Abstract
The adipocytokine leptin has distinct functions regulating vascular tone, inflammation, and collateral artery growth. Arteriogenesis is an inflammatory process and provides a mechanism to overcome the effects of vascular obstruction. We, therefore, tested the effects of leptin in hypoperfused rat brain (three-vessel occlusion). Systemic leptin administration for 1 week after occlusion surgery increased cerebral hemodynamic reserve similar to granulocyte–macrophage colony-stimulating factor (GM-CSF), as indicated by improved CO2 reactivity (vehicle 0.53%±0.26% versus leptin 1.05%±0.6% per mm Hg arterial pCO2, P<0.05). Infusion of microspheres under maximal vasodilation failed to show a positive effect of leptin on cerebral perfusion (vehicle 64.9%±4.5% versus leptin 66.3%±7.0%, occluded/nonoccluded hemisphere). Acute treatment with GM-CSF led to a significant increased CO2 reactivity and cerebral perfusion (79.2%±8.1% versus 64.9%±4.5%, P<0.05). Vasoconstrictive response of isolated rat carotid artery rings, after phenylephrine was attenuated at 24 hours following preincubation with leptin, was unaffected by removal of endothelium but abrogated by coculture with N-(omega)-nitro--arginine methylester, pointing toward an inducible nitric oxide synthase-mediated mechanism. In chronic cerebral hypoperfusion, acute leptin treatment restored the hemodynamic reserve of the cerebral vasculature through its effects on vascular tone, while leaving vascular outward remodeling unaffected. Our results, for the first time, reveal a protective role of leptin on vascular function in hemodynamically compromised brain tissue.
Keywords: arteriogenesis, cerebral hypoperfusion, collateral artery growth, leptin, three-vessel occlusion, vascular reactivity
Introduction
Collateral artery growth (arteriogenesis) is a natural escape mechanism capable of overcoming the detrimental effects of arterial obstruction and occlusion. Vascular growth and proliferation are inflammatory processes mediated by monocyte/macrophage-derived cytokines and growth factors (Fujii et al, 2006). Stimulation of arteriogenesis by granulocyte–macrophage colony-stimulating factor (GM-CSF) (Schneeloch et al, 2004) in a nonfatal hypoperfusion model of the rat brain (Busch et al, 2003) led to significant proliferation of the ipsilateral posterior artery (posterior cerebral arteries, PCA), an important preexisting cerebral collateral pathway (Buschmann et al, 2003). We recently reported that the adaptive proliferation of preexisting collateral pathways is an effective rescue system against detrimental effects of tissue ischemia in the rat brain (Schneeloch et al, 2004).
The adipocytokine leptin has proinflammatory properties acting through the Ob receptor on peripheral tissue such as endothelial cells or monocytes (Sanchez-Margalet and Martin-Romero, 2001). High plasma levels of leptin are associated with cardiovascular disease, probably based on peripheral leptin resistance (Knudson et al, 2005). The adipocytokine has been described to improve endothelial function in obese patients, improving vasodilatory reserve and counterbalancing the negative cardiovascular effects of increased body weight (Schindler et al, 2006). We have previously shown a positive effect of leptin on reactive hyperemic blood flow in a rabbit model (Schirmer et al, 2004). Leptin was shown to induce C-reactive protein in human endothelial cells (Singh et al, 2007), to activate peripheral blood mononuclear cells through the Janus kinase/signal transducers and activators of transcription pathway (Sanchez-Margalet and Martin-Romero, 2001), and to result in production of proinflammatory, angiogenic cytokines (Aleffi et al, 2005). Furthermore, leptin has been described to stimulate endothelial cell proliferation and angiogenesis (Bouloumie et al, 1998; Sierra-Honigmann et al, 1998).
In this study, we tested the hypothesis whether leptin has beneficial effects on cerebral perfusion in an established oligemia model of the brain by improving vascular function and stimulating monocyte-mediated arteriogenesis.
