Abstract
Spreading depression (SD) involves coordinated depolarizations of neurons and glia that propagate through the brain tissue. Repetitive SD-like events are common following human ischemic strokes, and are believed to contribute to the enlargement of infarct volume. Accumulation of Zn2+ is also implicated in ischemic neuronal injury. Synaptic glutamate release contributes to SD propagation, and because Zn2+ is costored with glutamate in some synaptic vesicles, we examined whether Zn2+ is released by SD and may therefore provide a significant source of Zn2+ in the postischemic period. Spreading depression-like events were generated in acutely prepared murine hippocampal slices by deprivation of oxygen and glucose (OGD), and Zn2+ release was detected extracellularly by a Zn2+-selective indicator FluoZin-3. Deprivation of oxygen and glucose-SD produced large FluoZin-3 increases that propagated with the event, and signals were abolished in tissues from ZnT3 knockout animals lacking synaptic Zn2+. Synaptic Zn2+ release was also maintained with repetitive SDs generated by microinjections of KCl under normoxic conditions. Intracellular Zn2+ accumulation in CA1 neurons, assessed using microinjection of FluoZin-3, showed significant increases following SD that was attributed to synaptic Zn2+ release. These results suggest that Zn2+ is released during SDs and could provide a significant source of Zn2+ that contributes to neurodegeneration in the postischemic period.
Keywords: brain slice, CA1, oxygen–glucose deprivation, spreading depression, zinc, ZnT3
Introduction
The phenomenon of spreading depression (SD) involves a wave of coordinated neuronal and glial depolarization that spreads in a regenerative manner across the brain tissue (Leao, 1944; Somjen, 2001). Spreading depression does not generally cause injury to the healthy brain, because ionic gradients are restored by the action of adenosine triphosphate-dependent transporters. However, SD can cause irreversible loss of membrane potential and neuronal injury if metabolic substrates are not sufficient for timely repolarization (Hossmann, 1996; Somjen, 2001). Spreading waves of depolarization that are very similar to SD are observed in animal models of stroke, and accumulating evidence suggests that these SD-like events have a causative role in the evolution of ischemic brain injury (Hartings et al, 2003; Strong et al, 2000). Recent long-term recording in patients with ischemic stroke has revealed that SD-like events occur repetitively for many hours and days after stroke onset (Dohmen et al, 2008), suggesting that approaches to limit the onset or consequences of these events may be of benefit at quite late time points.
We recently examined factors contributing to the onset of SD in brain slices, and found that Zn2+ accumulation can contribute to the initiation of forms of SD that are relevant for ischemia. Chelation of Zn2+ could delay or prevent SD-like events generated by either combined oxygen and glucose deprivation (OGD-SD) or the Na+/K+/ATPase inhibitor ouabain (ouabain-SD), and these effects were more prominent when adenosine A1 receptor activation was enhanced (Dietz et al, 2008). Zn2+ is present at high levels in the brain, located in intracellular binding proteins, and also concentrated into many nerve terminals by the activity of the ZnT3 transporter (Frederickson et al, 2005; Palmiter et al, 1996). A large body of literature has shown that disruption of Zn2+ homeostasis can cause neuronal injury, by disruption of both glycolytic and mitochondrial functions, and actions on a range of other intracellular targets (Choi and Koh, 1998; Dineley et al, 2003; Galasso and Dyck, 2007; Sensi et al, 2009). Reducing Zn2+ accumulation has also been shown to limit neuronal injury in animal focal and global ischemia models (Calderone et al, 2004; Galasso and Dyck, 2007; Koh et al, 1996; Lee et al, 2002). In addition to preventing a number of deleterious intracellular signaling cascades, it is possible that limiting the onset of SD-like events could contribute to beneficial effects of Zn2+ chelation in some stroke models.
Previous work has identified glutamate release as a contributor to SD propagation, based on the effectiveness of glutamate receptor blockers and microdialysis measurements of extracellular glutamate accumulation (Fabricius et al, 1993; Somjen, 2001). Although the regulation of glutamate release during SD is not well understood, it is likely that depolarization of nerve terminals owing to K+ elevations contributes. In this study, we examined whether significant amounts of Zn2+ may be released from nerve terminals during SD. Zn2+ is costored with glutamate in many hippocampal and neocortical synaptic vesicles (Frederickson et al, 2005). High levels of Zn2+ can be visualized in nerve terminals using histochemical staining methods, and Zn2+ release can be detected following nerve stimulation (Frederickson et al, 2005; Sensi et al, 2009), including studies using the Zn2+-sensitive indicator FluoZin-3 (see Qian and Noebels (2005, 2006), but also see Kay (2003)). Knockout (KO) of ZnT3 prevents accumulation of Zn2+ in synaptic terminals without influencing other essential Zn2+ stores (Cole et al, 1999), and prevents fluorescence signals attributed to synaptic Zn2+ release (Qian and Noebels, 2006).
We used fluorescence imaging of Zn2+ and ZnT3 KO tissues to show in this study that SD and related events indeed cause large Zn2+ releases that appear because of the release from synaptic vesicles, and that this source of Zn2+ contributes to accumulation in postsynaptic neurons. Releasable Zn2+ stores were not readily depleted with repetitive SD trials, and these results therefore identify SD and related spreading depolarizations as new and potentially significant sources of Zn2+ for neuronal injury in the postischemic period.
Materials and methods
Slice Preparation
Acute brain slices were prepared from mice as described previously (Dietz et al, 2008). All procedures were carried out in accordance with the National Institute of Health guidelines for the humane treatment of laboratory animals, and the protocol for these procedures was reviewed annually by the Institutional Animal Care and Use Committee at the University of New Mexico School of Medicine. After cutting in ice-cold solution and then holding for 1 hour at 35°C, artificial cerebrospinal fluid (ACSF) was changed, and slices were held at room temperature until used for recording. Individual slices were then transferred to the recording chamber and superfused with oxygenated ACSF at 2 mL/min.
Mice homozygous for deletion of the ZnT3 gene were originally obtained from Dr Richard Palmiter (Cole et al, 1999) and backcrossed onto C57Bl/6 for more than 13 generations. A homozygous breeding colony of ZnT3 KO animals was then established at the University of New Mexico. Initial studies of intracellular and extracellular Zn2+ transients were conducted in slices obtained from wild-type FVB/N mice, as were used for previous studies of Zn2+ and SD initiation (Dietz et al, 2008). As no differences were noted in SD-FluoZin-3 responses between wild-type C57Bl/6 and FVB/N mice, responses from ZnT3 KO tissues were either compared with responses from populations of wild-type C57Bl/6 tissues or pooled populations of C57Bl/6 and FVB/N tissues, as noted in figure legends. Wild-type animals were obtained from Harlan Laboratories (Bar Harbor, ME, USA), and all animals were housed under standard conditions (12-hour/12-hour light–dark cycle) before killing at 4 to 6 weeks of age.
Spreading Depression Initiation and Recording
Extracellular measurements of direct current (DC) potentials were made using borosilicate glass microelectrodes, filled with ACSF (∼5 MΩ) and placed in the stratum radiatum ∼50 μm below the surface of the slice. Spreading depression was generated by a localized microinjection of KCl, delivered using brief pressure pulses (10 to 40 psi, 100 to 250 milliseconds, 1 mol/L solution) from a conventional patch electrode (3 to 5 ΩM) placed under the surface of the slice, ∼350 μm from the recording electrode. Oxygen–glucose deprivation was used to generate the closely related propagating event (OGD-SD). In both cases (SD and OGD-SD), the passage of the coordinated, spreading depolarization was identified as a sharp decrease in DC potential, as described previously (Somjen, 2001). In some experiments, the spread of SD or OGD-SD was monitored using intrinsic optical signals (IOSs), generated by transmission of red (>575 nm) light.
