Abstract
The authors describe a selection of normal findings and common naturally occurring lesions in the reproductive system of female macaques, including changes in the ovaries, uterus, cervix, vagina, and mammary glands. Normal features of immature ovaries, uteri, and mammary glands are described. Common non-neoplastic lesions in the ovaries include cortical mineralization, polyovular follicles, cysts, ovarian surface epithelial hyperplasia, and ectopic ovarian tissue. Ovarian neoplasms include granulosa cell tumors, teratomas, and ovarian surface epithelial tumors. Common non-neoplastic uterine findings include loss of features of normal cyclicity, abnormal bleeding, adenomyosis, endometriosis, epithelial plaques, and pregnancy-associated vascular remodeling. Hyperplastic and neoplastic lesions of the uterus include endometrial polyps, leiomyomas, and rarely endometrial hyperplasia and endometrial adenocarcinoma. Vaginitis is common. Cervical lesions include endocervical squamous metaplasia, polyps, and papillomavirus-associated lesions. Lesions in the mammary gland are most often proliferative and range from ductal hyperplasia to invasive carcinoma. Challenges to interpretation include the normal or pathologic absence of menstrual cyclicity and the potential misinterpretation of sporadic lesions, such as epithelial plaques or papillomavirus-associated lesions. Interpretation of normal and pathologic findings is best accomplished with knowledge of the life stage, reproductive history, and hormonal status of the animal.
Keywords: primate pathology, female reproductive pathology, uterus, ovary, mammary gland
INTRODUCTION
This review is intended to provide the reader with a brief descriptive and photographic reference to a few of the more common spontaneous lesions in the reproductive tract, pituitary gland, and mammary gland of female macaques. For further discussion of reproductive pathology in female nonhuman primates, the reader is also referred to the recent work of Cooper and Gabrielson (2007) and other articles on the subject (DiGiacomo 1977; Wilkinson et al. 2008). Neoplasms of the reproductive system in macaques have been the subject of several reviews (e.g., Beniashvilli 1989). The few reference books on the spontaneous pathology of nonhuman primates also include information on neoplasms and background findings in the reproductive tract (Benirschke, Garner, and Jones 1978; Takayama, Fukushima, and Thorgeirsson 2000). Two types of change are of greater importance and pose difficulty in interpretation for the toxicologic pathologist, namely, those that relate to abnormalities of the menstrual cycle and potentially treatment related hyperplastic lesions of the endometrium that have relevance to cancer risk. Accordingly, we have empha-sized those two areas.
Tissues presented in this review have been collected from animals in breeding colonies and control and treated groups from Wake Forest University, the California National Primate Research Center, Covance Laboratories, Schering-Plough (formerly Organon), and GlaxoSmithKline. Because the age and reproductive status of populations at each site differ substantially, estimates of lesion incidence are not included. Furthermore, some of the “findings” reported here are normal but are included because they create challenges to interpretation. All procedures involving animals at each institution were conducted in compliance with applicable regulations, including Institutional Animal Care and Use Committee oversight.
PITUITUITARY GLAND
Pituitary Adenomas
Pituitary adenomas are the most common neoplasm in older cynomolgus macaques (Remick et al. 2006) and have also been reported to be common in rhesus macaques (Chalifoux, MacKey, and King 1983). These benign neoplasms are generally clinically silent and found incidentally at necropsy. These neoplasms are most commonly immunoreactive for prolactin or adrenocorticotropic hormone (Figures 1A, 1B), but multihormonal neoplasms are common. Prolactin-staining neoplasms are occasionally associated with galactorrhea (Daviau and Trupkiewicz 2001). In these characteristics, they are similar to those pituitary adenomas seen most commonly in human beings and also may be similarly associated with type 2 diabetes (Remick et al. 2006).
FIGURE 1.
(A) Pituitary microadenoma in an aged cynomolgus macaque. H&E. (B) Pituitary adenoma in a twenty-five-year-old cynomolgus macaque. Immunohistochemistry for adrenocorticotropic hormone; hematoxylin counterstain. (C) Gonadotroph hypertrophy in the pars distalis of an adult ovariectomized cynomolgus macaque. H&E. (D) Follicle-stimulating hormone immunostain, hematoxylin counterstain.
Hypertrophy of Gonadotrophs
We have noted the presence of hypertrophied gonadotroph cells in the pituitary of cynomolgus monkeys after ovariectomy, similar to the so-called gonadectomy cells seen in ovariectomized rats. These cells occur diffusely within the pars distalis of ovariectomized animals and stain positively using immunohistochemistry with antibodies directed against follicle stimulating hormone (Figures 1C, 1D).
OVARY
Immaturity
Because female macaques have high individual variability for pubertal onset between the ages of 2.5 and 4, a critical assessment of ovarian morphology is essential to the correct interpretation of changes elsewhere in the reproductive system. Both ovaries of young animals should be examined grossly and histologically for the presence of corpora lutea or remnants, indicating prior ovulation. The ovaries of immature animals are not completely quiescent but typically contain follicles in a range of maturation stages, including antral follicles, which may produce estradiol in early preovulatory animals (Wood, Hester, and Cline 2007). However, these early pubertal ovaries lack the corpora lutea typical of late-pubertal or adult-cycling animals. Immaturity and states of hypothalamo-pituitary-gonadal axis dysfunction are discussed further under the section titled “Uterus” below.
Non-neoplastic Lesions
Ovarian Cysts
Cysts in and around the ovary are common incidental findings in macaques of all ages (Figure 2A). Cysts can arise from the rete ovarii, embryonic remnants, or cycling ovarian structures (follicles, corpora lutea). In our experience, cysts adjacent to the ovary are most common and likely arise from the mesonephric tubules (extraovarian rete ovarii) and/or mesonephric ducts. These cysts can range from microscopic dilation of the rete ovarii to macroscopically evident structures, and solitary cysts typically are of no functional consequence. Follicular cysts can arise as the consequence of disturbances of pituitary hormone production (abnormal release of gonadotropins). In contrast to solitary cysts, polycystic ovaries likely represent a distinct disease entity similar to that described in women; it is described below under “Disorders of Cycle Regulation.”
FIGURE 2.
(A) Gross appearance of a para-ovarian cyst in a nineteen-year-old cynomolgus monkey. (B) Ectopic ovarian tissue on the uterine serosal surface of an adult, normally cycling cynomolgus monkey. Note the presence of primary and atretic follicles in a dense ovarian-type stroma. H&E. (C) Multifocal mineralization in the ovary of a 2.5-year-old cynomolgus monkey. H&E. (D) Polyovular follicles in the ovary of a 2.5-year-old cynomolgus monkey. H&E. (E) Papillary hyperplasia of the ovarian surface epithelium in a 7.5-year-old cynomolgus monkey. H&E. (F) Focal smooth muscle metaplasia of the ovarian stroma in the ovary of an 18-year-old cynomolgus monkey; the absence of oocytes and fibrosis of the adjacent stroma reflects the age of the animal. H&E. (G, H, I) Ovarian deciduosis in a 16-year-old cynomolgus monkey, forming a polypoid mass projecting above the ovarian surface. (G) H&E. (H) anti-smooth muscle actin. (I) H&E at higher magnification. Typical endometrial lymphocytes were present (arrow).
Ectopic Ovarian Tissue
In macaques, ectopic ovarian tissue can occasionally be observed within the broad ligament, on the serosal surface of the uterus, or within the myometrium (Figure 2B). Recently, Kuwamura et al. (2006) reported the presence of ectopic uterine ovarian tissue in 9 out of 118 (7.6%) cynomolgus monkeys ranging from 3 to 7 years of age. Generally such lesions are not grossly visible and are found only incidentally during micro-scopic investigation of the uterus. The anomaly consists of ovarian stromal tissue with varying appearance of primordial, primary, and/or atretic follicles. Since no signs of trauma or inflammatory changes are generally observed in relation to this lesion, the presence of uterine ovarian tissue is considered to have a congenital background (Payan and Gilbert 1987). The parametrial location of this anomaly supports the hypothesis of its embryologic origin. When using ovariectomized macaques as an experimental model, care must be taken to confirm completeness of the ovariectomy using postsurgical serum estradiol, progesterone, and/or gonadotropin concentrations (Cline, Register, and Clarkson 2002A).
Cortical Mineralization
Multifocal mineralization of the ovarian cortex is a common incidental finding of no known significance in macaques (Majeed and Gopinath 1980). These lesions appear to arise from degenerate follicles, specifically, oocytes, and thus most likely represent dystrophic mineralization of atretic follicles (Figure 2C). We have encountered this change most commonly in young adult animals.
Polyovular Follicles
The presence of two or more oocytes within a developing follicle is commonly observed in rhesus macaques (Figure 2D) (Koering 1983). The presence and number of polyovular follicles can vary from animal to animal, but such follicles can be abundant in an individual macaque (Van Wagenen and Simpson 1973). Polyovular follicles occur in a wide range of mammalian species (Mossman and Duke 1973). The number of polyovular follicles has been demonstrated to increase in the rhesus macaque following administration of follicle stimulating hormone (FSH) (Van Wagenen and Simpson 1973). In mice, inhibition of oocyte nest breakdown resulting in multioocyte follicles has been proposed as an indicator of endocrine disrupting effects by estrogens during development (Chen et al. 2007). Although no similar association has been shown for primates, recording of polyovular follicles is recommended in toxicology studies.
Hyperplasia of the Ovarian Surface Epithelium
Minor degrees of hyperplasia of the surface epithelium of the ovary (Figure 2E) are common in macaques and have little known significance. However, the ovarian surface epithelium expresses sex steroid receptors, and there is evidence for hormonal regulation of this tissue; we have shown that progestogenic oral contraceptive treatment, which lowers ovarian cancer risk in women, increases ovarian surface epithelial apoptosis and upregulates transforming growth factor beta 2, which serves as an inhibitor of epithelial proliferation (Rodriguez et al. 2002, 1998). More recently, Wright et al. (2008) have developed a model in rhesus monkeys for inducing injury and repair of the ovarian surface epithelium. Thus, while the frequency of ovarian epithelial neoplasms in macaques is low, insights may be gained into regulation of this tissue.
Endometriosis
The ovary is the most common site of extrauterine endometriosis in macaques. In one retrospective study of rhesus monkeys, more than 80% of the animals with endometriosis had ovarian involvement (Fanton, Hubbard, and Wood 1986). Typical features of endometriosis are described below in the section “Uterus.”
Ovarian Smooth Muscle Metaplasia
We have rarely observed smooth muscle metaplasia in the ovarian cortex of aged cynomolgus monkeys (Figure 2F). In the human ovary, smooth muscle metaplasia is thought to originate mainly from either metaplastic endometrial stromal cells in endometriotic foci or from metaplastic ovarian stromal cells closely related to endometriotic lesions (Fukunaga 2000). There is evidence that endometrial decidualized stromal cells can express and upregulate smooth muscle actin during the process of decidualization and throughout pregnancy (Miehe et al. 2005). Thus, metaplasia could possibly be a type of ovarian endometriosis.
Ovarian “Deciduosis”
A rare observation in the cynomolgus monkey is the finding of ectopic decidual tissue within the ovarian cortex. This lesion, also referred to as “deciduosis” or “extrauterine decidual change,” is described to be a quasiphysiologic process that can arise in the subcoelomic mesenchyme as the result of the progesterone stimulus of pregnancy or after exogenous progesterone administration in humans (Nascimento, Hornstein, and Crum 2006). The fact that this observation is rare in cynomol-gus monkeys, while it is common in women, lies in the fact that the lesion in general is observed during or after pregnancy only. Pregnant cynomolgus monkeys are seldom used in toxicity studies. Ovarian ectopic decidual change can take the form of single decidualized cells, nodules, or a confluent sheet of decid-ualized cells occasionally with admixed smooth muscle cells, sometimes forming a polypoid projection from the ovarian surface (see Figures 2G–2I)
Ovarian Neoplasms
Sex Cord-Stromal Tumors/Granulosa Cell Tumors
The most common neoplasm of the ovary in macaques is the granulosa cell tumor (Beniashvilli 1989). These neoplasms have not been hormonally characterized in all reported cases but are considered to be estrogen producing, as in other species (Lauszus et al. 2001; Gocze et al. 1997). These neoplasms are most often unilateral, well demarcated, and tan and may be multilobulated (Figure 3A). Histologically they consist of sheets of small polygonal cells arranged into indistinct clusters by a dense collagenous matrix, with varying formation of cords and follicle-like struc-tures (Figure 3B). Endometrial hyperplasia, endometrial polyps, and/or vaginal keratinization may accompany these neoplasms as a result of elevated serum estradiol (Figure 3C).
FIGURE 3.
Ovarian neoplasia. (A–C) Granulosa cell tumor from a twenty-six-year-old cynomolgus monkey with an estrogen-producing ovarian granulosa cell tumor, with the functional consequence of uterine enlargement by endometrial hyperplasia and myometrial hypertrophy. (A) Gross specimen. The uterus weighed more than 70 grams, compared to <2 grams for an ovariectomized animal or 7 grams for a cycling animal. (B) Typical histologic appearance of ovarian granulosa cell tumors. H&E. (C) Simple endometrial hyperplasia induced by estrogens of tumoral origin. H&E. (D–F) Benign ovarian teratoma in an 8.5-year-old cynomolgus monkey. (D) Subgross histologic appearance of this cystic neoplasm. H&E. (E, F) Multiple tissue types including hair, bone, fat, and glandular epithelium. H&E.