Materials and methods
Animal Model
Experiments were carried out with permission of state authorities according to the German Law for Protection of Animals and to the Animal Ethical Committee of the University of Amsterdam, The Netherlands, conforming to the National Institute of Health Guidelines for Care and Use of Laboratory Animals (NIH Publication No. 85-23 revised 1996).
A nonlethal brain hypoperfusion model (three-vessel occlusion, 3-VO) was used to investigate cerebral arteriogenesis, as previously described (Busch et al, 2003). In brief, 53 adult male Sprague–Dawley rats (weight: 270 to 350 g) were anesthetized with 0.8% to 1.5% halothane in 70%:30% nitrous oxide/oxygen. Vertebral arteries were coagulated by paravertebral access, as proposed by Pulsinelli and Brierley (1979; Pulsinelli et al, 1983), and the left common carotid artery was exposed by ventral cervical midline incision and ligated under simultaneous laser Doppler flowmetry (PeriSoft; PeriMed, Järfälla, Sweden). After surgery, wounds were closed and infiltrated with Bupivacaine (0.25%). After recovery from anesthesia, animals were returned to their cages in pairs of two, with free access to chow and water, and allowed to move freely. For measurements of the cerebrovascular reserve, 8 animals were used as nonoperated control rats (control group, no 3-VO), 9 animals were studied after 3-VO following application of Ringer's solution (vehicle group), 10 animals were studied after 3-VO following application of leptin (300 μg/kg per day; R&D Systems, Wiesbaden, Germany; leptin-treated group), and additional 10 animals were studied after 3-VO following application of GM-CSF (40 μg/kg per day; Leucomax, Schering-Plough, USA; GM-CSF-treated group). Solutes were applied by subcutaneous injections every day for 7 days.
For the microsphere perfusion studies, separate animals were used (n=16). Five animals were studied after 3-VO following application of Ringer's solution (vehicle group), six animals were studied after 3-VO following application of leptin (300 μg/kg per day; R&D Systems; leptin-treated group), and additional five animals were studied after 3-VO following application of GM-CSF (40 μg/kg per day; Leucomax; GM-CSF-treated group). Solutes were applied by subcutaneous injections every day for 7 days. For the carotid ring studies, separate animals (n=5) were used per condition. Carotid rings from one animal were distributed over the different conditions.
Hemodynamic Measurements after 7 Days
Changes of the cerebrovascular reserve were tested by ventilating animals with 6% CO2 and recording the associated alterations in cerebral blood flow. After 1 week, recovery animals were fixed in the same way and laser Doppler flow were measured at same sites. Animals were reanesthetized with 3.5% to 4% halothane and maintained with 0.8% to 1.5% halothane in 70%:30% nitrous oxide/oxygen. After tracheotomy, rats were paralyzed with pancuronium bromide (0.2 mg/kg/h) and mechanically ventilated with a small animal respirator. For testing changes of the cerebrovascular reserve, arterial pCO2 levels of the animals was raised by connecting the input of the animal respirator for ∼6 minutes to a certified gas bottle, containing 6% CO2, 30% oxygen, and 64% nitrous oxide and kept on the same level of anesthesia. Body temperature was kept constant at 37°C, using a feedback-controlled heating pad, and blood gases were measured in arterial samples, drawn from a femoral artery. During ventilation with 6% CO2, cerebral blood flow in both hemispheres was measured by laser Doppler flowmetry in each animal for at least three times and expressed as percent increase of blood flow in parietal cortex per mm Hg arterial pCO2 change. Between each measurement, arterial pCO2 levels were allowed to return to baseline (Busch et al, 2003).
To quantify directly the effects of vascular remodeling on cerebral perfusion, a microsphere perfusion method was used as previously described (Hoefer et al, 2001). In brief, differently labeled fluorescent microspheres were injected into the right common carotid artery under conditions of adenosine-induced maximal vasodilation at different pressure levels, 7 days after 3-VO. After weighing and tissue lysis, microspheres per gram of tissue were counted using a flow cytometer. Perfusion was expressed as percentage left (ligated carotid artery) versus right hemisphere.