Zn2+ Indicator Measurements
Extracellular Zn2+ measurements were made using the charged form of the Zn2+-sensitive indicator FluoZin-3, using approaches developed by Kay (2003) and Qian and Noebels (2005, 2006) and used in our recent work (Dietz et al, 2008). FluoZin-3 was dissolved in ACSF at 1.5 to 2 μmol/L and superfused over the slice at 35°C. Background fluorescence was then reduced by the addition of a Zn2+-selective chelator CaEDTA (1 mmol/L) in all experiments, except where indicated otherwise. Responses are reported as %ΔF/F0, because Zn2+ binds to multiple components of the recording buffer (Rumschik et al, 2009), making absolute calibration from addition of exogenous Zn2+ standards problematic. Fluorescence increases because of the addition of FluoZin-3 were very similar in wild-type and ZnT3 KO slices, and addition of CaEDTA reduced fluorescence to very similar levels in both wild-type and ZnT3 KO tissues (see Supplementary Figure 1). FluoZin-3 was excited at 495 nm, and emission was detected at 535/50 nm using a monochromator-based imaging system (Till Photonics, Rochester, NY, USA).
Intracellular Zn2+ measurements were made from single CA1 pyramidal neurons. Procedures for intracellular recording/indicator injection were carried out as described previously (Dietz et al, 2008) using electrodes tip filled with 1 mmol/L FluoZin-3 for recordings from slices superfused at 32°C. As high-resistance intracellular electrodes were used, a lower final indicator concentration was achieved by loading into neurons with the passage of hyperpolarizing current (300 to 800 pA, 20 minutes), and the recording/filling electrode was then slowly withdrawn in 0.5 μm steps from the neuron (Dietz et al, 2008). As high-resistance intracellular recording/filling electrodes were used, this withdrawal procedure did not result in significant indicator leak or extracellular accumulation of FluoZin-3. Signals from cell bodies were background subtracted using adjacent regions in the cell body layer, to take into account changes in autofluorescence caused by SD events.
Reagents and Solutions
Slice cutting solution contained (in mmol/L): 3 KCl, 1.25 NaH2PO4, 6 MgSO4, 26 NaHCO3, 0.2 CaCl2, 10 glucose, 220 sucrose, and 0.43 ketamine. Artificial cerebrospinal fluid contained (in mmol/L): 126 NaCl, 3 KCl, 1.25 NaH2PO4, 1 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 glucose and was equilibrated with 95%O2/5%CO2. Both cutting and recording solutions were 315 to 320 mOsm. In OGD experiments, ACSF was modified by equimolar replacement of glucose with sucrose, and extensively equilibrated with 95%N2/5%CO2. FluoZin-3 was obtained from Invitrogen (Carlsbad, CA, USA), and all other reagents were obtained from Sigma (St Louis, MO, USA).
Statistical Analysis
Data are reported as mean±s.e.m. Significant differences between group data were evaluated using unpaired or paired Student's t-tests, with correction for multiple comparisons where appropriate.
Results
Synaptic Zn2+ Release Following Oxygen–Glucose Deprivation-Spreading Depression
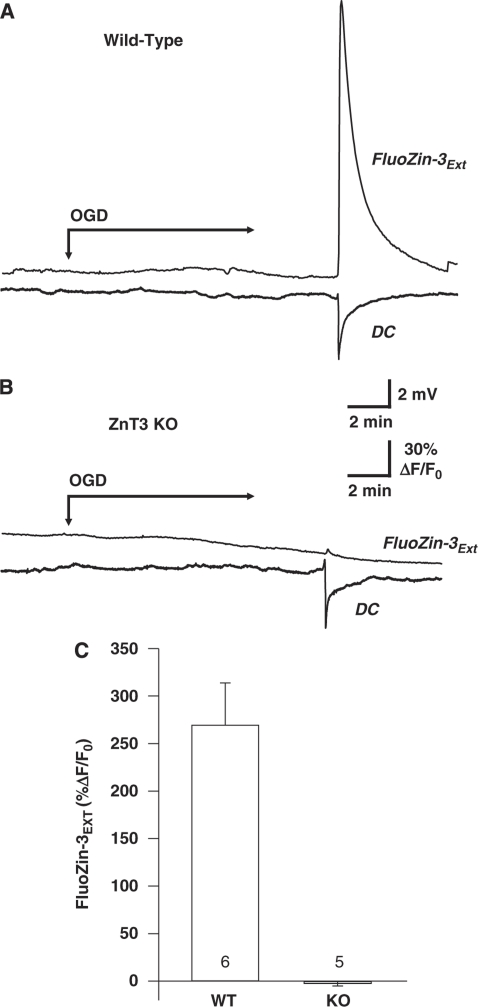
Figure 1A shows a representative example of Zn2+ increases associated with SD-like events in the OGD model (OGD-SD). Oxygen–glucose deprivation-SD was detected as a sharp DC shift after ∼10 minutes OGD exposure, and was accompanied by a large increase in the fluorescence of extracellular FluoZin-3.
Figure 1.
Synaptic Zn2+ release during OGD-SD. (A) OGD-SD generated a substantial extracellular FluoZin-3 increase in a wild-type C57Bl/6 preparation. Representative traces that show FluoZin-3 response from a region of interest in the stratum radiatum, aligned to the electrical (DC) recording of SD using an extracellular electrode at the same location. OGD was initiated at the arrow and present throughout the remainder of the experiment. (B) Identical recording conditions as described in panel A, except in a slice from a ZnT3 KO animal, which lacks synaptic Zn2+. SD was still generated under these conditions (as indicated by the sharp DC shift); however, the FluoZin-3 response was abolished. (C) Mean FluoZin-3 responses generated by OGD-SD from wild-type C57Bl/6 (n=6) and ZnT3 KO slices (n=5), indicating a synaptic origin of Zn2+ release during OGD-SD. KO, knockout; OGD-SD, oxygen–glucose deprivation-spreading depression.
Extracellular Zn2+ accumulation was detected using the charged form of FluoZin-3 (1.5 to 2 μmol/L), which is confined to the extracellular space. As described previously (Qian and Noebels, 2005, 2006), a Zn2+ chelator with high affinity but slow Zn2+ binding kinetics (CaEDTA, 1 mmol/L) was also included to improve signal–noise in the initial measurements. This chelator is effective for these studies because it does not reduce extracellular Ca2+ concentrations (unlike EDTA), but effectively reduces the large background extracellular Zn2+ fluorescence signal, because of the higher affinity of this cation than the prebound Ca2+ (KDZn=10−16 mol/L, KDCa=10−10 mol/L) (see Supplementary Figure 1). Owing to the time taken for exchange of Zn2+ with prebound Ca2+ on the EDTA molecule (Kay, 2004), extracellular Zn2+ is readily detected as FluoZin-3 transients before sequestration by CaEDTA.