Teratomas
Mature teratomas are by far the most common germ cell tumors in women. In nonhuman primates, ovarian teratomas have been reported in rhesus monkeys (Chalifoux 1993), the cynomolgus monkey (Kaspareit et al. 2007; Toyosawa et al. 2000), and other species. For cynomolgus monkeys, the incidence of this neoplasm is estimated at <0.1% (Toyosawa et al. 2000). In our collection, we have encountered unilateral mature benign teratomas in the ovaries of sexually mature cynomolgus monkeys as young as four years of age. In these cases, the affected ovary was enlarged by a cystic mass filled with caseous material (Figures 3D–3F). Microscopically, tumors are situated within the ovary, surrounded by normal ovarian tissue (follicles, corpora lutea, and stroma). In one case, a young corpus luteum was noted next to the tumor, suggesting normal ovarian cyclicity of the tumor-bearing ovary in this animal. Histologically these tumors consist of one or more cysts lined by epithelium, most often keratinized. In the wall of the cyst, skin adnexa, such as sebaceous glands, hair shafts, sweat glands, and a variety of other mature tissue elements, are present. Structures from other germ cell layers have been identified, such as adipose tissue, smooth and skeletal muscle, cartilage, bone, and epithelium with goblet cells.
Ovarian Surface Epithelial Tumors
In women, ovarian surface epithelial neoplasms are the most common tumor type (70%) and are almost always malignant (Seidman, Russell, and Kurman 2002). In contrast, epithelial ovarian tumors may be less common than nonepithelial neoplasms in macaques; a recent review of the literature identified ten reported surface epithelial tumors in macaques, of which six were benign, an additional six carcinomas not otherwise specified, and in contrast, twelve granulosa cell tumors and fifteen germ cell tumors (Moore et al.2003). This finding that tumors of the surface epithelium do not predominate is consistent with our experience.
UTERUS
Non-neoplastic Lesions
Disorders of Cycle Regulation
Irregular Uterine Bleeding
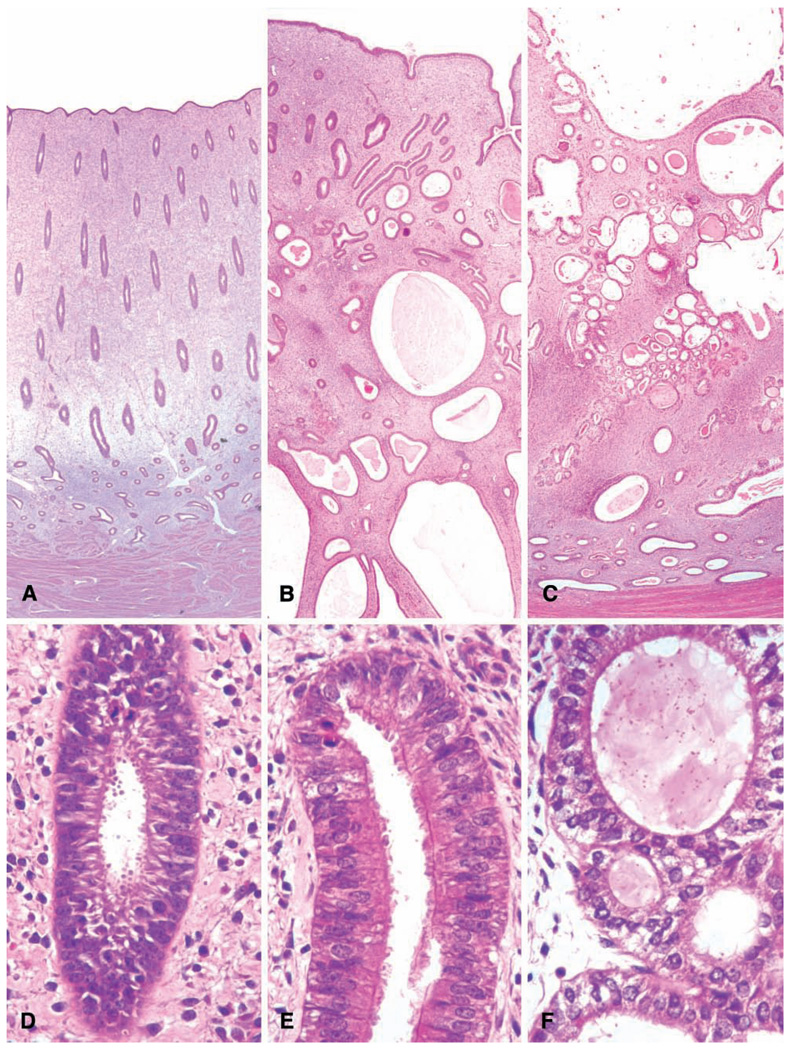
Varying degrees of irregular endometrial bleeding are a common occurrence in macaques, as in women. Recent large observational studies in women have indicated that regular monthly menstrual cycles throughout life are the exception rather than the rule (Gorrindo et al. 2007); thus, observers should not be surprised to see high interindividual variation in menstrual patterns in macaques. In particular, irregular patterns of endometrial bleeding are common in the adolescent macaques typically used in toxicology studies (Resko et al. 1982 and our unpublished observations). These animals are typically at or around menarche and display long, irregular anovulatory cycles; in this regard, they are similar to adolescent human beings (Strickland and Wall 2003). In macaques, this type of uterine bleeding is usually observed only histologically and is characterized by the presence of hemorrhage in the endometrium or uterine lumen, with the endometrium displaying varying degrees of a proliferative phase pattern (Figures 4A–4C). This is similar to the glandular and stromal breakdown with hemorrhage that is commonly observed during anovulatory cycles around menarche in humans (Strickland and Wall 2003). It may be distinguished from normal menstruation by the lack of a secretory phase endometrium, the presence of mitoses in the endometrial glands and stroma, and absence of full endometrial shedding. In addition, there is a lack of a regressing corpus luteum, and often the ovary will display one or more small to medium-sized variably atretic tertiary follicles, as is common in early pubertal or perimenarchal macaques (Van Wagenen and Simpson 1973; Vidal, unpublished observation).
FIGURE 4.
Three syndromes of ovary-dependent dysregulation of the endometrium. (A–C) Abnormal endometrial bleeding in a pubertal macaque; note the absence of a corpus luteum (A) and hemorrhage in the functionalis without prior luteal differentiation (B). In panel (C), the absence of subnuclear vacuoles or stromal decidualization indicate that the bleeding is out of cycle. (D–F) Suppression of ovarian function in a 21.5-year-old cynomolgus monkey, with quiescent ovarian and endometrial morphology. (G–I) Polycystic ovarian syndrome in a thirteen-year-old cynomolgus monkey, characterized by polycystic ovaries and endometrial hyperplasia. This pattern should not be confused with (A).
Anovulatory Cycles
A great challenge in the interpretation of the primate endometrium is the common occurrence of menstrual cycle irregularity or suppression. Anovulatory cycles occur in both young macaques as well as in mature cycling adults. The cycles following menarche are often irregular and prolonged and lack a distinct luteal phase, suggesting a lack of ovulation. In one study, only 15% of rhesus macaques ovulated in the first five cycles following menarche (Resko et al. 1982). In the wild, macaques are highly social animals living in troops, in which social stressors normally lead to reproductive suppression of sub ordinate females. In a controlled laboratory setting of small social groups, Adams, Kaplan, and Koritnik (1985) demonstrated that this interactive social stress caused a much higher proportion of anovulatory cycles in subordinate animals, such that only 54% of subordinates had normal ovulatory cycles relative to 88% of dominant animals. Accordingly, when evaluating the uteri of any cohort of macaques, some proportion of the endometria will lack distinct features of any particular stage of the menstrual cycle, or they may incompletely express a follicular-phase or luteal-phase morphology (Figures 4D–4F). This is especially common in non clinical toxicology safety testing, as the animals are often young and may have only recently reached menarche. A reasonable approach to characterizing such tissues is to describe them as “inactive” with further comment regarding any features of the menstrual cycle that may be present. Although this is a common developmental feature, it may still be considered noteworthy. Because suppression of ovarian function is stress induced through the hypothalamo-pituitary-gonadal axis, a higher proportion of inactive uteri within a given treatment group might indicate treatment-associated stress or illness.
Polycystic Ovary Syndrome
In contrast to solitary cysts, multiple cystic follicles present bilaterally may represent polycystic ovary syndrome (PCOS). This syndrome of anovulation, hyper androgenism, and infertility is common in women with a prevalence estimated to be as high as 10% of reproductive-age women (Bulun and Adashi 2002). The syndrome can be reproduced in macaques by exposure in utero to excess androgen (Zhou et al. 2005), and we have recently reported a spontaneous case of PCOS in a cynomolgus macaque (Figures 4G–4I) (Arifin et al. 2008). Features of the syndrome include loss of menstrual cyclicity, hyperandrogenemia, and increased abdominal fat deposition. Because observational and hormonal measures are needed to identify the clinical syndrome, it may be an under recognized naturally occurring disorder in macaques.
Endometriosis
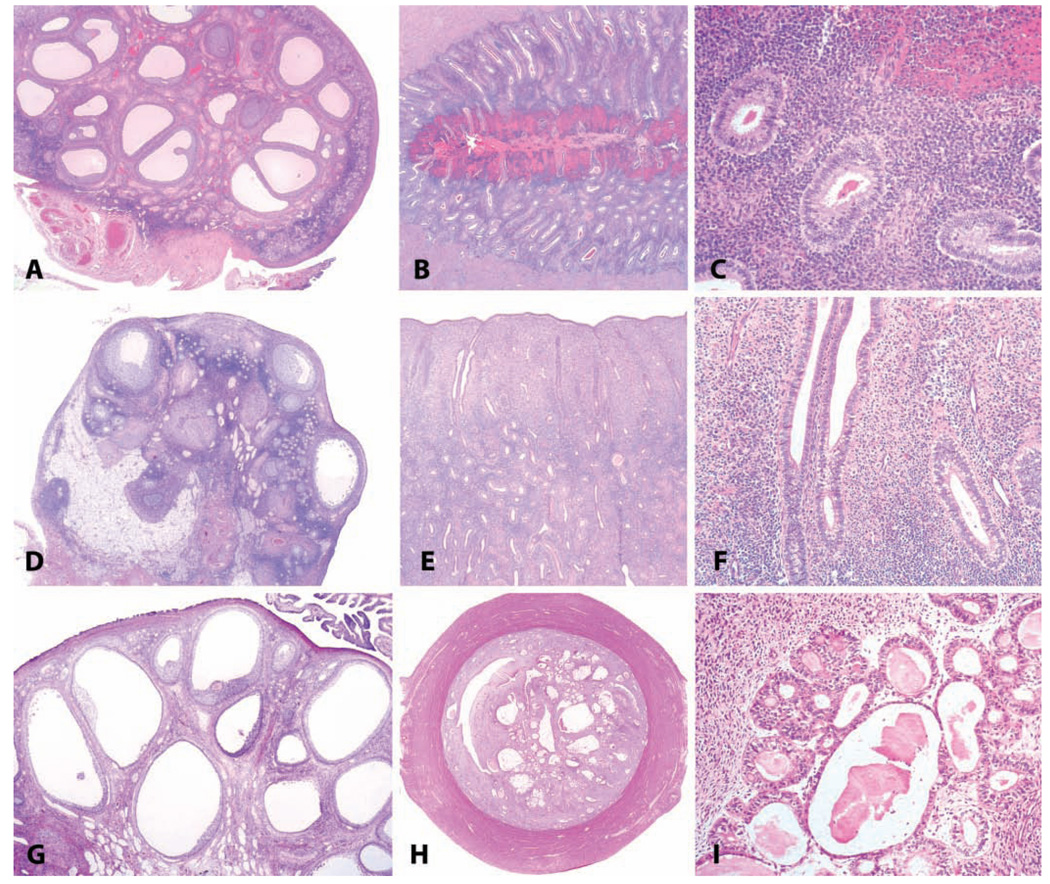
Endometriosis is one of the most common disorders of macaques, occurring with an incidence as high as 30% in sexually mature females in some colonies of rhesus (Zondervan et al. 2004) and cynomolgus (Ami, Suzaki, and Goto 1993) macaques. In a large cohort of 1,600 animals under long-term observation at the Wisconsin Primate Center, the incidence in animals greater than 10 years of age was 31.4% (Zondervan et al. 2004). The classical gross appearance of endometriosis is of a dark red to brown cystic lesion in the caudal abdomen, filled with turbid brown fluid (Figure 5A). Lesions most commonly affect the uterus, colon, and ovaries (Fanton, Hubbard, and Wood 1986). However, endometriotic lesions can vary widely in size, color, and distribution and may in some cases resemble scar tissue or metastatic neoplasia within the abdomen (Figure 5B). Cytologically, the fluid within an endometriotic cyst consists of degenerate red and white blood cells, cellular debris, and activated macrophages containing cellular debris and hemosiderin. Histologically, endometriosis consists of glandular epithelium resembling that of the endometrium, surrounded by dense endometrial stroma, and evidence of current or past hemorrhage (Figures 5C, 5D, 5E). Although endometriotic signs and lesions are known to wax and wane with the menstrual cycle, estrogen receptor expression in endometriotic lesions changes less during the menstrual cycle than that of the endometrium proper, indicating some degree of dysregulation or autonomy (Sternfeld, West, and Brenner 1988).