Visualization of Cerebral Angioarchitecture
Cerebrovascular anatomy was studied by a modification (Maeda et al, 1998) of the latex perfusion method of Coyle (Chicago Latex Product no. 563, Crystal Lake, IL, USA) (Coyle, 1984; Coyle and Heistad, 1991; Coyle and Panzenbeck, 1990). After perfusion under maximal vasodilatation, brains were carefully removed and fixed by immersion in 4% paraformaldehyde. External diameter of both internal carotid arteries, anterior cerebral arteries, middle cerebral arteries, the posterior communicating arteries, and both PCA were measured, using a stereo-zoom microscope with a calibrated eyepiece micrometer.
Vascular Reactivity of Carotid Artery Segments
Experiments on vascular reactivity were conducted as described before (Mulders et al, 2006). In brief, carotid artery segments were incubated for 24 hours in MEM199 culture medium containing 100 U/ml penicillin and 100 μg/ml streptomycin in a humidified atmosphere (5% CO2:95% O2) at 37°C in the absence or presence of leptin (R&D Systems) or vehicle. Cumulative concentration response curves for phenylephrine were calibrated for carotid artery segments after 30 minutes of preincubation with the nitric oxide synthase (NOS)-inhibitor N (omega)-nitro--arginine methylester (-NAME; 100 μmol) as well as with the vehicle (distilled water). In selected preparations, the endothelium was removed after 24 hours of incubation with leptin or vehicle, by inserting a polyethylene catheter into the lumen and gently rubbing the vascular segments. Endothelial integrity was checked for all preparations as previously described (Mulders et al, 2006). Isometric force of contraction was measured continuously, and data are presented as percentage of the third KCl (100 mmol/L) induced contraction.
Data Analysis
All data are given as mean±s.e.m. as the number of experiments has been provided in each experimental group. Changes of laser Doppler flow results are expressed as percentage of the mean baseline value. The Kolmogorov–Smirnov test was used to verify normal distribution within various experimental groups. Group differences for hemodynamic, morphologic, and general physiologic measurements were analyzed for statistical significance using analysis of variance. Post hoc comparisons were performed using Bonferroni correction. Statistical significance was assumed at P-value <0.05.
Results
Metabolic Effects of Leptin Treatment
To document the efficacy of leptin treatment, animals were weighed every day. Leptin treatment significantly reduced total body weight by 13% (P<0.001) after 1 week, as compared with control animals (see Table 1).
Table 1. Total body weight of the four groups at baseline and after 1 week therapy.
| Control (no 3-VO, n=8) | Vehicle (n=9) | Leptin (n=10) | GM-CSF (n=10) | |
|---|---|---|---|---|
| Weight before 3-VO (g) | 320±6 | 316±8 | 317±6 | 315±0 |
| Weight after 1 week (g) | 320±2 | 298±12 (−5.7%) | 275±6* (−13%) | 307±10 (−2.5%) |
3-VO, three-vessel occlusion; GM-CSF, granulocyte–macrophage colony stimulating factor.
Percent change in body weight is indicated in parentheses. Leptin treatment significantly reduced total body weight compared with baseline values before treatment (*P<0.001).