To test the hypothesis that Zn2+ is synaptically released during OGD-SD, mice lacking synaptic Zn2+ (ZnT3 KO mice; see Cole et al (1999)) were used. ZnT3 is a Zn2+ transporter localized selectively to synaptic vesicles (Cole et al, 1999; Palmiter et al, 1996), which is responsible for transporting cytosolic Zn2+ into synaptic vesicles. We previously showed that Zn2+ accumulation can contribute to the onset of OGD-SD, and described conditions of high adenosine A1 receptor activation in which chelation of Zn2+ was sufficient to completely prevent the onset of SD (Dietz et al, 2008). In these experiments, exogenous A1 receptor agonists were not applied, to ensure that OGD-SD was still generated and allow consequences of these events on Zn2+ release to be determined. Under these conditions, OGD-SD was generated in all wild-type and ZnT3 KO tissues (mean onset time 10.09±1.01 minutes in ZnT3 KO versus 8.97±0.50 minutes in wild type, P=0.37); however, Figure 1B shows that FluoZin-3 increases were abolished in a paired slice obtained from a ZnT3 KO animal. Figure 1C shows mean data from a group of such experiments. Instead of fluorescence increases, small decreases were observed following the propagation of OGD-SD in ZnT3 KO tissues, which were caused by changes in autofluorescence and/or tissue swelling (see below).
During OGD exposures, small decreases in FluoZin-3 fluorescence were observed before the onset of OGD-SD, but these were not significantly different between wild-type and ZnT3 KO preparations (−5.45%±1.52% versus −6.83%±1.53% ΔF/F0, respectively, P=0.54, n=6 each). As CaEDTA was present throughout these studies, it is unlikely that this is caused by decreases in extracellular Zn2+ levels, and is likely caused by small, uniform changes in tissue optical properties before the onset of OGD-SD (see below). No FluoZin-3 fluorescence increases were observed before the onset of OGD-SD, regardless of whether CaEDTA was present or not (n=17 and n=5, respectively).
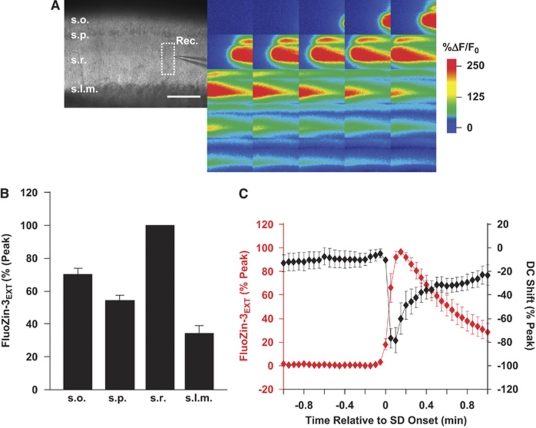
Figure 2 illustrates the wave-like spread of FluoZin-3 increases, and examines the relationship with the onset of OGD-SD. A movie file showing the spread of fluorescence increases is available in Supplementary Data. Figure 2A shows that the fluorescence increase propagated slowly across the slice (mean 3.68±0.55 mm/min, n=11). This rate was very similar to the propagation rate of OGD-SD observed in this model with intrinsic optical methods in a separate population of slices (4.82±0.70 mm/min, n=9, P=0.22), and consistent with many previous reports for the propagation rate of SD and related events generated in hypoxia or ischemia models (see Somjen, 2001). FluoZin-3 increases were also largest in the stratum radiatum (Figure 2B), a region that is expected to contain high concentrations of Zn2+-containing excitatory synaptic inputs (Qian and Noebels, 2006) and to be the site of initial current sink during SD initiation (Kunkler et al, 2005). Figure 2C shows that the onset of Zn2+ increases was coincident with that of OGD-SD measured in the same slices, suggesting that Zn2+ increases occur together with the spread of OGD-SD, rather than significantly leading or following the response.
Figure 2.
Propagation of extracellular Zn2+ increases following OGD-SD. (A) Spread of FluoZin-3 increases across a wild-type C57Bl/6 slice during OGD-SD. The bright field image (left panel) shows the orientation of the slice (s.o., stratum oriens; s.p., stratum pyramidale; s.r., stratum radiatum; s.l.m., stratum lacunosum-moleculare). The color panels are a series of 25 images taken at 3-second intervals from this slice, showing the spread of the event. Scale bar, 200 μm. (B) Summary data of regional differences in FluoZin-3 increases generated by OGD-SD from the different hippocampal subfields of four wild-type C57Bl/6 preparations. Greatest increases were seen in s.r. and s.o. (C) Coincidence between the onset of FluoZin-3 increases and SD in the same population of slices from panel B. FluoZin-3 and DC recordings are each both normalized to peak responses and plotted against the time to SD onset (t=0), identified from the DC shift. OGD-SD, oxygen–glucose deprivation-spreading depression.
Relative Amplitude of Signals
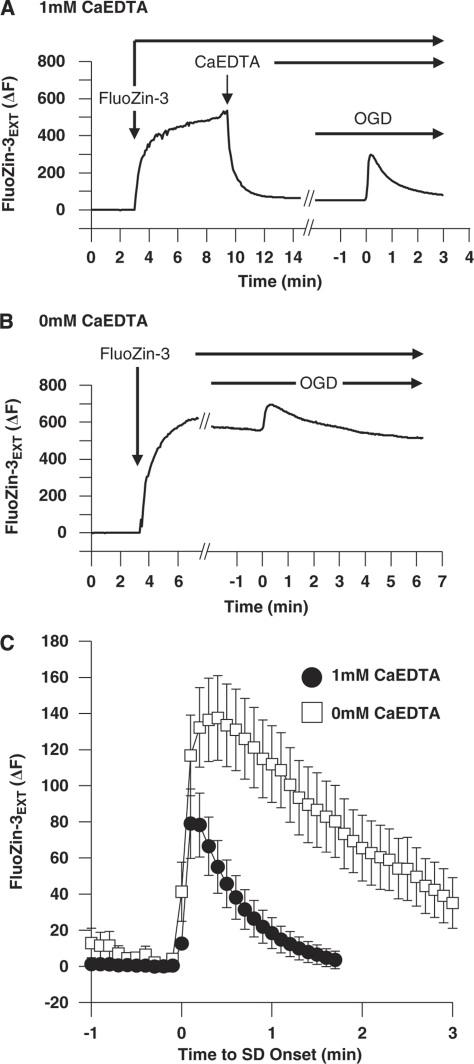
As noted above, the methods used to evaluate Zn2+ release follow previously published work, in which a Zn2+ chelator with high affinity but slow Zn2+ binding kinetics (CaEDTA) was used to improve signal–noise (Qian and Noebels, 2005). We tested the hypothesis that this chelator greatly reduced the time course of FluoZin-3 transients from OGD-SD by comparing results from parallel studies in the absence of CaEDTA. Figures 3A and 3B illustrate the greatly enhanced background FluoZin-3 fluorescence in the absence of CaEDTA, and shows fluorescence transients generated by OGD-SD superimposed on these different background fluorescence levels. Figure 3C shows mean data from a set of such experiments, and shows a substantial increase in mean time constant of decay of transients (0.61±0.11 minutes versus 2.03±0.25 minutes, with and without CaEDTA, respectively, n=5, P=0.0023). The peak amplitude of average raw fluorescence transients showed a trend toward an increase without CaEDTA, although this was not statistically significant in this data set (136.46±23.12 versus 82.43±18.47 arbitrary fluorescence units, 0 mmol/L versus 1 mmol/L CaEDTA, respectively, P>0.10, n=5 each). However, because of the very different background fluorescence levels, there was a substantial increase in the signal–noise ratio of signals when CaEDTA was included (86.13±35.43 (0 mmol/L CaEDTA) versus 241.28±41.04 (1 mmol/L CaEDTA) (%ΔF/F0), P=0.022, n=5 each). As described previously (Dietz et al, 2008), CaEDTA did not interfere with OGD-SD initiation (7.38±0.93 versus 7.80±0.34, with CaEDTA and without CaEDTA, respectively n=5, P=0.65) or propagation (2.86±0.62 mm/min versus 3.68±0.94 mm/min, respectively, P=0.49, n=5 each).
