Wallis M. Growth hormone and prolactin—Molecular and functional evolution. J Mammary Gland Biol Neoplasia. 2002;7:291–312. doi: 10.1023/a:1022804817104. [DOI] [PubMed] [Google Scholar]
- Foth D, Cline JM. Effects of mammalian and plant estrogens on mammary glands and uteri of macaques. Am J Clin Nutr. 1998;68(6 Suppl.):1413S–1417S. doi: 10.1093/ajcn/68.6.1413S. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Lasley BL, Natarajan K, Tarantal AF. Effects of exogenous estrogenic agents on pubertal growth and reproductive system maturation in female rhesus monkeys. Toxicol Sci. 2003;74:103–113. doi: 10.1093/toxsci/kfg090. [DOI] [PubMed] [Google Scholar]
- Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone and progesterone levels in relation to breast cancer risk. J Steroid Biochem Mol Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Howard B, Ashworth A. Signalling pathways implicated in early mammary gland morphogenesis and breast cancer. PLoS Genet. 2006;2:ell2. doi: 10.1371/journal.pgen.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5:119–137. doi: 10.1023/a:1026487120779. [DOI] [PubMed] [Google Scholar]
- Isaksson E, Wang H, Sahlin L, von Schoultz B, Cline JM, von Schoultz E. Effects of long-term HRT and tamoxifen on the expression of progesterone receptors A and B in breast tissue from surgically postmenopausal cynomolgus macaques. Breast Cancer Res Treat. 2003;79:233–239. doi: 10.1023/a:1023925906199. [DOI] [PubMed] [Google Scholar]
- Isaksson E, Wang H, Sahlin L, von Schoultz B, Masironi B, von Schoultz E, Cline JM. Expression of estrogen receptors (alpha, beta) and insulin-like growth factor-I in breast tissue from surgically postmenopausal cynomolgus macaques after long-term treatment with HRT and tamoxifen. Breast. 2002;11:295–300. doi: 10.1054/brst.2002.0422. [DOI] [PubMed] [Google Scholar]
- Jaffe BD, Evans TA, Howell S, Westergaard GC, Snoy PJ, Higley JD. Left versus right nipple preference in free-ranging infant rhesus macaques (Macaca mulatta) Dev Psychobiol. 2006;48:266–272. doi: 10.1002/dev.20128. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jenness R. The composition of human milk. Semin Perinatal. 1979;3:225–239. [PubMed] [Google Scholar]
- Jolicoeur F. Intrauterine breast development and the mammary myoepithelial lineage. J Mammary Gland Biol Neoplasia. 2005;10:199–210. doi: 10.1007/s10911-005-9581-9. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Binart N, Lucas B, Bouchard B, Goffin V. Implications of multiple phenotypes observed in prolactin receptor knockout mice. Front Neuroendocrinal. 2001;22:140–145. doi: 10.1006/frne.2001.0212. [DOI] [PubMed] [Google Scholar]
- Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- Khan SA, Bhandare D, Chatterton RT., Jr The local hormonal environment and related biomarkers in the normal breast. Endocr Relat Cancer. 2005;12:497–510. doi: 10.1677/erc.1.00732. [DOI] [PubMed] [Google Scholar]
- Kleinberg DL. Early mammary development: Growth hormone and IGF-1. J Mammary Gland Biol Neoplasia. 1997;2:49–57. doi: 10.1023/a:1026373513521. [DOI] [PubMed] [Google Scholar]
- Kleinberg DL, Niemann W, Flamm E, Cooper P, Babitsky G, Valensi Q. Primate mammary development. Effects of hypophysectomy, prolactin inhibition, and growth hormone administration. J Clin Invest. 1985;75:1943–1950. doi: 10.1172/JCI111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg DL, Todd J, Niemann W. Prolactin stimulation of alpha-lactalbumin in normal primate mammary gland. J Clin Endocrinol Metab. 1978;47:435–441. doi: 10.1210/jcem-47-2-435. [DOI] [PubMed] [Google Scholar]
- Lazala C, Saenger P. Pubertal gynecomastia. J Pediatr Endocrinol Metab. 2002;15:553–560. doi: 10.1515/jpem.2002.15.5.553. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B, Keen CL, Glazier CE, Anderson J. A longitudinal study of rhesus monkey (Macaca mulatta) milk composition: Trace elements, minerals, protein, carbohydrate, and fat. Pediatr Res. 1984;18:911–914. doi: 10.1203/00006450-198409000-00023. [DOI] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- MacPherson E, Montagna W. The mammary glands of rhesus monkeys. Journal of Investigative Dermatology. 1974;63:17–18. doi: 10.1111/1523-1747.ep12677294. [DOI] [PubMed] [Google Scholar]