Cerebral Vascular Remodeling after Three-Vessel Occlusion
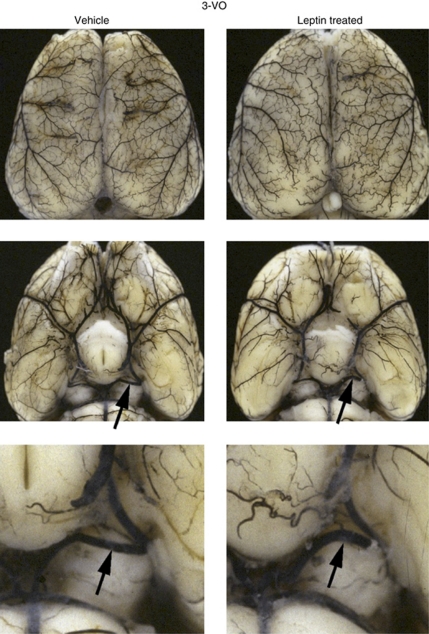
Changes of cerebral angioarchitecture after 3-VO was examined using the cerebrovascular latex perfusion method. The PCA is the main collateral pathway of the brain at the level of the circle of Willis. At 1 week after 3-VO, the external diameter of the PCA, ipsilateral to the carotid occlusion increased significantly, as compared with nonischemic control rats, whereas no change was noted contralaterally (Table 2; Figure 1). Application of leptin for 1 week did not significantly increase the external diameter of the ipsilateral PCA. Similarly, the anterior cerebral vasculature system was not affected by the treatment with leptin, and the cerebral collateral system of the brain surface (Heubner's anastomoses) did not show a significant difference after 1 week of leptin treatment, compared with untreated control rats.
Table 2. Changes in external vascular diameter (μm) of anterior and posterior cerebral arteries: main collateral pathways after three-vessel occlusion (3-VO).
|
Anterior cerebral artery |
Posterior cerebral artery |
|||
|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | |
| Nonischemic control | 251±14 | 290±19 | 187±10 | 196±11 |
| 1 Week 3-VO | ||||
| Vehicle (n=5) | 279±14 | 237±9 | 250±11‡ | 199±12 |
| Leptin (n=6) | 286±12 | 224±12 | 281±11 | 184±9 |
| GM-CSF (n=5) | 322±12 | 311±15* (+31%) | 317±13* | 231±10 |
GM-CSF, granulocyte–macrophage colony stimulating factor.
After latex infusion, external diameter of main collateral pathways was determined using stereo-zoom microscopy (‡P<0.05 compared with nonischemic control; *P<0.05 compared with vehicle 3-VO).
Figure 1.
Cerebral angioarchitecture. Visualization of the cerebral angioarchitecture using the latex method after maximal vasodilation. Dorsal view of brains investigated 1 week after three-vessel occlusion (3-VO) in vehicle- and leptin-treated animals. Leptin treatment leads to a moderate but not significant enlargement of ipsilateral PCA (arrows) compared with vehicle-treated animals.
Hemodynamic Effects of Leptin Treatment
The hemodynamic effects of 3-VO and consecutive treatment with leptin or GM-CSF were tested by measuring alterations of blood flow during ventilation with 6% CO2. Arterial pCO2 increased during controlled ventilation with 6% CO2 similarly in all four groups, that is, by 21±2 mm Hg in control rats, 26±2 mm Hg in vehicle-treated animals, 25±2 mm Hg in GM-CSF-treated animals, and 24±2 mm Hg in leptin-treated animals at 1 week after 3-VO (P=NS).
At 1 week after 3-VO, total cerebral reactivity to CO2 was significantly reduced in vehicle-treated animals compared with nonischemic control rats. Treatment with leptin or GM-CSF, however, led to restoration of the total cerebrovascular reactivity. Total CO2 reactivity is expressed as the average cerebrovascular reactivity of both hemispheres.
On the side of ligation of the common carotid artery (ipsilaterally), cerebrovascular reactivity after 1 week was significantly reduced in vehicle-treated animals. After leptin or GM-CSF treatment, the ipsilateral hemodynamic reserve of the brain recovered to the level of nonoperated animals compared with nonoperated control mice (see Table 3).
Table 3. Carbon dioxide reactivity of cerebral blood flow in nonoperated controls, in vehicle-, leptin-, and GM-CSF-treated animals after three-vessel occlusion (3-VO).
|
Carbon dioxide reactivity (% per mm Hg apCO2) |
Microsphere perfusion (% occluded/non-occluded hemisphere) | |||
|---|---|---|---|---|
| Total | Ipsilateral | Contralateral | ||
| Nonischemic control (n=8) | 1.29±0.10 | 1.48±0.15 | 1.10±0.09 | |
| 1 Week 3-VO | ||||
| Vehicle (n=9) | 0.54±0.09# | 0.42±0.08# | 0.65±0.12# | 64.9±1.4 |
| Leptin (n=10) | 1.05±0.20 | 1.11±0.24* | 0.99±0.24 | 66.3±2.2 |
| GM-CSF (n=10) | 1.13±0.25 | 1.25±0.28* | 1.12±0.41 | 79.2±2.6‡ |
GM-CSF, granulocyte–macrophage colony stimulating factor.