Figure 3.
Effects of the selective Zn2+ chelator CaEDTA on FluoZin-3 transients. (A) FluoZin-3 transients in the presence of CaEDTA. Representative trace from a wild-type C57Bl/6 slice showing the change in raw fluorescence during FluoZin-3 and CaEDTA wash-in. Subsequent application of OGD generated a FluoZin-3 transient superimposed on low-background fluorescence (plotted after axis break, time relative to OGD-SD onset). (B) Same conditions as described in panel A, but without coapplication of CaEDTA. The representative trace shows the wash-in of FluoZin-3, and the subsequent FluoZin-3 transient during OGD-SD occurs on a substantially elevated fluorescence baseline. (C) Mean FluoZin-3 transients during OGD-SD. CaEDTA significantly accelerated the rate of decay and reduced the peak amplitude of FluoZin-3 transients (raw fluorescence transients, n=5 each). OGD-SD, oxygen–glucose deprivation-spreading depression.
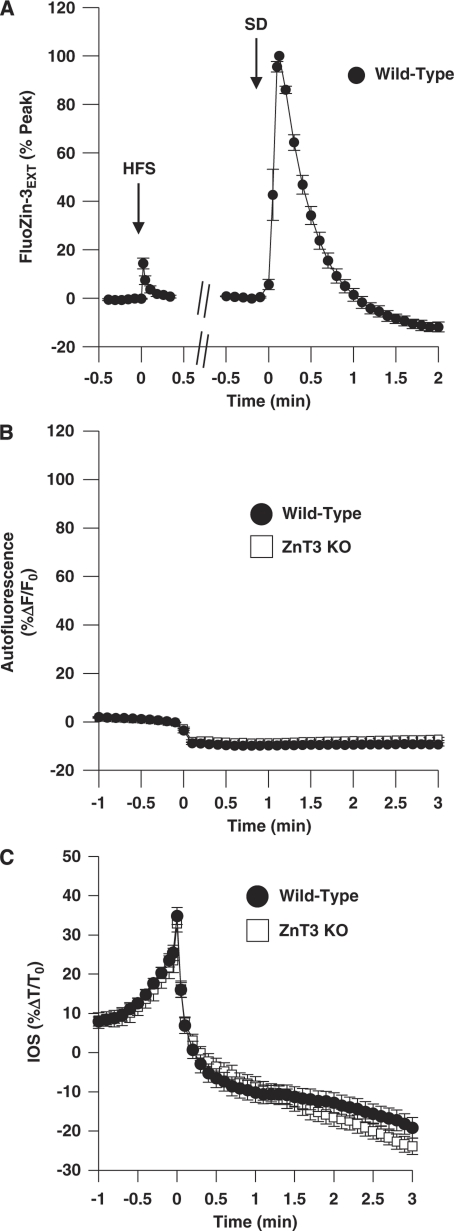
Figure 4A compares extracellular FluoZin-3 responses to electrical stimulation of synaptic inputs with OGD-SD in the same six preparations. Responses to relatively short trains of high-frequency stimulation (50 Hz, up to 200-millisecond trains) were not reliably detected with the charge-coupled device (CCD) methods used in this study, but Figure 4A shows robust extracellular FluoZin-3 transients with 1-second train (50 Hz). After recovery from synaptic stimulation (10 minutes), the same slices showed much larger extracellular FluoZin-3 increases during OGD-SD. When the areas under the curve were compared, FluoZin-3 signals were ∼50-fold larger following OGD-SD (1.6±0.71 versus 85.77±12.36 (%ΔF/F0) minutes, n=6).
Figure 4.
Factors influencing the amplitude of extracellular FluoZin-3 transients during OGD-SD. (A) Comparison of extracellular FluoZin-3 signals generated by strong electrical stimulation of Schaffer collateral inputs (50 Hz, 1 second), followed by OGD-SD in the same preparation. Data are collected from wild-type FVB/N slices, and are normalized to OGD-SD peak responses in each slice (n=6). (B) Autofluorescence changes are small and opposite in sign to FluoZin-3 responses. Signals are from unlabeled preparations (n=6, both groups), excited at the same wavelength (495 nm) as used in FluoZin-3 studies. Error bars are smaller than the symbol size, and are overlapping for wild-type C57Bl/6 (filled circles) and ZnT3 KO tissues (open squares). (C) Tissue swelling associated with SD is not responsible for difference in FluoZin-3 signals between wild-type and ZnT3 KO preparations. Mean intrinsic optical signals (see the ‘Materials and methods' section) from 6 wild-type C57Bl/6 and 6 ZnT3 KO preparations. KO, knockout; OGD-SD, oxygen–glucose deprivation-spreading depression.
As has been noted by others, autofluorescence signals could interfere with FluoZin-3 signals (Kay, 2003; Qian and Noebels, 2005). However, Figure 4B shows that differences in background autofluorescence changes are identical in wild-type and ZnT3 KO tissues, and as such cannot be responsible for the conclusions regarding synaptic Zn2+ release made above. Figure 4B shows the time courses of autofluorescence signals recorded in paired sets of wild-type and ZnT3 KO slices that were not bathed in FluoZin-3. These signals are recorded at the same wavelength as used for FluoZin-3 studies (495 nm), and show small decreases that are caused by a combination of flavoprotein autofluorescence changes (Shuttleworth et al, 2003) and tissue swelling that accompanies SD. If differences in baseline fluorescence between unloaded tissues and FluoZin-3 fluorescence are taken into account, the decrease in autofluorescence is calculated to lead to an average underestimation of FluoZin-3 transients of 28.08%±5.00% (n=4). Figure 4C shows that tissue swelling responses (assessed by changes in transmitted light signals, rather than autofluorescence) were also identical in wild-type and ZnT3 KO tissues.
Taken together, results from Figures 3 and 4 imply that: (1) Zn2+ release signals are large, but underestimated because of slow binding to CaEDTA and by concomitant decreases in background autofluorescence and (2) differences in autofluorescence changes or tissue swelling do not underlie the difference between wild-type and ZnT3 KO data described above. These results reinforce the idea that FluoZin-3 increases following OGD-SD are indeed caused by synaptic Zn2+ release.