- Martel C, Meiner MH, Gagne D, Simard J, Labrie F. Widespread tissue distribution of steroid sulfatase, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase (3 beta-HSD), 17 beta-HSD 5 alpha-reductase and aromatase activities in the rhesus monkey. Mol Cell Endocrinol. 1994;104:103–111. doi: 10.1016/0303-7207(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Meyer JS. Cell proliferation in normal human breast ducts, fibroadenomas, and other ductal hyperplasias measured by nuclear labeling with tritiated thymidine. Effects of menstrual phase, age, and oral contraceptive hormones. Hum Pathol. 1977;8:67–81. doi: 10.1016/s0046-8177(77)80066-x. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Clark JA, Myers PH, Korach KS. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor alpha. Endocrinology. 2002;143:2357–2365. doi: 10.1210/endo.143.6.8836. [DOI] [PubMed] [Google Scholar]
- Neville MC. Regulation of mammary development and lactation. In: Neville MC, Neifert MR, editors. Lactation: Physiology, nutrition and breast-feeding. New York: Plenum; 1983. pp. 103–140. [Google Scholar]
- Ng ST, Zhou J, Adesanya OO, Wang J, LeRoith D, Bondy CA. Growth hormone treatment induces mammary gland hyperplasia in aging primates. Nat Med. 1997;3:1141–1144. doi: 10.1038/nm1097-1141. [DOI] [PubMed] [Google Scholar]
- Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J Clin Endocrinol Metab. 1996;81:1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- Perry DL, Spedick JM, McCoy TP, Adams MR, Franke AA, Cline JM. Dietary soy protein containing isoflavonoids does not adversely affect the reproductive tract of male cynomolgus macaques (Macaca fascicularis) J Nutr. 2007;137:1390–1394. doi: 10.1093/jn/137.6.1309. [DOI] [PubMed] [Google Scholar]
- Raccurt M, Lobie PE, Moudilou E, Garcia-Caballero T, Frappart L, Morel G, Mertani HC. High stromal and epithelial human GH gene expression is associated with proliferative disorders of the mammary gland. J Endocrinol. 2002;175:307–318. doi: 10.1677/joe.0.1750307. [DOI] [PubMed] [Google Scholar]
- Remick AK, Wood CE, Cann JA, Gee MK, Feiste EA, Kock ND, Cline JM. Histologic and immunohistochemical characterization of spontaneous pituitary adenomas in fourteen cynomolgus macaques (Macaca fascicularis) Vet Pathol. 2006;43:484–493. doi: 10.1354/vp.43-4-484. [DOI] [PubMed] [Google Scholar]
- Ruan W, Kleinberg DL. Insulin-like growth factor I is essential for terminal end bud formation and ductal morphogenesis during mammary development. Endocrinology. 1999;140:5075–5081. doi: 10.1210/endo.140.11.7095. [DOI] [PubMed] [Google Scholar]
- Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000;27:17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- Russo J, Russo IH. Experimentally induced mammary tumors in rats. Breast Cancer Res Treat. 1996;39:7–20. doi: 10.1007/BF01806074. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, Easton D, Forbes JF, Key T, Hankinson SE, Howell A, Ingle J. Critical assessment of new risk factors for breast cancer: Considerations for development of an improved risk prediction model. Endocr Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Estrogen-progestin replacement and risk of breast cancer. Jama. 2000;284:691–694. [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikoski P, Register TC, Lees CJ, Lundeen S, Hutchison J, Brown KH, Cline JM. Effects of two novel selective estrogen receptor modulators, raloxifene, tamoxifen, and ethinyl estradiol on the uterus, vagina and breast in ovariectomized cynomolgus monkeys (Macaca fascicularis) Am J Obstet Gynecol. 2007;196:75–82. doi: 10.1016/j.ajog.2006.09.038. [DOI] [PubMed] [Google Scholar]
- Speert H. The normal and experimental development of the mammary gland of the rhesus monkey with some pathologic correlations. Contributions to Embryology, The Carnegie Institute of Washington. 1948;32:9–65. [Google Scholar]
- Sternlicht MD. Key stages in mammary gland development: The cues that regulate ductal branching morphogenesis. Breast Cancer Res. 2006;8:201. doi: 10.1186/bcr1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stute P, Register TC, Blair RM, Cline JM. Effects of tibolone on estrogen biosynthesis in the mammary tissue of postmenopausal monkeys. Menopause. 2006;13:232–240. doi: 10.1097/01.gme.0000198487.55456.0e. [DOI] [PubMed] [Google Scholar]