Blood flow was measured during ventilation with 6% CO2 by laser Doppler flowmetry (LDF) of the ipsilateral and contralateral hemispheres. The CO2 reactivity is expressed as percent change of LDF per mm Hg increase of arterial pCO2. Total CO2 reactivity is the average cerebrovascular reactivity of both hemispheres. Note suppression of CO2 reactivity at 1 week of 3-VO (#P<0.05 vehicle-treated versus nonischemic control group), followed by almost complete recovery after leptin and GM-CSF treatment (*P<0.05 compared with vehicle treatment). Only GM-CSF treatment lead to a significant improved microsphere perfusion under maximal vasodilation (‡P<0.05, vehicle- compared with leptin-treated animals).
Microsphere perfusion of the brain under maximal vasodilation failed to show an effect of leptin on cerebral perfusion. Conversely, GM-CSF treatment led to a significant improvement of perfusion under maximal vasodilation measured with the microsphere method.
Effects of Leptin on Vascular Tone
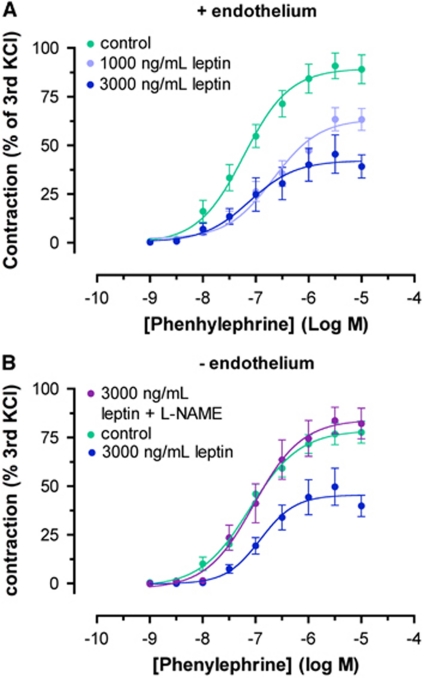
We hypothesized that leptin treatment of carotid artery rings would result in an increased vasodilatory response toward methylcholine, but preincubation with different concentrations of leptin did not have an effect on the methylcholine-induced vasodilation. However, preincubation of carotid artery segments with leptin for 24 hours significantly damped cumulative concentration response curves for phenylephrine, showing a weakened contractile capacity of the carotid artery (Figure 2A). To elucidate the mechanisms behind this leptin-induced shift of vascular tone toward a more dilated state, we removed the endothelium from the carotid arteries, thereby abolishing any effects of endothelium-derived vasodilatory factors such as endothelial NOS. As this did not have any effect, we then added -NAME to the vessel, thereby inhibiting endothelium-independent inducible NOS (iNOS). This measure completely suppressed the effect of leptin, pointing toward a significant iNOS-mediated mechanism (Figure 2B).
Figure 2.
Vasoreactivity after leptin incubation ex-vivo. Carotid rings were preincubated with leptin for 24 hours, and cumulative concentration response curves were calculated for the contractile reaction toward phenylephrine. (A) Note the delay in the phenylephrine-induced contractile response after 24-hour leptin incubation. (B) -NAME (N-(omega)-nitro--arginine methylester) treatment of endothelium-removed carotid artery rings completely abrogated the effects of leptin incubation, pointing toward an inducible nitric oxide synthase-mediated mechanism.