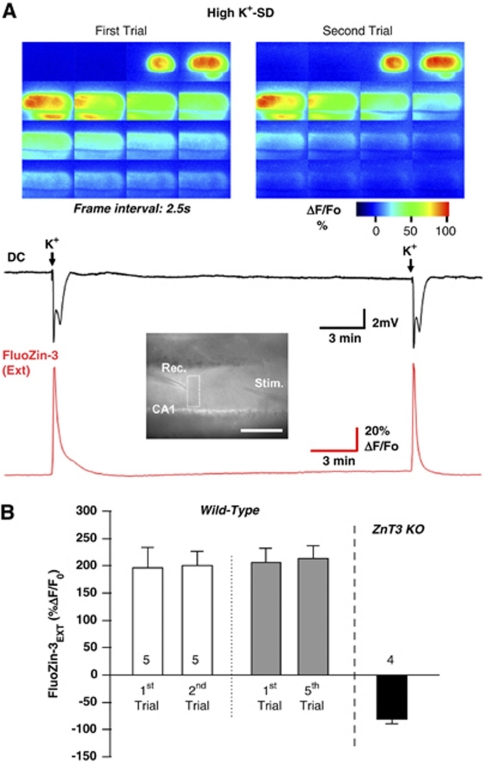
Responses to High K+-Spreading Depression
Repetitive SD-like events have been proposed as an important contributor to the spread of ischemic infarcts in animal models (Hartings et al, 2003; Hossmann, 1996), and recent clinical recordings strongly support this idea for human ischemic stroke (Dohmen et al, 2008; Strong et al, 2002). To determine whether Zn2+ release pools are significantly depleted by a single SD event, or alternatively whether significant Zn2+ release could occur during repetitive SD events, we examined responses to local microinjections of KCl (high K+-SD, see the ‘Materials and methods' section). This stimulus produces SD which can be delivered repetitively without neuronal injury in superfused slices.
Figure 5A shows FluoZin-3 measurements in response to a pair of high K+-SD responses. Similar to the OGD stimulus, K+-SD induced a large increase in FluoZin-3 fluorescence coincident with electrical SD recordings, which propagated with characteristics consistent with SD (4.05±0.30 mm/min, n=5). The second SD (triggered 15 minutes after the first) resulted in a very similar electrical SD and FluoZin-3 response. In a population of five slices (Figure 5B), there was no significant decrease in amplitude of FluoZin-3 transients, and no change in the propagation rate of the responses (4.05±0.30 mm/min versus 4.09±0.37 mm/min, P=0.93, respectively).
Figure 5.
Zn2+ release following high K+-SD. (A) Representative images and plots of extracellular FluoZin-3 and DC recordings during two episodes of high K+-SD in the same wild-type C57Bl/6 preparation. Similar to OGD-SD, a large propagating FluoZin-3 increase was observed with SD. Repetitive SDs could be generated with the high K+-stimulus, and little decrease in the peak amplitude of FluoZin-3 increases was observed. The location of the K+-ejection pipette and the recording sites are indicated in the bright field image, and the color panels show the spread of FluoZin-3 increases during the two SD events. Arrows on DC recording indicate when the microinjection of high K+ was administered. Elapsed time of each montages is ∼1 minute. (B) Summary data showing no significant decrease in peak FluoZin-3 increases with pairs of repetitive high K+-SD trials (wild-type C57Bl/6, n=5), or when comparing the first and fifth SDs in a separate series of experiments. Abolition of FluoZin-3 increases was observed in ZnT3 KO tissues indicating synaptic release of Zn2+ during high K+-SD. KO, knockout; OGD-SD, oxygen–glucose deprivation-spreading depression.
An additional set of experiments was performed to determine whether additional rounds of SD might lead to some progressive decrease in the amplitude of responses. Five SDs were generated in each slice (15-minute interval between trials), and FluoZin-3 was included during the first and fifth trials. Intrinsic optical imaging was used to verify that reproducible SDs were generated throughout the series (see Supplementary Figure 2), and also showed that the propagation rate of the events was not altered (4.54±0.16 mm/min versus 4.61±0.22 mm/min for the first and fifth events, respectively, P=0.77, n=5).
Although there was no demonstrable change in the amplitude of FluoZin-3 transients, it was noted that the first transient recovered somewhat more slowly to baseline than in the second trial, although there was not a significant difference in the area of responses (29.36±6.51 versus 22.20±2.97 (%ΔF/F0) minutes, first versus second, respectively, P=0.25, n=5 each). This may hint at a small additional, delayed Zn2+ release during the first event, but responses may also be influenced by tissue swelling that does not recover fully following the first SD. The fact that there was no progressive acceleration in recovery rate when the second or fifth SDs were compared (Supplementary Figure 2), provides some support for the latter possibility.
Figure 5B also shows that FluoZin-3 increases generated by high K+-SD are abolished in ZnT3 KO preparations. As with OGD-SD in ZnT3 KO tissues, a small propagating fluorescence decrease is noted, which is attributed to optical (autofluorescence and swelling) changes that occur coincident with SD (see above). These observations imply that the first SD event does not deplete available synaptic Zn2+ pools, and that repetitive SDs could lead to repetitive, large extracellular Zn2+ increases in the postischemic period.
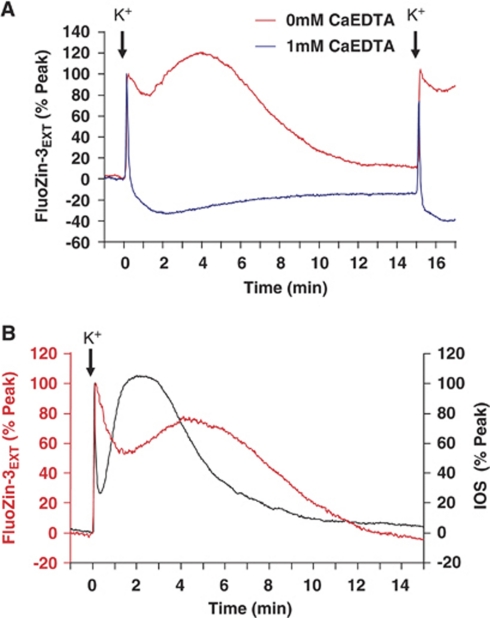
Chelator and Optical Effects on High K+-Spreading Depression Responses
Figure 6 shows that (like the situation with OGD-SD), the duration of Zn2+ accumulation is significantly underestimated because of CaEDTA binding. If CaEDTA is omitted, Zn2+ increases persist for ∼10 minutes after each SD and the increases also invariably appear biphasic, with a second peak occurring ∼4 to 6 minutes after the onset of SD. These biphasic signals could be the result of two distinct phases of Zn2+ release, but could also be caused by interruption of a monophasic Zn2+ signal by an optical artifact. Figure 6B provides some support for the second possibility. In these studies, the kinetics of FluoZin-3 signals were directly compared with IOSs, as determined from transmission signals in the unlabeled tissue (after FluoZin-3 washout in the same tissues). The IOS is attributed to rapid swelling of neurons associated with the propagation of SD and passive accumulation of extracellular K+ by astrocytes (Zhou et al, 2010). As can be seen, a large, delayed swelling response occurred at ∼2 minutes after SD, and corresponded to the time course of the dip in the sustained FluoZin-3 increase. It seemed that the second large peak in IOS interrupted the decay in FluoZin-3 response, and indeed this was observed in all four preparations examined, with the delayed IOS peak interrupting (at mean 2.39 minutes, n=4) the decay in FluoZin-3 decline (peaks at 0.11±0.02 minutes and 4.53±0.61 minutes, n=4) in the same preparations. These findings do not rule out a second delayed phase of Zn2+ release, but they are consistent with the possibility that Zn2+ increases following high K+-SD could involve a single large release event, which can be rapidly terminated by CaEDTA. Detection of prolonged increases (without CaEDTA) may be interrupted by tissue swelling before a slow decline to baseline.
Figure 6.