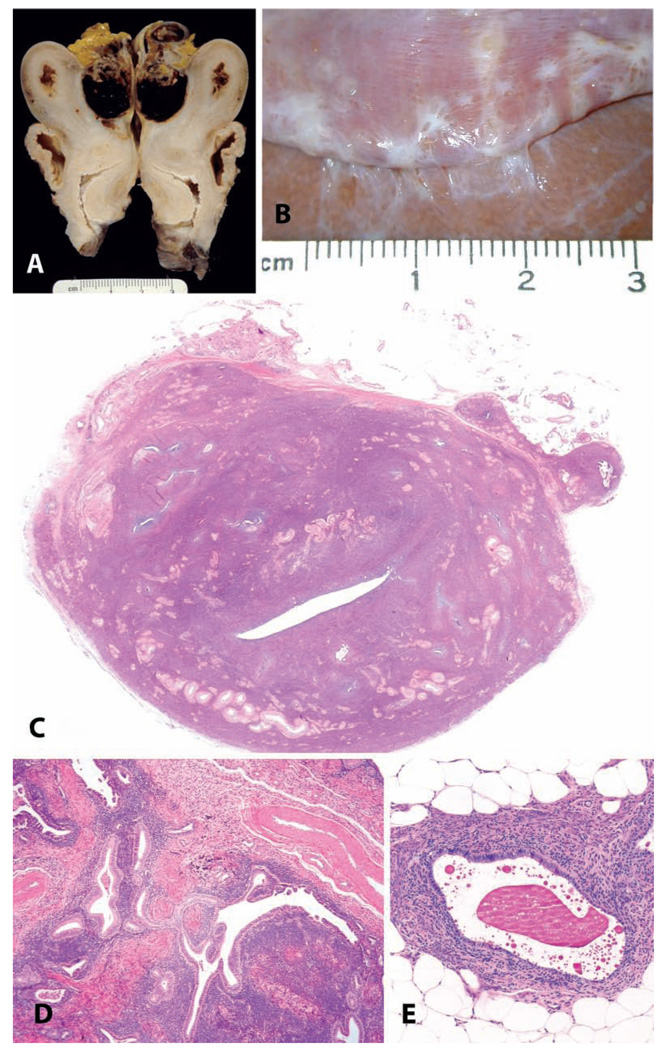
FIGURE 5.
Endometriosis and adenomyosis. (A and B) Variation in the gross appearance of endometriosis in macaques. (A) Typical blood-filled cyst caudal to the uterus in an adult female cynomolgus monkey. (B) Plaque-like pale lesions causing adhesions between the liver and diaphragm in a nineteen-year-old cynomolgus monkey. (C) Transverse section of the uterus of a twenty-four-year-old cynomolgus monkey with both adenomyosis and endometriosis, causing asymmetry of the uterus. H&E. (D and E) Typical histologic appearance of endometriosis, including endometrial glands, stroma, and hemorrhage or hemosiderin. H&E.
Statistically significant risk factors for endometriosis in a case-control study in rhesus macaques included hysterotomy (ten-fold risk) and long-term estrogen treatment (six-fold risk) (Hadfield et al. 1997). Whole-body irradiation has also been associated with a higher incidence of endometriosis (Fanton and Golden 1991).
In the late 1990s, an intriguing observation was made of a possible link between dioxin exposure and endometriosis (Rier 2002; Rier, Coe, et al. 2001; Rier et al. 1993, Rier, Turner, et al. 2001); also, treatment with dioxin facilitates the successful growth of endometrial explants (Yang, Agarwal, and Foster 2000). However, the causative link in this regard has recently been called into question (Guo 2004). More recently, other intriguing possibilities have emerged, including a strong association with developmental lead exposure (Krugner-Higby et al. 2003) and altered intestinal microflora in animals with endometriosis (Bailey and Coe 2002). Endometriosis in the macaque may have a similar biochemical basis to the disease in man, where it has been postulated that a polymorphism in the expression of N-acetyltransferase may influence risk (Fakis et al. 2007).
Adenomyosis
Adenomyosis in the uterus is defined as the abnormal presence of endometrial tissue within the myometrium, in a location clearly distinct from the endometrium proper. Small clusters of adenomyosis consisting of a few isolated endometrial glands surrounded by scant endometrial stroma are common incidental findings in macaques; more extensive lesions that displace and deform the myometrium are seen less commonly but do occur spontaneously in untreated animals (Figure 5C). The incidence of adenomyosis is difficult to assess because it often occurs concurrently with endometriosis. It is also our experience that an increased incidence of adenomyosis can be found in chronic studies with compounds displaying significant stimulating effects on the myometrium, such as estrogens (Baskin, Smith, and Marx 2002). Because of the hypertrophy of the myometrium, weak areas (especially those where fibrous tissue and vessels are present) can develop within the muscular layer, and in such areas, endometrial tissue can become entrapped within the myometrium.
Pregnancy-associated Vascular Remodeling
The extraordinary growth of the fetus, placenta, and uterus during the 150-day gestation period of macaques requires an accompanying increase in blood flow and enlargement of the uterine blood vessels. Placental trophoblasts invade along the endometrial and myometrial blood vessels in late pregnancy, causing extensive remodeling of the vessel wall and perivascular connective tissue (Blankenship, Enders, and King 1993). Thus pregnancy leaves a long-lasting imprint on the vasculature of the uterus, consisting of an expanded zone of loose connective tissue around myometrial blood vessels, particularly, the veins (Figure 6). We have found that this change may persist for more than six years after the last pregnancy and is a more persistent reliable indicator of true pregnancy status in cynomolgus macaques than rhesus macaques (Cline and Bain 1995). In addition to the myometrial connective tissue changes, inter mediate trophoblasts can persist within the endometrial spiral arteries and can be observed for many months following pregnancy (personal observation, Vidal). Similar changes have been described in the uteri of women (Manning 1974).
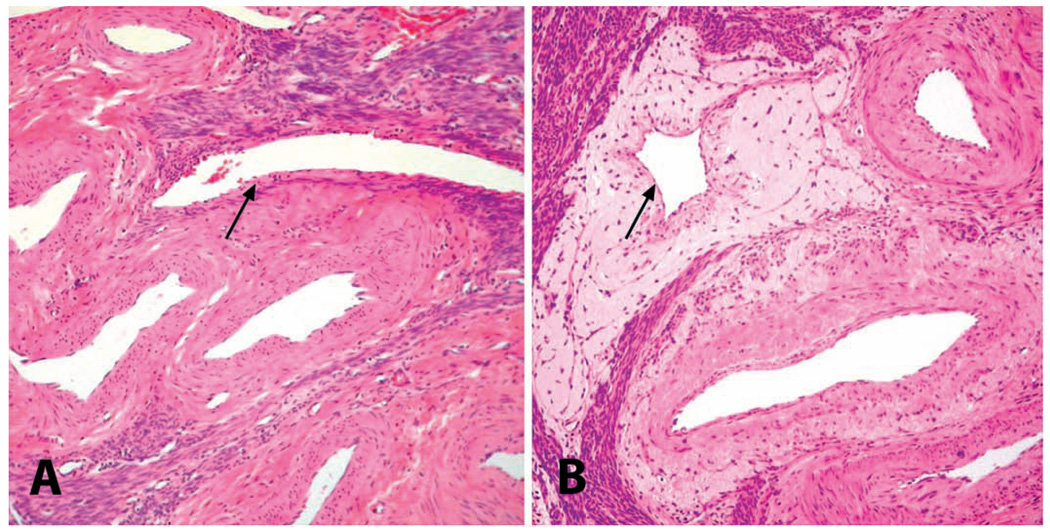
FIGURE 6.
Uteri of adult cynomolgus monkeys. Myometrial vasculature of (A) a nulliparous animal and (B) a multiparous animal, demonstrating the perivenous accumulation of loose extracellular matrix indicative of prior pregnancy. (A) Myometrial vasculature of a nulliparous animal; note the nearly invisible wall of myometrial vein (arrow). (B) Similar area from a multiparous animal, showing perivascular accumulation of pale eosinophilic extracellular matrix surrounding a myometrial vein (arrow). H&E.
The Epithelial Plaque
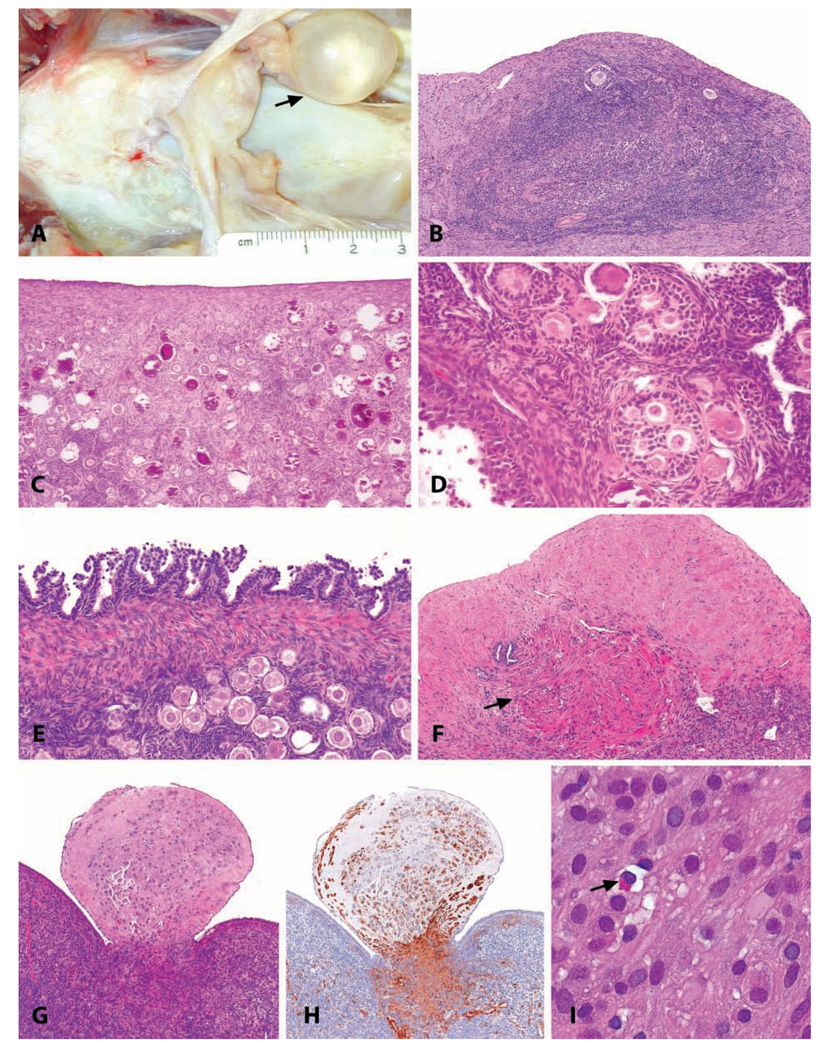
Macaques have a uniquely florid epithelial proliferative response of the endometrial surface in early implantation, not occurring in human beings or other laboratory animal species. This change consists of distinctive plaque-like structures on facing walls of the endometrium, which efface the normal simple columnar surface epithelium and extend into the superficial endometrial stroma. When related to pregnancy, this localized epithelial proliferation is only temporary, and the structure regresses after a few weeks, mainly by apoptosis. This proliferative change, known as the epithelial plaque, is of most interest when it occurs occasionally during the luteal phase in the absence of an embryo (Kaspareit et al. 2004). The epithelial plaque response can be induced experimentally by combined estrogen and progestogen treatment accompanied by trauma to the endometrium and has been used as a model of early implantation (Ghosh, Bell, and Sengupta 2004; Ghosh and Sengupta 1989). The epithelial cells composing the plaques are generally large, pleomorphic, and polygonal, with anaplastic nuclear features resembling a carcinoma. Features distinguishing this change from endometrial carcinoma are often its simultaneous but noncontiguous presence on opposing walls of the endometrium and the distinctive surface orientation of the epithelial proliferative change without invasion into the deeper endometrium (Figure 7).