Discussion
This study shows that the adipocytokine leptin improves cerebrovascular reactivity in a nonlethal model of hypoperfused rat brain, but did not induce significant positive outward remodeling of the cerebral angioarchitecture. Conversely, leptin did not significantly enhance cerebral perfusion, as assessed by microsphere infusion under maximal vasodilation. The adipocytokine endothelium independently attenuated the vasoconstrictive response of carotid arteries toward phenylephrine, which was inhibited by -NAME, suggesting an iNOS-mediated effect of leptin on collateral vasotonus.
Leptin and Collateral Artery Growth
We have previously shown that cerebral arteriogenesis can be stimulated by systemic therapy with GM-CSF, which leads to a significant increase in proliferation and outward remodeling of preexisting cerebral collateral pathways, alongside an improved cerebrovascular CO2 reactivity (Buschmann et al, 2003). The effects of GM-CSF on cerebral perfusion, as assessed under conditions of maximal vasodilation, clearly indicate that both GM-CSF and leptin vascular reactivity, as well as proliferation in a nonlethal rat model of cerebral hypoperfusion, are of importance. Microsphere perfusion experiments confirmed CO2 reactivity data and hence the proarteriogenic role of GM-CSF. Treatment with leptin resulted in a significantly improved CO2 reactivity, but only in a moderate, not significant, outward enlargement of main collateral conductance arteries. Perfusion measurements under maximal vasodilation revealed that these changes in hemodynamics are not due to vascular proliferation. Cytokine effects on vasotonus and remodeling may differ between distinct vascular territories. We reported earlier the effects of leptin on reactive hyperemia in a rabbit hindlimb model (Schirmer et al, 2004). In the latter study, leptin improved hindlimb blood flow during hyperemia, but left collateral artery growth unaffected. Completely abolishing vascular reactivity by inducing maximal vasodilation yields reliable measurements of arterial growth. However, more functional approaches, such as the measurement of cerebrovascular reactivity, can provide valuable insights into clinically relevant mechanisms such as endothelial function.
Leptin and Vascular Function
The vasodilatory capacity of the cerebral circulation is of importance in various physiologic and pathophysiologic conditions. The capacity of the vasculature to respond directly to acute alterations in tissue perfusion is relevant to prevent detrimental effects of arterial stenoses and keep cerebral perfusion above critical levels. Insights into the mechanisms of vascular response can increase our understanding of disease processes. Vasodilation is a fundamental escape mechanism in conditions of tissue hypoperfusion. Under clinical conditions, measurement of cerebrovascular reactivity is a useful tool for risk assessment in patients with severe extracranial stenoses. The pathophysiologic importance of leptin and hyperleptinemia in patients are discussed controversially. Data from (healthy) animal models have shown a vasodilatory effect of leptin (Vecchione et al, 2003). Although obesity is known to negatively affect coronary endothelial function, elevated leptin levels may have a positive effect on endothelial function in obese patients (Schindler et al, 2006). Knudson et al (2005) described leptin resistance in prediabetic subjects with a high risk of atherosclerosis and coronary heart disease. At the same time, recent investigations showed that elevated plasma leptin levels were associated with insulin resistance, inflammation, and disturbance of hemostasis, and were associated with an increased risk of atherosclerosis and coronary heart disease (Schindler et al, 2006). Furthermore, leptin is known to be involved in neointima formation (Bodary et al, 2007).
Our data suggest a beneficial effect of leptin on the vasculature, attenuating vasoconstriction and improving vasodilatory capacity. Vascular dysfunction in the clinical situation may, at least to some extent, be due to leptin resistance, which is found in disease conditions such as diabetes or hypertension.