Factors influencing the amplitude of FluoZin-3 transients during high K+-SD. (A) Like the situation with OGD-SD, omission of CaEDTA resulted in substantially longer-lasting FluoZin-3 transients and revealed an additional component to the decay phase. Responses to pairs of high K+-SD (15-minute interval) are shown in 1 mmol/L CaEDTA (blue) and 0 mmol/L CaEDTA (red). (B) Intrinsic optical signal changes are synchronous with the interruption of the FluoZin-3 decay. The figure shows superimposition of responses from a slice exposed to high-K+ bathed in FluoZin-3, followed by a washout period and a second exposure to high-K+ measuring the intrinsic optical signal (IOS) from the same slice (in the absence of FluoZin-3, see the ‘Materials and methods' section). A large delayed swelling response reported by the IOS (peak ∼2 minutes) appeared to interrupt the slow decay in the FluoZin-3 signal. OGD-SD, oxygen–glucose deprivation-spreading depression.
Intracellular Accumulation of Zn2+ Following Spreading Depression
We examined whether Zn2+ released following SD is accumulated by postsynaptic CA1 neurons. If this is the case, it could provide a significant source of Zn2+ that could contribute to neuronal vulnerability and/or potentially contribute to preconditioning (see the ‘Discussion' section). Postsynaptic Zn2+ increases were assessed following a single-neuron injection of FluoZin-3 using sharp microelectrodes, rather than using bulk loading with membrane-permeable indicators, to minimize compartmentalization of indicator and difficulty with effective bulk loading of slices from 4- to-6-week-old mice. The recording electrode was withdrawn before the onset of SD.
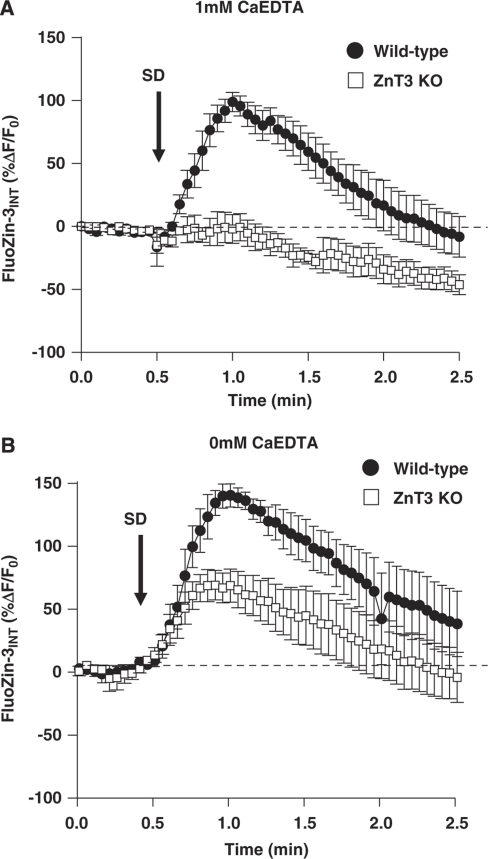
Figure 7 shows mean intracellular FluoZin-3 increases from two sets of experiments using high K+ as a stimulus to trigger SD. In the first set, the recording conditions were identical to those used in Figure 5 wherein CaEDTA was included in the superfusate to remove contaminating Zn2+ and thereby improve signal–noise of extracellular FluoZin-3 increases generated by SD. In this study, the same approach was used to test the possible contributions of extracellular contaminating Zn2+ to intracellular FluoZin-3 responses following SD. As can be seen in wild-type preparations, there were robust intracellular Zn2+ increases in wild-type neurons. In contrast, there was not a significant intracellular transient in ZnT3 KO slices, implying that the main source of Zn2+ available for entry after SD derived from presynaptic vesicles under these conditions.
Figure 7.
Intracellular accumulation of synaptically released Zn2+ following high K+-SD. Each plot shows mean intracellular FluoZin-3 increases from single CA1 neurons loaded with FluoZin-3 using sharp microelectrodes (see the ‘Materials and methods' section). SD was generated by localized high K+ microinjection, as shown in Figures 5 and 6. (A) In CaEDTA, a large increase in intracellular Zn2+ accumulation can be seen in wild-type tissues (n=5, pooled C57Bl/6 and FVB/N), but not in ZnT3 KO tissues (n=5). (B) When experiments were repeated without CaEDTA, intracellular FluoZin-3 increases were observed in both wild-type (n=5, pooled C57Bl/6 and FVB/N) and ZnT3 KO tissues (n=5) indicating that contaminating extracellular Zn2+ also accumulates postsynaptically following high K+-SD. KO, knockout; SD, spreading depression.
Figure 7B shows a parallel set of experiments, conducted without CaEDTA in the superfusate. In these experiments, signals in wild-type preparations were larger than observed without CaEDTA (P<0.03, n=5), and a significant postsynaptic transient is demonstrable in ZnT3 KO tissues. It is possible that CaEDTA could contribute to depletion of Zn2+ that is released intracellularly because of oxidative processes (Frederickson et al, 2002). However, as discussed below, an alternative interpretation of these findings is that (if CaEDTA is not included) there is sufficient contaminating Zn2+ to cause significant postsynaptic accumulation after SD, even without the additional source of coordinated release of Zn2+ from synaptic vesicles.
Discussion
General
This study provides the first evidence that SD causes a coordinated release of Zn2+ from nerve terminals, which depolarize as the SD event propagates across a brain region. Zn2+ release following OGD or localized high K+ stimulation propagated with characteristics entirely consistent with SD, with the wavefront of FluoZin-3 increases occurring coincidently with the DC potential shift and progressing at ∼2 to 5 mm/min across the slice. A similar release was observed with two different types of initiating stimuli, suggesting that the Zn2+ release could contribute to consequences of SD associated with diverse conditions, including brain ischemia, trauma, and migraine aura. A single SD event did not deplete the pool of releasable Zn2+, and Zn2+ released during SD was accumulated in postsynaptic neurons. Thus, repetitive SDs that occur following ischemia could provide a major source of Zn2+, which in turn could contribute to the progression of ischemic brain injury.
Synaptic Zn2+ Release
Zn2+ is accumulated in synaptic vesicles by the ZnT3 transporter (Palmiter et al, 1996), and evidence has accumulated that Zn2+ release is observed following electrical stimulation of synaptic inputs (Frederickson et al, 2005; Sensi et al, 2009), including from studies using extracellular FluoZin-3 in hippocampal slices (Qian and Noebels, 2005, 2006). Despite the fact that ZnT3 KO lack synaptic Zn2+, basic aspects of excitatory transmission and brain function appear relatively normal (Cole et al, 1999, 2001), but a recent report has described cognitive loss in aged ZnT3 KO animals (Adlard et al, 2010). In this study, SD was still generated in ZnT3 KO tissues and appeared indistinguishable from SD in wild-type tissues, consistent with previous conclusions that glutamateric transmission is not substantially impaired in these tissues. The observation that FluoZin-3 increases owing to SD were abolished in ZnT3 KO tissues implies that these signals detected by the indicator are caused by Zn2+ derived from synaptic vesicles. The pattern of release was also consistent with synaptic release, because largest increases were observed in regions involved in SD and with high synaptic density (the stratum oriens and radiatum, Figure 2B).
Although detection of Zn2+ in the extracellular space is usually interpreted to imply that Zn2+ is released and serves as a neurotransmitter/modulator (Qian and Noebels, 2005; Sensi et al, 2009), it has also been suggested that Zn2+ is not normally released, but remains loosely bound to extracellular binding sites exposed during stimulation (Kay, 2003). In the latter case, FluoZin-3 increases could occur as the high-affinity indicator in the extracellular space artificially removes Zn2+ from binding sites within vesicles, to give the appearance of vesicular release. However, the experiments in Figure 6 do not use extracellular indicators that might interfere with release, and yet, Zn2+ increases were detected in postsynaptic neurons following intracellular indicator injections. The fact that intracellular Zn2+ transients were abolished in ZnT3 KO tissues (Figure 6A) strongly suggests that Zn2+ moves across the synapse and accumulates postsynaptically. It is noted that SD is an exceptionally strong stimulus, involving coordinated and likely severe depolarization of a large population of nerve terminals, and the conclusions drawn in this study may not apply to more moderate synaptic stimuli. However, similar conclusions of transsynaptic flux of Zn2+ have recently been made from bulk loading of Newport green into area CA1 and electrical stimulation of afferent fibers (Suh, 2009).