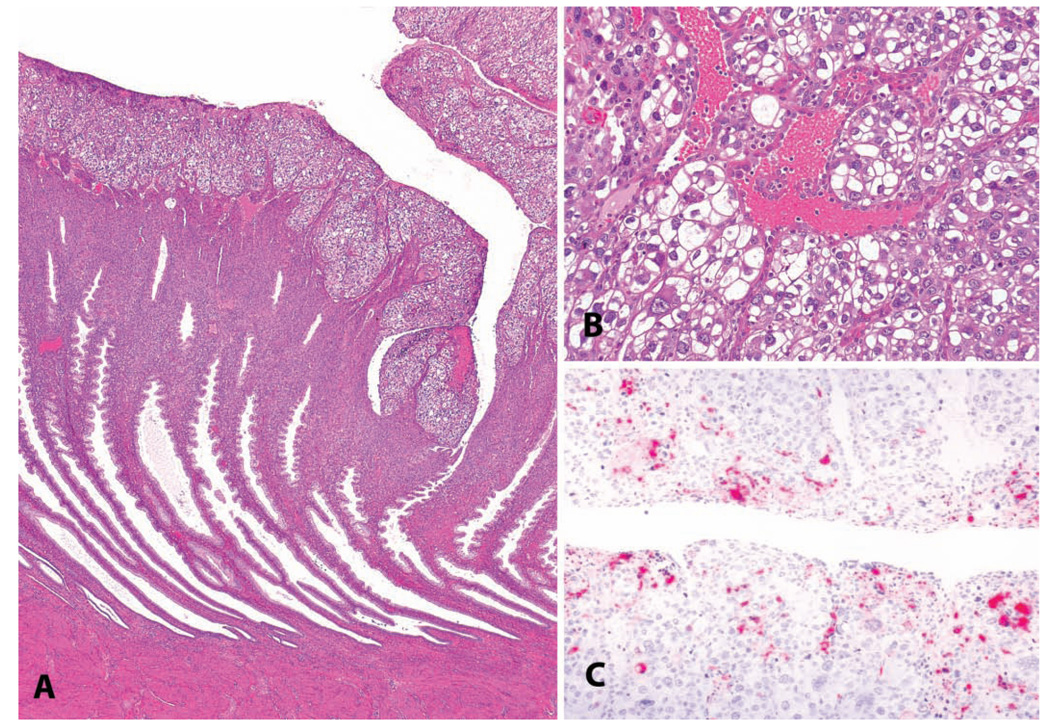
FIGURE 7.
An epithelial plaque in a 3.5-year-old cynomolgus monkey. (A) Low-magnification photomicrograph demonstrating the surface orientation and concurrent endometrial glandular features of the luteal phase. (B) Higher magnification. H&E. (C) Immunohistochemistry for cleaved caspase 3, indicating apoptosis, in a regressing plaque. Vector red chromogen; hematoxylin counterstain.
Hyperplastic and Neoplastic Changes of the Uterus
Leiomyomas
Uterine leiomyomas or “fibroids” are common, benign neoplasms in adult and aging macaques (Kaspareit et al. 2007; McClure 1973; Seibold and Wolf 1973). They may be solitary or multiple (Figures 8A–8C) and consist of a well demarcated, expansile mass of smooth muscle cells with varying amounts of dense collagenous matrix. A malignant variant (leiomyosarcoma) has been reported (Cook, Rogers, and Sowers 2004), but in general, these neoplasms are benign. Like the normal myometrium, these neoplasms express estrogen and progesterone receptors and are hormone responsive. They may result in abnormal uterine bleeding or interfere with fertility, as in women.
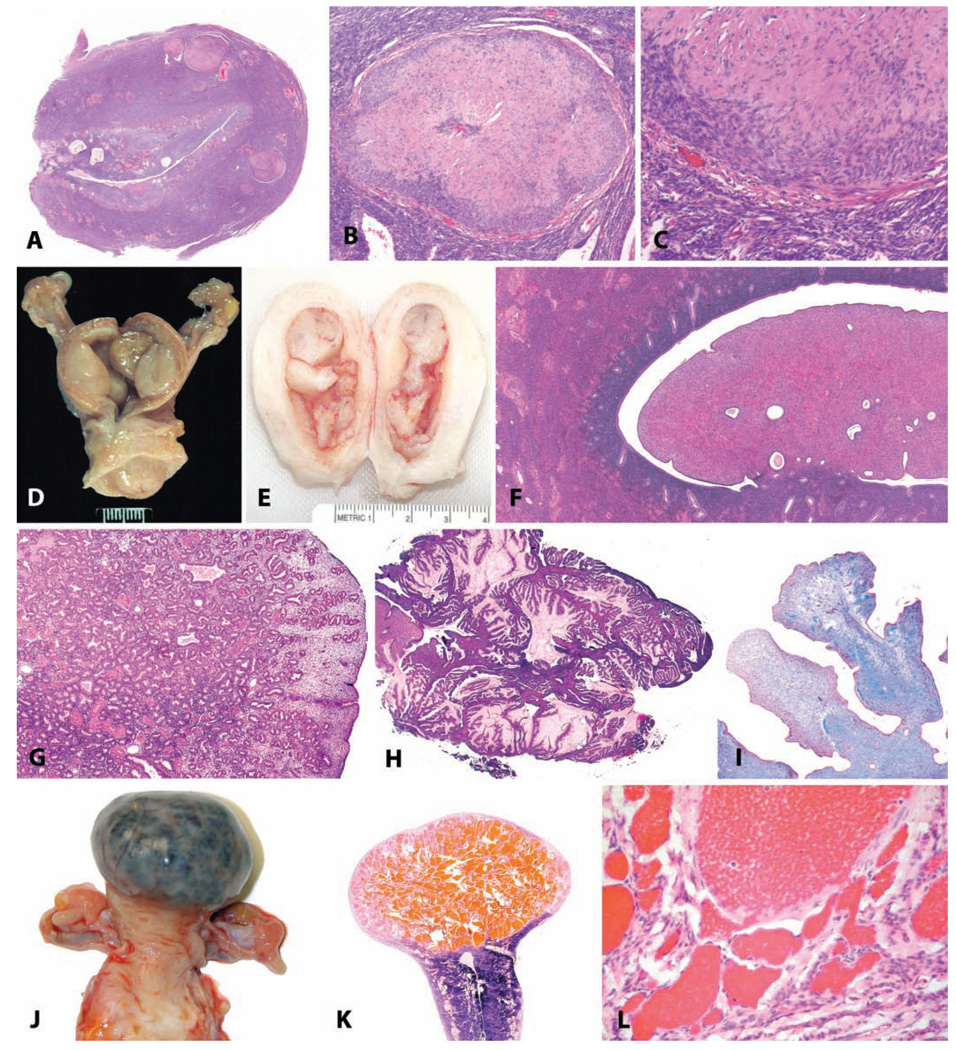
FIGURE 8.
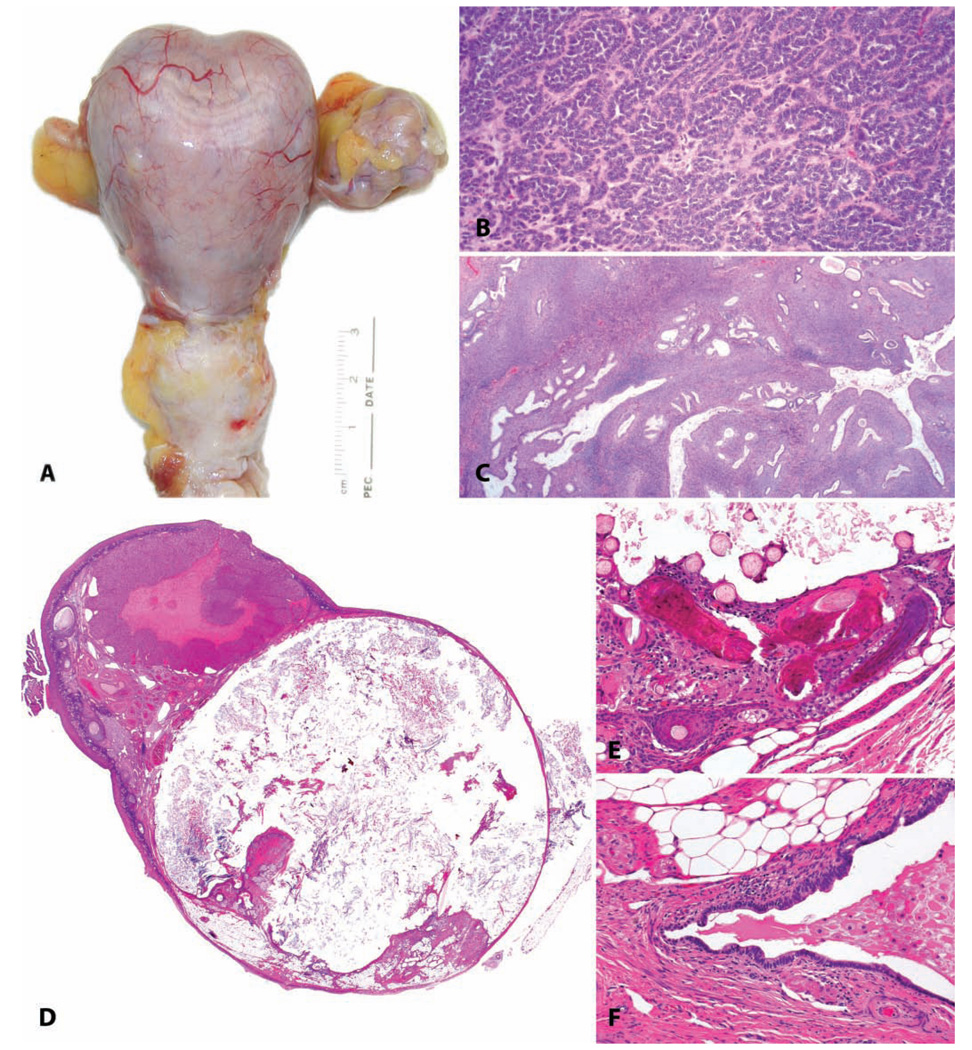
Benign uterine neoplasms. (A) Longitudinal section through the uterus of a thirty-year-old rhesus macaque; within the myometrium there are four leiomyomas. H&E. (B and C) Higher magnification demonstrating smooth muscle morphology of the neoplastic cells. H&E. (D and E) Gross photographs of endometrial polyps in aged rhesus macaques; the polyps in (D) are sessile, and those in (E) are pedunculated. (F) Endometrial polyp consisting primarily of stromal tissue in a fourteen-year-old cynomolgus monkey. H&E. (G) Endometrial polyp in an adult rhesus monkey consisting primarily of glandular tissue. H&E. (H) Endometrial polyp in an adult rhesus monkey, with mucous differentiation. H&E. (I) Fibrosis within a polyp in an adult cynomolgus monkey; Masson’s trichrome stain. (J) Gross photograph of a myometrial cavernous hemangioma in a twenty-two-year-old cynomolgus monkey. (K) Subgross appearance. (L) Higher magnification. H&E.
Endometrial Polyps
Endometrial polyps occur in approximately one out of four women over the age of forty (Sherman, Mazur, and Kurman 2002), and they also occur spontaneously in female macaques. The prevalence of polyps is increased in animals treated with estrogens or tamoxifen. Spontaneous endometrial polyps have been observed in both rhesus and cynomolgus macaques at the California National Primate Research Center and often present clinically with irregular and occasionally persistent bleeding (unpublished observations, Vidal and Tarara). In our experience, endometrial polyps in cynomolgus macaques occur at a relatively young age (approximately nine to ten years old, with a range of six to thirteen years), and a similar finding has been reported by other investigators (Kaspareit et al. 2007). This is in contrast to our observations in the rhesus in which polyps have been observed in older, often postmenopausal animals (approximately twenty-five years with a range of eleven to thirty-two years). Macroscopically, endometrial polyps often distend the uterine lumen and may be sessile or pedunculated with one or more polyps occurring in an individual uterus (Figures 8D–8I). These polyps have abundant stroma consisting of a wellvascularized to fibrotic core resembling normal endometrial stroma with varying numbers of glands scattered throughout and generally a single layer of columnar to cuboidal epithelium. Hyperplastic, metaplastic, or malignant changes may occur rarely within a polyp. In some cases in rhesus macaques, ovar-ian findings (granulosa cell tumor, variably luteinized follicular cyst) may have been a source of estrogenic stimulus for development of the polyp.
Uterine Hemangiomas
We have identified three myometrial hemangiomas in aged cynomolgus macaques. These benign neoplasms were present within the fundic myometrium and varied from 0.5 to 3 cm in diameter. Grossly, the lesions were dark red to purple and, in the case of the largest tumor, deformed the contour of the uterus. Histologically, the neoplasms were expansile with clearly demarcated margins and were composed of variably sized blood-filled cavities lined by well-differentiated endothelium (Figures 8J–8L). Uterine hemangiomas are uncommon in human beings (Zaloudek and Hendrickson 2002) and are rarely reported in other species.
Endometrial Hyperplasia
Hyperplasia of the endometrium is induced in cynomolgus and rhesus macaques by treatment with estrogens (Baskin, Smith, and Marx 2002; Cline, Register, and Clarkson 2002A; Cline et al. 2001), as is the case in women (Steiner et al. 2007). Endometrial hyperplasia in macaques also occurs in association with granulosa cell tumors (Figure 3C) and polycystic ovarian syndrome (Arifin et al. 2008) (Figures 4H and 4I).