Mechanisms of the Effects on Vasotonus
Only a few hormones have been studied as extensively as leptin in the recent years, but current data on its potential vasodilatory effects and underlying mechanisms are still poorly understood. Our results suggest that the effects of leptin are endothelium independent. Momin et al (2006) also showed an endothelium independent effect of leptin (Momin et al, 2006). The fact that treatment of the endothelium-free carotid rings with -NAME abrogated the effects of leptin suggests that the underlying mechanism is iNOS related. Interestingly, a recent study has shown that leptin prevents angiotensin II-induced vasoconstriction and stimulates nitric oxide production in vascular smooth muscle cells, also through stimulation of iNOS expression (Rodriguez et al, 2007). This effect was attenuated in hypertensive rats (Rodriguez et al, 2006). In contrast, earlier reports have described an endothelium-dependent effect of leptin-induced vasodilation (Vecchione et al, 2002). Other reports have suggested an nitric oxide-independent but smooth muscle cell hyperpolarization-mediated mechanism (Momin et al, 2006). The exact vascular effects of leptin are potentially specific to the vascular bed, as suggested by Lembo et al (2000). Possibly, endothelial-independent, iNOS-mediated effects, as described in this study, are distinctive players in the cerebral circulation, whereas the effects of leptin on large arteries in the peripheral or coronary circulation are endothelium dependent.
Conclusion
This study demonstrates significant effects of the adipocytokine leptin on the cerebrovascular tone under oligemic conditions of the brain. Leptin treatment improved cerebrovascular reactivity on 3-VO in vivo, attenuated carotid artery contractility in vitro, but failed to induce enhancement of cerebral perfusion and a significant improvement of arteriogenesis. These cerebrovascular effects of the adipocytokine leptin contribute to the understanding of its function in cerebrovascular regulation, suggesting further studies to clarify the involved pathogenetic mechanisms in more detail.
The authors declare no conflict of interest.
References
- Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, Vizzutti F, Anania FA, Milani S, Rombouts K, Laffi G, Pinzani M, Marra F. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG, Jr, Eitzman DT. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 2007;27:70–76. doi: 10.1161/01.ATV.0000252068.89775.ee. [DOI] [PubMed] [Google Scholar]
- Bouloumie A, Drexler HCA, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- Busch HJ, Buschmann IR, Mies G, Bode C, Hossmann KA. Arteriogenesis in hypoperfused rat brain. J Cereb Blood Flow Metab. 2003;23:621–628. doi: 10.1097/01.WCB.0000057741.00152.E4. [DOI] [PubMed] [Google Scholar]
- Buschmann IR, Busch HJ, Mies G, Hossmann KA. Therapeutic induction of arteriogenesis in hypoperfused rat brain via granulocyte-macrophage colony-stimulating factor. Circulation. 2003;108:610–615. doi: 10.1161/01.CIR.0000074209.17561.99. [DOI] [PubMed] [Google Scholar]
- Coyle P. Diameter and length changes in cerebral collaterals after middle cerebral artery occlusion in the young rat. Anat Rec. 1984;210:357–364. doi: 10.1002/ar.1092100211. [DOI] [PubMed] [Google Scholar]
- Coyle P, Heistad DD. Development of collaterals in the cerebral circulation. Blood Vessels. 1991;28:183–189. doi: 10.1159/000158860. [DOI] [PubMed] [Google Scholar]
- Coyle P, Panzenbeck MJ. Collateral development after carotid artery occlusion in Fischer 344 rats. Stroke. 1990;21:316–321. doi: 10.1161/01.str.21.2.316. [DOI] [PubMed] [Google Scholar]
- Fujii T, Yonemitsu Y, Onimaru M, Tanii M, Nakano T, Egashira K, Takehara T, Inoue M, Hasegawa M, Kuwano H, Sueishi K. Nonendothelial mesenchymal cell-derived MCP-1 is required for FGF-2-mediated therapeutic neovascularization: critical role of the inflammatory/arteriogenic pathway. Arterioscler Thromb Vasc Biol. 2006;26:2483–2489. doi: 10.1161/01.ATV.0000244684.23499.bf. [DOI] [PubMed] [Google Scholar]