Other Potential Sources for Extracellular Zn2+ Increases During Oxygen–Glucose Deprivation
A previous study has reported that OGD and subsequent reperfusion in rat hippocampal slices produces slowly developing and sustained extracellular Zn2+ increases (Wei et al, 2004), which appear very different from the abrupt transient increases we describe in this study following the passage of SD. The different kinetics are consistent with different sources of Zn2+, because in the previous study, it was concluded that release was not from synaptic vesicles, but rather was caused by oxidative release from intracellular binding proteins. The slice preparation methods and recording conditions between the two studies are quite different, and seem sufficient to prevent SD from being generated in the earlier study. It is also possible that the differences in recording conditions made oxidative release of Zn2+ less prominent in our recording conditions, because significant Zn2+ increases were not observed before SD in the current study, including in experiments in which CaEDTA was not included in the recording media. However, it seems likely that in the intact brain, extracellular Zn2+ levels in peri-infarct areas could increase because of a combination of oxidative release from damaged neurons, together with periodic large synaptic Zn2+ release events as SDs sweep through a brain region. Zn2+ elevations have been reported in microdialysis studies of forebrain ischemia and middle cerebral artery occlusion in rats (Kitamura et al, 2006a, 2006b); however, studies with higher temporal resolution will be required to determine whether there is pulsitile Zn2+ release (due to SD) superimposed on an elevated Zn2+ baseline in different regions surrounding an ischemic infarct.
Contaminating Zn2+
Zn2+ is likely present at low levels in most in vitro recording solutions, as a contaminant of salts and also leached from glass and possibly stimulating electrodes (Kay, 2004). One approach to remove the contributions of exogenous Zn2+ is to use solutions containing CaEDTA, a chelator that binds Zn2+ with much higher affinity than Ca2+ (Kay, 2004). CaEDTA reduces background FluoZin-3 fluorescence to a similar degree in wild-type and ZnT3 KO tissues (Supplementary Figure 1), suggesting that synaptically derived Zn2+ does not contribute significantly to basal Zn2+ levels in the superfusate. Similar to previous descriptions of electrically evoked FluoZin-3 transients in hippocampal slice (Qian and Noebels, 2005), removing contaminating Zn2+ improved the signal–noise ratio of FluoZin-3 transients following SD, and was therefore used in most of the experiments described in this study. However, some experiments were conducted without CaEDTA to determine the possible contribution of contaminating Zn2+ to postsynaptic Zn2+ increases after SD (Figure 6B).
Ca2+ loading of CA1 pyramidal neurons is expected to occur following SD by a reverse operation of sodium–calcium exchangers, multiple voltage-dependent channels, and possibly other nonselective cation channels (Dietz et al, 2008; Somjen, 2001). Many of these pathways will also flux Zn2+ (Weiss et al, 2000), and thus any Zn2+ that contaminates the recording solutions is likely to enter postsynaptic neurons following SD, together with Zn2+ derived from the coordinated release from synaptic vesicles. Results of Figure 6B support this conclusion. When CaEDTA was present, significant FluoZin-3 increases were observed in wild-type neurons but abolished in ZnT3 KO tissues. This implies that synaptic release leads to accumulation close to influx sites that is rapid enough to lead to significant influx before displacement of Ca2+ from the chelator. When CaEDTA was not used, FluoZin-3 increases were larger, and importantly, were also significant in cells from ZnT3 KO animals. This strongly suggests that contaminating Zn2+ can enter during the large postsynaptic depolarization accompanying SD, together with Zn2+ derived from synaptic vesicles. These observations may have implications for other studies of Zn2+ accumulation, if significant amounts of free Zn2+ are present in recording solutions and enter during postsynaptic activation.
Possible Consequences of Synaptic Zn2+ Release
Repetitive SD events accompany and appear to contribute to enlargement of ischemic infarcts (Dohmen et al, 2008; Hartings et al, 2003; Strong et al, 2000). Zn2+ chelation has been shown to reduce injury following global ischemia (Calderone et al, 2004; Galasso and Dyck, 2007; Koh et al, 1996), and a large body of literature has identified mechanisms coupling Zn2+ accumulation to neuronal injury (Choi and Koh, 1998; Dineley et al, 2003; Sensi et al, 2009; Weiss et al, 2000). Therefore, it is tempting to speculate that large Zn2+ increases that occur following SD contribute to the progression of ischemic injuries. Zn2+ increases could lead to disruption of mitochondrial function and/or glycolysis (Sensi et al, 2009; Sheline et al, 2000) and could serve as an upstream trigger of deleterious neuronal Ca2+ overload (Medvedeva et al, 2009; Vander Jagt et al, 2009).
The fact that the vesicular release pool is not depleted by a single SD event implies that there is either small fractional release and/or effective refilling of vesicular Zn2+ pools between repetitive SD events. Therefore, SDs could provide a source for substantial Zn2+ release throughout the postischemic period, because repetitive SDs have been recorded for tens of hours, or even days following experimental or clinical strokes (Dohmen et al, 2008; Hartings et al, 2003; Strong et al, 2002).
Although most studies have concentrated on deleterious functions of Zn2+ elevations, it is also possible that a bolus of Zn2+ release could limit neuronal excitability and prevent damage under some circumstances. For example, Zn2+ is an effective inhibitor of NMDA-type glutamate receptors (Sensi et al, 2009) and limiting flux through these channels after SD has passed through a brain region could limit the activation of excitotoxic processes. Spreading depression is also known to confer ischemic preconditioning, and Zn2+ release during the event might contribute to this process, through mechanisms that have recently been described for Zn2+ in cultured cortical neurons (Aras et al, 2009).
From these considerations, opposing effects of Zn2+ may underlie conflicting reports of the consequences of Zn2+ chelation on infarction following focal ischemia, in which CaEDTA has been reported to both significantly delay (Lee et al, 2002) and aggravate injury (Kitamura et al, 2006c). It is possible SD-dependent Zn2+ release close to an infarct core may contribute to injury, whereas important beneficial effects may be derived from Zn2+ released as SD sweeps further through peri-infarct tissue. Understanding the relative contribution of Zn2+ to injury at different times following stroke should also be helpful to determine whether extracellular Zn2+ levels can be effectively targeted to improve stroke outcome.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by NIH grants NS051288 (CWS.), T32HL007736 (REC), and DK073446 (CTS).