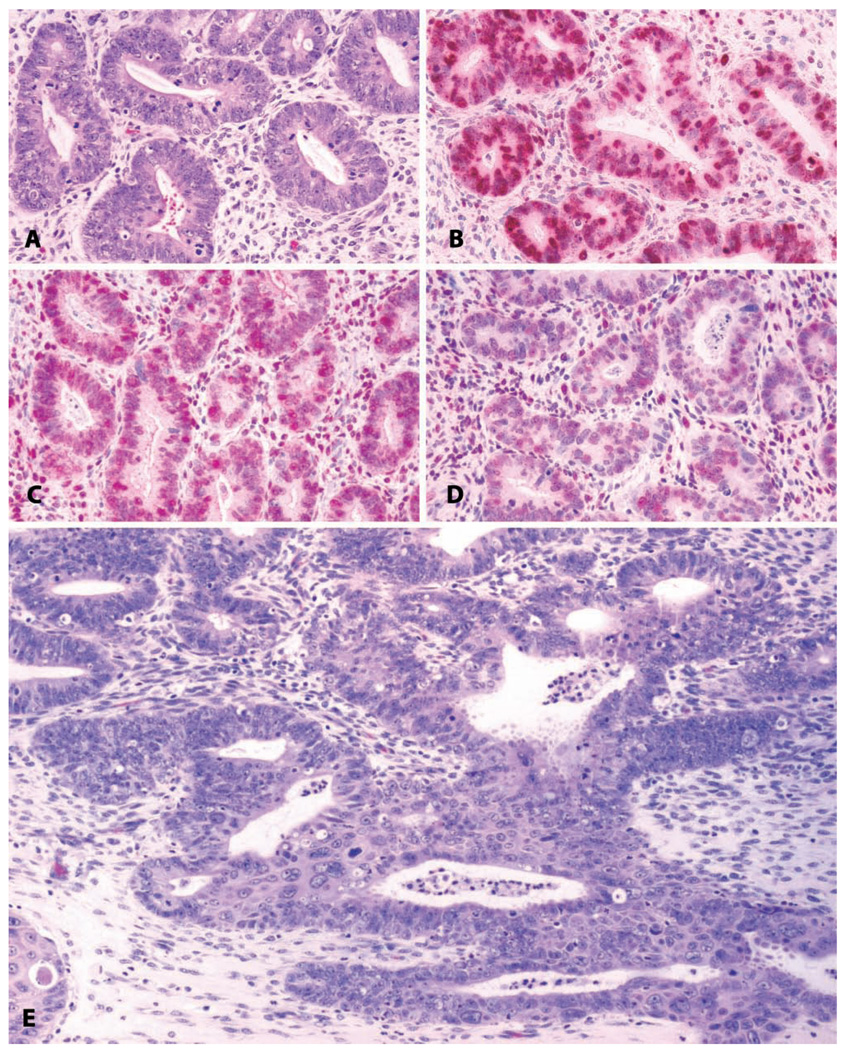
In the evaluation of the abnormally thickened human endometrium, the histologic appearance has historically been classified as simple hyperplasia (Figures 9B and 9E) or complex hyperplasia (Figures 9C, 9F, and Figures 10A–10D) (Ronnett and Kurman 2002). Both simple and complex hyperplasia have an increased gland-to-stroma ratio, disordered architecture, glandular epithelial pseudostratification, and increased numbers of glandular epithelial mitoses. Either may also be associated with epithelial atypia, consisting of loss of epithelial cell polarity, increased nucleus-to-cytoplasm ratio, nuclear clearing with clumping of chromatin at the membrane, and a round to irregular nuclear contour. Complex hyperplasia with atypia has the strongest association with endometrial cancer risk in women (Kurman, Kaminski, and Norris 1985). A more recent system of evaluating hyperplastic lesions in the endometrium replaces the category of complex hyperplasia with atypia with endometrial intraepithelial neoplasia (EIN) (Mutter et al. 2007).
FIGURE 9.
Uterus: normal and pathologic proliferative changes. (A and D) Normal follicular phase endometrium with edema of the functionalis and orderly glandular proliferation. (B and E) Simple endometrial hyperplasia with disorganized glandular structures, cystic change, and ciliary metaplasia of the glandular epithelium. (C and F) Complex endometrial hyperplasia, with “back-to-back” crowding of endometrial glands.
FIGURE 10.
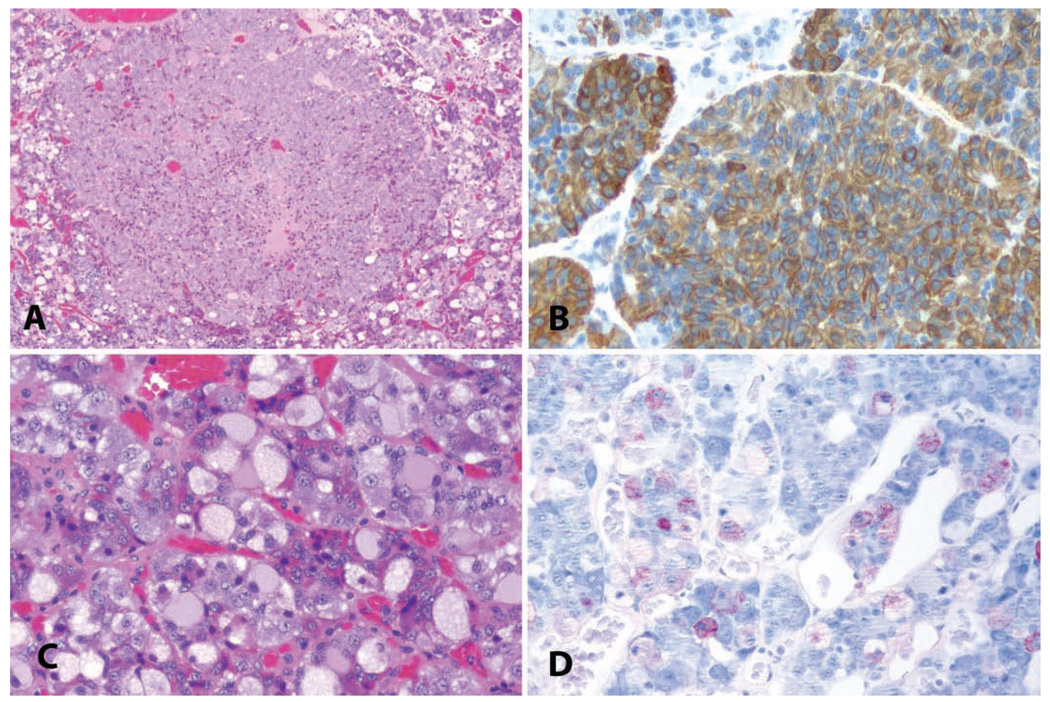
Uterus: Hyperplasia with progression to invasive endometrial carcinoma. (A) Endometrial glandular hyperplasia in a thirty-two-year-old rhesus macaque chronically treated with estradiol by subcutaneous implant. “Complex hyperplasia” or “endometrial intraepithelial neoplasia.” H&E. (B) Corresponding area stained for proliferating cells; anti-Ki-67 antibody, Vector red chromogen, and hematoxylin counterstain. (C) Corresponding area stained for estrogen receptor alpha; anti-ER antibody, Vector red chromogen, and hematoxylin counterstain. (D) Corresponding area stained for progesterone receptors; anti-PR antibody, Vector red chromogen, and hematoxylin counterstain. (E) Adjacent region of the same slide; highly pleomorphic and invasive endometrial adenocarcinoma. H&E.
In light of the evolving classification of endometrial hyperplasias in human beings, it is incumbent upon the toxicologic pathologist to carefully describe and correctly interpret endometrial changes in laboratory animals. When extrapolating findings in macaques in a regulatory setting to likely outcomes in human beings, there is a tendency for regulators to regard any use of the term hyperplasia as representing complex hyperplasia with atypia, when this may not be the case. There are also differences in the traditional lexicon of MD pathologists and veterinary pathologists, for example, in the case of cystic atrophy and cystic endometrial hyperplasia, respectively, for a lesion in which the endometrium is thickened by cystic dilatation of the glands without marked glandular proliferation. When evaluating proliferative changes, we recommend the use of normal endometrium, follicular phase for endometrial proliferation with features of the normal follicular phase, such as parallel straight or tortuous glands with zonal edema of the functionalis (Figure 9A); simple hyperplasia for disorganized endometrial proliferation without glandular crowding (Figure 9B); and complex hyperplasia for endometrial proliferation with glandular crowding (Figure 9C). Atypia should be indicated when present. We recommend the use of cystic change (evident in Figures 9B and 9C) to avoid the implication of risk that might be associated with the term cystic hyperplasia.
Endometrial Adenocarcinoma
Despite the readiness with which endometrial hyperplasia may be induced in monkeys (Baskin, Smith, and Marx 2002; Cline, Register, and Clarkson 2002A; Cline et al. 2001), and the clear association between endometrial hyperplasia and endometrial cancer risk in human beings (Kurman, Kaminski, and Norris 1985), endometrial adenocarcinomas appear to be exceedingly rare as a spontaneous lesion in monkeys. A single case report has been made in the literature of endometrial adenocarcinoma in a rhesus monkey, in association with pyometra and endometriosis (Strozier et al. 1972). This apparent low incidence may be indicative of a true low incidence or may be an artifact of the low numbers of animals under observation; in human populations, the rate of endometrial cancer is approximately twenty-five cases per one hundred thousand women per year (Jemal et al. 2007). We are aware of two additional unreported cases in aged rhesus macaques (unpublished observation, Cline; Figure 10E). The case depicted is from a twenty-four-year-old rhesus macaque with a history of prior estrogen treatment.
Trophoblastic Neoplasms
Malignancies of placental origin or with placental differentiation are relatively common in human beings but rare in domestic or laboratory species. Among nonhuman primates, choriocarcinoma and trophoblastic tumors have been reported in the uterus and ovaries (Cooper, Shih, and Gabrielson 2005; Giusti et al. 2005). These neoplasms have characteristically anaplastic morphology, invasive or expansile growth patterns, and varying degrees of necrosis and, in the case of trophoblastic neoplasms, may be wildly pleomorphic. Three tumor types form the majority of human cases; choriocarcinomas are distinguished by central hemorrhage, distinct populations of uninucleate and syncitial trophoblastic cells, and expansile invasion at the tumor margin. Placental site trophoblastic tumors have more pleomorphic infiltrative cells and more infiltrative growth. Epithelioid trophoblastic tumors consist of small, round, uniform cells with an expansile growth pattern and typically have a “geographic” pattern of necrotic and viable tissue (Shih, Mazur, and Kurman 2002).
Other Incidental Uterine Findings
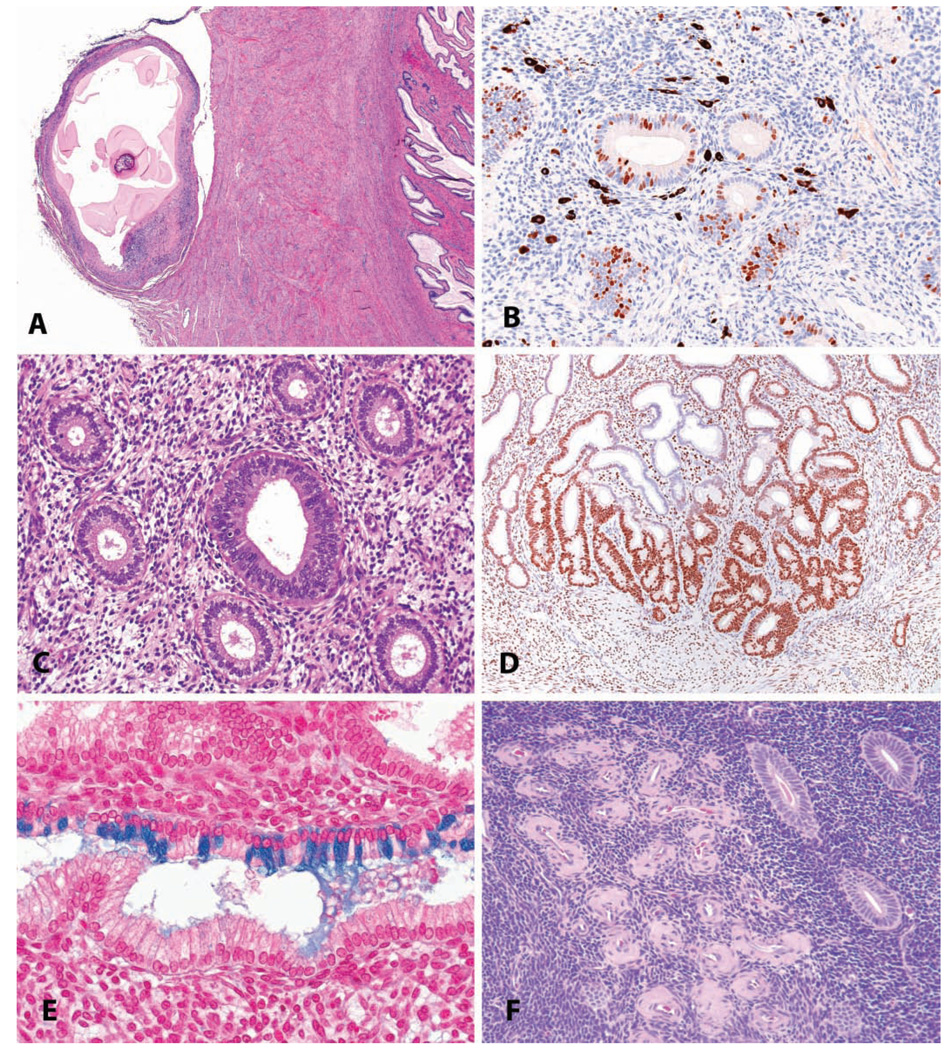
A variety of incidental changes may be seen in the uterus; a few selected examples are shown in Figure 11. Parasitic lesions are uncommon, given the widespread use of ivermectin treatment during quarantine; however, remnants of metazoan parasites, including cestode larvae, filarids, and pentastome larvae, are occasionally encountered (Figure 11A). Melanosis is a common lesion of no significance in the endometrium, as in other sites; its primary importance is as an interpretive challenge, with differential possibilities including hemosiderin or lipofuscin (Figure 11B). As noted above, irregular menstrual cycles are the rule rather than the exception, and in such animals with, for example, insufficient luteal phase progesterone (Figure 11C), there may be disagreement between glandular and stromal morphology. Focal glandular hyperplasias are occasionally seen as incidental findings and thus may or may not be treatment related (Figure 11D). Mucinous metaplasia of endometrial glands may rarely be observed (Figure 11E). The metaplastic cells in mucinous metaplasia have the same characteristics as endocervical cells. The cells in the lesion are tall and columnar and secrete mucins, which differentiate them histochemically from the normal glandular secretory cells. In general, epithelial metaplastic changes in the monkey endometrium are far less common than in women, where a variety of types are reported. Hyaline perivascular deposits within the stroma of the endometrium (Figure 11F) are common in older animals.