- Hoefer IE, van Royen N, Buschmann IR, Piek JJ, Schaper W. Time course of arteriogenesis following femoral artery occlusion in the rabbit. Cardiovasc Res. 2001;49:609–617. doi: 10.1016/s0008-6363(00)00243-1. [DOI] [PubMed] [Google Scholar]
- Knudson JD, Dincer UD, Zhang C, Swafford AN, Jr, Koshida R, Picchi A, Focardi M, Dick GM, Tune JD. Leptin receptors are expressed in coronary arteries, and hyperleptinemia causes significant coronary endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H48–H56. doi: 10.1152/ajpheart.01159.2004. [DOI] [PubMed] [Google Scholar]
- Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- Maeda K, Hata R, Hossmann K-A. Differences in the cerebrovascular anatomy of C57Black/6 and SV129 mice. Neuroreport. 1998;9:1317–1319. doi: 10.1097/00001756-199805110-00012. [DOI] [PubMed] [Google Scholar]
- Momin AU, Melikian N, Shah AM, Grieve DJ, Wheatcroft SB, John L, El Gamel A, Desai JB, Nelson T, Driver C, Sherwood RA, Kearney MT. Leptin is an endothelial-independent vasodilator in humans with coronary artery disease: evidence for tissue specificity of leptin resistance. Eur Heart J. 2006;27:2294–2299. doi: 10.1093/eurheartj/ehi831. [DOI] [PubMed] [Google Scholar]
- Mulders AC, Hendriks-Balk MC, Mathy MJ, Michel MC, Alewijnse AE, Peters SL. Sphingosine kinase-dependent activation of endothelial nitric oxide synthase by angiotensin II. Arterioscler Thromb Vasc Biol. 2006;26:2043–2048. doi: 10.1161/01.ATV.0000237569.95046.b9. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Levy DE, Duffy TE. Cerebral blood flow in the four-vessel occlusion rat model. Stroke. 1983;14:832–834. doi: 10.1161/01.str.14.5.832. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Fortuno A, Gomez-Ambrosi J, Zalba G, Diez J, Fruhbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–331. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Fruhbeck G, Gomez-Ambrosi J, Catalan V, Sainz N, Diez J, Zalba G, Fortuno A. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction is blunted in spontaneously hypertensive rats. J Hypertens. 2006;24:1589–1597. doi: 10.1097/01.hjh.0000239295.17636.6e. [DOI] [PubMed] [Google Scholar]
- Sanchez-Margalet V, Martin-Romero C. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell Immunol. 2001;211:30–36. doi: 10.1006/cimm.2001.1815. [DOI] [PubMed] [Google Scholar]
- Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, Sayre J, Dahlbom M, Licinio J, Schelbert HR. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–1195. doi: 10.1016/j.jacc.2005.10.062. [DOI] [PubMed] [Google Scholar]
- Schirmer SH, Buschmann IR, Jost MM, Hoefer IE, Grundmann S, Andert JP, Ulusans S, Bode C, Piek JJ, van Royen N. Differential effects of MCP-1 and leptin on collateral flow and arteriogenesis. Cardiovasc Res. 2004;64:356–364. doi: 10.1016/j.cardiores.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Schneeloch E, Mies G, Busch HJ, Buschmann IR, Hossmann KA. Granulocyte-macrophage colony-stimulating factor-induced arteriogenesis reduces energy failure in hemodynamic stroke. Proc Natl Acad Sci USA. 2004;101:12730–12735. doi: 10.1073/pnas.0404880101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Singh P, Hoffmann M, Wolk R, Shamsuzzaman AS, Somers VK. Leptin induces C-reactive protein expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:e302–e307. doi: 10.1161/ATVBAHA.107.148353. [DOI] [PubMed] [Google Scholar]
- Vecchione C, Aretini A, Maffei A, Marino G, Selvetella G, Poulet R, Trimarco V, Frati G, Lembo G. Cooperation between insulin and leptin in the modulation of vascular tone. Hypertension. 2003;42:166–170. doi: 10.1161/01.HYP.0000082806.73530.68. [DOI] [PubMed] [Google Scholar]
- Vecchione C, Maffei A, Colella S, Aretini A, Poulet R, Frati G, Gentile MT, Fratta L, Trimarco V, Trimarco B, Lembo G. Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes. 2002;51:168–173. doi: 10.2337/diabetes.51.1.168. [DOI] [PubMed] [Google Scholar]