- Stute P, Wood CE, Kaplan JR, Cline JM. Cyclic changes in the mammary gland of cynomolgus macaques. Fertil Steril. 2004;82(Suppl. 3):1160–1170. doi: 10.1016/j.fertnstert.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Suparto IH, Williams JK, Cline JM, Anthony MS, Fox JL. Contrasting effects of two hormone replacement therapies on the cardiovascular and mammary gland outcomes in surgically postmenopausal monkeys. Am J Obstet Gynecol. 2003;188:1132–1140. doi: 10.1067/mob.2003.237. [DOI] [PubMed] [Google Scholar]
- Tanner JM. The assessment of growth and development in children. Arch Dis Child. 1952;27:10–33. doi: 10.1136/adc.27.131.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubura A, Hatano T, Hayama S, Morii S. Immunophenotypic difference of keratin expression in normal mammary glandular cells from five different species. Acta Anatomica. 1991;140:287–293. doi: 10.1159/000147071. [DOI] [PubMed] [Google Scholar]
- Vogel PM, Georgiade NG, Fetter BF, Vogel FS, McCarty KS., Jr The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol. 1981;104:23–34. [PMC free article] [PubMed] [Google Scholar]
- Walker ML, Gordon TP, Wilson ME. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod. 1983;29:841–848. doi: 10.1095/biolreprod29.4.841. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Walker ML, Pope NS, Gordon TP. Prolonged lactational infertility in adolescent rhesus monkeys. Biology of Reproduction. 1988;38:163–174. doi: 10.1095/biolreprod38.1.163. [DOI] [PubMed] [Google Scholar]
- Wood CE, Appt SE, Clarkson TB, Franke AA, Lees CJ, Doerge DR, Cline JM. Effects of high-dose soy isoflavones and equol on reproductive tissues in female cynomolgus monkeys. Biol Reprod. 2006;75:477–486. doi: 10.1095/biolreprod.106.052142. [DOI] [PubMed] [Google Scholar]
- Wood CE, Clarkson TB, Chen H, Xu X, Veenstra TD, Cline JM. Comparative effects of oral conjugated equine estrogens and micronized 17-beta estradiol on breast proliferation: A retrospective analysis. Menopause. 2008 doi: 10.1097/gme.0b013e318168f0ad. In press. [DOI] [PubMed] [Google Scholar]
- Wood CE, Hester JM, Cline JM. Mammary gland development in early pubertal female macaques. Toxicol Pathol. 2007;35:795–805. doi: 10.1080/01926230701584213. [DOI] [PubMed] [Google Scholar]
- Wood CE, Kaplan JR, Stute P, Cline JM. Effects of soy on the mammary glands of premenopausal female monkeys. Fertil Steril. 2006;85(Suppl. 1):1179–1186. doi: 10.1016/j.fertnstert.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, Cline JM. Soy isoflavonoid effects on endogenous estrogen metabolism in postmenopausal female monkeys. Carcinogenesis. 2007;28:801–808. doi: 10.1093/carcin/bgl163. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, Anthony MS, Kock ND, Cline JM. Breast and uterine effects of soy isoflavones and conjugated equine estrogens in postmenopausal female monkeys. J Clin Endocrinol Metab. 2004;89:3462–3468. doi: 10.1210/jc.2003-032067. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, Franke AA, Anthony MS, Cline JM. Dietary soy isoflavones inhibit estrogen effects in the postmenopausal breast. Cancer Res. 2006;66:1241–1249. doi: 10.1158/0008-5472.CAN-05-2067. [DOI] [PubMed] [Google Scholar]
- Wood CE, Register TC, Lees CJ, Chen H, Kimrey S, Cline JM. Effects of estradiol with micronized progesterone or medroxyprogesterone acetate on risk markers for breast cancer in postmenopausal monkeys. Breast Cancer Res Treat. 2007;101:125–134. doi: 10.1007/s10549-006-9276-y. [DOI] [PubMed] [Google Scholar]
- Wood CE, Sitruk-Ware RL, Tsong YY, Register TC, Lees CJ, Cline JM. Effects of estradiol with oral or intravaginal progesterone on risk markers for breast cancer in a postmenopausal monkey model. Menopause. 2007;14:639–647. doi: 10.1097/01.gme.0000247017.41007.80. [DOI] [PubMed] [Google Scholar]
- Wood CE, Usborne AL, Starost ME, Tarara RP, Hill LR, Wilkinson LM, Geisinger KR, Feiste EA, Cline JM. Hyperplastic and neoplastic lesions of the mammary gland in macaques. Vet Pathol. 2006;43:471–483. doi: 10.1354/vp.43-4-471. [DOI] [PubMed] [Google Scholar]
- Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- Zhou J, Ng S, Adesanya-Famuiya O, Anderson K, Bondy CA. Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression. FASEB J. 2000;14:1725–1730. doi: 10.1096/fj.99-0863com. [DOI] [PubMed] [Google Scholar]