FIGURE 11.
Uterus: incidental findings. (A) Parasitic remnant manifest as a fibrous/mineralized cyst on the cervical serosa in a four-year-old cynomolgus monkey. H&E. (B) Endometrial melanosis in a four-year-old cynomolgus monkey, shown in an immunohistochemical stain for the proliferation marker. Immunohistochemistry: diaminobenzidine chromogen, hematoxylin counterstain. (C) “Luteal phase defect” in an adult cynomolgus monkey, with a mixture of follicular-phase features (round glandular lumens, pseudostratification) and luteal phase features (stro-mal proliferation and hypertrophy, glandular subnuclear vacuoles). (D) Focal glandular hyperplasia, basalis, in an adult cynomolgus monkey. Progesterone receptor immunostain, diaminobenzidine chromogen, hematoxylin counterstain. (E). Endometrial glandular mucous metaplasia in an adult cynomolgus monkey. Alcian blue stain. (F) Focal endometrial hyaline perivascular deposits in an aged cynomolgus monkey. H&E.
CERVIX AND VAGINA
Vaginitis and Cervicitis
The vagina of macaques normally contains lymphoid aggregates and follicles, which have been the subject of extensive study because of their relevance to defense against simian immunodeficiency virus transmission (Miller and Lu 2003). Thus, the presence of lymphoid aggregates in the vagina is not an abnormal finding, and the distinction between normal and inflamed vaginal tissue is somewhat subjective (Figures 12A–12D). Furthermore, some animals are conformationally or behaviorally predisposed to contamination of the vagina by feces, hair, or other foreign material. Therefore, gross evaluation of the vaginal lumen for foreign material and gross lesions is an important part of the necropsy examination, as it may inform later histologic observations.
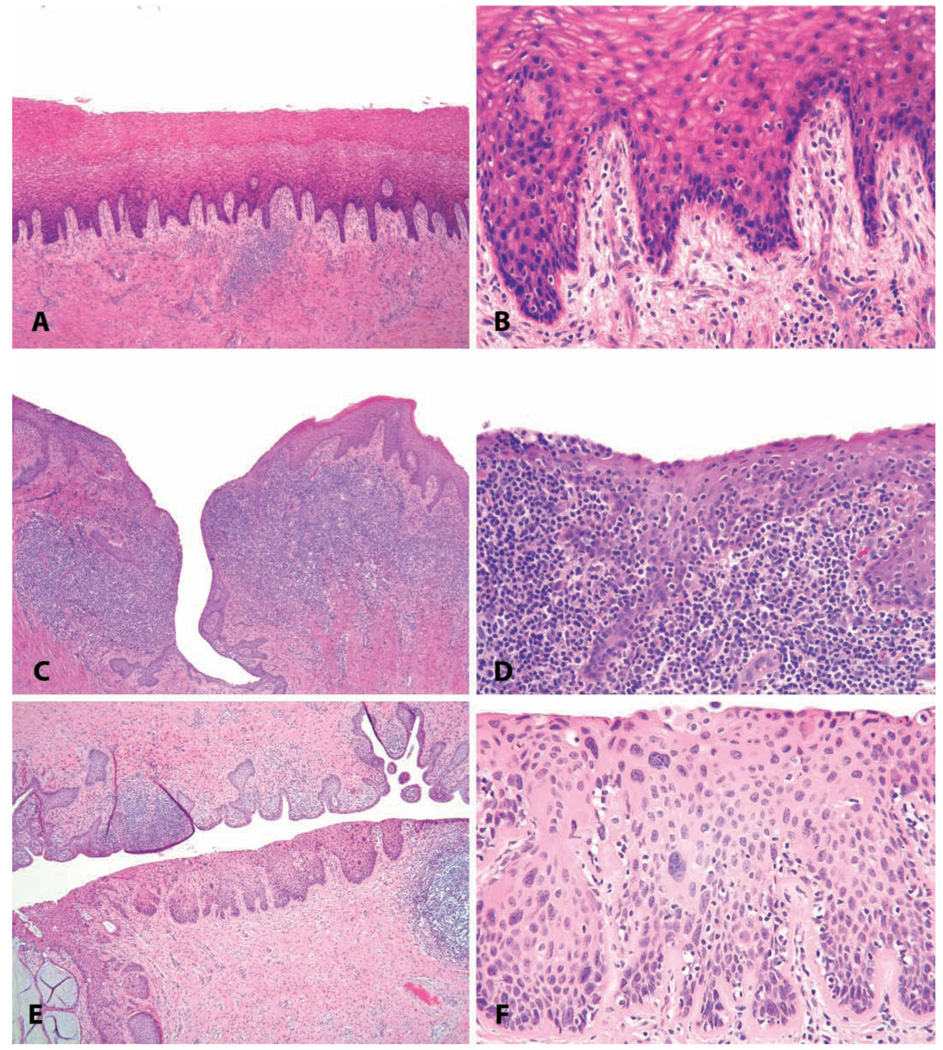
FIGURE 12.
Common vaginal lesions. (A–D) Vaginitis. (A and B) Minimal lymphocytic vaginitis, in a 22.5-year-old intact cycling animal with vaginal keratinization. (C and D) Severe chronic vaginitis with lymphoid follicular hyperplasia in a 7.5-year-old ovariectomized cynomolgus monkey (note atrophy of the surface epithelium and lack of keratinization). (E and F) Papillomavirus-induced in situ neoplasic lesion in a twenty-three-year-old cynomolgus monkey (cervical intraepithelial neoplasia, grade 2). H&E.
Endocervical Polyps
As described elsewhere in this volume, the cervical os of macaques normally has a protruding anterior shelf, as in women, which should not be misinterpreted as a polyp. However, both endocervical and endometrial polyps are commonly found in the endocervical channel and may protrude into the vagina.
Endocervical Squamous Metaplasia
Squamous metaplasia of the endocervical glands is a common incidental finding in peripubertal and estrogen-treated macaques. Because peripubertal animals are in a state of relative estrogen excess normally, some degree of cervical squamous metaplasia in two- to four-year-old animals is considered a normal finding (see the article by Wood in this volume).
Papillomavirus-induced Lesions
Papillomavirus (PV)-associated cervical dysplasia and neoplasia (cervical intraepithelial neoplasia, or CIN) are occasionally encountered in adult female macaques with a history of breeding activity (Figures 12E–12F) (Wood et al. 2004, 2007). The primary importance of these lesions in toxicologic pathology lies in their potential to confound the interpretation of compound-related changes. As in women, virtually all CIN lesions in female macaques are associated with infection with genital PV. Polymerase chain reaction screening has identified a background genital PV prevalence as high as 35% in colony acquired adult female cynomolgus macaques (Wood et al. 2007), a prevalence similar to that for human PV infection in sexually active younger women (Schiffman et al. 2007). The spectrum of lesions induced by PVs in macaques includes benign papillomas; CIN grades 1, 2, and 3; and invasive carcinoma. As in women, higher-grade lesions are most common in the cervical transformation zone. Further details are given in the article by Wood in this volume.
EXTERNAL GENITALIA (VULVA, CLITORIS, AND SEX SKIN)
Sex Skin
Rhesus and cynomolgus macaques have species-specific patterns of perineal sex skin swelling and erythema (Baulu 1976; Duran-Reynals, Bunting, and Wagenen 1950). Among rhesus macaques, there is seasonal variability in the prominence of sex skin swelling, with more regular cyclicity in fall and winter (Ghosh and Sengupta 1992). Among cynomolgus macaques, there is pronounced individual and regional variation in sex skin prominence in subpopulations from the widely dispersed island habitats in Southeast Asia. In many cynomolgus macaques, ovulation is “cryptic,” with little external evidence of reproductive status (Engelhardt et al. 2005). In other individual animals, there is clear correlation of external sex skin swelling and erythema with ovarian features and hormonal profiles. In still other animals, there is persistent perineal swelling that is present continuously without reference to menstrual cycle or hormonal features. Thus measurement of sex skin features is not generally useful in assessing cycle stage, particularly in cynomolgus macaques.
Papillomas
Cutaneous papillomas may be seen in animals infected with macaque PVs (Cooper and Gabrielson 2007). The relevance of these lesions to the more serious CIN lesions is uncertain, but it is likely that the exophytic benign lesions and precancerous lesions are caused by separate virus subtypes.
Clitoromegaly
We have observed clitoral enlargement along with a genealized anabolic effect on muscle mass in female cynomolgus monkeys given the synthetic androgen nandrolone (unpublished observation, Cline).
MAMMARY GLAND
Immaturity
The rapidly occurring, but highly variable, proliferative changes in the macaque mammary gland described elsewhere in this monograph are a source of much confusion in the interpretation of studies using young animals. The growing margin of the glandular tissue in young animals contains terminal end buds (solid epithelial structures approximately 200 microns in diameter, often surrounded by loose connective tissue) and immature ductal and lobuloalveolar units (small branching arrays of tubular structures). The terminal end buds in particular may resemble focal ductal hyperplasias or carcinoma in situ lesions. These normal structures may be distinguished from abnormal proliferative lesions by their location at the margin of the gland and by the lack of surrounding well-differentiated lobular structures (Wood, Hester, and Cline 2007).
Cystic Change
Fibrocystic disease has an autopsy prevalence of more than 50% in clinically normal women (Sarnelli and Squartini 1991) and is of uncertain significance for breast cancer risk (Marchant 2002). Cystic dilatation of mammary ducts and lobules is common in older macaques (Figure 13A) and, in our studies, does not correlate with hormonal treatment (unpublished observation, Cline).
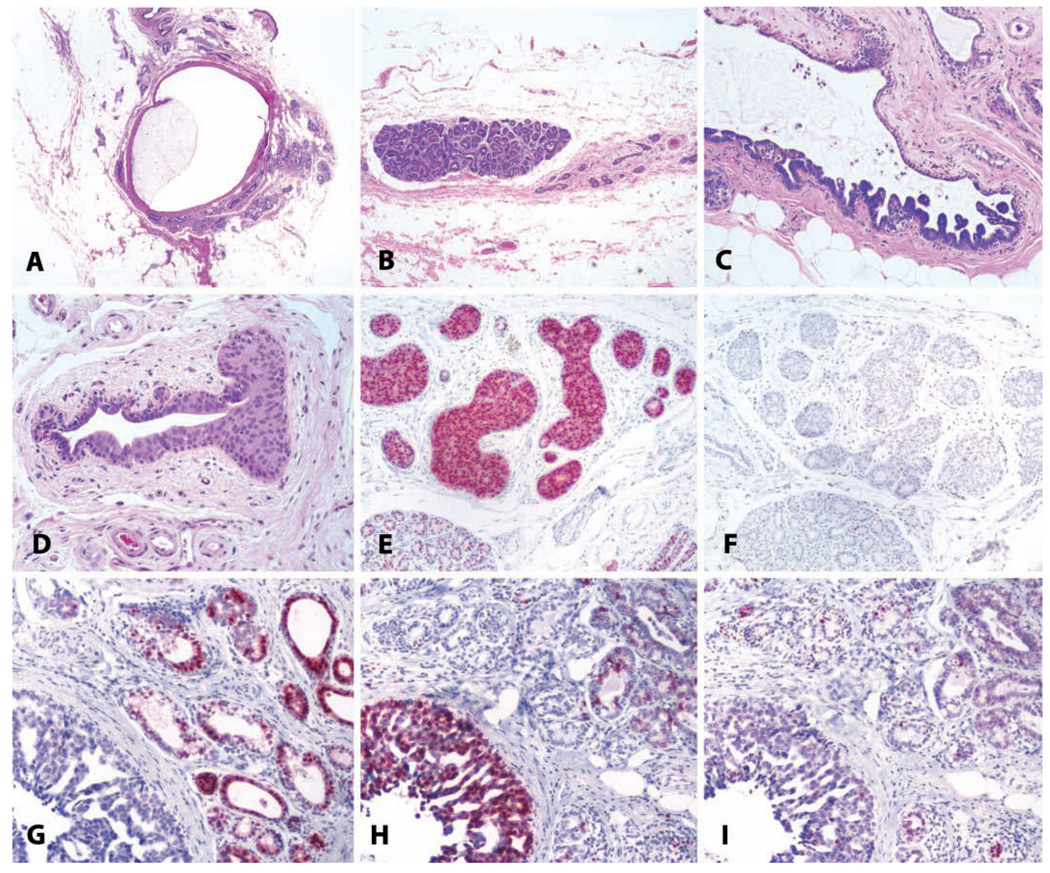
FIGURE 13.