Supplementary Material
References
- Adlard PA, Parncutt JM, Finkelstein DI, Bush AI. Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer's disease. J Neurosci. 2010;30:1631–1636. doi: 10.1523/JNEUROSCI.5255-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras MA, Hara H, Hartnett KA, Kandler K, Aizenman E. Protein kinase C regulation of neuronal zinc signaling mediates survival during preconditioning. J Neurochem. 2009;110:106–117. doi: 10.1111/j.1471-4159.2009.06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone A, Jover T, Mashiko T, Noh KM, Tanaka H, Bennett MV, Zukin RS. Late calcium EDTA rescues hippocampal CA1 neurons from global ischemia-induced death. J Neurosci. 2004;24:9903–9913. doi: 10.1523/JNEUROSCI.1713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Koh JY. Zinc and brain injury. Annu Rev Neurosci. 1998;21:347–375. doi: 10.1146/annurev.neuro.21.1.347. [DOI] [PubMed] [Google Scholar]
- Cole TB, Martyanova A, Palmiter RD. Removing zinc from synaptic vesicles does not impair spatial learning, memory, or sensorimotor functions in the mouse. Brain Res. 2001;891:253–265. doi: 10.1016/s0006-8993(00)03220-0. [DOI] [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz RM, Weiss JH, Shuttleworth CW. Zn2+ influx is critical for some forms of spreading depression in brain slices. J Neurosci. 2008;28:8014–8024. doi: 10.1523/JNEUROSCI.0765-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineley KE, Votyakova TV, Reynolds IJ. Zinc inhibition of cellular energy production: implications for mitochondria and neurodegeneration. J Neurochem. 2003;85:563–570. doi: 10.1046/j.1471-4159.2003.01678.x. [DOI] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- Fabricius M, Jensen LH, Lauritzen M. Microdialysis of interstitial amino acids during spreading depression and anoxic depolarization in rat neocortex. Brain Res. 1993;612:61–69. doi: 10.1016/0006-8993(93)91644-8. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nat Rev. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Suh SW, Koh JY, Cha YK, Thompson RB, LaBuda CJ, Balaji RV, Cuajungco MP. Depletion of intracellular zinc from neurons by use of an extracellular chelator in vivo and in vitro. J Histochem Cytochem. 2002;50:1659–1662. doi: 10.1177/002215540205001210. [DOI] [PubMed] [Google Scholar]
- Galasso SL, Dyck RH. The role of zinc in cerebral ischemia. Mol Med. 2007;13:380–387. doi: 10.2119/2007-00044.Galasso. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartings JA, Rolli ML, Lu XC, Tortella FC. Delayed secondary phase of peri-infarct depolarizations after focal cerebral ischemia: relation to infarct growth and neuroprotection. J Neurosci. 2003;23:11602–11610. doi: 10.1523/JNEUROSCI.23-37-11602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossmann KA. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev. 1996;8:195–208. [PubMed] [Google Scholar]
- Kay AR. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn. J Neurosci. 2003;23:6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. Detecting and minimizing zinc contamination in physiological solutions. BMC Physiol. 2004;4:4. doi: 10.1186/1472-6793-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Iida Y, Abe J, Mifune M, Kasuya F, Ohta M, Igarashi K, Saito Y, Saji H. In vivo measurement of presynaptic Zn2+ release during forebrain ischemia in rats. Biol Pharm Bull. 2006a;29:821–823. doi: 10.1248/bpb.29.821. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Iida Y, Abe J, Mifune M, Kasuya F, Ohta M, Igarashi K, Saito Y, Saji H. Release of vesicular Zn2+ in a rat transient middle cerebral artery occlusion model. Brain Res Bull. 2006b;69:622–625. doi: 10.1016/j.brainresbull.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kitamura Y, Iida Y, Abe J, Ueda M, Mifune M, Kasuya F, Ohta M, Igarashi K, Saito Y, Saji H. Protective effect of zinc against ischemic neuronal injury in a middle cerebral artery occlusion model. J Pharmacol Sci. 2006c;100:142–148. doi: 10.1254/jphs.fp0050805. [DOI] [PubMed] [Google Scholar]
- Koh JY, Suh SW, Gwag BJ, He YY, Hsu CY, Choi DW. The role of zinc in selective neuronal death after transient global cerebral ischemia. Science. 1996;272:1013–1016. doi: 10.1126/science.272.5264.1013. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Hulse RE, Schmitt MW, Nicholson C, Kraig RP. Optical current source density analysis in hippocampal organotypic culture shows that spreading depression occurs with uniquely reversing currents. J Neurosci. 2005;25:3952–3961. doi: 10.1523/JNEUROSCI.0491-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leao AAP. Spreading depression of activity in the cerebral cortex. J Neurophysiol. 1944;7:359–390. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Park KH, He YY, Hsu CY, Choi DW. Zinc translocation accelerates infarction after mild transient focal ischemia. Neuroscience. 2002;115:871–878. doi: 10.1016/s0306-4522(02)00513-4. [DOI] [PubMed] [Google Scholar]
- Medvedeva YV, Lin B, Shuttleworth CW, Weiss JH. Intracellular Zn2+ accumulation contributes to synaptic failure, mitochondrial depolarization, and cell death in an acute slice oxygen-glucose deprivation model of ischemia. J Neurosci. 2009;29:1105–1114. doi: 10.1523/JNEUROSCI.4604-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol. 2005;566:747–758. doi: 10.1113/jphysiol.2005.089276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Noebels JL. Exocytosis of vesicular zinc reveals persistent depression of neurotransmitter release during metabotropic glutamate receptor long-term depression at the hippocampal CA3-CA1 synapse. J Neurosci. 2006;26:6089–6095. doi: 10.1523/JNEUROSCI.0475-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumschik SM, Nydegger I, Zhao J, Kay AR. The interplay between inorganic phosphate and amino acids determines zinc solubility in brain slices. J Neurochem. 2009;108:1300–1308. doi: 10.1111/j.1471-4159.2009.05880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nat Rev. 2009;10:780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- Sheline CT, Behrens MM, Choi DW. Zinc-induced cortical neuronal death: contribution of energy failure attributable to loss of NAD(+) and inhibition of glycolysis. J Neurosci. 2000;20:3139–3146. doi: 10.1523/JNEUROSCI.20-09-03139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CW, Brennan AM, Connor JA. NAD(P)H fluorescence imaging of postsynaptic neuronal activation in murine hippocampal slices. J Neurosci. 2003;23:3196–3208. doi: 10.1523/JNEUROSCI.23-08-03196.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, Parkin MC, Lauritzen M. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33:2738–2743. doi: 10.1161/01.str.0000043073.69602.09. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Smith SE, Whittington DJ, Meldrum BS, Parsons AA, Krupinski J, Hunter AJ, Patel S, Robertson C. Factors influencing the frequency of fluorescence transients as markers of peri-infarct depolarizations in focal cerebral ischemia. Stroke. 2000;31:214–222. doi: 10.1161/01.str.31.1.214. [DOI] [PubMed] [Google Scholar]
- Suh SW. Detection of zinc translocation into apical dendrite of CA1 pyramidal neuron after electrical stimulation. J Neurosci Methods. 2009;177:1–13. doi: 10.1016/j.jneumeth.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Vander Jagt TA, Connor JA, Weiss JH, Shuttleworth CW. Intracellular Zn(2+) increases contribute to the progression of excitotoxic Ca(2+) increases in apical dendrites of CA1 pyramidal neurons. Neuroscience. 2009;159:104–114. doi: 10.1016/j.neuroscience.2008.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Hough CJ, Li Y, Sarvey JM. Characterization of extracellular accumulation of Zn2+ during ischemia and reperfusion of hippocampus slices in rat. Neuroscience. 2004;125:867–877. doi: 10.1016/j.neuroscience.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Weiss JH, Sensi SL, Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- Zhou N, Gordon GR, Feighan D, Macvicar BA. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cereb Cortex. 2010;20:2614–2624. doi: 10.1093/cercor/bhq018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.