Benign and malignant breast lesions. (A) Cystic change in a sixteen-year-old cynomolgus monkey, with marked dilatation of a large duct. H&E. (B) Focal lobular hyperplasia in the mammary gland of an adult cynomolgus monkey. Note the atrophy of other glandular elements on the right side of the photo. H&E. (C) Papillary ductal hyperplasia in an adult cynomolgus monkey. H&E. (D) Ductal hyperplasia with atypia in an eighteen-year-old cynomolgus monkey. H&E. (E and F) The same lesion as in (D) stained for estrogen receptor and the proliferation marker Ki67, respectively. Note overexpression of estrogen receptor. (G, H, and I) Ductal carcinoma in a five-year-old rhesus macaque, immunostained for (G) estrogen receptor, (H) proliferating cells/Ki67, and (I) progesterone receptor. Vector red chromogen; hematoxylin counterstain.
Focal Lobular Hyperplasia
Focal hyperplasia of mammary lobules occurs commonly in macaques and consists of one or more rounded, enlarged, expansile lobules against a background of atrophic lobules (Cameron and Faulkin 1974; Warner 1979). These enlarged lobules may be numerous in individual animals (Figure 13B) and are also present in nonatrophic glands but are less conspicuous than in atrophic glands. We have also used the term focal lobular proliferation to name this lesion in order to avoid confusion with risk-associated lesions in women that have a different morphology (Cline 2007); however, we believe that the term focal lobular hyperplasia should be used, because it is the most clearly descriptive of the change. These lesions have elevated expression of proliferation markers relative to adjacent tissue but also have relatively normal lobular architecture and cellular phenotypes, including myoepithelial cells, and they continue to express sex steroid receptors. The behavior of these lesions is unknown, but in our experience, they do not progress to neoplasia. Focal lobular hyperplasia should also be distinguished from atypical lobular hyperplasia, which consists of irregularly enlarged acini with at least two layers of luminal epithelial cells and variable cystic dilation by secretory material. This latter lesion may represent a precursor to lobular carcinoma in situ, which includes both architectural and cytologic atypia.
Ductal Hyperplasia
Ductal hyperplasias in cynomolgus monkeys span a diverse morphologic range from columnar cell lesions of the terminal ductal lobular units to focal micropapillary intraductal lesions (Figure 13C) and more extensive atypical lesions approaching ductal carcinoma in situ (Figures 13D–13F) (Wood et al. 2006). The background prevalence of these lesions is around 3% in middle-aged adult macaques but increases with age (Cline 2007). Among women, ductal hyperplasias occur in approximately 20% of women undergoing reduction mammoplasty (Dotto et al. 2008) and are associated with increased risk of subsequent breast cancer (Ashbeck et al. 2007). Other types of ductal hyperplasias, such as columnar cell change, have been associated in women with adjacent coexisting malignancy and may represent distinct precursor lesions (Abdel-Fatah et al. 2007). The similar lesions found in macaques may thus deserve particular scrutiny.
Mammary Gland Cancers
Mammary gland carcinomas include both ductal carcinoma in situ and invasive ductal carcinoma, including metastatic disease (Wood et al. 2006). Among women, most breast neoplasms are invasive and of ductal origin, but a wide range of morphologies has been described. The full range of tumor types seen in human beings has not yet been described in macaques, although major types of tumor growth patterns such as comedocarcinoma, cribriform, and micropapillary types have been noted (Wood et al. 2006). The most common broad morphologic classifications are ductal carcinoma in situ, lobular carcinoma in situ, and invasive ductal carcinoma (Figures 13G–13I). Mammary cancers in macaques may express estrogen and progesterone receptors, and high-grade tumors may overexpress immunoreactive HER2/neu; neoplasms of ductal origin express e-cadherin (Wood et al. 2006).
Abbreviations
- PCOS
polycystic ovary syndrome
- CIN
cervical intraepithelial neoplasia
- PV
papillomavirus
REFERENCES
- Abdel-Fatah TM, Powe DG, Hodi Z, Lee AH, Reis-Filho JS, Ellis IO. High frequency of coexistence of columnar cell lesions, lobular neoplasia, and low grade ductal carcinoma in situ with invasive tubular carcinoma and invasive lobular carcinoma. Am J Surg Pathol. 2007;31:417–426. doi: 10.1097/01.pas.0000213368.41251.b9. [DOI] [PubMed] [Google Scholar]
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiol Behav. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Ami Y, Suzaki Y, Goto N. Endometriosis in cynomolgus monkeys retired from breeding. J Vet Med Sci. 1993;55:7–11. doi: 10.1292/jvms.55.7. [DOI] [PubMed] [Google Scholar]
- Arifin E, Shively CA, Register TC, Cline JM. Polycystic ovary syndrome with endometrial hyperplasia in a cynomolgus monkey (Macaca fascicularis) Veterinary Pathology. doi: 10.1354/vp.45-4-512. in press. [DOI] [PubMed] [Google Scholar]
- Ashbeck EL, Rosenberg RD, Stauber PM, Key CR. Benign breast biopsy diagnosis and subsequent risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:467–472. doi: 10.1158/1055-9965.EPI-06-0394. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Coe CL. Endometriosis is associated with an altered profile of intestinal microflora in female rhesus monkeys. Hum Reprod. 2002;17:1704–1708. doi: 10.1093/humrep/17.7.1704. [DOI] [PubMed] [Google Scholar]
- Baskin GB, Smith SM, Marx PA. Endometrial hyperplasia, polyps, and adenomyosis associated with unopposed estrogen in rhesus monkeys (Macaca mulatta) Vet Pathol. 2002;39:572–575. doi: 10.1354/vp.39-5-572. [DOI] [PubMed] [Google Scholar]
- Baulu J. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Horm Behav. 1976;7:481–494. doi: 10.1016/0018-506x(76)90019-2. [DOI] [PubMed] [Google Scholar]
- Beniashvilli DS. An overview of the world literature on spontaneous tumors in nonhuman primates. J Med Primatol. 1989;18:423–437. [PubMed] [Google Scholar]
- Benirschke K, Garner F, Jones T, editors. Pathology of laboratory animals. New York: Springer-Verlag; 1978. [Google Scholar]
- Blankenship TN, Enders AC, King BF. Trophoblastic invasion and modification of uterine veins during placental development in macaques. Cell Tissue Res. 1993;274:135–144. doi: 10.1007/BF00327994. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Adashi EY. The physiology and pathology of the female reproductive axis. In: Larsen PR, Kronenberg H, Melmed S, Polonsky K, editors. Williams Textbook of Endocrinology. Philadelphia: W. B. Saunders; 2002. pp. 627–632. [Google Scholar]
- Cameron AM, Faulkin LT., Jr Subgross evaluation of the non-human primate mammary gland: method and initial observations. J Med Primatol. 1974;3:298–310. doi: 10.1159/000460031. [DOI] [PubMed] [Google Scholar]
- Chalifoux LV. Ovarian teratoma, Macaca mulatta. In: Jones TC, Mohr U, Hunt RD, editors. Nonhuman Primates II. Berlin: Springer-Verlag; 1993. [Google Scholar]
- Chalifoux LV, MacKey JJ, King NW. A sparsely granulated, nonsecreting adenoma of the pars intermedia associated with galactorrhea in a male rhesus monkey (Macaca mulatta) Vet Pathol. 1983;20:541–547. doi: 10.1177/030098588302000505. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest break down and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Cline JM. Assessing the mammary gland of nonhuman primates: Effects of endogenous hormones and exogenous hormonal agents and growth factors. Birth Defects Res B Dev Reprod Toxicol. 2007;80:126–146. doi: 10.1002/bdrb.20112. [DOI] [PubMed] [Google Scholar]
- Cline JM, Bain FT. Uterine vascular changes indicating prior pregnancy in macaques. Veterinary Pathology. 1995;32:585. [Google Scholar]
- Cline JM, Register TC, Clarkson TB. Comparative effects of tibolone and conjugated equine estrogens with and without medroxyprogesterone acetate on the reproductive tract of female cynomolgus monkeys. Menopause. 2002a;9:242–252. doi: 10.1097/00042192-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Cline JM, Register TC, Clarkson TB. Effects of tibolone and hormone replacement therapy on the breast of cynomolgus monkeys. Menopause. 2002b;9:422–449. doi: 10.1097/00042192-200211000-00007. [DOI] [PubMed] [Google Scholar]
- Cline JM, Soderqvist G, Register TC, Williams JK, Adams MR, Von Schoultz B. Assessment of hormonally active agents in the reproductive tract of female nonhuman primates. Toxicol Pathol. 2001;29:84–90. doi: 10.1080/019262301301418883. [DOI] [PubMed] [Google Scholar]
- Cook AL, Rogers TD, Sowers M. Spontaneous uterine leiomyosarcoma in a rhesus macaque. Contemp Top Lab Anim Sci. 2004;43:47–49. [PubMed] [Google Scholar]
- Cooper TK, Gabrielson KL. Spontaneous lesions in the reproductive tract and mammary gland of female non-human primates. Birth Defects Res B Dev Reprod Toxicol. 2007;80:149–170. doi: 10.1002/bdrb.20105. [DOI] [PubMed] [Google Scholar]
- Cooper TK, Shih IM, Gabrielson KL. Uterine epithelioid trophoblastic tumour in a redtailed guenon (Cercopithecus ascanius) J Comp Pathol. 2005;133:218–222. doi: 10.1016/j.jcpa.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Daviau JS, Trupkiewicz JG. Pituitary adenoma with galactorrhea in an adult male cynomolgus macaque (Macaca fascicularis) Contemp Top Lab Anim Sci. 2001;40:57–59. [PubMed] [Google Scholar]
- DiGiacomo RF. Gynecologic pathology in the rhesus monkey (Macaca mulatta): II. Findings in laboratory and free-ranging monkeys. Vet Pathol. 1977;14:539–546. doi: 10.1177/030098587701400601. [DOI] [PubMed] [Google Scholar]
- Dotto J, Kluk M, Geramizadeh B, Tavassoli FA. Frequency of clinically occult intraepithelial and invasive neoplasia in reduction mammoplasty specimens: A study of 516 cases. Int J Surg Pathol. 2008;16:25–30. doi: 10.1177/1066896907307176. [DOI] [PubMed] [Google Scholar]
- Duran-Reynals F, Bunting H, Wagenen G. Studies on the sex skin of Macaca mulatta. Ann N Y Acad Sci. 1950;52:1006–1014. doi: 10.1111/j.1749-6632.1950.tb53997.x. [DOI] [PubMed] [Google Scholar]
- Engelhardt A, Hodges JK, Niemitz C, Heistermann M. Female sexual behavior, but not sex skin swelling, reliably indicates the timing of the fertile phase in wild long-tailed macaques (Macaca fascicularis) Horm Behav. 2005;47:195–204. doi: 10.1016/j.yhbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Fakis G, Boukouvala S, Kawamura A, Kennedy S. Description of a novel polymorphic gene encoding for arylamine N-acetyltransferase in the rhesus macaque (Macaca mulatta), a model animal for endometriosis. Pharmacogenet Genomics. 2007;17:181–188. doi: 10.1097/FPC.0b013e328011e3ad. [DOI] [PubMed] [Google Scholar]
- Fanton JW, Golden JG. Radiation-induced endometriosis in Macaca mulatta. Radiat Res. 1991;126:141–146. [PubMed] [Google Scholar]
- Fanton JW, Hubbard GB, Wood DH. Endometriosis: clinical and pathologic findings in 70 rhesus monkeys. Am J Vet Res. 1986;47:1537–1541. [PubMed] [Google Scholar]
- Fukunaga M. Smooth muscle metaplasia in ovarian endometriosis. Histopathology. 2000;36:348–352. doi: 10.1046/j.1365-2559.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Bell SC, Sengupta J. Immunohistological localization of insulin-like growth factor binding protein-1 in primary implantation sites and trauma-induced deciduomal tissues of the rhesus monkey. Placenta. 2004;25:197–207. doi: 10.1016/j.placenta.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Sengupta J. Endometrial responses to a deciduogenic stimulus in ovariectomized rhesus monkeys treated with oestrogen and progesterone. J Endocrinol. 1989;120:51–58. doi: 10.1677/joe.0.1200051. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Sengupta J. Patterns of ovulation, conception and preimplantation embryo development during the breeding season in rhesus monkeys kept under semi-natural conditions. Acta Endocrinol (Copenh) 1992;127:168–173. doi: 10.1530/acta.0.1270168. [DOI] [PubMed] [Google Scholar]
- Giusti AM, Terron A, Belluco S, Scanziani E, Carcangiu ML. Ovarian epithelioid trophoblastic tumor in a cynomolgus monkey. Vet Pathol. 2005;42:223–226. doi: 10.1354/vp.42-2-223. [DOI] [PubMed] [Google Scholar]
- Gocze PM, Beamer WG, de Jong FH, Freeman DA. Hormone synthesis and responsiveness of spontaneous granulosa cell tumors in (SWR x SWXJ-9) F1 mice. Gynecol Oncol. 1997;65:143–148. doi: 10.1006/gyno.1997.4635. [DOI] [PubMed] [Google Scholar]
- Gorrindo T, Lu Y, Pincus S, Riley A, Simon JA, Singer BH, Weinstein M. Lifelong menstrual histories are typically erratic and trending: A taxonomy. Menopause. 2007;14:74–88. doi: 10.1097/01.gme.0000227853.19979.7f. [DOI] [PubMed] [Google Scholar]
- Guo SW. The link between exposure to dioxin and endometriosis: a critical reappraisal of primate data. Gynecol Obstet Invest. 2004;57:157–173. doi: 10.1159/000076374. [DOI] [PubMed] [Google Scholar]
- Hadfield RM, Yudkin PL, Coe CL, Scheffler J, Uno H, Barlow DH, Kemnitz JW, Kennedy SH. Risk factors for endometriosis in the rhesus monkey (Macaca mulatta): a case-control study. Hum Reprod Update. 1997;3:109–115. doi: 10.1093/humupd/3.2.109. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- Kaspareit J, Friderichs-Gromoll S, Buse E, Habermann G. Spontaneous neoplasms observed in cynomolgus monkeys (Macaca fascicularis) during a 15-year period. Exp Toxicol Pathol. 2007;59:163–169. doi: 10.1016/j.etp.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Kaspareit J, Friderichs-Gromoll S, Buse E, Habermann G, Vogel F. Spontaneous epithelial plaques in the uterus of a non-pregnant cynomolgus monkey (Macaca fascicularis) Exp Toxicol Pathol. 2004;56:9–12. doi: 10.1016/j.etp.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Koering MJ. Preantral follicle development during the menstrual cycle in the Macaca mulatta ovary. Am J Anat. 1983;166:429–443. doi: 10.1002/aja.1001660405. [DOI] [PubMed] [Google Scholar]
- Krugner-Higby L, Rosenstein A, Handschke L, Luck M, Laughlin NK, Mahvi D, Gendron A. Inguinal hernias, endometriosis, and other adverse outcomes in rhesus monkeys following lead exposure. Neurotoxicol Teratol. 2003;25:561–570. doi: 10.1016/s0892-0362(03)00076-x. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kuwamura Y, Kakehi K, Hirakawa K, Miyajima H. Ectopic uterine ovarian tissue in cynomolgus monkeys. Toxicol Pathol. 2006;34:220–222. doi: 10.1080/01926230600695482. [DOI] [PubMed] [Google Scholar]
- Lauszus FF, Petersen AC, Greisen J, Jakobsen A. Granulosa cell tumor of the ovary: a population-based study of 37 women with stage I disease. Gynecol Oncol. 2001;81:456–460. doi: 10.1006/gyno.2001.6183. [DOI] [PubMed] [Google Scholar]
- Majeed SK, Gopinath C. Calcification in the adrenals and ovaries of monkeys. Lab Anim. 1980;14:363–365. doi: 10.1258/002367780781071166. [DOI] [PubMed] [Google Scholar]
- Manning PJ. The staining of elastic tissue and related fibres in uterine blood vessels. Med Lab Technol. 1974;31:115–125. [PubMed] [Google Scholar]
- Marchant DJ. Benign breast disease. Obstet Gynecol Clin North Am. 2002;29:1–20. doi: 10.1016/s0889-8545(03)00048-2. [DOI] [PubMed] [Google Scholar]
- McClure HM. Tumors in nonhuman primates: observations during a six-year period in the Yerkes primate center colony. Am J Phys Anthropol. 1973;38:425–429. doi: 10.1002/ajpa.1330380243. [DOI] [PubMed] [Google Scholar]
- Miehe U, Neumaier-Wagner P, Kadyrov M, Goyal P, Alfer J, Rath W, Huppertz B. Concerted upregulation of CLP36 and smooth muscle actin protein expression in human endometrium during decidualization. Cells Tissues Organs. 2005;179:109–114. doi: 10.1159/000085002. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Lu FX. Anti-HIV and -SIV immunity in the vagina. Int Rev Immunol. 2003;22:65–76. doi: 10.1080/08830180305230. [DOI] [PubMed] [Google Scholar]
- Moore CM, Hubbard GB, Leland MM, Dunn BG, Best RG. Spontaneous ovarian tumors in twelve baboons: a review of ovarian neoplasms in non-human primates. J Med Primatol. 2003;32:48–56. doi: 10.1034/j.1600-0684.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Mossman HW, Duke KL. Comparative morphology of the mammalian ovary. Madison, WI: University of Wisconsin Press; 1973. [Google Scholar]
- Mutter GL, Zaino RJ, Baak JP, Bentley RC, Robboy SJ. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol. 2007;26:103–114. doi: 10.1097/PGP.0b013e31802e4696. [DOI] [PubMed] [Google Scholar]
- Nascimento AF, Hornstein MD, Crum CP. Benign conditions of the ovary. In: Crum CP, Lee KR, editors. Diagnostic Gynecologic and Obstetric Pathology. Philadelphia: Elsevier-Saunders; 2006. pp. 713–752. [Google Scholar]
- Payan HM, Gilbert EF. Mesenteric cyst-ovarian implant syndrome. Arch Pathol Lab Med. 1987;111:282–284. [PubMed] [Google Scholar]
- Remick AK, Wood CE, Cann JA, Gee MK, Feiste EA, Kock ND, Cline JM. Histologic and immunohistochemical characterization of spontaneous pituitary adenomas in fourteen cynomolgus macaques (Macaca fascicularis) Vet Pathol. 2006;43:484–493. doi: 10.1354/vp.43-4-484. [DOI] [PubMed] [Google Scholar]
- Resko JA, Goy RW, Robinson JA, Norman RL. The pubescent rhesus monkey: Some characteristics of the menstrual cycle. Biol Reprod. 1982;27:354–361. doi: 10.1095/biolreprod27.2.354. [DOI] [PubMed] [Google Scholar]
- Rier SE. The potential role of exposure to environmental toxicants in the pathophysiology of endometriosis. Ann N Y Acad Sci. 2002;955:201–212. doi: 10.1111/j.1749-6632.2002.tb02781.x. discussion 230–32, 396–406. [DOI] [PubMed] [Google Scholar]
- Rier SE, Coe CL, Lemieux AM, Martin DC, Morris R, Lucier GW, Clark GC. Increased tumor necrosis factor-alpha production by peripheral blood leukocytes from TCDD-exposed rhesus monkeys. Toxicol Sci. 2001;60:327–337. doi: 10.1093/toxsci/60.2.327. [DOI] [PubMed] [Google Scholar]
- Rier SE, Martin DC, Bowman RE, Dmowski WP, Becker JL. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl Toxicol. 1993;21:433–441. doi: 10.1006/faat.1993.1119. [DOI] [PubMed] [Google Scholar]
- Rier SE, Turner WE, Martin DC, Morris R, Lucier GW, Clark GC. Serum levels of TCDD and dioxin-like chemicals in Rhesus monkeys chronically exposed to dioxin: correlation of increased serum PCB levels with endometriosis. Toxicol Sci. 2001;59:147–159. doi: 10.1093/toxsci/59.1.147. [DOI] [PubMed] [Google Scholar]
- Rodriguez GC, Nagarsheth NP, Lee KL, Bentley RC, Walmer DK, Cline M, Whitaker RS, Isner P, Berchuck A, Dodge RK, Hughes CL. Progestin-induced apoptosis in the Macaque ovarian epithelium: differential regulation of transforming growth factor-beta. J Natl Cancer Inst. 2002;94:50–60. doi: 10.1093/jnci/94.1.50. [DOI] [PubMed] [Google Scholar]
- Rodriguez GC, Walmer DK, Cline M, Krigman H, Lessey BA, Whitaker RS, Dodge R, Hughes CL. Effect of progestin on the ovarian epithelium of macaques: cancer prevention through apoptosis? J Soc Gynecol Investig. 1998;5:271–276. doi: 10.1016/s1071-5576(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Ronnett BM, Kurman RJ.Kurman RJ.Precursor lesions of endometrial carcinoma Blaustein's Pathology of the Female Genital Tract 2002New York: Springer-Verlag; 467–500 [Google Scholar]
- Sarnelli R, Squartini F. Fibrocystic condition and “at risk” lesions in asymptomatic breasts: A morphologic study of postmenopausal women. Clin Exp Obstet Gynecol. 1991;18:271–279. [PubMed] [Google Scholar]
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- Seibold HR, Wolf RH. Neoplasms and proliferative lesions in 1065 nonhuman primate necropsies. Lab Anim Sci. 1973;23:533–539. [PubMed] [Google Scholar]
- Seidman CE, Russell MD, Kurman RJ. Surface epithelial tumors of the ovary. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. New York: Springer-Verlag; 2002. pp. 791–904. [Google Scholar]
- Sherman ME, Mazur MT, Kurman RJ. Benign diseases of the endometrium. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. New York: Springer-Verlag; 2002. pp. 421–466. [Google Scholar]
- Shih L, Mazur MT, Kurman RJ. Gestational trophoblastic disease and related lesions. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. New York: Springer-Verlag; 2002. pp. 1193–1247. [Google Scholar]
- Steiner AZ, Xiang M, Mack WJ, Shoupe D, Felix JC, Lobo RA, Hodis HN. Unopposed estradiol therapy in postmenopausal women: results from two randomized trials. Obstet Gynecol. 2007;109:581–587. doi: 10.1097/01.AOG.0000251518.56369.eb. [DOI] [PubMed] [Google Scholar]
- Sternfeld MD, West NB, Brenner RM. Immunocytochemistry of the estrogen receptor in spontaneous endometriosis in rhesus macaques. Fertil Steril. 1988;49:342–348. [PubMed] [Google Scholar]
- Strickland JL, Wall JW. Abnormal uterine bleeding in adolescents. Obstet Gynecol Clin North Am. 2003;30:321–335. doi: 10.1016/s0889-8545(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Strozier LM, McClure HM, Keeling ME, Cummins LB. Endometrial adenocarcinoma, endometriosis, and pyometra in a rhesus monkey. J Am Vet Med Assoc. 1972;161:704–706. [PubMed] [Google Scholar]
- Takayama S, Fukushima S, Thorgeirsson UP. Atlas of spontaneous and chemically induced tumors in nonhuman primates. Switzerland: Karger AG, Basel; 2000. [Google Scholar]
- Toyosawa K, Okimoto K, Koujitani T, Kikawa E. Choriocarcinoma and teratoma in the ovary of a cynomolgus monkey. Vet Pathol. 2000;37:186–188. doi: 10.1354/vp.37-2-186. [DOI] [PubMed] [Google Scholar]
- Van Wagenen G, Simpson ME. New Haven, CT: Yale University Press; 1973. Postnatal development of the ovary in Homo sapiens and Macaca mulatta and induction of ovulation in the macaque. [Google Scholar]
- Warner M. Mammary pathology. In: Bowden D, editor. Aging in Nonhuman Primates. New York: Van Nostrand Reinhold; 1979. pp. 210–227. [Google Scholar]
- Wilkinson M, Walters S, Smith T, Wilkinson A. Reproductive abnormalities in aged female Macaca fascicularis. J Med Primatol. 2008;37(Suppl 1):88–93. doi: 10.1111/j.1600-0684.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- Wood CE, Borgerink H, Register TC, Scott L, Cline JM. Cervical and vaginal epithelial neoplasms in cynomolgus monkeys. Vet Pathol. 2004;41:108–115. doi: 10.1354/vp.41-2-108. [DOI] [PubMed] [Google Scholar]
- Wood CE, Chen Z, Cline JM, Miller BE, Burk RD. Characterization and experimental transmission of an oncogenic papillomavirus in female macaques. J Virol. 2007;81:6339–6345. doi: 10.1128/JVI.00233-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE, Hester JM, Cline JM. Mammary gland development in early pubertal female macaques. Toxicol Pathol. 2007;35:795–805. doi: 10.1080/01926230701584213. [DOI] [PubMed] [Google Scholar]
- Wood CE, Usborne AL, Starost MF, Tarara RP, Hill LR, Wilkinson LM, Geisinger KR, Feiste EA, Cline JM. Hyperplastic and neoplastic lesions of the mammary gland in macaques. Vet Pathol. 2006;43:471–483. doi: 10.1354/vp.43-4-471. [DOI] [PubMed] [Google Scholar]
- Wright JW, Pejovic T, Fanton J, Stouffer RL. Induction of proliferation in the primate ovarian surface epithelium in vivo. Hun Reprod. 2008;23:129–138. doi: 10.1093/humrep/dem347. [DOI] [PubMed] [Google Scholar]
- Yang JZ, Agarwal SK, Foster WG. Subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin modulates the pathophysiology of endometriosis in the cynomolgus monkey. Toxicol Sci. 2000;56:374–381. doi: 10.1093/toxsci/56.2.374. [DOI] [PubMed] [Google Scholar]
- Zaloudek C, Hendrickson MR. Mesenchymal tumors of the uterus. In: Kurman RJ, editor. Blaustein's Pathology of the Female Reproductive Tract. New York: Springer-Verlag; 2002. pp. 561–615. [Google Scholar]
- Zhou R, Bird IM, Dumesic DA, Abbott DH. Adrenal hyperandrogenism is induced by fetal androgen excess in a rhesus monkey model of polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:6630–6637. doi: 10.1210/jc.2005-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Weeks DE, Colman R, Cardon LR, Hadfield R, Schleffler J, Trainor AG, Coe CL, Kemnitz JW, Kennedy SH. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum Reprod. 2004;19:448–455. doi: 10.1093/humrep/deh052. [DOI] [PubMed] [Google Scholar]